Abstract
Eight women who were in treatment for breast cancer (n = 4) or breast cancer survivors (n = 4), presenting with 1 or more of 4 symptoms (chronic pain, fatigue, hot flashes, and sleep difficulties), were given 4 to 5 sessions of self-hypnosis training for symptom management. Analyses revealed (a) significant pre- to posttreatment decreases in pain intensity, fatigue, and sleep problems and (b) that pain intensity continued to decrease from posttreatment to 6-month follow-up. Although there was a slight increase in fatigue severity and sleep problems from posttreatment to 6-month follow-up, the follow-up scores did not return to pretreatment levels. The findings provide initial support for using hypnosis to manage symptoms in women who are breast cancer survivors. Clinical trials evaluating hypnosis efficacy over and above other treatments are warranted.
Women who are receiving or have received breast cancer treatment often report problems with a number of symptoms, including ongoing pain, fatigue, sleep difficulties, and hot flashes (Bardwell & Ancoli-Israel, 2008; Ewertz & Jensen, 2011; Minton & Stone, 2008). For example, in a recent survey study, Jensen and colleagues found that about half of a sample of breast cancer survivors reported ongoing pain that was significantly associated with physical dysfunction, psychological dysfunction, and sleep problems (Jensen et al., 2010). In a large (N = 2,645) survey of women who were within 4 years of their initial breast cancer diagnosis, Bardwell and colleagues found that 39% had elevated scores on a measure of insomnia (Bardwell et al., 2008). Minton and Stone (2008) recently completed a systematic review of studies reporting the frequency of fatigue in disease-free breast cancer survivors and found that the rates varied from 10% to close to 50%, depending on the sample and method of fatigue measurement used in each study. However, in those studies that compared breast cancer survivors with women who have not had breast cancer, women with breast cancer consistently reported fatigue with significantly greater frequency. Harris and colleagues compared the frequency of menopausal symptoms in a group of 110 breast cancer survivors with a control sample of women who did not have a history of cancer and found that the survivor group was over twice as likely (73% versus 32%) to report menopausal symptoms, and that hot flashes were by far the most common symptom reported (96% in the survivor group); although insomnia (38%) was also very common (Harris, Remington, Trentham-Dietz, Allen, & Newcomb, 2002). Although a number of medications exist that can reduce the severity of pain, fatigue, sleep difficulties, and hot flashes, many of these medications have significant adverse effects (e.g., tolerance, constipation, and sedation for opioid analgesics), and no treatment has proven effective for all patients. Thus, there is a need to identify and evaluate additional treatments for these symptoms in women with breast cancer.
Research suggests that self-hypnosis training has the potential to help women with breast cancer or a history of breast cancer reduce or manage the effects of symptoms such as pain and fatigue. For example, controlled trials demonstrate that hypnosis treatments can reduce pain in patients with many different pain conditions (Jensen et al., 2005; Patterson & Jensen, 2003), including pain reported by women with breast cancer who are in active treatment (Butler et al., 2009; Montgomery et al., 2007; Spiegel & Bloom, 1983). Hypnosis has also been shown to reduce both postsurgical pain and fatigue in women with cancer who have had breast surgery (Montgomery et al., 2007), and one case study reported significant improvement in fatigue following a hypnotic intervention in two women with breast cancer undergoing radiotherapy (Schnur & Montgomery, 2008). A randomized study comparing standard medical care to an intervention that combined cognitive-behavioral therapy and hypnosis (CBTH) to treat radiotherapy-related fatigue found that those who received the CBTH intervention did not report increases in fatigue over the course of treatment, while those who received standard care reported increases in fatigue as treatment progressed (Montgomery et al., 2009).
There is also evidence that hypnotic treatment may reduce the frequency and severity of hot flashes and may also improve sleep quality in breast cancer survivors. For example, Elkins and colleagues reported that 16 breast cancer survivors who were given four weekly sessions of hypnosis treatment targeting a decrease in hot flashes reported 59% and 70% decreases in daily and weekly hot-flash scores, respectively (Elkins, Marcus, Stearns, & Hasan Rajab, 2007). In a follow-up randomized trial comparing hypnosis treatment with standard care in 51 breast cancer survivors, Elkins and colleagues found that the hypnosis treatment resulted in significant decreases in the frequency and severity of hot flashes as well as significant improvements in sleep quality (Elkins et al., 2008). These findings are consistent with a case-series study reporting improvement in sleep quality with hypnosis treatments (e.g., Becker, 1993).
The purpose of the current case series was to further explore the possibility that training in self-hypnosis may reduce the frequency or severity of pain, fatigue, hot flashes, and sleep problems in women undergoing breast cancer treatment or who are breast cancer survivors and to estimate the effect sizes of treatment benefit. Clinicians at a university-based cancer wellness center were informed about the study and invited to refer women who might be interested in learning self-hypnosis strategies for managing any one or more of four symptoms (pain, fatigue, hot flashes, sleep difficulties) to the study. Measures assessing primary (i.e., the four targeted symptoms for those participants presenting with the symptom) and secondary (depression, anxiety, multiple-symptom reporting) were assessed at pretreatment, posttreatment, and at 1-, 3-, and 6-month follow-up. Frequency of use of self-hypnosis was also assessed at the 6-month follow-up assessment point. The primary hypothesis was that there would be significant pre- to posttreatment reductions in the four primary outcome variables in those participants reporting problems with the symptom being examined. In addition, we planned exploratory tests to determine if treatment was associated with improvements in the secondary outcome variables, and if any improvements found would maintain for up to 6 months following treatment.
Method
Participants
Eight women receiving treatment for breast cancer or who were breast cancer survivors were referred to the study and provided written consent to participate. The University of Washington Institutional Review Board approved the study. Five of the study participants presented with ongoing bothersome pain, 5 with fatigue, 5 with sleep difficulties, and 2 with hot flashes. Pretreatment, posttreatment, and 1-, 3-, and 6-month follow-up data were available for all 8 participants.
The average age of the study participants was 58.5 years (SD = 7.5 years; range = 46–65). Two of the participants had some college, and the other 6 were college graduates. All of the participants were white. Six were married, 1 was divorced, and another reported that she was single and had never been married. Four of the participants were in active treatment for cancer, and the other 4 were breast cancer survivors who had completed treatment. The participants reported having had surgery (n = 8), radiation therapy (n = 7), chemotherapy (n = 5), and endocrine (anti-estrogen) therapy (n = 3) to treat their cancer. Of the 4 participants who were not receiving cancer treatment during the course of this study, 2 had not received any treatment for at least 6 months, and 2 had not received any treatment for at least 2 years. Four of the participants reported that they were post-menopausal, 3 reported that they were going through menopause at the time of the study, and 1 reported that she had not yet gone through menopause.
