Abstract
Background
Cerebral hyperperfusion syndrome (CHS), characterized by severe ipsilateral headache, seizures, and intracranial hemorrhage, is a rare, poorly understood complication that can be fatal following carotid endarterectomy (CEA). The purpose of the study was to determine the factors associated with CHS as captured in the Vascular Quality Initiative.
Methods
Analysis was conducted on 51,001 procedures captured from the CEA module of the Vascular Quality Initiative from 2003 to 2015. Preoperative, operative, and postoperative variables were considered for inclusion in logistic regression analyses to determine possible associations with CHS. The relative contribution of each variable to the overall model was determined using dominance analysis.
Results
The mean age was 70.2 ± 6 9.4 years; there were 39.6% female patients, 93.1% of white race, with 29.6% of CEAs being performed for symptomatic status. The overall rate of CHS was 0.18% (n = 94), with 55.1% occurring in asymptomatic and 44.9% occurring in symptomatic patients with an associated mortality rate of 38.2%. Multivariable analysis including preoperative variables showed that female gender (odds ratio [OR], 1.65; 95% confidence interval [CI], 1.09–2.51; P = .019), <1 month major ipsilateral stroke (OR, 5.36; 95% CI, 2.35–12.22; P < .001), coronary artery disease (OR, 1.77; 95% CI, 1.15–2.71; P = .009), and contralateral stenosis ≥70% (OR, 1.54; 95% CI, 1.00–2.36; P = .050) were independently associated with CHS and that <1 month major stroke was the most important contributor to the model. With the additional inclusion of operative and postoperative variables, female gender (OR, 1.75; 95% CI, 1.14–2.67; P = .010), <1 month ipsilateral major stroke (OR, 3.20; 95% CI, 1.32–7.74; P = .010), urgency (OR, 2.25; 95% CI, 1.38–3.67; P = .001), re-exploration (OR, 2.98; 95% CI, 1.27–6.97; P = .012), postoperative hypertension (OR, 4.09; 95% CI, 2.65–6.32; P < .001), postoperative hypotension (OR, 3.21; 95% CI, 1.97–5.24; P < .001), dysrhythmias (OR, 3.23; 95% CI, 1.64–6.38; P = .001), and postoperative myocardial infarction (OR, 2.84; 95% CI, 1.21–6.67; P = .017) were significantly associated with CHS, with postoperative blood pressure lability and cardiac complications having the strongest associations with CHS.
Conclusions
The risk of CHS was highest in female patients and in those with a recent major stroke, coronary artery disease, and contralateral stenosis ≥70%. In addition, in adjusting for operative and postoperative variables, CHS was most significantly associated with postoperative blood pressure lability and cardiac complications. These data lend insight into a high-risk population for this devastating complication.
Cerebral hyperperfusion syndrome (CHS) following carotid endarterectomy (CEA) was originally described by Sundt and colleagues in 1981 and is characterized by severe ipsilateral headache, seizures, and intracranial hemorrhage.1,2 The pathophysiologic mechanism is derived from the “normal perfusion breakthrough” theory, in which it was noted that cerebral edema and hemorrhage occurred after excision of arteriovenous malformation.3 This phenomenon is thought to be due to chronically vasodilated intracranial vessels that lose their capacity to constrict or to autoregulate in the presence of chronic cerebral ischemia.4 Because of its rare occurrence, with a cited incidence of 0% to 3% in the literature, it remains a poorly understood phenomenon, yet it is a complication with devastating implications, associated with up to a 50% mortality rate.2,5 We sought to determine the clinical factors associated with the development of CHS following CEA in participants from the national Vascular Quality Initiative (VQI).
METHODS
Description of the data set
Data were obtained from the CEA module of the national VQI database after approval was obtained from the National Research Advisory Council. The VQI, which originated in northern New England in 2002,6 is a prospective registry composed of 17 regions within the country and 358 centers in 46 states in addition to Ontario; it was created for the purpose of improving vascular surgical outcomes. In addition to including detailed demographic data, the VQI collects granular data regarding intraoperative and postoperative variables, making it the ideal data set to study vascular surgical outcomes. To ensure accurate entry of all eligible cases, registry data are compared with hospital claims data annually, minimizing missing cases and enhancing quality control. Additional details of the database have been published previously.6–8 This project was reviewed by the University of Pennsylvania Institutional Review Board and determined to qualify as a Quality Improvement Project and as such did not meet the requirements for formal review and qualified for waiver of informed consent.
Ascertainment of CHS and definition of variables
CHS was defined in the VQI as seizures associated with headache or any hemorrhage noted on computed tomography scan or magnetic resonance imaging and was coded as a binary variable. Urgency referred to CEA performed within 24 hours of admission or if the patient could not be discharged before operation; transfer was defined as whether the patient was transferred from another hospital or rehabilitation unit; coronary artery disease (CAD) encompassed prior myocardial infarction and stable and unstable angina; and postoperative hypertension and hypotension were defined by the need to use medications to treat postoperative hypertension and hypotension. Minor stroke is nondisabling, whereas major stroke is characterized by disability causing nonindependent living status.
