Abstract
Many cancer survivors experience menopausal symptoms, including female survivors taking aromatase inhibitors or with a history of oophorectomy or chemotherapy, and male survivors who received or are receiving androgen-ablative therapies. Sexual dysfunction is also common in cancer survivors. Sexual dysfunction and menopause-related symptoms can increase distress and have a significant negative impact on quality of life. This portion of the NCCN Guidelines for Survivorship provide recommendations for screening, evaluation, and treatment of sexual dysfunction and menopausal symptoms to help healthcare professionals who work with survivors of adult-onset cancer in the posttreatment period.
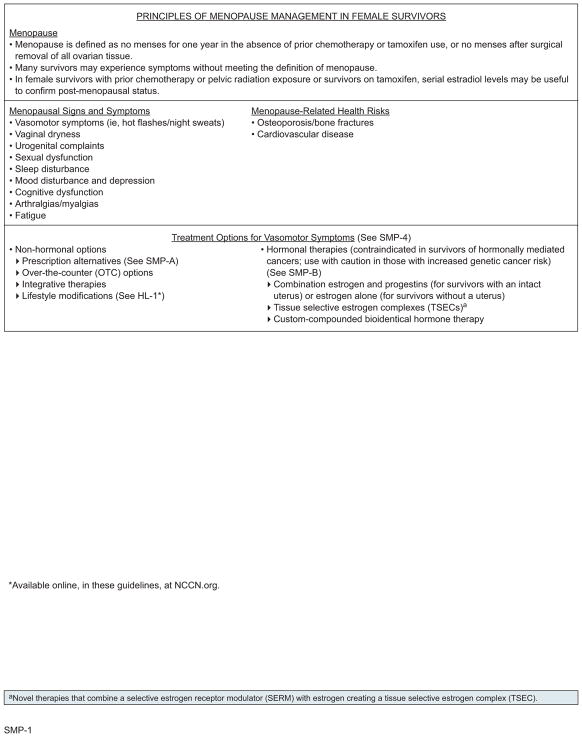
Menopause
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Survivorship define menopause as no menses for 1 year in the absence of prior chemotherapy or tamoxifen use, or no menses after surgical removal of all ovarian tissue. Healthy women reach menopause at a mean age of 51 years, with 95% reaching menopause between 45 and 55 years.1 Many cancer survivors experience menopausal symptoms without meeting the definition of menopause, including female survivors on aromatase inhibitors or with a history of oophorectomy or chemotherapy and male survivors who received or are receiving androgen ablative therapies (ie, androgen deprivation therapy [ADT]). These symptoms can include hot flashes/night sweats, vaginal dryness, urinary complaints, sexual dysfunction, sleep disturbance, mood disturbance, depression, cognitive dysfunction, arthralgias/myalgias, and fatigue; these menopausal symptoms can occur in both men and women. Males may also experience gynecomastia, decreased testicle size, and thinning of body hair. Menopausal symptoms can have a profound impact on quality of life (QoL).2,3
Menopausal symptoms in cancer survivors have been most extensively studied in female survivors after breast cancer treatment, with hot flashes reported occurring in approximately 46% to 73% of survivors.2,4–6 In one study of breast cancer survivors diagnosed at age ≤40 years, 46% reported hot flashes, 51% reported vaginal dryness, and 39% reported dyspareunia. 6 Similarly, approximately 50% to 80% of men on ADT experience hot flashes, which can persist after treatment.7–12 The incidence of gynecomastia in men on ADT varies with the method of ADT used and can be as high as 80% in those on estrogen therapy.9,13
Premenopausal cancer survivors who have received chemotherapy may experience transient or permanent menopause.14–16 If appropriate and desired, referral for fertility preservation should be considered before chemotherapy, because studies report that 33% to 73% of premenopausal women treated for breast cancer become perimenopausal or postmenopausal after treatment.2 Younger survivors with irregular menses may have primary ovarian insufficiency and may develop menopausal symptoms. 17 These women may or may not be fertile and should be counseled about the possibility of pregnancy despite amenorrhea.
MENOPAUSE-RELATED SYMPTOMS (Females)
MENOPAUSE-RELATED SYMPTOMS (Males)
MENOPAUSE-RELATED SYMPTOMS (Females and Males)
MENOPAUSE-RELATED SYMPTOMS (Females)
MENOPAUSE-RELATED SYMPTOMS (Males)
MENOPAUSE-RELATED SYMPTOMS (Females and Males)
MENOPAUSE-RELATED SYMPTOMS (Females)
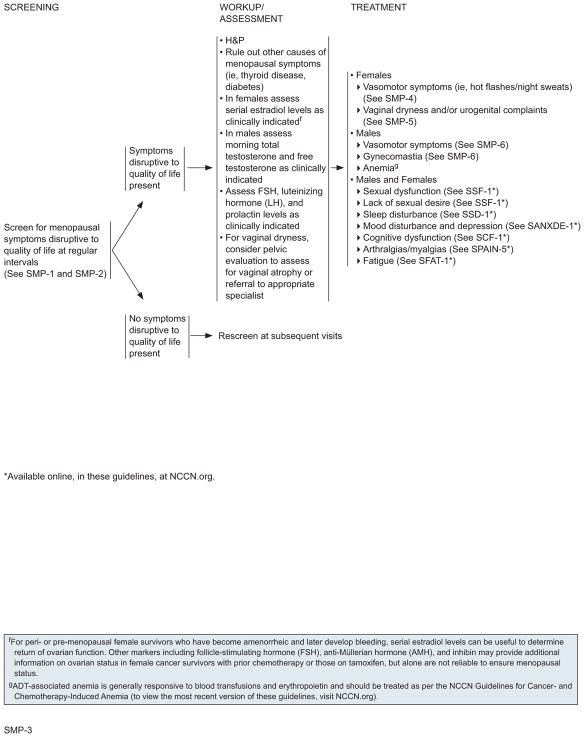
Assessment and Evaluation for Menopausal Symptoms
Survivors with menopausal symptoms disruptive to their QoL should be assessed and treated for medical causes of their symptoms such as thyroid disease and diabetes. Laboratory evaluation includes estradiol, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin, as clinically indicated. FSH is not a reliable marker of menopausal status in female survivors with prior chemotherapy or pelvic radiation exposure or in female survivors on tamoxifen. In male survivors, morning total testosterone and free testosterone may also be checked if hypogonadism is suspected.18 For women with complaints of vaginal dryness, a pelvic evaluation should be performed to assess for vaginal atrophy and can be accomplished by referral to an appropriate specialist.