Intervention Protocol
The intervention protocol consisted of four to five sessions of self-hypnosis training in symptom management, usually administered weekly but sometimes less frequently, depending upon the availability and desires of the participants. We allowed for variability in the number of treatment sessions depending on what both the clinician and participant thought was needed; 6 participants had four treatment sessions and 2 participants had five. All of the sessions were based on a standardized protocol used in previous self-hypnosis training clinical trials (Jensen et al., 2008; Jensen, Barber, Romano, Hanley, et al., 2009; Jensen, Barber, Romano, Molton, et al., 2009; Jensen et al., 2005) but modified for the population and symptoms that were the focus of this study. Modifications included: (a) inclusion of a script that had suggestions for fatigue management for those participants presenting with fatigue (Jensen, 2011); (b) inclusion of a script that had suggestions to reduce the frequency and severity of hot flashes based in large part on the script used by Elkins and colleagues in their randomized trial (Elkins et al., 2008) for those participants presenting with hot flashes; (c) inclusion of a script that (1) provided information and recommendations regarding basic sleep hygiene, (2) provided information in the use of self-hypnotic strategies for getting to sleep at night, and (3) had suggestions for increased ability to get to sleep and for experiencing a deep and restful sleep at night (Jensen, 2011). All treatment was provided by the first author (MPJ).
Prehypnosis Cue, Audio Recording of Sessions, and Induction
All of the hypnotic sessions were preceded by a cue (“Take a deep breath … hold it … hold it for a moment … and let it out. Notice how comfortable that feels …”) and were audio recorded. The audio recordings of the sessions were burned onto CDs and given to the participants. Participants were encouraged to listen to at least one audio recording of the sessions on a daily basis during treatment and as often as they wished following treatment. They were encouraged to listen to the most recent hypnosis session recording that they were given; although they were also allowed to listen to whichever session they chose. Because each session began with a cue, it became linked via classical conditioning to the participants’ responses to the hypnotic induction and suggestions each time they listened to a recording. Participants were also instructed to practice self-hypnosis regularly throughout the day without the CDs, even for just a few minutes at a time, and to begin each of these practice sessions with the self-hypnosis cue to further enhance any associations between the cue and response to hypnosis. Following the cue, a standardized hypnotic induction was provided that began with suggestions for perceived relaxation in specific muscle groups followed by suggestions for experiencing being in a “special place” (Jensen, 2011). The induction took about 10 minutes.
The hypnotic suggestions that followed the induction depended on the participants’ symptom presentation. If they presented with only one of the target symptoms, standardized suggestions for either or both (a) reductions in that symptom (e.g., hypnotic analgesia for pain, decrease in the frequency of hot flashes for participants presenting with hot flashes) or (b) increases in experiences or symptoms inconsistent with the symptom (e.g., comfort for pain, ability to easily fall asleep for sleep difficulties) were given (see below for examples of the specific suggestions used). These suggestions were repeated at each of the subsequent sessions. If any participant presented with more than one of the four target symptoms, she was asked to select the symptom to target during each treatment sessions (e.g., the participant could choose to hear suggestions managing fatigue in the first session, managing pain in the second, managing sleep in the third, and then managing pain again in the final session; the symptom targeted at each session was up to the participant). All of the suggestions for symptom improvement were standardized and script-driven in order to enhance replicability in the event that a clinical trial testing for the efficacy of the treatment was deemed warranted.
Pain Management Suggestions
The scripts used to provide pain management suggestions were based on those used in previously completed clinical trials of self-hypnosis training for pain management (Jensen, 2011; Jensen, Barber, Romano, Hanley, et al., 2009; Jensen, Barber, Romano, Molton, et al., 2009; Jensen, et al., 2005). They included suggestions for (a) decreased pain, (b) deep relaxation, (c) hypnotic analgesia, (d) decreased pain unpleasantness, and (e) sensory substitution. The pain management scripts used in this study have been previously published (Jensen, 2011; Jensen, Barber, Romano, Hanley, et al., 2009; Jensen, Barber, Romano, Molton, et al., 2009).
Fatigue Management Suggestions
The script used for fatigue management included suggestions to be aware of the natural cycles of feeling energy after periods of rest and reframing feelings of fatigue as feelings of “rest” and relaxation (Jensen, 2011). Key portions of this script are presented below.
And you can remember that the natural state of the body is to feel energized after periods of rest, and to let you know when it is an appropriate time to rest, and then after you rest, to feel energized again. A natural pattern and rhythm … rest … energy … rest … energy. You know this so well … it is how your mind and body have worked since even before you were born. What might be useful to remember … and you may find this very useful indeed … is that you can allow yourself periods of rest when it is appropriate … and then after these periods of rest you can really experience a sense of energy … a sense of being able to accomplish what it is you want to accomplish. … In fact, you have been resting for a number of minutes already today … just now … so that you can know that when you are done with this session, you can wake up and feel rested and energized. … Just feel good. … And then, when it is time for you take a brief rest, you can allow yourself to do so … it need not be a long rest … just enough to let your body and mind pull together the resources to feel energetic again … maybe 5 minutes, maybe even just 1 minute. You can find a comfortable place … take a nice deep breath and hold it … hold it … and let it go … close your eyes and just let your mind and body rest … and when you wake up you will feel rested and energized. … This is called pacing … only going for as long as is appropriate … and giving yourself brief periods of rest so that when you are awake … you feel energized … fatigue becomes a thing of the past … you can focus your body and mind … and when you need to feel more energy … just take a nice, brief, rest. It might be twice a day on some days … it might be every hour on other days … only you really know what is best for you … but you can pace yourself with periods of rest followed by periods of feeling so energetic … so that when you are awake, you feel energized, alert, focused … and the periods of rest … when you use them … feel so restful … relaxing … all of the time you feel good. … Sometimes relaxed … sometimes energized … as you and your body need … and take a moment now, to picture yourself … sometime in the future … pacing yourself well … resting … and energized … resting … and energized … always feeling good. Really picture yourself … and then bring those skills back … and use them, now, and every day … so you can feel awake and full of energy when you wish.
Hot Flashes Management Suggestions
The script used for hot flash management was as adapted from the intervention used by Elkins and colleagues in their randomized trial of hypnosis for hot flash management (Elkins et al., 2008). Portions of the transcript used in the present study for managing hot flashes are presented below.
As you are learning to use hypnosis to remain more comfortable and relaxed everyday … and here finding a coolness and comfort. Now going to a place where it is cool and so comfortable … finding that you are in the mountains [Note: Individualized mental imagery for relaxation and coolness may be inserted here and used instead of mountain imagery depending on the patient preference. The same phrases for coolness should be used, however.] … It is cool here, in fact there is snow all around. … The air is very cool and it is pleasant to notice the white snow on the trees and the ground. … You might want to take a deep breath of the crisp, cool air … and feeling cool waves of comfort flowing over you and through you … and feel more refreshed. … It is the kind of day when the cool air and cold snow feels very good. … This is a very beautiful place and perhaps you can see a lake in the distance … there is a path before you and you might enjoy walking down that mountain path.
With every step you take, feeling the cool air, the fresh cool feeling of feeling the air on your face, your forehead, and a gentle breeze across your ears. It is just so pleasant there and just notice the coolness while standing at the top of this snow-covered mountain … cool waves of comfort flowing over you and through you … and while you are there it is possible that your mind could drift to other times when you have felt such a coolness and comfort. Perhaps the coolness of standing in front of an air conditioner … feeling the cold air … or the coolness one could experience when opening a refrigerator or having a cool drink of ice water when one is really thirsty and feeling the cool feeling of the water that is clear and clean and so refreshing.
Sleep Management
The script used for improved sleep quality began with a script providing basic information about the associations between brain activity and sleep, in order to establish the rationale for using self-hypnotic strategies for altering brain activity, and hence influencing sleep (Jensen, 2011). A portion of the script that presents this information is presented below.