Statistical analysis
A retrospective analysis of the prospectively collected data set was performed. Summary statistics of demographic data were performed; continuous variables were compared using Student t-test, and the frequency of categorical variables was compared using χ2 test. Univariate logistic regression was performed to determine the factors associated with CHS. Any variable reaching a statistical significance of P < .10 from the univariate analysis was considered for inclusion into the multivariable model, in which a forward selection approach was undertaken using P = .25 as the cutoff for inclusion for all variables. Because of prior studies that have implicated a recent neurologic event as a significant predictor for CHS,9 we derived multiple multivariable models to include various types of prior ipsilateral neurologic events. These exploratory analyses included <1 month neurologic event (transient ischemic attack [TIA] or stroke), <1 month TIA or minor stroke, <1 month major stroke, and ipsilateral TIA or stroke, defined as ocular or cortical or combined. The multivariable model associated with the neurologic event with the greatest area under the curve (AUC) was selected. Based on prior data implicating contralateral severe stenosis and contralateral occlusion as risk factors for CHS,2,5 we hypothesized a priori that the risk of CHS would be higher in those participants with ipsilateral severe carotid stenosis as well as contralateral severe carotid stenosis or occlusion and tested for effect modification. We ultimately derived two multivariable models because of the desire to develop one that included preoperative variables that would affect a surgeon’s decision to operate as well as a model that included preoperative, operative, and postoperative variables. In deriving the multivariable models, we considered the following prior neurologic events for inclusion: <1 month neurologic event (TIA or stroke), <1 month TIA or minor stroke, <1 month major stroke, and ipsilateral TIA or stroke, defined as ocular or cortical or combined; <1 month major stroke was associated with the greatest AUC and therefore was considered in the multivariable model for CHS.
The goodness of fit for both models was confirmed by the Hosmer-Lemeshow test as has been previously described.10,11 To determine the relative importance of each variable as it contributed to the multivariable model, dominance analysis was performed as previously described.12,13 In brief, dominance analysis is a statistical approach to determine the relative contribution of each predictor in the model to the outcome of interest, in this case, CHS. This is performed by determining each variable’s contribution to the overall variance of the model. The statistical threshold for significance for all analyses was P < .05. All analyses were performed using Stata version 12 (StataCorp LP, College Station, Tex).
RESULTS
There were a total of 51,001 CEA procedures from 2003 to 2015 included in this analysis. The overall mean age of the cohort was 70.2 ± 9.4 years; it consisted of 39.6% female patients, 93.1% of white race, and 34.7% with diabetes. Hypertension was present in 88.7%, and CAD was present in 28.6%. There were a total of 94 patients (0.18%) who suffered from CHS, which was associated with a 38.2% mortality rate. There was a statistically significant higher transfer rate among those with CHS (11.2% vs 5.1%; P = .009). There was a significantly higher rate of CAD in those with CHS (40.4% vs 28.6%; P = .014). Patients with CHS had a higher rate of preoperative beta blocker use (73.0% vs 62.3%; P = .037). For additional summary statistics of the demographics of the overall cohort as well as for the CHS vs no-CHS patients, refer to Table I.
Table I.
Summary statistics of demographics and clinical characteristics
| Variable | Overall (N = 51,001) | No CHS (n = 50,907) | CHS (n = 94) | P value |
|---|---|---|---|---|
| Age, years | 70.2 ± 9.4 | 70.2 ± 9.4 | 71.9 ± 11.2 | .081 |
| Female gender | 39.6 | 39.6 | 48.9 | .066 |
| White race | 93.1 | 93.1 | 97.9 | .615 |
| Transfer status | 5.2 | 5.1 | 11.2 | .009 |
| Diabetes | 34.7 | 34.7 | 38.3 | .465 |
| BMI | 28.3 ± 5.6 | 28.3 ± 5.6 | 29.0 ± 5.8 | .316 |
| CHF | 9.8 | 9.8 | 11.2 | .662 |
| Hypertension | 88.7 | 88.7 | 94.4 | .091 |
| CAD | 28.6 | 28.6 | 40.4 | .014 |
| Smoking | – | – | – | .299 |
| Former | 47.6 | 47.6 | 40.9 | – |
| Current | 28.6 | 28.6 | 29.0 | – |
| Renal function | – | – | – | .956 |
| Cr <1.8 mg/dL | 92.9 | 92.9 | 92.1 | – |
| Cr ≥1.8 mg/dL | 6.1 | 6.1 | 6.7 | – |
| Dialysis | 1.0 | 1.0 | 1.1 | – |
| COPD | 21.8 | 21.8 | 25.8 | .355 |
| ACE inhibitor/ARB | 51.1 | 51.1 | 55.2 | .662 |
| Beta blocker | 62.3 | 62.3 | 73.0 | .037 |
| Statin | 78.9 | 78.9 | 82.0 | .803 |
| Aspirin | 82.9 | 82.9 | 82.0 | .936 |
| Anticoagulant | 6.4 | 6.4 | 9.0 | .329 |
ACE, Angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CHS, cerebral hyperperfusion syndrome; COPD, chronic obstructive pulmonary disease; Cr, creatinine.