For perimenopausal or premenopausal female survivors who have become amenorrheic and later develop bleeding, serial estradiol levels can be useful to determine return of ovarian function. Other markers, including FSH, anti-Mullerian hormone (AMH), and inhibin, may provide additional information on ovarian status in female cancer survivors with prior chemotherapy or those on tamoxifen, but alone are not reliable to ensure menopausal status.19,20
Management of Menopausal Symptoms in Female Survivors
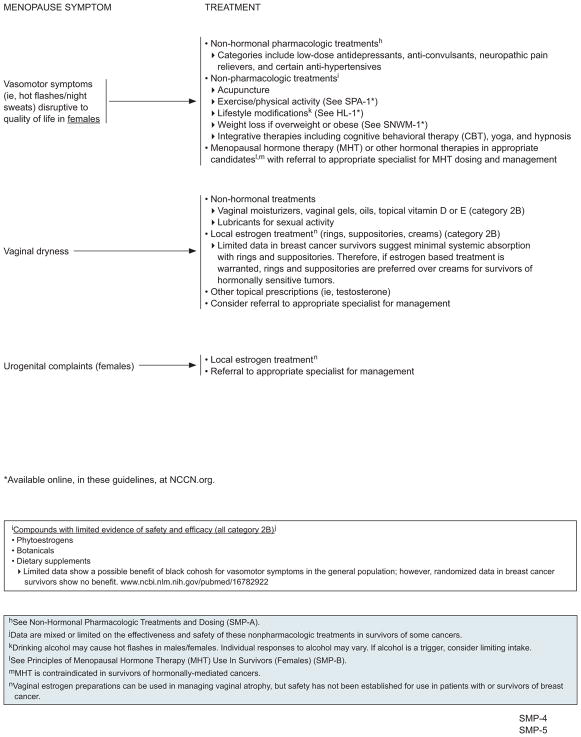
Management of sexual dysfunction, lack of sexual desire, sleep disturbance, mood disturbance, depression, cognitive dysfunction, fatigue, and arthralgias/myalgias is described in other sections of these NCCN Guidelines (visit NCCN.org for the complete version of these guidelines). Management of hot flashes, vaginal dryness, and urogenital complaints associated with menopause are described in the following sections. The panel prefers the use of nonhormonal options as first-line therapy for survivors with menopausal symptoms disruptive to QoL, but hormonal therapies can also be used after consideration of the risks and benefits to an individual survivor.
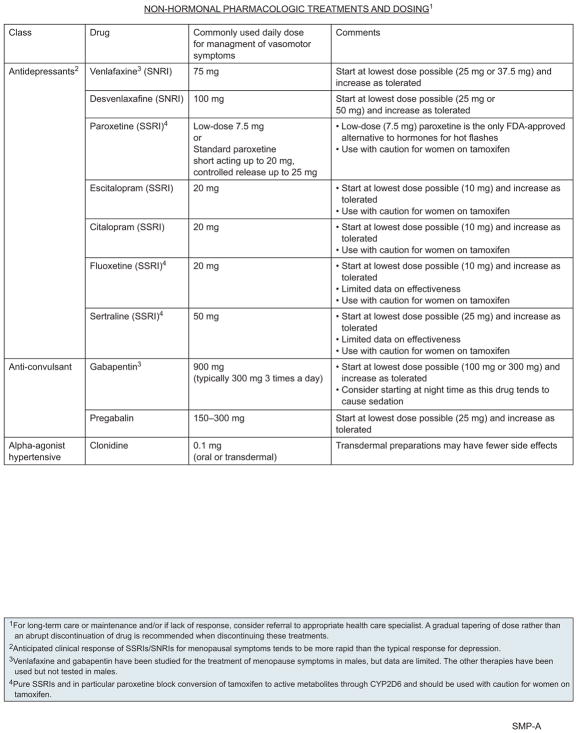
Nonhormonal Pharmacologic Treatment of Hot Flashes
For the management of hot flashes, nonhormonal pharmacologic options include low-dose antidepressants, anticonvulsants, neuropathic pain relievers, and certain antihypertensives.21–24
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been shown to improve vasomotor symptoms in the general population, although the degree of symptom reduction may be smaller than with hormonal treatments.25–27 A randomized clinical trial in healthy postmenopausal women showed that low-dose paroxetine reduces the frequency and severity of hot flashes.27 Small studies have shown that SSRIs and SNRIs also reduce the severity and frequency of hot flashes in female cancer and survivor populations.28–37 One study was a randomized, double-blind, placebo-controlled study of 80 survivors with gynecologic cancers.29 Results showed that 7.5 mg daily of paroxetine reduced the frequency and severity of vasomotor symptoms and the number of resultant nighttime awakenings.
However, pure SSRIs, and in particular paroxetine, should be used with caution in women taking tamoxifen, because these drugs block the conversion of tamoxifen to active metabolites through inhibition of cytochrome P450 2D6 (CYP2D6).38 However, analysis of a large database that included almost 17,000 breast cancer survivors found no evidence of an increase in cancer recurrence in women on concurrent tamoxifen and antidepressants, including paroxetine.39 In contrast, a study of 2,430 breast cancer survivors found an increased risk of cancer death in those taking tamoxifen and an SSRI.40 The NCCN Panel recommends alternative therapy if available, although no definitive conclusion can be drawn regarding the impact of the interaction between pure SSRIs and tamoxifen. Doses of antidepressants required for improvements in vasomotor symptoms are typically much lower than those needed for depression, and the response is typically faster; side effects include dry mouth, decreased appetite, fatigue, nausea, constipation, and possible sexual dysfunction. On discontinuation, SNRIs and SSRIs should be gradually tapered to minimize withdrawal symptoms.
The anticonvulsants gabapentin and pregabalin have also been shown to improve menopause-related vasomotor symptoms in the general population and in female cancer survivors.41–46 For example, one trial of 420 breast cancer survivors who experienced ≥2 hot flashes per day found that 900 mg/d of gabapentin decreased the hot flash severity score by 46% at 8 weeks compared with a 15% reduction in the placebo group.45 As with antidepressants, doses of anticonvulsants used in this setting are lower than in other settings. Side effects of anticonvulsants include somnolence, so they may be particularly useful when given at bedtime in patients who experience hot flash–disturbing sleep.
Small studies provide evidence that the alphaagonist antihypertensive clonidine can reduce hot flashes in some healthy postmenopausal women.47,48 Randomized controlled trials (RCTs) in breast cancer survivors also show that clonidine can reduce hot flash frequency and severity in postmenopausal women taking tamoxifen49,50; side effects include sleep difficulties, dry mouth, fatigue, dizziness, and nausea.
Several studies have compared nonhormonal pharmacologic treatments. For example, venlafaxine has been compared with clonidine in breast cancer survivors.51–53 Results of these studies have varied, but it appears that venlafaxine may have a faster effect, but is less well tolerated, than clonidine. A randomized crossover study compared venlafaxine with gabapentin in breast cancer survivors46 and found that both treatments resulted in similar reductions in hot flash severity. However, 68% of participants indicated a preference for venlafaxine compared with gabapentin (32%).