You may be aware that during a good night’s sleep the brain goes through different stages. First, when you are awake, your brain is quite active. Your brain cells are firing and talking with each other; back and forth. [Note: Some patients may need to be told what a brain cell is.] … As a person starts to become relaxed before going to sleep, fewer brain cells in the brain are “firing.” The brain starts to “calm down” and you start to feel more relaxed. … From this stage, people usually first drift into a light sleep. … Soon, people usually move … into a deeper stage of sleep. … Once the very slow brain activity reaches 50% or more, the person has entered the deepest stage of sleep, … [then] … the brain starts to become active again. … Each stage of sleep seems to be important to our health. …. Another fact to be aware of is that people need different amounts of sleep. … Also, our need for sleep seems to decrease a little as we get older. … Another thing that is not necessarily a problem is waking up at night. We all start to wake up more at night as we get older. The important thing is if you are able to get back to sleep when you do wake up, and if you feel rested in the morning.
So it is important to understand that it is fine to need less sleep, and to wake up at night. It is also important to understand that your sleep depends on the activity in your brain. And here is what is interesting: you can control the activity in your brain using the self-hypnosis skills that you have been learning. What do you think is happening in your brain during the inductions that we do in the clinic, and that you are practicing at home? [Let the patient respond. If the patient says that the brain “slows down” during hypnosis, agree enthusiastically (“That’s right!”). If the patient is not sure or does not say something about the brain becoming more relaxed or slowing activity, proceed to explain what is known about brain activity during hypnosis.]
During hypnosis, and in response to hypnotic inductions, the brain starts to slow down, much like it does just before a person falls asleep. … During hypnosis, the brain is not asleep; it is just in a state that is very similar to that stage just prior to falling asleep. There is much more slow activity.
Next, basic sleep hygiene information was presented, because improving sleep hygiene is associated with improved sleep (Jensen, 2011). The sleep hygiene information that was covered included activities helpful for (a) preparing the body for sleep (avoid drugs that interfere with sleep, avoid daytime naps, exercise regularly, wake up at the same time every day, etc.); (b) preparing the mind for sleep (develop a presleep ritual, use bed only for sleep and sex, use self-hypnosis strategies to facilitate drifting off to sleep); and (c) preparing the bedroom for sleep (sleep in a cool room with warm blankets, turn any clock to the wall, etc.).
Patients were then told that once they were in bed and ready to go to sleep, they could use their favorite hypnotic induction (e.g., relaxation induction, “safe place” induction; see Jensen, 2011) to facilitate a brain state that is “similar to the pattern of activity the brain shows right before it drifts to sleep” (e.g., Crawford, 1990) or use either of two additional strategies for facilitating the transition from being awake to being asleep. The first of these is to utilize the natural processes that occur as one relaxes into sleep (muscle “twitches” and hypnogogic images) by simply noticing them when they occur. The “twitches” can be counted as they occur, and patients told that as they start to drift into sleep they will lose count, so they can note this and start the count over. Patients who would prefer to pay attention to hypnogogic images can do so with a suggestion that they can become absorbed by these images.
The second additional strategy is a variant of the “3-2-1” technique (Enqvist, personal communication, April 7, 2006). This technique is similar to the relaxation or “safe place” induction in that it gives the patient something to focus on that is inconsistent with thinking, planning, or feeling anxious—all of which are associated with brain activity that is inconsistent with drifting off into sleep. A script presenting the instructions for this technique follows.
The goal of the 3-2-1 technique is the same as the other self-hypnotic inductions: to give you something interesting to focus your attention on and experience as your mind slows down. Here is how it works. First, just listen for three things. Any three things that you hear: the noise of your breathing—one; or maybe a sound of a far-off airplane—two; or maybe the sound of your skin against the sheet—three. Any three things at all. They can even be the same thing. Just listen for, hear, and then count three things. It’s that simple.
Next, feel three things. Any three things. The feeling of the sheet against the skin—one. An interesting tingling sensation in the limbs—two. Cool or maybe warm air on the face—three. It does not matter what they are. Any three things will do. They can be different or the same. Just feel them and count them, 1, 2, 3.
And then, see three things. Allow three images to come into the mind. Just let them appear, on their own. A rose—one. A blue sky—two. Some third image; it does matter what it is, maybe a beach—three. Any three images. Then, after you have seen the third thing, go back, and hear two things, and count them in the mind. Then feel two things. Then see two things. Then hear one thing, feel one thing, and see one thing.
And then start again. Hear three things, feel three things, see three things. Then hear two things, feel two things, see two things. Then hear, feel, and see one thing. And back to three.
As the mind is experiencing what it hears, feels, and sees, as it starts to drift to sleep, you will likely lose count. That is fine; just start over. Hear, feel, and see three things. Hear, feel, and see two things. Hear, feel, and see one thing. You can use this strategy and discover what interesting things you can experience as you drift into a deep, restful sleep.
Additional Suggestions
Following the suggestions given for symptom management, additional nonstandardized suggestions were given that were specifically tailored for each participant. These suggestions were added to the protocol in order to both (a) increase each participant’s interest and investment in the procedures even further and (b) take advantage of the opportunity for the hypnotic sessions to help the participants achieve additional personal goals that were not necessarily directly related to the four targeted symptoms. The specific suggestions made during this portion of the session were created by the clinician (i.e., they were not scripted) based on that session’s pretreatment discussion with the participant regarding the goals of the session. Examples of the goals selected by the participants for these suggestions were increased confidence, increased sense of calm, and increased focus on valued goals and activities.
Self-Hypnosis and Posthypnotic Suggestions
Following the last hypnotic suggestion of each session, participants were given posthypnotic suggestions to extend the benefits of treatment beyond the treatment sessions (Jensen, 2011). These included suggestions for (a) an ability to practice and easily experience self-hypnosis again outside of the treatment sessions, (b) an increased ability to experience both hypnosis and symptom improvement over time and with practice, and (c) the extension of the benefits of hypnosis and self-hypnosis beyond the treatment and practice sessions.
Measures and Assessment Procedures
Primary Outcome Measures
The primary outcome measures of this study assessed the four target symptoms: pain intensity, fatigue, hot flash frequency, and sleep quality. All outcome measures were assessed by a research study assistant (i.e., not the clinician, to reduce the chances that a desire to please the clinician might affect the outcome ratings) via telephone interviews. Pain intensity was assessed by asking participants to rate their least, worst, and average pain over the past week on 0–10 Numerical Rating Scales (NRS; 0 = No pain to 10 = Pain as intense as you can imagine). These scores were then averaged into a single composite score representing characteristic pain. The use of such composite measures of pain ratings has been recommended as a way to increase reliability in pain clinical trials with limited power due to low sample sizes (Jensen, Turner, Romano, & Fisher, 1999). Self-report of pain intensity is recognized by experts as the most appropriate primary outcome measure in most analgesic clinical trials (Turk et al., 2003). In addition, the 0–10 NRS has been recommended as a useful measure of this pain domain because of (a) strong evidence for its validity as evidenced by its strong association with other measures of pain intensity and responsivity to analgesic treatment, (b) understandability and ease of use, and (c) ease of administration and scoring (Jensen, 2010).