P value represents the comparison of continuous and categorical variables by CHS status.
Categorical variables are presented as %. Continuous variables are presented as mean ± standard deviation.
With regard to their prior surgical history and preoperative neurologic status, the CHS patients had a higher rate of ipsilateral cortical stroke (23.6% vs 12.0%; P = .001) as well as a higher rate of ipsilateral ocular or cortical stroke (27.0% vs 13.4%; P < .001). With regard to timing of the neurologic event, patients with CHS had a higher rate of <1 month ipsilateral TIA or stroke (26.0% vs 23.2%; P = .005) as well as a significantly higher rate of <1 month ipsilateral major stroke (7.9% vs 1.4%; P < .001). The history of vertebrobasilar TIA or stroke was also higher in CHS patients (6.7% vs 2.3%; P = .005). The presence of contralateral severe stenosis, defined as ≥70% or occlusion, was also greater in the CHS group (38.2% vs 28.7%; P = .048). There was no difference in ipsilateral stenosis severity between the CHS and no-CHS groups (P = .232). There was also no difference in the rate of ipsilateral ocular or cortical TIA between groups (18.0% vs 16.7%; P = .745). Preoperative anticoagulant use was no different between CHS and no-CHS groups (9.0% vs 6.4%; P = .329). Details of this univariate analysis are displayed in Table II.
Table II.
Summary statistics (%) of prior surgical history and preoperative neurologic status
| Variable | Overall (N = 51,001) | No CHS (n = 50,907) | CHS (n = 94) | P value |
|---|---|---|---|---|
| History of CEA or CAS | 15.5 | 15.5 | 15.7 | .950 |
| History of ipsilateral CEA | 2.2 | 2.2 | 1.1 | .488 |
| History of ipsilateral CAS | 0.3 | 0.3 | 0.0 | .691 |
| Ipsilateral ocular TIA | 8.1 | 8.1 | 5.6 | .384 |
| Ipsilateral cortical TIA | 9.7 | 9.7 | 12.4 | .400 |
| Ipsilateral ocular or cortical TIA | 16.7 | 16.7 | 18.0 | .745 |
| Ipsilateral ocular stroke | 1.8 | 1.8 | 3.4 | .247 |
| Ipsilateral cortical stroke | 12.0 | 12.0 | 23.6 | .001 |
| Ipsilateral ocular or cortical stroke | 13.4 | 13.4 | 27.0 | <.001 |
| Ipsilateral TIA or stroke | 29.6 | 29.6 | 44.9 | .002 |
| Ipsilateral <1 month TIA or stroke | 23.2 | 23.2 | 26.0 | .005 |
| Ipsilateral <1 month TIA or minor stroke | 21.8 | 21.8 | 28.1 | .154 |
| Ipsilateral <1 month major stroke | 1.4 | 1.4 | 7.9 | <.001 |
| History of vertebrobasilar TIA or stroke | 2.3 | 2.3 | 6.7 | .005 |
| Ipsilateral most severe stenosis | .232 | |||
| <50% | 1.6 | 1.6 | 1.1 | |
| ≥50%–60% | 2.5 | 2.5 | 1.1 | |
| ≥60%–70% | 5.4 | 5.4 | 4.5 | |
| ≥70%–80% | 27.2 | 27.2 | 20.2 | |
| ≥80% | 61.6 | 61.6 | 68.5 | |
| Occluded | 1.8 | 1.8 | 4.5 | |
| Ipsilateral most severe stenosis ≥70% | 90.8 | 90.8 | 93.3 | .416 |
| Contralateral stenosis ≥70% or occlusion | 28.8 | 28.7 | 38.2 | .048 |
CAS, Carotid artery stenting; CEA, carotid endarterectomy; CHS, cerebral hyperperfusion syndrome; TIA, transient ischemic attack.
With regard to operative and postoperative variables, urgency status was higher among CHS patients (28.1% vs 12.7%; P < .001), as was the need for re-exploration (6.8% vs 1.9%; P = .001). Mean procedure time was greater in the CHS group (143.5 ± 9.3 vs 118.9 ± 52.4 minutes; P < .001). Blood pressure lability proved to be an important postoperative predictor, with the CHS patients exhibiting a higher rate of postoperative hypertension (46.1% vs 17.0%; P < .001) as well as a higher rate of postoperative hypotension (29.2% vs 10.9%; P < .001). Indeed, the need for any blood pressure medication was notably high in the CHS group (62.9% vs 27.0%; P < .001). Postoperative cardiac issues were also higher in the CHS group, with CHS patients exhibiting a higher rate of dysrhythmias (13.5% vs 2.0%; P < .001) as well as a higher rate of postoperative myocardial infarction (7.9% vs 0.9%; P < .001). Postoperative anticoagulant use was no different between CHS and no-CHS patients (7.9% vs 6.9%; P = .707). Details of the summary analysis of operative and postoperative variables are provided in Table III.