Nonpharmacologic Treatment of Hot Flashes
Nonpharmacologic treatments, including acupuncture, exercise/physical activity, yoga, lifestyle modifications, weight loss if overweight or obese, hypnosis, and cognitive behavioral therapy (CBT) may help survivors manage hot flashes.21,23,24,54–59 Phytoestrogens, botanicals, and dietary supplements can also be used (category 2B for all); however, data are mixed or limited on the effectiveness and safety of these particular treatments in the general menopausal population and in cancer survivors.22,60–67 Vitamin E has been thought to have marginal improvement in vasomotor symptoms in both general menopause and patients with breast cancer, but data are limited and have shown mixed results.68 Limited data show a possible benefit of black cohosh for vasomotor symptoms in the general population.69–71 However, randomized data in breast cancer survivors show no benefit.72
Acupuncture is used as a treatment for hot flashes in the general population, although evidence supporting its benefit is limited in the noncancer setting. 73,74 Several studies in women with cancer or female survivors have shown acupuncture to be a safe and effective option for managing vasomotor symptoms,75–78 3 of which compared acupuncture with either venlafaxine or gabapentin and found acupuncture to be equivalent to or better than drug treatment.75,77,78
Yoga may also help survivors manage hot flashes. A randomized trial in 355 healthy perimenopausal and postmenopausal women found that yoga improved QoL associated with menopause, including an improvement in the vasomotor symptom domain. 79 Another RCT showed that yoga improved sleep but did not affect the frequency of symptomatic burden of vasomotor symptoms.80
Evidence that exercise/physical activity helps manage hot flashes in postmenopausal women is inconclusive. 21,79,81–87 An RCT of 261 perimenopausal and postmenopausal women found no difference in the frequency of hot flashes between those randomized to an exercise intervention and to the control group.82 A similar trial involving 248 women also found that physical activity did not improve vasomotor symptoms.85 Studies in the survivorship and cancer populations are limited and do not support a role for the use of physical activity specifically to improve hot flash symptoms.88 Despite the lack of data suggesting a benefit for vasomotor symptoms, the NCCN Panel believes that physical activity should be recommended in menopausal cancer survivors given the many beneficial effects on overall health.
Other lifestyle modifications may also help minimize vasomotor symptoms. In the Women’s Health Initiative (WHI) Dietary Modification trial of 17,473 postmenopausal women not taking menopausal hormone therapy (MHT), those who lost ≥10% of their body weight were more likely to eliminate hot flash symptoms than those who maintained their body weight.56 Data in breast cancer survivors also suggest that weight loss may help alleviate hot flashes in this population.57,59 A longitudinal study in 761 women showed that those who quit smoking saw improvements in the frequency and severity of hot flashes compared with those who continued to smoke.89 Although studies of this sort have not been performed in survivor populations, data suggest that survivors who are current smokers are more likely to experience hot flashes.90 Individual vasomotor responses to alcohol vary.91 If alcohol triggers hot flashes in an individual survivor, limiting intake should be recommended.
Evidence suggests that CBT may reduce vasomotor symptoms in the general population92,93 and has been studied for the management of vasomotor symptoms in cancer and survivor populations. In one trial, patients with breast cancer were randomized to either receive CBT, CBT plus an exercise intervention, or a control group.88 Results suggested that CBT lessened the perceived burden of hot flashes. Another study randomized 96 women with menopausal symptoms after breast cancer treatment to a group CBT intervention or usual care group,94 and found that the hot flashes and night sweats problem rating was significantly reduced in the CBT arm.
Hormonal Treatment of Hot Flashes
MHT is the most effective treatment for the management of vasomotor symptoms in postmenopausal women.1,95–99 However, use of long-term MHT is controversial because the associated health risks are thought to outweigh potential benefits. In the past, MHT was typically given to postmenopausal women not only to treat vasomotor symptoms, but with the thought that MHT was effective at preventing heart disease. The best data examining health benefits and risks came from the large WHI study which showed that estrogen alone in postmenopausal women with prior hysterectomy was associated with an increased risk of stroke, a decreased risk of hip fracture, and had no effect on coronary heart disease or breast cancer incidence.100 In the WHI, estrogen plus progestin in postmenopausal women with a uterus was associated with a decreased risk of colorectal cancer (CRC) and hip fracture and an increased risk of stroke, pulmonary embolism, and invasive breast cancer.101 The study participants also had a higher rate of death from lung cancer during the intervention and were diagnosed with more advanced stages of CRC during the intervention and follow-up than those who received placebo.102–104 MHT was also associated with an increase in breast cancer incidence, and the cancers were more likely to be lymph node–positive.105,106 However, the absolute number of trial participants diagnosed with breast cancer was small, and the absolute risk was low. A systematic review of randomized double-blinded studies of MHT versus placebo found no evidence that MHT affects the incidence of CRC, but found that MHT increases the risk of breast cancer and death from lung cancer in postmenopausal women taking estrogen and progestins combined.107
Data from retrospective studies and an incomplete RCT suggest that MHT is safe to use in survivors of early-stage endometrial cancer.108–112 In breast cancer survivors, the data are inconclusive because the only 2 RCTs of MHT in this population had conflicting results. The HABITS trial found an increased risk of breast cancer recurrence with the use of MHT, with a cumulative incidence at 5 years of 22.2% in the MHT arm and 8.0% in the control arm.113 In the Stockholm trial, no difference was seen in breast cancer recurrence after 10.8 years of follow-up.114
Overall, based on these data, the panel believes that MHT can be used in appropriate female cancer survivors. Alternatives to MHT should typically be tried first, and patients should be referred to an appropriate specialist for dosing and management of MHT. MHT is contraindicated in survivors with a history of hormonally mediated cancers. Other contraindications for survivors mirror those for the general population and include a history of abnormal vaginal bleeding, active or recent history of thromboembolic event, pregnancy, and active liver disease. In addition, MHT should be used with caution in survivors with coronary heart disease or hypertension, in current smokers, and in those with an increased genetic cancer risk. In general, the lowest dose possible to control symptoms should be used, and treatment should be individualized based on risks.
Hormonal treatments for the relief of hot flashes in women include combination estrogen and progestins (for survivors with an intact uterus) or estrogen alone (for those without a uterus). Different local and systemic formulations of hormones exist including oral, transdermal, vaginal ring, and an intrauterine device. Estrogen transdermal formulations may be preferred over other formulations due to lower rates of venous thromboembolism (VTE) and stroke.115 Micronized progestin may be preferred over medroxyprogesterone acetate (MPA) due to lower rates of VTE and breast cancer risk. Other hormonal options for treating hot flashes include novel therapies that combine a selective estrogen receptor modulator (SERM) with estrogen, creating a tissue selective estrogen complex, one of which contains a conjugated estrogen and the SERM bazedoxifene116 and is FDA-approved for treating menopausal symptoms in healthy postmenopausal women. Custom compounded bioidentical hormones are not recommended because data supporting claims that they are safer and more effective than standard hormones are lacking.117,118 Young cancer survivors experiencing menopause at an early age can consider oral contraceptives or MHT for symptom relief and potential cardiac and bone benefits as long as not contraindicated.