Fatigue was measured using 10 items from the Patient Reported Outcomes Measurement Information System (PROMIS) fatigue item bank (Cella et al., 2010). A strength of all the PROMIS item banks, including the 10 fatigue items administered in this study, is that any combination of items can be transformed into a T score (mean of 50, standard deviation of 10) that is anchored to the mean levels of the domain found in a healthy U.S. general population (Cella et al., 2010). Thus, a patient with a score of 70 (or a sample mean with an average score of 70) is reporting a level of fatigue that is two standard deviations above the mean of the PROMIS healthy normative U.S. sample.
Hot flash frequency was measured using an adaptation of a daily diary assessing hot flash frequency and severity used in a clinical trial, and that demonstrated responsivity to treatment in that trial (Goodwin et al., 2008). The diary begins with a very detailed operational definition of mild, moderate, severe, and very severe hot flashes, describing each in terms of duration (e.g., for very severe, “Lasting up to 45 minutes”), physical symptoms (e.g., for moderate, “Head, neck, ears, or whole body felt warm; tense, tight muscles; clammy [wet skin]; a change in heart rate or rhythm [heart speeds up to changes beat]; some sweating; dry mouth”), emotional symptoms (e.g., for severe, “Embarrassment, anxiety, feelings of having a panic attack”), and action needed (e.g., for mild, “Usually no action is needed”). Participants are then asked to indicate the number of each category of hot flash that she experienced in the past 24 hours. However, because the procedures of the current study allowed for only a single assessment, 7-days’ worth of diary ratings could not be obtained. Therefore, the diary measure was modified to allow participants to provide estimates of the number of each category of hot flash she experienced in the past 7 days. The total number of hot flashes reported in the past week was used as the primary measure of this outcome domain.
Sleep quality was assessed using the 6-item Medical Outcomes Study (MOS) Sleep Problem Index–I (Hays & Stewart, 1992). This measure contains items that ask about difficulties with getting to sleep, getting enough sleep to feel rested, awakening short of breath or with a headache, awakening and having difficulty falling back to sleep, staying awake during the day, and getting the amount of needed sleep. The Sleep Problem Index has demonstrated adequate internal consistency (Cronbach’s alpha = .78) and has shown a strong association (r = .97) with the nine-item MOS Sleep Problem Index–II (Hays & Stewart, 1992), which has shown validity as a measure of sleep quality through its responsivity to treatment in numerous clinical trials (e.g., Rudwaleit et al., 2011; Russell et al., 2009). The six-item version of this scale was administered in the current study in an effort to minimize assessment burden.
Secondary Outcome Measures
Evidence indicates that hypnotic treatments can have benefits beyond those that are specifically targeted by the hypnotic suggestions (Jensen et al., 2006). In order to determine if the hypnotic intervention examined in this study had such additional benefits on pain interference (for those reporting pain as a problem) and for depression, anxiety, or general symptom reporting for all of the participants, we assessed each of these domains at each assessment point.
To assess pain interference, we administered a modified version of the seven-item Interference Scale of the Brief Pain Inventory (BPI; Daut, Cleeland, & Flanery, 1983). The original BPI Interference Scale asks respondents to indicate the extent that pain interferes with seven basic activities (general activity, mood, walking, normal work, relations with other people, sleep, and enjoyment of life) on 0 to 10 scales (0 = Does not interfere and 10 = Completely interferes). The walking item has been modified for populations of individuals who are disabled and who do not necessarily ambulate to assess the extent to which pain interferes with “mobility (ability to get around).” The modified version of the BPI Interference Scale has strong evidence for its validity and reliability in samples of individuals with physical disabilities (Osborne, Raichle, Jensen, Ehde, & Kraft, 2006; Raichle, Osborne, Jensen, & Cardenas, 2006).
We administered the Patient Health Questionnaire Depression Scale (PHQ–9) to assess depressive symptoms (Kroenke et al., 2009). The PHQ–9 items match the nine Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM–IV; American Psychiatric Association, 1994) symptoms that make up the diagnostic criteria for major depression, and each one is rated on a 4-point frequency scale (0 = Not at all, 3 = Nearly every day) over the past two weeks. A great deal of research supports the validity and reliability of the PHQ–9 in many patient populations (Kroenke et al., 2010), including populations of patients with cancer (Thekkumpurath et al., 2011). The PHQ–9 can vary from 0 to 27, with cutoff points of 5, 10, 15, and 20 representing mild, moderate, moderately severe, and severe depression, respectively.
Anxiety was assessed using the Generalized Anxiety Disorder–7 (GAD–7; Spitzer, Kroenke, Williams, & Lowe, 2006). The GAD–7 assesses the frequency of general anxiety symptoms (feeling nervous, unable to stop worrying, worrying too much, trouble relaxing, restlessness, irritability, feeling afraid) on 4-point Likert scales (0 = Not at all to 3 = Nearly every day) over the past week. Cutoffs for the 0–21 range of the scale are 5, 10, and 15 for mild, moderate, and severe anxiety, respectively (Spitzer et al., 2006). A great deal of research supports the validity and reliability of the GAD-7 and includes support for its responsivity to treatments that reduce anxiety (Kroenke et al., 2010).
General symptom reporting was assessed using the Patient Health Questionnaire Somatic Scale (PHQ–15; Kroenke, Spitzer, & Williams, 2002). This measure asks respondents to rate the extent to which they have been bothered (on a 3-point scale from 0 = Not bothered to 3 = Bothered a lot) by 15 different symptoms (e.g., stomach pain, back pain, dizziness, shortness of breath) in the past week. The score can range from 0 to 30, and cutoff points of 5, 10 and 15 are used to represent mild, moderate, and severe somatic symptoms, respectively. Research supports the validity of the PHQ–15 for identifying patients with somatoform disorder and for detecting change in somatization in clinical trials (Kroenke et al., 2010).
Frequency of Self-Hypnosis Practice
Finally, as a behavioral measure of perceived helpfulness of treatment, at the 6-month follow-up point, participants were asked to indicate how frequently they (a) listened to the CDs provided with treatment and (b) practiced self-hypnosis on their own without the CDs over the past 30 days.
Data Analysis
The primary study hypothesis (that there would be a significant reduction in the symptoms targeted by the hypnotic suggestions) could be tested for the measures of pain intensity, fatigue, and sleep problems, as there were enough participants presenting with these problems (n = 5 each) to allow us to estimate these effects. Because data were available at all time points for all eight participants, we used a series of repeated measures analyses of variance (ANOVAs) to test for the effects of treatment, followed by univariate tests of the scores between each time point if a significant omnibus time effect emerged from these analyses. In each case, we limited the analysis sample to those participants presenting with the targeted symptoms. Because only two participants reported problems with hot flashes, descriptive analyses were performed to understand the changes, if any, reported in hot flash frequency for these participants. ANOVAs followed by univariate tests were also used to test for any changes in the secondary outcome variables of pain interference, depression, anxiety, and general symptom reporting. Data from the five participants reporting pain difficulties were used for the analyses examining pain interference, and data on all eight study participants were used in the analyses examining depression, anxiety, and general symptom reporting. Descriptive analyses were used to examine the frequency of self-hypnosis practice at the 6-month follow-up point.
Results
The means and standard deviations for the primary and secondary outcome variables assessed at each assessment point (pretreatment, posttreatment, 1-, 3- and 6-month follow-up) are presented in Table 1. The effect sizes (Cohen’s d) for pre- to posttreatment decreases in outcome ranged from 1.47 (for characteristic pain intensity) to 2.05 (for fatigue). Pretreatment to 6-month follow-up effect sizes were even larger for pain intensity (4.32) but decreased some, relative to the pre-to posttreatment effect sizes, for both fatigue (1.03) and sleep quality (1.05), reflecting continued improvement (decreases) in pain but some return towards pretreatment levels for both fatigue and sleep. However, even the pretreatment to follow-up effect sizes were greater than the cutoff suggested by Cohen (1988) as indicating a “large” effect.