Table III.
Summary statistics of operative and postoperative variables
| Variable | Overall (N = 51,001) | No CHS (n = 50,907) | CHS (n = 94) | P value |
|---|---|---|---|---|
| Urgency | 12.8 | 12.7 | 28.1 | <.001 |
| Local/regional | 9.1 | 9.1 | 10.1 | .734 |
| Patch angioplasty | 87.2 | 87.2 | 86.5 | .850 |
| Shunt | 54.3 | 54.3 | 50.0 | .418 |
| Re-exploration | 1.9 | 1.9 | 6.8 | .001 |
| Procedure time, minutes | 118.9 ± 52.4 | 118.9 ± 52.4 | 143.5 ± 9.3 | <.001 |
| Postoperative hypertension | 17.0 | 17.0 | 46.1 | <.001 |
| Postoperative hypotension | 10.9 | 10.9 | 29.2 | <.001 |
| Any IV medication | 27.1 | 27.0 | 62.9 | <.001 |
| Dysrhythmia | 2.0 | 2.0 | 13.5 | <.001 |
| Postoperative MI | 0.9 | 0.9 | 7.9 | <.001 |
| Discharge anticoagulant | 6.8 | 6.9 | 7.9 | .707 |
Any IV medication, Intravenous (IV) medication used to treat hypertension or hypotension; CHS, cerebral hyperperfusion syndrome; Local/regional, localdlocal anesthetic and IV sedation, regionaldcervical plexus block, epidural, or spinal; MI, myocardial infarction.
Categorical variables are presented as %. Continuous variables are presented as mean ± standard deviation.
The univariable logistic regression showed a significantly increased odds of CHS in patients of female gender (odds ratio [OR], 1.56; 95% confidence interval [CI], 1.03–2.36; P = .037), those who were transferred (OR, 2.34; 95% CI, 1.21–4.51; P = .012), those with CAD (OR, 1.69; 95% CI, 1.11–2.59; P = .015), and those receiving preoperative beta blockers (OR, 1.64; 95% CI, 1.02–2.61; P = .039). Similar to the χ2 analysis, the odds of CHS was higher in those with a history of ipsilateral cortical stroke (OR, 2.26; 95% CI, 1.38–3.68; P = .001), ipsilateral ocular or cortical stroke (OR, 2.39; 95% CI, 1.50–3.82; P < .001), <1 month ipsilateral TIA or stroke (OR, 1.85; 95% CI, 1.20–2.86; P = .005), and <1 month major stroke (OR, 5.92; 95% CI, 2.73–12.86; P < .001). There was also a higher odds of CHS among those with a history of vertebrobasilar TIA or stroke (OR, 2.16; 95% CI, 1.33–3.51; P = .002) as well as among those with a contralateral stenosis ≥70% or occlusion (OR, 1.53; 95% CI, 0.61–3.23; P = .050). Urgency was associated with an increased odds of CHS (OR, 2.68; 95% CI, 1.68–4.25; P < .001), as were re-exploration (OR, 3.87; 95% CI, 1.68–8.88; P = .001) and procedure time >2 hours (OR, 1.83; 95% CI, 1.16–2.88; P = .010). The results of the univariate logistic regression are summarized in the Supplementary Table (online only).
Preoperative variables model
Female gender remained independently associated with an increased odds of CHS (OR, 1.65; 95% CI, 1.09–2.51; P = .019). A <1 month major ipsilateral stroke was associated with a significant increase in odds of CHS in the adjusted model (OR, 5.36; 95% CI, 2.35–12.22; P < .001), as were CAD (OR, 1.77; 95% CI, 1.15–2.71; P = .009) and contralateral stenosis ≥70% (OR, 1.54; 95% CI, 1.00–2.36; P = .050). The results of this multivariable logistic regression analysis are summarized in Table IV. The relative importance of each variable in the multivariable model was then determined by dominance analysis, the results of which are summarized in Fig 1.12,13 A <1 month ipsilateral major stroke had the strongest association to CHS, accounting for 32% of the overall model variance, with CAD as the second most important variable, accounting for 16% of the model variance. Female gender, white race, and contralateral stenosis ≥70% were the next most important variables, all of which were also significant in the multivariable model.
Table IV.
Multivariable logistic regression for the association between cerebral hyperperfusion syndrome (CHS) and preoperative variables
| Variable (N = 50,120) | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.02 | 1.00–1.04 | .115 |
| Female gender | 1.65 | 1.09–2.51 | .019 |
| White race | 3.47 | 0.85–14.14 | .083 |
| Transfer status | 1.75 | 0.87–3.53 | .115 |
| CAD | 1.77 | 1.15–2.71 | .009 |
| Ipsilateral <1 month major stroke | 5.36 | 2.35–12.22 | <.001 |
| Contralateral stenosis ≥70% or occlusion | 1.54 | 1.00–2.36 | .050 |
CAD, Coronary artery disease; CI, confidence interval; OR, odds ratio. Area under curve = 0.6519.