Treatment of Vaginal Dryness
Vaginal dryness can be treated with over-the-counter vaginal moisturizers, gels, oils, and topicals for comfort and topical vitamin D or E.119,120 Lubricants can be used for sexual activity.121,122 Local hormonal treatments can also be used,101,123–127 although some controversy exists regarding their safety in survivors of hormone-dependent cancers.128 However, evidence suggests that local estrogen does not increase the risk of breast cancer recurrence.129 Vaginal estrogen preparations include rings, suppositories, and creams, and they have been shown to be effective for managing symptoms of vaginal dryness in menopausal women.127,130 Limited data in breast cancer survivors suggest minimal systemic absorption with rings and suppositories and are therefore preferred for survivors with hormone-sensitive tumors if estrogen-based treatment is warranted.128,131 Other topical hormone prescriptions (ie, testosterone) can also be considered, but data regarding safety or effectiveness are limited. One RCT of 441 survivors of breast or gynecologic cancer showed that vaginal dehydroepiandrosterone (DHEA) led to significant improvements in sexual desire, arousal, pain, and overall sexual function.132 In this trial, clinically important systemic estrogenic activity was not evident, and the treatment was safe and well tolerated.
Overall, the decision to use local hormones should be individualized with a discussion of the possible risks and benefits. Referral to an appropriate specialist for management can also be considered.
Treatment of Urogenital Complaints
Women sometimes present with urogenital complaints associated with menopause, such as urogenital atrophy and urinary incontinence. The NCCN Panel recommends treatment with local vaginal estrogen and referral to an appropriate specialist.130,133 See “Treatment of Vaginal Dryness,” (previous section) for a discussion on the safety of vaginal estrogen.
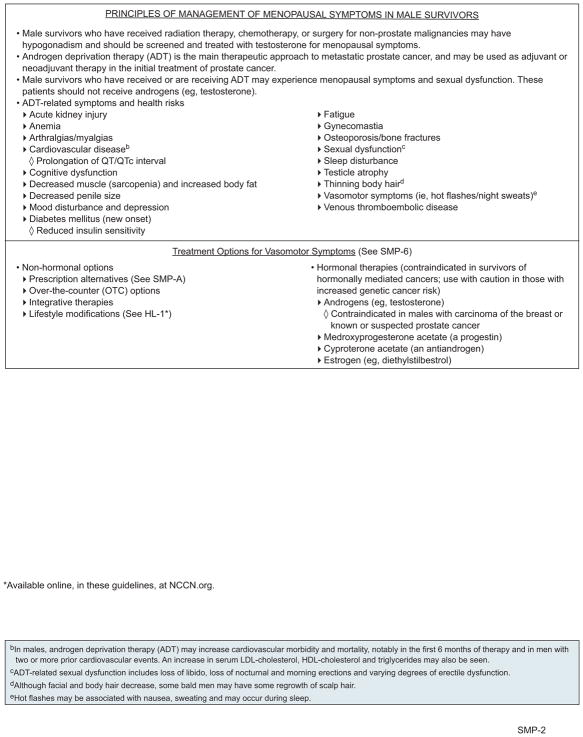
Management of ADT-Related Symptoms in Male Survivors
Prostate cancer survivors may be on ADT for 2 to 3 years without evidence of disease (see the NCCN Guidelines for Prostate Cancer, available at NCCN. org), and may experience many symptoms, including hot flashes, gynecomastia, and anemia.
Vasomotor Symptoms
For vasomotor symptoms disruptive to QoL in men, alternative ADT options, such as intermittent ADT or antiandrogen monotherapy, can be tried if deemed appropriate by the treating oncologist (see NCCN Guidelines for Prostate Cancer).
Androgens (eg, testosterone) are used as MHT for the relief of hot flashes in men who have hypogonadism and are cured of prostate cancer or who have hypogonadism from chemotherapy or radiation for other malignancies. However, androgens are contraindicated in men with advanced prostate malignancy on ADT. Hormonal options for the relief of hot flashes in survivors on ADT include MPA, estrogen, and cyproterone acetate.134–137
Nonhormonal options include the SSRI venlafaxine and the anticonvulsant gabapentin. Gabapentin has been shown to be safe and moderately effective at controlling hot flashes in men with prostate cancer in 2 RCTs.138–140 Case reports and small pilot studies have shown that venlafaxine may improve hot flash symptoms in men with prostate cancer undergoing ADT.141
As in female cancer survivors, men with ADT-related symptoms can try nonpharmacologic treatments, including acupuncture, exercise/physical activity, yoga, lifestyle modifications, weight loss if overweight or obese, hypnosis, and CBT. Small studies in prostate cancer survivors with a history of ADT have also found that acupuncture is effective at controlling hot flashes in this population.142,143 A study of 68 patients with prostate cancer on ADT also found that CBT reduced the perceived burden of hot flashes compared with usual care.144
Also as in women with vasomotor symptoms, phytoestrogens, botanicals, and dietary supplements are often used in men (category 2B for all). However, data are very limited on the effectiveness and safety of these nonpharmacologic treatments in survivors on ADT.145 Furthermore, there are concerns that supplemental vitamin E may increase the risk for prostate cancer.146,147
Gynecomastia
Gynecomastia and breast pain can be treated in men on ADT by prophylactic radiation (must be delivered before development of breast tissue), tamoxifen, or reduction mammoplasty.13,148,149
Anemia
Anemia in men on ADT is generally responsive to erythropoietin (EPO) and blood transfusions. These men can be treated as per the NCCN Guidelines for Cancer- and Chemotherapy-Induced Anemia (available at NCCN.org).
Sexual Dysfunction
Cancer treatment, especially hormonal therapy and therapy directed towards the pelvis, can often impair sexual function. In addition, depression and anxiety, which are common in survivors, can contribute to sexual problems. Thus, sexual dysfunction is common in survivors and can cause increased distress and have a significant negative impact on QoL.150–155 Nonetheless, sexual function is often not discussed with cancer survivors156–160; reasons for this include a lack of training of healthcare professionals, discomfort of providers and/or survivors with the topic, survivors’ perception of discomfort from the provider, and insufficient time during visits for discussion.150 However, effective strategies for treating both female and male sexual dysfunction exist, making these discussions a critical part of survivorship care.