Table 1.
Means and Standard Deviations for the Primary and Secondary Outcome Variables at Pretreatment, Posttreatment, 1-Month, 3-Month, and 6-Month Follow-Up
| Outcome Variable (Measure) | n | Pretreatment Mean (SD) |
Posttreatment Mean (SD) |
1-Month Follow-up Mean (SD) |
3-Month Follow-up Mean (SD) |
6-Month Follow-up Mean (SD) |
F (df) for time effect | Pre-post effect size (Cohen’s d) |
|---|---|---|---|---|---|---|---|---|
| Primary outcome variables | ||||||||
| Characteristic pain | 5 | 5.13a (0.77) | 4.00b (0.47) | 2.73bc (1.71) | 2.53cd (1.26) | 1.80bd (1.79) | 6.44* | 1.47 |
| Fatigue (PROMIS) | 5 | 61.93a (5.60) | 50.46b (7.00) | 51.82ab (10.35) | 61.45ab (9.24) | 56.15ab (8.36) | 6.75* | 2.05 |
| Sleep problems (MOS) | 5 | 50.00a (12.69) | 30.67bc (11.16) | 29.33bc (13.62) | 38.67ab (22.44) | 36.67ab (17.00) | 5.85* | 1.52 |
| Secondary outcome variables | ||||||||
| Pain interference (BPI) | 5 | 3.34a (3.12) | 1.14a (1.19) | 1.37a (1.33) | 0.69a (0.72) | 0.94a (0.91) | 2.71† | 0.71 |
| Depression (PHQ-9) | 8 | 6.25a (3.38) | 3.63b (2.33) | 4.38ab (3.70) | 6.63a (4.32) | 4.88ab (3.23) | 2.07 | 0.78 |
| Anxiety (GAD-7) | 8 | 6.38ab (3.81) | 4.63ac (5.42) | 4.88a (4.88) | 7.75bc (4.46) | 4.13c (4.36) | 2.50† | 0.46 |
| Symptom frequency | 8 | 8.25a (3.43) | 6.63ab (3.33) | 5.50b (2.56) | 6.88ab (2.30) | 5.75ab (3.01) | 1.52 | 0.47 |
Note. F values reported are associated with a time effect, and effect sizes reported are associated with the pre- to posttreatment change in outcome.
Means with different subscripts are significantly (p < .05) different from one another. BPI = Brief Pain Inventory; MOS = Medical Outcome study, PHQ-9 = Patient Health Questionnaire-9 depression scale; GAD-7 = Generalized Anxiety Disorder-7 scale.
An effect size (d) that is 0.80 is considered large, effect sizes of 0.50 are considered medium, and those of 0.20 are considered small (Cohen, 1988).
p < .10.
p < .01.
Figures 1, 2, 3, and 4 present graphs of the primary outcome variable scores at each assessment point for each individual participant who reported problems with pain, fatigue, sleep, and hot flashes at baseline, respectively. These graphs make it possible to evaluate the differences in the participants’ patterns of response to treatment that cannot be seen by examining the average scores alone. The immediate pre- to posttreatment decreases in pain intensity for all five participants presenting with pain can be seen, with continued improvements in pain from posttreatment to 1-month posttreatment for 4 of the 5 participants. More variability in changes in pain can be seen after both the 1-month (e.g., 1 participant’s pain decreased, 3 stayed the same, and 1 increased), and 3-month follow-ups; although at the 6-month assessment, each of the participants’ pain scores were less than they were at baseline, and 2 of the participants reported that they were experiencing no pain at this follow-up point.
Figure 1.
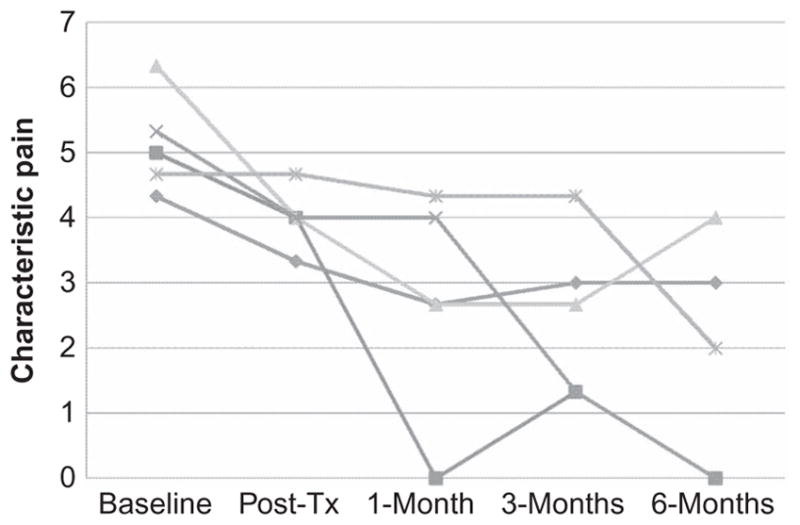
Characteristic pain intensity scores at each assessment point for the five participants who presented with pain as a problem.
Figure 2.
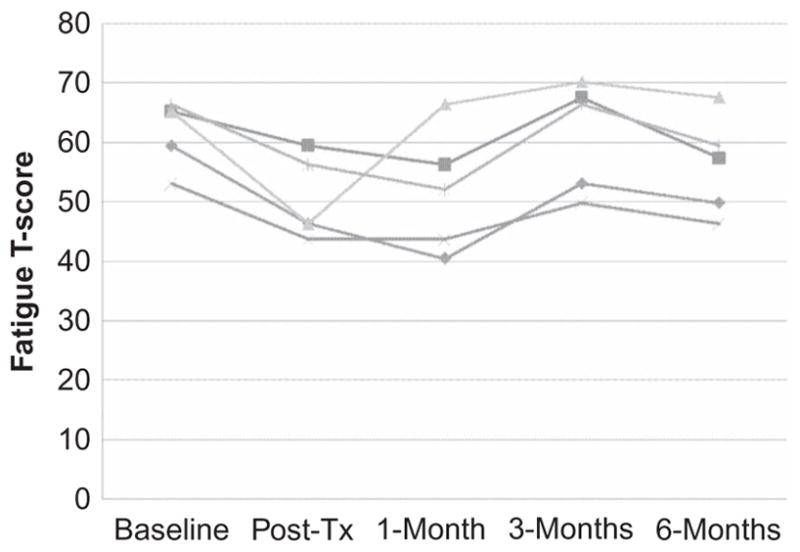
PROMIS Fatigue item bank T scores at each assessment for the five participants who presented with fatigue as a problem.
Figure 3.