Fig 1.
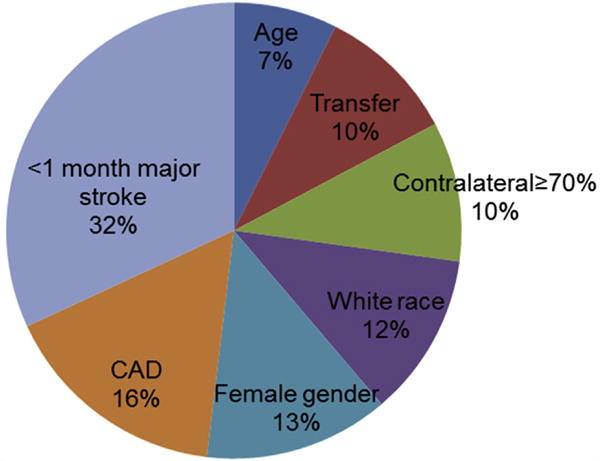
Relative association of preoperative variables to cerebral hyperperfusion syndrome (CHS). CAD, Coronary artery disease.
Preoperative, operative, and postoperative variables model
Female gender was again significantly associated with CHS in the adjusted model (OR, 1.75; 95% CI, 1.14–2.67; P = .010). White race showed a significant association with CHS (OR, 4.07; 95% CI, 1.01–16.60; P = .050). A <1 month major ipsilateral stroke was associated with an increased odds of CHS (OR, 3.20; 95% CI, 1.32–7.74; P = .010), as were urgency (OR, 2.25; 95% CI, 1.38–3.67; P = .001) and re-exploration (OR, 2.97; 95% CI, 1.27–6.97; P = .012). Postoperative blood pressure lability was associated with CHS, with postoperative hypertension (OR, 4.10; 95% CI, 2.65–6.32; P < .001) as well as postoperative hypotension (OR, 3.21; 95% CI, 1.97–5.24; P < .001) being independently associated with CHS. Cardiac complications were also associated with CHS, with patients with dysrhythmia (OR, 3.23; 95% CI, 1.64–6.38; P = .001) as well as postoperative myocardial infarction (OR, 2.84; 95% CI 1.21–6.66; P = .017) exhibiting a higher odds of CHS. The results of the multivariable logistic regression including preoperative, operative, and postoperative variables are summarized in Table V. The variable with the strongest association in this model was postoperative hypertension, accounting for 27% of the overall variance (Fig 2), with postoperative hypotension as the second most important, accounting for 15%. Cardiac-related complications were significantly associated as well, with dysrhythmia accounting for 12% of the model fit and postoperative myocardial infarction contributing 8%; <1 month major stroke, urgency, female gender, and re-exploration were also significant factors in the model.
Table V.
Multivariable logistic regression for the association between cerebral hyperperfusion syndrome (CHS) and preoperative, operative, and postoperative variables
| Variable (N = 49,845) | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.02 | 0.99–1.04 | .188 |
| Female gender | 1.75 | 1.14–2.67 | .010 |
| White race | 4.07 | 1.01–16.68 | .051 |
| CAD | 1.38 | 0.88–2.16 | .159 |
| Beta blocker | 1.34 | 0.83–2.18 | .234 |
| Ipsilateral <1 month major stroke | 3.20 | 1.32–7.74 | .010 |
| Contralateral stenosis ≥70% or occlusion | 1.47 | 0.95–2.27 | .082 |
| Urgency | 2.25 | 1.38–3.67 | .001 |
| Re-exploration | 2.97 | 1.27–6.97 | .012 |
| Procedure time >2 hours | 1.58 | 0.99–2.52 | .055 |
| Postoperative hypertension | 4.10 | 2.65–6.32 | <.001 |
| Postoperative hypotension | 3.21 | 1.97–5.24 | <.001 |
| Dysrhythmia | 3.23 | 1.64–6.38 | .001 |
| Postoperative MI | 2.84 | 1.21–6.66 | .017 |
CAD, Coronary artery disease; CI, confidence interval; MI, myocardial infarction; OR, odds ratio.
Area under curve (AUC) = 0.7850.
Fig 2.

Relative association of preoperative, operative, and postoperative variables to cerebral hyperperfusion syndrome (CHS). CAD, Coronary artery disease; MI, myocardial infarction.
For both multivariable models, we considered a possible interaction between ipsilateral ≥70% stenosis and contralateral ≥70% stenosis or occlusion. Having a contralateral ≥70% stenosis or occlusion did not affect the association between ipsilateral ≥70% stenosis and the occurrence of CHS (P = .3159 and P .3001, respectively). In comparison of the two models (preoperative vs preoperative, operative and postoperative variables), the addition of operative and postoperative variables contributed to the model significantly, as denoted by the greater AUC (AUC = 0.7850 vs 0.6519; P < .001; Fig 3, A). Whereas postoperative blood pressure lability and cardiac complications constituted a significant proportion of the fit of the overall model (62%; Fig 2), postoperative variables alone did not account for all of the model, as evidenced by a smaller AUC (0.7297 vs 0.7850; P = .006; Fig 3, B), suggesting that preoperative characteristics of the patient were still important to serve as a milieu for development of CHS.