Female Sexual Dysfunction
Female sexual problems relate to issues of sexual desire, arousal, orgasm, and pain.161–163 Sexual dysfunction after cancer treatment is common in female survivors. 154,164–170 A survey of 221 survivors of vaginal and cervical cancers found that the prevalence of sexual problems was significantly higher among survivors than among age- and race-matched controls from the National Health and Social Life Survey (mean number of problems, 2.6 vs 1.1; P<.001).168 A survey of survivors of ovarian germ cell tumors and age-, race-, and education-matched controls found that survivors reported a significant decrease in sexual pleasure.171
Female sexual dysfunction varies with cancer site and treatment modalities.165,166 For example, survivors of cervical cancer treated with radiotherapy had worse sexual functioning scores (for arousal, lubrication, orgasm, pain, and satisfaction) than those treated with surgery, whose sexual functioning was similar to that of age- and race-matched noncancer controls.165 A systematic review of sexual functioning in cervical cancer survivors found similar results, except that no differences in orgasm/satisfaction were observed.172 Chemotherapy seems to be linked to female sexual dysfunction in breast cancer survivors,166 possibly related to the prevalence of chemotherapy-induced menopause in this population.162 Furthermore, body-image changes related to breast cancer surgery and reconstruction can affect women’s sexual health and well-being.173 In addition, survivors with a history of hematopoietic stem cell transplant (HSCT) may have multiple types of sexual dysfunction, even 5 to 10 years after diagnosis.174–176 Some of the sexual dysfunction associated with HSCT is related to graft-versus-host disease (GVHD), which can result in vaginal fibrosis, stenosis, mucosal changes, vaginal irritation, bleeding, and increased sensitivity of genital tissues.175,177 In addition, high-dose corticosteroids used for chronic GVHD can increase emotional lability and depression, affecting feelings of attractiveness, sexual activity, and sexual QoL.
Male Sexual Dysfunction
The NIH Consensus Conference on Impotence defined impotence as “male erectile dysfunction, that is, the inability to achieve or maintain an erection sufficient for satisfactory sexual performance.” 178 In fact, impotence and erectile dysfunction (ED) are not synonymous. Impotence can involve problems of sexual desire, orgasm, or ejaculation, which are not necessarily linked with achieving or maintaining an erection.179
ED occurs frequently in the general population and increases with age.180 In one community-based study, 33% of men aged ≥75 years reported moderate ED or worse.181 ED is also very common in male cancer survivors. Anticancer treatment modalities used in a variety of cancers have the potential to damage blood vessels, leading to a reduction in blood circulation to the penis and/or damage to the autonomic nervous system. Thus, higher rates of ED are seen in cancer survivors than in the general population. The prevalence of ED in survivors of CRC has been reported to range from 45% to 75%,151,182,183 and has been reported in up to 90% of survivors of prostate cancer.184–188
Male cancer survivors exposed to radiation or chemotherapy often experience hypogonadism, usually primary hypogonadism. Hypogonadism in men refers to a decrease in the production of sperm and/or testosterone. Primary hypogonadism is the result of testicular failure. In these men, testosterone levels and sperm counts are below normal, and serum LH and FSH are above normal. Secondary hypogonadism is a disease of the pituitary or hypothalamus. In men with secondary hypogonadism, serum testosterone levels and sperm counts are subnormal and serum LH and FSH levels are normal or reduced. Adult-onset hypogonadism is characterized by a deficiency of testosterone and a failure of the body to produce an adequate compensatory response. In these men, low testosterone levels are associated with normal or low levels of gonadotropins, suggesting physiologic failure of both the testicles and hypothalamic-pituitary system.
Evaluation and Assessment of Sexual Function
All adult cancer survivors, regardless of gender identity and sexual orientation, should be asked about their sexual function at regular intervals by inquiring about any concerns or distress regarding sexual function, sexual activity, sexual relationships, or sex life. Cancer survivors who report distress should be evaluated further. Inquiries into treatment-related infertility should be made if indicated, with referrals as appropriate. ASCO’s recently updated clinical practice guidelines on fertility preservation for patients with cancer have more information on the topic.189 It is important for providers to be aware that fertility issues can be addressed in the survivorship phase, whether or not they were addressed before treatment.190–192 A discussion regarding the need for contraception may also be helpful in some cases, because the incidence of unplanned pregnancies is approximately 3 times higher in cancer survivors than in the general population.193
Survivors for whom screening does not indicate an issue with sexual function should be rescreened at subsequent visits. For survivors with sexual function concerns who do not wish to discuss them at the current visit, referral can be made to a sexual health specialist if the patient is interested. These survivors should also be reevaluated and engaged in discussions about the potential impact of treatment on sexual function at future visits.
For survivors who want to discuss their sexual function further, screening tools can be considered, several of which are available for both men and women. For women, options include the Brief Sexual Symptom Checklist for Women, the Arizona Sexual Experiences Scale (ASEX), the Female Sexual Function Index (FSFI), and a breast cancer-specific adaptation of the FSFI (FSFI-BC).194–197 For men, the Sexual Health Inventory for Men, the Sexual Quality of Life Questionnaire–Men, and the PROMIS Sexual Function and Satisfaction Measures–Male are examples. 180,198,199 The FSFI has been validated in patients with cancer and cancer survivors.200,201 The FSFI and ASEX were also identified in a systematic review as tools that have acceptable psychometric properties in patients with breast cancer.202 The other tools have not been validated in cancer or survivor populations.
Survivors with concerns about their sexual function should undergo a more thorough evaluation, including screening for possible psychosocial problems or mental health issues (ie, anxiety, depression, relationship issues, body image concerns, drug or alcohol use) that can contribute to sexual dysfunction. It is also important to identify prescription and over-the-counter medications (especially hormone therapy, narcotics, beta blockers, and SSRIs) that could be a contributing factor. Traditional risk factors for sexual dysfunction, such as cardiovascular disease, diabetes, obesity, smoking, and alcohol abuse, should also be assessed, as well as the patients’ oncologic and treatment history. In addition, the impact of cancer and its treatment on sexual function should be explored further. Finally, for men, total morning testosterone should be measured, if indicated by concerns regarding hypogonadism.18
Interventions for Female Sexual Dysfunction
Female sexual dysfunction is often multifactorial in nature. Therefore, treatment of sexual dysfunction often requires a multidimensional treatment plan that addresses the underlying issues, which can be physiologic (eg, menopause, illness), disease-induced, medication-induced, psychologic (eg, anxiety, depression), and interpersonal. Informed patient and physician decision-making is the standard for guiding treatment decisions for treatment. Referrals to specialists (ie, psychotherapy, sexual/couples counseling, gynecologic care, sexual health specialist) should be made if appropriate and available.
Overall, the evidence base for interventions to treat female sexual dysfunction in survivors is weak and high-quality studies are needed.203,204 Based on evidence from other populations, evidence from survivors when available, recommendations from the American Congress of Obstetricians and Gynecologists (ACOG),161 and consensus among the NCCN Survivorship Panel, the panel made recommendations for treatment of female sexual dysfunction in survivors. The panel recommends that treatment be guided by the specific type of problem. Treatments depend on the type of sexual dysfunction and may include both over-the-counter and prescription options, as well as pelvic physical therapy and integrative therapies. When prescription medications are being considered, the risks and benefits should be discussed or the survivor should be referred to an appropriate healthcare provider (eg, sexual health specialist) for prescription and/or treatment. The evidence base for each recommendation is described herein.