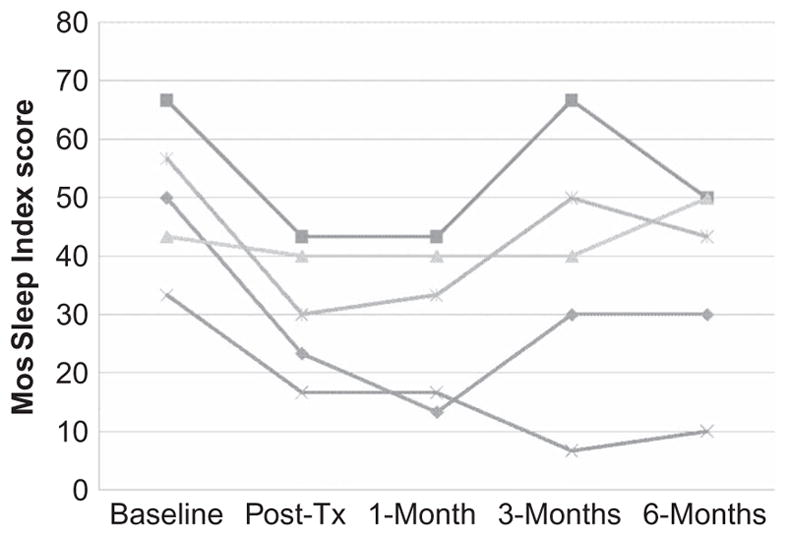
MOS Sleep Problem Index scores at each assessment point for the five participants who presented with sleep problems at baseline.
Figure 4.
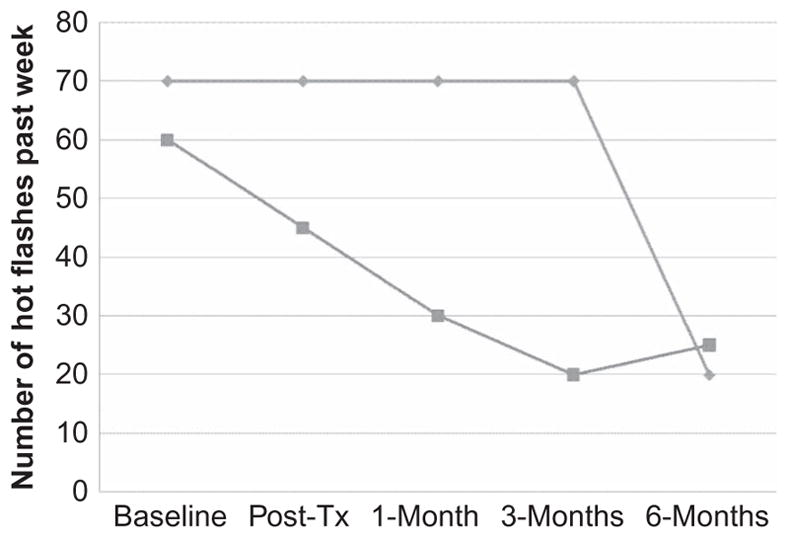
Number of hot flashes during the past week at each assessment point for the two participants who presented with hot flashes as a problem.
With respect to fatigue, the participants showed a similar pattern of responding (see Figure 2). They all reported pre- to posttreatment improvements that continued to the 1-month follow-up point. There was a return to pretreatment fatigue levels for these participants at the 3-month assessment point for three of the participants, but all participants reported improvements in fatigue from the 3- to the 6-month assessment point. One participant reported a marked decrease in fatigue pre- to posttreatment that returned to pretreatment levels by the 1-month follow-up point and then generally stayed the same through the 6-month assessment point.
The pattern of improvements in sleep appeared more similar to those for pain intensity than to those for fatigue. There was a decrease in sleep problems for all 5 of the participants presenting with sleep difficulties from pretreatment to posttreatment; although the improvements for one of the participants was very modest. These improvements maintained for 4 of the participants and sleep improved for the fifth participant to the 1-month assessment point. Like pain, there was more variability in sleep quality scores following the 1-month follow-up point, with 3 participants reporting an increase in sleep problems (2 of these reached or almost reached pretreatment levels at the 3-month follow-up), 1 reporting little change, and 1 reporting continued improvement. This variability continued to the 6-month assessment, with 3 reporting improvements and 2 reporting a worsening in sleep problems. Even with this variability, however, 4 of the 5 participants reported fewer sleep difficulties at the 6-month follow-up assessment than they did at pretreatment.
Of the 2 participants presenting with problems with hot flashes, 1 reported no changes in the number of hot flashes in the last week from pre- to posttreatment (both frequencies were 70), while the other reported a 25% decrease in the frequency of hot flashes (from 60 to 45; see Figure 4). The lack of change in hot flashes for the first participant maintained through the 3-month follow-up point, but there was a substantial decrease (to only 20 per week) at the 6-month follow-up for this participant. For the participant who reported a pre- to posttreatment decrease in the number of hot flashes in the past week, this improvement continued through the 1-month (30 hot flashes in the last week) and 3-month (20 hot flashes) assessment points and then was maintained at 6-month (25 hot flashes) follow-up.
No statistically significant time effects emerged for any of the secondary outcome variables, although there was a nonsignificant trend (p < .10) for improvements in pain interference and anxiety over time (see Table 1). The absolute decrease in pain interference (from 3.34/10 at baseline to 1.14/10 at posttreatment, and maintaining between 0.69 and 1.37 through the 6-month follow-up) was substantial, but significant differences in pain interference scores between each assessment point did not emerge due to the high variability in these scores and the limited power to detect such effects due to the low sample size. The pre- to posttreatment effect sizes for improvement in the secondary outcome measures were in the moderate range (Cohen’s d = 0.46 and 0.47) for anxiety and general symptom reporting, and in the moderate to large range (Cohen’s d = 0.71 and 0.78) for depression and pain interference.
At 6 months, only 1 participant reported that she was still listening to one of the CDs provided during treatment. She reported that she usually listened to the CD twice a day. This participant presented at baseline with problems with both sleep and fatigue (but not pain or hot flashes). She did not show the largest decreases in fatigue (pretreatment to 6-month follow-up PROMIS fatigue scores were 66.44 and 59.53) or improvements in sleep quality (MOS Sleep Index scores of 56.67 to 43.33), but she did improve about one standard deviation unit (relative to this sample’s baseline standard deviation; about one half a standard deviation unit relative to normative population standard deviations for the PROMIS fatigue scale) in both outcomes. However, even though only 1 participant reported that she continued to listen to the CDs of the treatment sessions, all eight of the participants reported that they continued to practice self-hypnosis on their own without a CD. The number of days they practiced self-hypnosis in the past 30 days ranged from 2 to 24, and the number of times they practiced on the days that they practiced was either once (n = 4 participants) or twice (n = 4 participants).
Discussion
Primary Hypothesis
The primary hypothesis was supported for the three outcome variables that could be tested, with statistically significant pre- to posttreatment decreases in characteristic pain, fatigue, and sleep problems and with effect sizes in the large range for all of these outcomes. Moreover, on average, characteristic pain intensity continued to improve through the 6-month posttreatment assessment point. Although the measures of fatigue and sleep problems regressed towards pretreatment values from the 1-month to the 3-month assessment point, there was improvement in both outcome measures from 3-months to 6-months. The scores for sleep problems and fatigue at the last follow-up point were below the baseline scores and the effect sizes for the pretreatment to 6-month follow-up differences in pain, fatigue, and sleep problems were all large. However, the 6-month follow-up scores for fatigue and sleep problems were not statistically significantly different from the baseline scores, perhaps due to low power associated with the small sample size.