Fig 3.
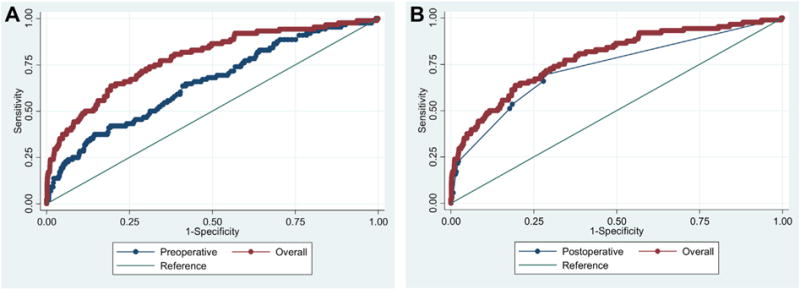
A, Receiver operating characteristic curves for preoperative vs overall model. Preoperative area under the curve (AUC) 0.6519 vs overall AUC 0.7850. B, Receiver operating characteristic curves for postoperative vs overall model. Postoperative AUC 0.7297 vs overall AUC 0.7850.
DISCUSSION
CHS is a devastating, poorly understood complication that can occur after CEA. This is the largest study to date of CHS that follows CEA using the national VQI data set. In this study, we demonstrated that when preoperative variables are considered, recent major stroke was associated with a more than fivefold increased odds of CHS. Female gender, CAD, and severe contralateral stenosis were also independently associated with CHS. In contrast, we demonstrated that with the inclusion of operative and postoperative variables, blood pressure lability was the most influential, accounting for 42% of the variance in the model, with postoperative hypertension associated with a 4.10-fold increased odds and postoperative hypotension associated with a 3.21-fold increased odds of CHS. Postoperative cardiac complications were also highly associated with CHS and accounted for 20% of the overall model, with dysrhythmia associated with a 3.23-fold increase in odds and myocardial infarction associated with a 2.84-fold increase in odds. In addition, urgency and re-exploration were independently associated with CHS.
The rationale for considering two models was to provide a model composed of patient-related factors that would be considered before one’s decision to bring the patient to the operating room as well as a model that encompassed operative and postoperative variables to further define associations with the CHS event. Whereas CHS has been described as occurring as early as postoperative day 0,5 it typically has a more delayed onset between postoperative days 3 and 6.10,11 Thus, the postoperative course and postoperative management of the patient are germane to development of CHS. Whereas postoperative blood pressure lability and cardiac complications were highly associated with CHS, they did not explain all of the variability in the model, indicating the importance of pre-existing comorbidities in the pathophysiologic process of CHS.
The importance of recent major stroke in CHS has a potentially significant impact on how symptomatic carotid stenosis patients are treated. In a recent analysis of pooled data from the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST), which included 5893 patients, the benefit from CEA was greatest in those patients who were randomized within 2 weeks after their last ischemic event, with a 30.2% reduction of absolute risk of stroke in those who were randomized within 2 weeks of their neurologic event, which was reduced to a third in those patients randomized >4 weeks after the event.14 The results from our study, however, suggest that recent major stroke is the most important preoperative risk factor for CHS. Indeed, short time interval between neurologic event and CEA was the most important risk factor for CHS in a prospective study of clinical predictors for CHS following CEA.9 Furthermore, one should take note that in the summary analysis from NASCET and ECST, the patients were eligible for entry in these trials if they had a relatively minor neurologic event, such as a recent TIA or a nondisabling stroke. The pooled analysis, therefore, does not provide information on those patients who have had a recent major stroke. The findings from our study show that patients undergoing urgent CEA <1 month major stroke are at a substantially increased odds for development of CHS. Further study should be performed to determine what the optimal time interval may be to prevent another stroke, yet minimizing the risk of CHS in symptomatic patients.
CAD was also a significant preoperative risk factor for CHS. Although this association has not been the emphasis in prior papers,9 the presence of underlying coronary disease could provide the substrate for the increased dysrhythmias and myocardial infarctions seen in CHS patients in this large cohort.
Female gender was also associated with a higher risk of CHS, which parallels the generally poorer outcomes in women compared with men after CEA. Women have been noted to have a higher perioperative risk of stroke as well as death after CEA,15,16 thus making the margin of benefit smaller in considering performance of a CEA in a woman with an asymptomatic carotid stenosis. Once again, this reflects the importance of considering sex-specific outcomes in weighing the risks and benefits of surgery.