Integrative therapies, including yoga and meditation, may be helpful for female survivors with sexual dysfunction.79,205 In addition, CBT has been shown to be effective at improving sexual functioning in breast cancer survivors.206
Vaginal moisturizers and gels, oils, and topical vitamin D or E can help alleviate symptoms such as vaginal dryness and sexual pain,120,207 although data on these over-the-counter products are limited in the general population. In one study of breast cancer survivors, the control group used a nonhormonal moisturizer and saw a transient improvement in vaginal symptoms.118 Topical anesthetics may help with vaginal pain as demonstrated in a study of 46 breast cancer survivors that found that application of lidocaine to the vulvar vestibule before vaginal penetration improved dyspareunia.208
Pelvic physical therapy (ie, pelvic floor muscle training) may improve sexual pain, arousal, lubrication, orgasm, and satisfaction. A small study of 34 survivors of gynecologic cancers found that pelvic floor training significantly improved sexual function.209
Vaginal dilators are an option for survivors with pain during sexual activity. In addition, they are used for survivors with vaginal stenosis from pelvic radiation. However, evidence for the effectiveness of dilators is limited.210
Several topical prescription medications can also be considered for female survivors with sexual dysfunction. For example, vaginal estrogen (pills, rings, or creams) has been shown to be effective in treating vaginal dryness, itching, discomfort, and painful intercourse in postmenopausal women.101,123–127 A study of 76 postmenopausal breast cancer survivors on aromatase inhibitor therapy found that intravaginal testosterone cream or an estradiol-releasing vaginal ring were safe and improved vaginal atrophy and sexual function.211
Vaginal androgens (ie, DHEA; also known as prasterone) can be considered for vaginal dryness or pain with sexual activity. Prasterone received FDA approval in 2016. Several studies have shown prasterone to be effective at reducing dyspareunia in postmenopausal women.212–216 However, a systematic review and meta-analysis published in 2015 concluded it is uncertain whether prasterone improves menopausal symptoms.217 An RCT of 441 survivors of breast or gynecologic cancer showed that vaginal DHEA led to significant improvements in sexual desire, arousal, pain, and overall sexual function.132 In this trial, clinically important systemic estrogenic activity was not evident, and the treatment was safe and well tolerated. Overall, safety data for the use of androgen-based therapy in survivors of hormonally mediated cancers are limited. The FDA label for prasterone warns that exogenous estrogens are contraindicated in women with a history of breast cancer.218
In 2013, the FDA approved the SERM ospemifene for treating moderate to severe dyspareunia in postmenopausal women without known or suspected breast cancer and without a history of breast cancer. 219 Ospemifene has been studied in several large trials of women with postmenopausal vulvar and vaginal atrophy and was found to effectively treat vaginal dryness and dyspareunia.220–222 No data in the survivor population are available. The NCCN Panel recommends consideration of ospemifene for dyspareunia in survivors of cancers that are not hormonally sensitive.
In August 2015, the FDA approved flibanserin to treat acquired, generalized hypoactive sexual desire disorder in premenopausal women.223 Meta-analyses have shown that flibanserin resulted in approximately 1 additional satisfying sexual event every 2 months in premenopausal women.224,225 This drug has not been studied in patients with cancer or survivors, but it is a reasonable option to discuss with premenopausal survivors with low or lack of desire, libido, or intimacy; other options for these survivors include bupropion and buspirone.226 These drugs have been studied in a few trials involving noncancer populations.227–229 Despite limited safety and efficacy data, these drugs may be considered as options for hypoactive sexual desire disorder.
Currently, the panel does not recommend the use of oral phosphodiesterase type 5 inhibitors (PDE5i) for female sexual dysfunction due to the lack of data regarding their effectiveness in women. Although thought to increase pelvic blood flow to the clitoris and vagina,230,231 PDE5i showed contradictory results in RCTs of various noncancer populations of women being treated for sexual arousal disorder.232–237 More research is needed before a recommendation can be made regarding the use of sildenafil for the treatment of female sexual dysfunction.
Interventions for Male Sexual Dysfunction
Using a consensus-based approach, the NCCN Survivorship Panel concluded that: 1) informed patient and physician decision-making is the standard for guiding treatment decisions for treatment of male sexual dysfunction; and 2) a psychological overlay frequently exists in patients with sexual dysfunction and may be even more pronounced in the face of cancer survivorship. Thus, treatment of male sexual dysfunction may require a multidimensional treatment plan that addresses the underlying issues. Referrals to specialists (ie, psychotherapy, sexual/couples counseling, urology, sexual health specialist) should be made if appropriate and available. Treatment of sexual dysfunction in male survivors should be guided by the specific type of problem.
Treatment for male sexual dysfunction should include modification of risk factors, such as smoking cessation, weight loss, increasing physical activity, and avoiding excess alcohol consumption. Several trials have shown that such lifestyle modifications can improve sexual function in men.238–241 In fact, one study found that PDE5i treatment with an aerobic activity program was more effective than PDE5i treatment alone in 60 men with ED.242 Evidence for these effects in patients with cancer and survivors is lacking.
In addition, treatment of psychosocial problems, with referral to sex and couples therapy as appropriate, can often alleviate symptoms of male sexual dysfunction.243–247 Small studies in survivors of prostate cancer suggest that these approaches can also be helpful in the survivorship population.248,249 Therapy is often offered in conjunction with medical therapy.
PDE5i treatment has been shown to improve the symptoms of ED and to be well tolerated.250,251 These drugs can also be used for problems with male orgasms (eg, less intensity, difficulty achieving). Many studies have also shown the efficacy and tolerability of PDE5i for treating ED in patients with cancer and survivors.252,253 Importantly, PDE5i is contraindicated in patients taking oral nitrates because together they can lead to a dangerous decrease in blood pressure. 254,255 The timing and dose of on-demand PDE5i should be started conservatively, and it should be titrated to maximum dose if needed.179 Patients should be monitored periodically for efficacy, side effects, and any significant change in health status. In addition to on-demand PDE5i treatment, studies have shown that daily, low-dose treatment with these drugs can be effective.256–259
If total morning testosterone is <300 ng/dL, then hypogonadism is diagnosed and testosterone therapy may relieve symptoms of ED, problems with ejaculation, or problems with orgasm.260 An RCT in 470 men aged >65 years with testosterone levels <275 ng/dL found that testosterone gel led to improvements in sexual function, desire, and activity.261,262 Other studies have shown that the addition of testosterone to PDE5i therapy in men with low serum testosterone levels helps improve ED.263–268 Testosterone therapy should not be used if contraindicated by the primary oncologic diagnosis (eg, prostate cancer on active surveillance, prostate cancer on ADT).