The improvement in the frequency of hot flashes could not be evaluated statistically, given that only two participants presented with problems with hot flashes. However, one of the two participants presenting with problems with hot flashes reported a 25% reduction in the number of hot flashes from pre- to posttreatment, which decreased another 25% from baseline levels at the 1-month assessment point, and this reduction was maintained at the 3- and 6-month assessment points. This finding is promising and consistent with the preliminary studies by Elkins suggesting that hypnosis may effectively reduce the frequency of hot flashes for some women (Elkins et al., 2008).
Secondary Hypothesis
The lack of significant effects on the secondary outcome variables that were not specifically targeted by the hypnotic suggestions is consistent with the possibility that hypnosis’ effects may depend, at least in part, on the specific hypnotic suggestions made; that is, hypnosis is more likely to benefit those symptoms that are addressed in the hypnotic suggestions than those symptoms that are not addressed. However, there was general (even if not statistically significant) improvement in all of the secondary outcome variables from pre- to posttreatment. This improvement could reflect either indirect effects (e.g., improvements in pain intensity may then be reflected by subsequent improvements in pain interference and distress) or possible direct, but less strong, effects of the hypnosis treatment on these variables. Future research with larger samples could determine if the improvements in these secondary outcome variables are reliable and, if so, could determine the extent to which they might be mediated by improvements in the targeted outcome variables.
Individual Patterns of Response
No 2 participants evidenced the same pattern of response to treatment. The greatest divergence in patterns between participants emerged after the 1-month follow-up point, when some showed a return to pretreatment levels for some of the outcome measures, some evidenced continued improvement, and some maintained the pretreatment to 1-month follow-up treatment gains they made. This finding is consistent with those from hypnosis clinical trials, which also show variability in how individuals respond to hypnotic treatment (e.g., Jensen, Barber, Romano, Hanley, et al., 2009; Jensen, Barber, Romano, Molton, et al., 2009). These findings also underscore the need to be flexible clinically and to not assume that early improvement necessarily means that the patient will maintain gains, or that a lack of treatment response early in treatment necessarily means that the patient will not benefit from hypnosis treatment in the long term.
Self-Hypnosis Practice
The fact that all of the participants in this study reported that they continued to practice self-hypnosis (even if, for 1 participant, on just 2 days out of the past 30) provides some evidence that they valued the skills they had learned. This result is consistent with the findings from previous research performed by our group, indicating ongoing use of self-hypnosis strategies by most individuals once they are learned (Jensen, Barber, Romano, Hanley, et al., 2009; Jensen, Barber, Romano, Molton, et al., 2009). The 1 participant who reported she practiced and listened to the audio recordings of the sessions regularly at 6-month posttreatment did not report the largest improvements in outcomes, but she was among those who appeared to benefit from treatment. It would be interesting in future research, using data from larger samples, to determine the extent to which frequency of self-hypnosis practice is associated with continued improvements or maintenance of treatment gains.
Limitations
There are a number of limitations of the current study. Primary among them is the lack of a treatment condition that controls for the effects of time, participation in a clinical trial, and expectancy effects. Other recently published trials that have included such control conditions have demonstrated the efficacy of self-hypnosis training on pain outcomes over and above the effects of these alternative explanations for treatment outcome, such as regression to the mean, therapist attention, and patient outcome expectancies (Jensen, Barber, Romano, Hanley, et al., 2009; Jensen, Barber, Romano, Molton, et al., 2009). However, that does not mean that the hypnotic intervention used in this study is necessarily specifically effective in women who are in breast cancer treatment or who are breast cancer survivors. Such a determination requires a randomized controlled trial.
A second major limitation was the small sample size. Only 2 participants presented with hot flashes, which did not allow us to perform statistical tests for differences in this outcome. Moreover, only 5 participants were available to test for pre- to posttreatment changes in the three other primary outcome variables and one of the secondary outcome variables, and only 8 participants were available to test for the effects of treatment on three of the secondary outcome variables. The small sample size severely limits our ability to detect treatment effects, especially on outcome variables that had medium and medium-to-large effect sizes but that may nonetheless represent meaningful change. Small sample sizes can also result in unreliable statistics, as they can be very sensitive to the effects of individual participants and outliers. A visual inspection of the individual outcome scores at each assessment point (Figures 1–4) does not indicate that any participant in this study was a large outlier. However, future research using larger samples is needed to help determine the reliability of the current findings.
Another limitation is the low pretreatment scores on all of the secondary outcome measures. The average pretreatment PBI Interference score (M = 3.34), PHQ–9 Depression score (M = 6.25), GAD–7 Anxiety score (M = 6.38), and PHQ–15 Somatization score (M = 8.25) were all in the mild range for these scales. This may have resulted in a floor effect, which limits the ability to detect treatment effects. Also, the study included women who were receiving treatment for breast cancer or who were breast cancer survivors. The results might not generalize to individuals who have a history of other types of cancer.
Finally, there are a number of factors that could potentially impact the outcomes assessed that were not measured or controlled for in the analyses. These include medications that are used for symptom management as well as medications commonly given to patients in cancer treatment that may impact symptoms (e.g., initiation or discontinuation of aromatase inhibitors or Tamoxifen). It is possible that medications (e.g., analgesics and sleep aids) the participants took—and that were not monitored as a part of this study—could potentially explain the improvements seen. As well, there may have been natural improvement in symptoms over time. Future research, including research that examines the efficacy of self-hypnosis training relative to standard care or conditions that control for the effects of patient outcome expectancy, time, and therapist attention, should assess and control for these factors when possible.
Summary and Conclusions
Despite the study’s limitations, the findings provide support for the potential efficacy of self-hypnosis training for the management of bothersome symptoms commonly reported by women who are in breast cancer treatment or who are breast cancer survivors. In particular, the findings suggest that self-hypnosis training may provide for substantial improvements in daily pain intensity and sleep problems. The study also provides examples of the kinds of suggestions clinicians might consider when treating cancer-related symptoms using hypnosis. Randomized trials comparing this intervention to interventions that control for the effects of time, therapist attention, and patient outcome expectancy are indicated.
Contributor Information
Mark P. Jensen, University of Washington School of Medicine, Seattle, USA.
Julie R. Gralow, Fred Hutchinson Cancer Research Center and University of Washington School of Medicine, Seattle, USA
Alan Braden, University of Washington School of Medicine, Seattle, USA.