Severe contralateral stenosis of ≥70% was also significantly associated with CHS. The idea of CHS risk being higher in those with decreased cerebral reserve has been published by multiple other studies2,9,17,18 and is supported by findings from this investigation. The significance of the relationship between postoperative blood pressure lability and CHS mirrors findings from other studies, which cite the importance of preventing hypertension to decrease intracranial hemorrhage after CEA.5,9,12,13,19 The combination of defective cerebral autoregulation, uncontrollable hypertension, and increased cerebral blood flow has been implicated in the pathophysiologic mechanism of CHS.1,17 This has repercussions for how patients are optimally managed after CEA. Patients are typically monitored in a stepdown unit or an intensive care unit for serial neurologic examinations as well as for blood pressure control.20 The data from this study support close hemodynamic monitoring of patients in the postoperative setting, as blood pressure lability was highly associated with CHS. In addition, in patients who have required intravenous hypertension treatment in the hospital, discharge with close home blood pressure management in the first week seems appropriate to avoid this complication, given the typical delayed presentation (postoperative days 3–6) of CHS.10 The importance of this cannot be overemphasized as a recent single-institution study of CEA patients demonstrated that the majority of readmissions (73.4% of unplanned readmissions) were due to a carotid-attributed cause, including stroke, headache, reperfusion, and hypertension, and that the median time for readmission was 4 days.21 As CAD was found to have a significant association with CHS, it is not surprising that there was also an association between CHS and postoperative dysrhythmias and myocardial infarction. One could posit that this could be due to hemodynamic instability associated with CHS, with its attendant effects on afterload and cardiac strain.22 Indeed, both postoperative hypertension and hypotension have been associated with cardiac complications in a VQI study using a regional data set.23 In sum, these findings justify close monitoring of CEA patients in the postoperative period.
Urgency also increased the risk of CHS, probably because of the high proportion of these patients having had recent major strokes or neurologic events, which has also previously been reported.9 The need for re-exploration was also associated with CHS; this could be a marker for increased ischemia duration, if the reason for re-exploration was due to a technical issue such as an intimal flap. There was also a trend toward procedural time >2 hours as a significant contributor to CHS. This has not been emphasized in prior studies and could be an area of future investigation.
There are limitations to our study. This is an observational, cross-sectional study; thus, cause and effect cannot be directly determined. This is particularly relevant with regard to the postoperative variables; blood pressure lability could have been part of the overall clinical picture of CHS, rather than a direct cause of CHS. Based on literature review, CHS has a prevalence of up to 3%; our prevalence of 0.18% may represent under-reporting. It is also possible that given the definition of CHS as seizures associated with headache or intracranial hemorrhage noted on computed tomography or magnetic resonance imaging, the definition of CHS may not have captured milder forms of CHS, such as severe headache. Furthermore, the low prevalence of CHS in this study could be due in part to the delayed presentation of CHS, whereby the patient may develop CHS after discharge and suffer from a mortal event that may not be captured as the cause of death. Because timing of the reperfusion event was not captured, we are not able to shed light on this as a reason for possible data omission. Furthermore, because the VQI defines CHS as a binary variable, we do not have the knowledge to separate CHS patients into milder or more severe forms, the latter of whom likely had an associated elevated mortality rate. Understanding why patients with mild CHS are able to recover, whereas patients with severe forms of CHS cannot, would lend insight into the pathophysiologic mechanism of CHS and should be the subject of future study.
Finally, there may be unmeasured confounders contributing to the results of our model. However, the VQI is an exhaustive database inclusive of many relevant variables for this outcome, all of which were considered as candidate variables in building the appropriate multivariable models.
We also note important strengths of this study. This is the largest cohort studied for CHS to date, and thus it offers the most comprehensive information regarding this rare and devastating complication. The VQI is a registry composed of academic and community hospitals across the United States as well as Ontario and thus provides a “real-world” view of CEA that lends itself to generalizability across all types of providers and practice settings.
CONCLUSIONS
This study shows that the risk of CHS is highest in those who are female, have suffered a recent major stroke, have CAD, and have a contralateral severe carotid stenosis. Postoperative blood pressure lability and cardiac complications were also highly associated with CHS. These findings help identify a subset of patients undergoing CEA who are at high risk for CHS. Future study should be devoted to better defining the exact timing and mechanisms behind the development of CHS to avoid this complication.