Other treatments may help with ED and ejaculation and orgasm issues. Although evidence in the general population is lacking,269 studies in prostate cancer survivors suggest that pelvic physical therapy (ie, pelvic floor muscle training) may improve sexual function in this population.270,271 Vibratory therapy may reduce problems with orgasm.272 Finally, SSRIs (paroxetine, sertraline, citalopram, fluoxetine) dosed daily or clomipramine dosed on-demand may relieve problems with ejaculation (dry, retrograde, delayed, or climacturia).273–276
NCCN Categories of Evidence and Consensus.
Category 1
Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A
Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B
Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3
Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
NCCN Survivorship Panel Members
-
*,a,d,fCrystal S. Denlinger, MD/Chair†
Fox Chase Cancer Center
-
*,b,f,iTara Sanft, MD/Vice-Chair†Þ
Yale Cancer Center/Smilow Cancer Hospital
-
a,c,jK. Scott Baker, MD, MS€ξ
Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance
-
aShrujal Baxi, MD, MPH†
Memorial Sloan Kettering Cancer Center
-
*,e,hGregory Broderick, MDω
Mayo Clinic Cancer Center
-
fWendy Demark-Wahnefried, PhD, RD≅
University of Alabama at Birmingham Comprehensive Cancer Center
-
b,cDebra L. Friedman, MD, MS€‡†
Vanderbilt-Ingram Cancer Center
-
*,e,hMindy Goldman, MDΩ
UCSF Helen Diller Family Comprehensive Cancer Center
-
a,f,jMelissa Hudson, MD€‡†
St. Jude Children’s Research Hospital/The University of Tennessee Health Science Center
-
fNazanin Khakpour, MD¶
Moffitt Cancer Center
-
cAllison King, MD€Ψ‡†
Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine
-
a,jDivya Koura, MD‡
UC San Diego Moores Cancer Center
-
c,iElizabeth Kvale, MD£
University of Alabama at Birmingham Comprehensive Cancer Center
-
d,eRobin M. Lally, PhD, RN, MS
Fred & Pamela Buffett Cancer Center
-
dTerry S. Langbaum, MAS¥
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins
-
b,c,e,f,g,hMichelle Melisko, MD†£
UCSF Helen Diller Family Comprehensive Cancer Center
-
*,jJose G. Montoya, MDΦ
Stanford Cancer Institute
-
b,dKathi Mooney, RN, PhD#†
Huntsman Cancer Institute at the University of Utah
-
*,aJavid J. Moslehi, MDλÞ
Vanderbilt-Ingram Cancer Center
-
b,h,iTracey O’Connor, MD†
Roswell Park Cancer Institute
-
a,fLinda Overholser, MD, MPHÞ
University of Colorado Cancer Center
-
f,hElectra D. Paskett, PhDε
The Ohio State University Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
-
c,iJeffrey Peppercorn, MD, MPH†
Massachusetts General Hospital Cancer Center
-
jM. Alma Rodriguez, MD‡†Þ
The University of Texas MD Anderson Cancer Center
-
a,eKathryn J. Ruddy, MD, MPH‡†
Mayo Clinic Cancer Center
-
hPaula Silverman, MD†
Case Comprehensive Cancer Center/University Hospitals Seidman Cancer Center and Cleveland Clinic Taussig Cancer Institute
-
dSophia Smith, PhD, MSW£
Duke Cancer Institute
-
*,d,gKaren L. Syrjala, PhDθ£
Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance
-
e,hAmye Tevaarwerk, MD‡
University of Wisconsin Carbone Cancer Center
-
*,gSusan G. Urba, MD†£
University of Michigan Comprehensive Cancer Center
-
eMark T. Wakabayashi, MD, MPHΩ
City of Hope Comprehensive Cancer Center
-
*,jPhyllis Zee, MD, PhDΨ
Robert H. Lurie Comprehensive Cancer Center of Northwestern University
NCCN Staff: Deborah A. Freedman-Cass, PhD; and Nicole R. McMillian, MS
KEY:
*Discussion Section Writing Committee
Subcommittees: aAnthracycline-Induced Cardiac Toxicity; bFatigue; cCognitive Function; dAnxiety and Depression; eSexual Function; fHealthy Lifestyles; gPain; hMenopause-Related Symptoms; iSleep Disorders; jImmunizations and Infections
(Please note: Underlining denotes the lead of the subcommittee)
Specialties: ξBone Marrow Transplantation; λCardiology; εEpidemiology; ΠExercise/Physiology; ΩGynecology/Gynecologic Oncology; ‡Hematology/Hematology Oncology; ΦInfectious Diseases; ÞInternal Medicine; †Medical Oncology; ΨNeurology/Neuro-Oncology; #Nursing;; ≅Nutrition Science/Dietician; ¥Patient Advocacy; €Pediatric Oncology; θPsychiatry, Psychology, Including Health Behavior; £Supportive Care Including Palliative, Pain Management, Pastoral Care, and Oncology Social Work; ¶Surgery/Surgical Oncology; ωUrology
Individual Disclosures for Survivorship Panel
| Panel Member | Clinical Research Support/Data Safety Monitoring Board | Scientific Advisory Boards, Consultant, or Expert Witness | Promotional Advisory Boards, Consultant, or Speakers Bureau | Date Completed |
|---|---|---|---|---|
| K. Scott Baker, MD, MS | Cincinnati Childrens Medical Center | None | None | 5/1/17 |
| Shrujal Baxi, MD, MPH | AstraZeneca Pharmaceuticals LP; and Bristol-Myers Squibb Company | AstraZeneca Pharmaceuticals LP; and Flatiron Health | None | 7/14/17 |
| Gregory Broderick, MD | None | Repros | AbbVie | 7/17/17 |
| Wendy Demark-Wahnefried, PhD, RD | ACS; AICR; and NCI | ASCO | None | 8/4/17 |
| Crystal S. Denlinger, MD | Advaxis; Astex Pharmaceuticals; Bristol-Myers Squibb Company; Eli Lilly and Company; Genentech, Inc.; Incyte; MedImmune Inc.; Merrimack Pharmaceuticals; OncoMed Pharmaceuticals; and Pfizer Inc. | Eli Lilly and Company; EMD Serono; and Merrimack Pharmaceuticals | None | 2/9/17 |
| Debra L. Friedman, MD, MS | None | NCI; and Rally Foundation | None | 3/8/17 |
| Mindy Goldman, MD | DSM for PLUM study; and Madorra | Pfizer Inc. | Lumetra9/22/16 | |
| Melissa Hudson, MD | None | Pfizer Inc. | None | 8/04/17 |
| Nazanin Khakpour, MD | None | None | None | 2/26/16 |
| Allison King, MD | None | None | None | 8/4/17 |
| Divya Koura, MD | None | None | None | 2/23/17 |
| Elizabeth Kvale, MDa | None | None | None | 1/20/17 |
| Robin M. Lally, PhD, RN, MSa,b | ACS | NIH/NINR Study Section; ONS; and ONS Foundation | None | 5/9/17 |
| Terry S. Langbaum, MAS | None | None | None | 8/8/17 |
| Michelle Melisko, MDb | Celldex Therapeutics; Galena Biopharma; Eli Lilly and Company; Novartis Pharmaceuticals Corporation; and Puma Biotechnology, Inc. | None | Agendia BV | 11/16/16 |