Kevin J. Gertz, University of Washington School of Medicine, Seattle, USA
Jesse R. Fann, Fred Hutchinson Cancer Research Center and University of Washington School of Medicine, Seattle, USA
Karen L. Syrjala, Fred Hutchinson Cancer Research Center and University of Washington School of Medicine, Seattle, USA
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Bardwell WA, Ancoli-Israel S. Breast cancer and fatigue. Journal of Clinical Sleep Medicine. 2008;3(1):61–71. doi: 10.1016/j.jsmc.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell WA, Profant J, Casden DR, Dimsdale JE, Ancoli-Israel S, Natarajan L, Pierce JP. The relative importance of specific risk factors for insomnia in women treated for early-stage breast cancer. Psychooncology. 2008;17(1):9–18. doi: 10.1002/pon.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker PM. Chronic insomnia: Outcome of hypnotherapeutic intervention in six cases. American Journal of Clinical Hypnosis. 1993;36:98–105. doi: 10.1080/00029157.1993.10403051. [DOI] [PubMed] [Google Scholar]
- Butler LD, Koopman C, Neri E, Giese-Davis J, Palesh O, Thorne-Yocam KA, Spiegel D. Effects of supportive-expressive group therapy on pain in women with metastatic breast cancer. Health Psychology. 2009;28:579–587. doi: 10.1037/a0016124. [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Hays R. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen K. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Crawford HJ. Cognitive and psychophysiological correlates of hypnotic responsiveness and hypnosis. In: Fass ML, Brown DP, editors. Creative mastery in hypnosis and hypnoanalysis: A festschrift for Erika Fromm. Hillsdale, NJ: Erlbaum; 1990. pp. 155–168. [Google Scholar]
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- Elkins G, Marcus J, Stearns V, Hasan Rajab M. Pilot evaluation of hypnosis for the treatment of hot flashes in breast cancer survivors. Psychooncology. 2007;16:487–492. doi: 10.1002/pon.1096. [DOI] [PubMed] [Google Scholar]
- Elkins G, Marcus J, Stearns V, Perfect M, Rajab MH, Ruud C, Keith T. Randomized trial of a hypnosis intervention for treatment of hot flashes among breast cancer survivors. Journal of Clinical Oncology. 2008;26:5022–5026. doi: 10.1200/JCO.2008.16.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncologica. 2011;50:187–193. doi: 10.3109/0284186X.2010.533190. [DOI] [PubMed] [Google Scholar]
- Goodwin JW, Green SJ, Moinpour CM, Bearden JD, 3rd, Giguere JK, Jiang CS, Albain KS. Phase III randomized placebo-controlled trial of two doses of megestrol acetate as treatment for menopausal symptoms in women with breast cancer: Southwest Oncology Group Study 9626. Journal of Clinical Oncology. 2008;26:1650–1656. doi: 10.1200/JCO.2006.10.6179. [DOI] [PubMed] [Google Scholar]
- Harris PF, Remington PL, Trentham-Dietz A, Allen CI, Newcomb PA. Prevalence and treatment of menopausal symptoms among breast cancer survivors. Journal of Pain and Symptom Management. 2002;23:501–509. doi: 10.1016/s0885-3924(02)00395-0. [DOI] [PubMed] [Google Scholar]
- Hays RD, Stewart AL. Sleep measures. In: Stewart AL, Ware JE, editors. Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Duke University Press; 1992. pp. 235–259. [Google Scholar]
- Jensen MP. Measurement of pain. In: Fishman SM, Ballantyne JC, Rathmell JP, editors. Bonica’s management of pain. 4. Media, PA: Williams & Wilkins; 2010. pp. 251–270. [Google Scholar]
- Jensen MP. Hypnosis for chronic pain management: Therapist guide. New York, NY: Oxford University Press; 2011. [Google Scholar]
- Jensen MP, Barber J, Hanley MA, Engel JM, Romano JM, Cardenas DD, Patterson DR. Long-term outcome of hypnotic-analgesia treatment for chronic pain in persons with disabilities. International Journal of Clinical and Experimental Hypnosis. 2008;56:156–169. doi: 10.1080/00207140701849486. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Barber J, Romano JM, Hanley MA, Raichle KA, Molton IR, Patterson DR. Effects of self-hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinal-cord injury. International Journal of Clinical and Experimental Hypnosis. 2009;57:239–268. doi: 10.1080/00207140902881007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Barber J, Romano JM, Molton IR, Raichle KA, Osborne TL, Patterson DR. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. International Journal of Clinical and Experimental Hypnosis. 2009;57:198–221. doi: 10.1080/00207140802665476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Chang HY, Lai YH, Syrjala KL, Fann JR, Gralow JR. Pain in long-term breast cancer survivors: Frequency, severity, and impact. Pain Medicine. 2010;11:1099–1106. doi: 10.1111/j.1526-4637.2010.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Hanley MA, Engel JM, Romano JM, Barber J, Cardenas DD, Patterson DR. Hypnotic analgesia for chronic pain in persons with disabilities: A case series. International Journal of Clinical and Experimental Hypnosis. 2005;53:198–228. doi: 10.1080/00207140590927545. [DOI] [PubMed] [Google Scholar]
- Jensen MP, McArthur KD, Barber J, Hanley MA, Engel JM, Romano JM, Patterson DR. Satisfaction with, and the beneficial side effects of, hypnotic analgesia. International Journal of Clinical and Experimental Hypnosis. 2006;54:432–447. doi: 10.1080/00207140600856798. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of Affective Disorders. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Zhong X, Theobald D, Wu J, Tu W, Carpenter JS. Somatic symptoms in patients with cancer experiencing pain or depression: Prevalence, disability, and health care use. Archives of Internal Medicine. 2010;170:1686–1694. doi: 10.1001/archinternmed.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Research Treatment. 2008;112(1):5–13. doi: 10.1007/s10549-007-9831-1. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Bovbjerg DH, Schnur JB, David D, Goldfarb A, Weltz CR, Silverstein JH. A randomized clinical trial of a brief hypnosis intervention to control side effects in breast surgery patients. Journal of the National Cancer Institute. 2007;99:1304–1312. doi: 10.1093/jnci/djm106. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Kangas M, David D, Hallquist MN, Green S, Bovbjerg DH, Schnur JB. Fatigue during breast cancer radiotherapy: An initial randomized study of cognitive-behavioral therapy plus hypnosis. Health Psychology. 2009;28:317–322. doi: 10.1037/a0013582. [DOI] [PubMed] [Google Scholar]
- Osborne TL, Raichle KA, Jensen MP, Ehde DM, Kraft G. The reliability and validity of pain interference measures in persons with multiple sclerosis. Journal of Pain and Symptom Management. 2006;32:217–229. doi: 10.1016/j.jpainsymman.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychological Bulletin. 2003;129:495–521. doi: 10.1037/0033-2909.129.4.495. [DOI] [PubMed] [Google Scholar]
- Raichle KA, Osborne TL, Jensen MP, Cardenas D. The reliability and validity of pain interference measures in persons with spinal cord injury. Journal of Pain. 2006;7:179–186. doi: 10.1016/j.jpain.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Rudwaleit M, Gooch K, Michel B, Herold M, Thorner A, Wong R, Kupper H. Adalimumab improves sleep and sleep quality in patients with active ankylosing spondylitis. Journal of Rheumatology. 2011;38(1):79–86. doi: 10.3899/jrheum.100213. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Crofford LJ, Leon T, Cappelleri JC, Bushmakin AG, Whalen E, Sadosky A. The effects of pregabalin on sleep disturbance symptoms among individuals with fibromyalgia syndrome. Sleep Medicine. 2009;10:604–610. doi: 10.1016/j.sleep.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Schnur JB, Montgomery GH. Hypnosis and cognitive-behavioral therapy during breast cancer radiotherapy: A case report. American Journal of Clinical Hypnosis. 2008;50:209–215. doi: 10.1080/00029157.2008.10401624. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Bloom JR. Pain in metastatic breast cancer. Cancer. 1983;52:341–345. doi: 10.1002/1097-0142(19830715)52:2<341::aid-cncr2820520227>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD–7. Archives of Internal Medicine. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Thekkumpurath P, Walker J, Butcher I, Hodges L, Kleiboer A, O’Connor M, Sharpe M. Screening for major depression in cancer outpatients: The diagnostic accuracy of the 9-item patient health questionnaire. Cancer. 2011;117:218–227. doi: 10.1002/cncr.25514. [DOI] [PubMed] [Google Scholar]
- Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]