Supplementary Material
Footnotes
Author conflict of interest: none.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: GW
Analysis and interpretation: GW, AB, RD, PG, CR, RF
Data collection: Not applicable
Writing the article: GW, AB
Critical revision of the article: GW, AB, RD, PG, CR, RF
Final approval of the article: GW, AB, RD, PG, CR, RF
Statistical analysis: GW, RD, PG
Obtained funding: Not applicable
Overall responsibility: GW
References
- 1.Sundt TM, Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM, Jr, O’Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981;56:533–43. [PubMed] [Google Scholar]
- 2.Reigel MM, Hollier LH, Sundt TM, Jr, Piepgras DG, Sharbrough FW, Cherry KJ. Cerebral hyperperfusion syndrome: a cause of neurologic dysfunction after carotid endarterectomy. J Vasc Surg. 1987;5:628–34. [PubMed] [Google Scholar]
- 3.Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–72. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 4.Andrews BT, Levy ML, Dillon W, Weinstein PR. Unilateral normal perfusion pressure breakthrough after carotid endarterectomy: case report. Neurosurgery. 1987;21:568–71. doi: 10.1227/00006123-198710000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Piepgras DG, Morgan MK, Sundt TM, Jr, Yanagihara T, Mussman LM. Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg. 1988;68:532–6. doi: 10.3171/jns.1988.68.4.0532. [DOI] [PubMed] [Google Scholar]
- 6.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101–2. [DOI] [PubMed] [Google Scholar]
- 7.DeMartino RR, Hoel AW, Beck AW, Hallett JW, Arya S, Upchurch GH, et al. Antiplatelet and statin treatment is not associated with reduced myocardial infarction after high-risk vascular procedures. J Vasc Surg. 2014;60:1721. doi: 10.1016/j.jvs.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoel AW, Nolan BW, Goodney PP, Zhao Y, Schanzer A, Stanley AC, et al. Variation in smoking cessation after vascular operations. J Vasc Surg. 2013;57:1338–44. doi: 10.1016/j.jvs.2012.10.130. quiz: 1344.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maas MB, Kwolek CJ, Hirsch JA, Jaff MR, Rordorf GA. Clinical risk predictors for cerebral hyperperfusion syndrome after carotid endarterectomy. J Neurol Neurosurg Psychiatr. 2013;84:569–72. doi: 10.1136/jnnp-2012-303659. [DOI] [PubMed] [Google Scholar]
- 10.Bouri S, Thapar A, Shalhoub J, Jayasooriya G, Fernando A, Franklin IJ, et al. Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg. 2011;41:229–37. doi: 10.1016/j.ejvs.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg. 2007;107:1130–6. doi: 10.3171/JNS-07/12/1130. [DOI] [PubMed] [Google Scholar]
- 12.Azen R, Budescu DV. The dominance analysis approach for comparing predictors in multiple regression. Psychol Methods. 2003;8:129–48. doi: 10.1037/1082-989x.8.2.129. [DOI] [PubMed] [Google Scholar]
- 13.Budescu DV. Dominance analysisda new approach to the problem of relative importance of predictors in multiple-regression. Psychol Bull. 1993;114:542–51. [Google Scholar]
- 14.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–24. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 15.Hertzer NR, O’Hara PJ, Mascha EJ, Krajewski LP, Sullivan TM, Beven EG. Early outcome assessment for 2228 consecutive carotid endarterectomy procedures: the Cleveland Clinic experience from 1989 to 1995. J Vasc Surg. 1997;26:1–10. doi: 10.1016/s0741-5214(97)70139-3. [DOI] [PubMed] [Google Scholar]
- 16.Sarac TP, Hertzer NR, Mascha EJ, O’Hara PJ, Krajewski LP, Clair DG, et al. Gender as a primary predictor of outcome after carotid endarterectomy. J Vasc Surg. 2002;35:748–53. doi: 10.1067/mva.2002.120375. [DOI] [PubMed] [Google Scholar]
- 17.Solomon RA, Loftus CM, Quest DO, Correll JW. Incidence and etiology of intracerebral hemorrhage following carotid endarterectomy. J Neurosurg. 1986;64:29–34. doi: 10.3171/jns.1986.64.1.0029. [DOI] [PubMed] [Google Scholar]
- 18.Wagner WH, Cossman DV, Farber A, Levin PM, Cohen JL. Hyperperfusion syndrome after carotid endarterectomy. Ann Vasc Surg. 2005;19:479–86. doi: 10.1007/s10016-005-4644-3. [DOI] [PubMed] [Google Scholar]
- 19.Kim KH, Lee CH, Son YJ, Yang HJ, Chung YS, Lee SH. Postcarotid endarterectomy cerebral hyperperfusion syndrome: is it preventable by strict blood pressure control? J Korean Neurosurg Soc. 2013;54:159–63. doi: 10.3340/jkns.2013.54.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noorani A, Sadat U, Gaunt ME. Cerebral hemodynamic changes following carotid endarterectomy: ‘cerebral hyperperfusion syndrome’. Expert Rev Neurother. 2010;10:217–23. doi: 10.1586/ern.10.2. [DOI] [PubMed] [Google Scholar]
- 21.Ho KJ, Madenci AL, Semel ME, McPhee JT, Nguyen LL, Ozaki CK, et al. Predictors and consequences of unplanned hospital readmission within 30 days of carotid endarterectomy. J Vasc Surg. 2014;60:77–84. doi: 10.1016/j.jvs.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 22.Orlowski JP, Vidt DG, Walker S, Haluska JF. The hemodynamic effects of intravenous labetalol for postoperative hypertension. Cleve Clin J Med. 1989;56:29–34. doi: 10.3949/ccjm.56.1.29. [DOI] [PubMed] [Google Scholar]
- 23.Tan TW, Eslami MH, Kalish JA, Eberhardt RT, Doros G, Goodney PP, et al. The need for treatment of hemodynamic instability following carotid endarterectomy is associated with increased perioperative and 1-year morbidity and mortality. J Vasc Surg. 2014;59:16–24.e1. doi: 10.1016/j.jvs.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