| Jose G. Montoya, MD | None | None | None | 9/15/16 |
| Kathi Mooney, RN, PhD | University of Utah | NCI | None | 7/31/17 |
| Javid J. Moslehi, MD | Accleron, Inc.; ARIAD Pharmaceuticals, Inc.; Bristol-Myers Squibb Company; Millennium Pharmaceuticals, Inc.; and Vertex Pharmaceuticals Incorporated | ARIAD Pharmaceuticals, Inc.; Millennium Pharmaceuticals, Inc.; and Novartis Pharmaceuticals Corporation | None | 9/23/16 |
| Tracey O’Connor, MD | None | None | None | 6/5/17 |
| Linda Overholser, MD, MPHb | None | GW Cancer Institute Survivorship Project | None | 10/31/16 |
| Electra D. Paskett, PhDa,b | Merck & Co., Inc. | None | None | 7/31/17 |
| Jeffrey Peppercorn, MD, MPHb | None | None | None | 9/2/16 |
| M. Alma Rodriguez, MD | Amgen Inc.; and Ortho Biotech Products, L.P. | None | None | 6/19/17 |
| Kathryn J. Ruddy, MD, MPH | None | None | None | 8/9/17 |
| Tara Sanft, MD | None | None | Biotheranostics | 8/7/17 |
| Paula Silverman, MD | None | None | None | 3/4/17 |
| Sophia Smith, PhD, MSW | Pfizer Inc. | None | None | 5/5/17 |
| Karen L. Syrjala, PhD | NCI; and National Marrow Donor Program/CIBMTR | None | None | 5/24/17 |
| Amye Tevaarwerk, MD | None | Epic Care Systems | None | 7/25/17 |
| Susan G. Urba, MD | None | Merck & Co., Inc. | None | 7/17/17 |
| Mark T. Wakabayashi, MD, MPH | None | None | None | 3/20/17 |
| Phyllis Zee, MD, PhDa | Jazz Pharmaceuticals; Philips; and Technogel | Eisai Inc.; Merck & Co., Inc.; and Philips | None | 7/30/17 |
The NCCN Guidelines Staff have no conflicts to disclose.
- Elizabeth Kvale, MD: Aspire Health Care
- Robin M. Lally, PhD, RN, MS: UnitedHealthcare
- Electra D. Paskett, PhD: Pfizer Inc.
- Phyllis Zee, MD, PhD: Wolters Kluwer
- Robin M. Lally, PhD, RN, MS: UnitedHealthcare
- Michelle Melisko, MD: Merrimack
- Linda Overholser, MD, MPH: Bristol-Myers Squibb Company; and Nuvasive, Inc
- Electra D. Paskett, PhD: Pfizer Inc.
- Jeffrey Peppercorn, MD, MPH: GlaxoSmithKline
Footnotes
Please Note
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult the NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The National Comprehensive Cancer Network ® (NCCN®) makes no representation or warranties of any kind regarding their content, use, or application and disclaims any responsibility for their applications or use in any way. The full NCCN Guidelines for Survivorship are not printed in this issue of JNCCN but can be accessed online at NCCN.org.
Disclosures for the NCCN Survivorship Panel
At the beginning of each NCCN Guidelines panel meeting, panel members review all potential conflicts of interest. NCCN, in keeping with its commitment to public transparency, publishes these disclosures for panel members, staff, and NCCN itself.
Individual disclosures for the NCCN Survivorship Panel members can be found on page 1163. (The most recent version of these guidelines and accompanying disclosures are available on the NCCN Web site at NCCN.org.)
These guidelines are also available on the Internet. For the latest update, visit NCCN.org.
References
- 1.Martin K, Barbieri R. [Accessed August 10, 2017];Treatment of menopausal symptoms with hormone therapy. Available at: https://www.uptodate.com/contents/treatment-of-menopausal-symptoms-with-hormone-therapy.
- 2.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 3.Nishiyama T, Kanazawa S, Watanabe R, et al. Influence of hot flashes on quality of life in patients with prostate cancer treated with androgen deprivation therapy. Int J Urol. 2004;11:735–741. doi: 10.1111/j.1442-2042.2004.00896.x. [DOI] [PubMed] [Google Scholar]
- 4.Chandwani KD, Heckler CE, Mohile SG, et al. Hot flashes severity, complementary and alternative medicine use, and self-rated health in women with breast cancer. Explore (NY) 2014;10:241–247. doi: 10.1016/j.explore.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HY, Jotwani AC, Lai YH, et al. Hot flashes in breast cancer survivors: frequency, severity and impact. Breast. 2016;27:116–121. doi: 10.1016/j.breast.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leining MG, Gelber S, Rosenberg R, et al. Menopausal-type symptoms in young breast cancer survivors. Ann Oncol. 2006;17:1777–1782. doi: 10.1093/annonc/mdl299. [DOI] [PubMed] [Google Scholar]
- 7.Charig CR, Rundle JS. Flushing. Long-term side effect of orchiectomy in treatment of prostatic carcinoma. Urology. 1989;33:175–178. doi: 10.1016/0090-4295(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 8.Freedland SJ, Eastham J, Shore N. Androgen deprivation therapy and estrogen deficiency induced adverse effects in the treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2009;12:333–338. doi: 10.1038/pcan.2009.35. [DOI] [PubMed] [Google Scholar]
- 9.Guise TA, Oefelein MG, Eastham JA, et al. Estrogenic side effects of androgen deprivation therapy. Rev Urol. 2007;9:163–180. [PMC free article] [PubMed] [Google Scholar]
- 10.Sarosdy MF, Schellhammer PF, Soloway MS, et al. Endocrine effects, efficacy and tolerability of a 10. 8-mg depot formulation of goserelin acetate administered every 13 weeks to patients with advanced prostate cancer. BJU Int. 1999;83:801–806. doi: 10.1046/j.1464-410x.1999.00028.x. [DOI] [PubMed] [Google Scholar]
- 11.Schow DA, Renfer LG, Rozanski TA, Thompson IM. Prevalence of hot flushes during and after neoadjuvant hormonal therapy for localized prostate cancer. South Med J. 1998;91:855–857. doi: 10.1097/00007611-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Walker LM, Tran S, Robinson JW. Luteinizing hormone–releasing hormone agonists: a quick reference for prevalence rates of potential adverse effects. Clin Genitourin Cancer. 2013;11:375–384. doi: 10.1016/j.clgc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Autorino R, Perdona S, D’ Armiento M, et al. Gynecomastia in patients with prostate cancer: update on treatment options. Prostate Cancer Prostatic Dis. 2006;9:109–114. doi: 10.1038/sj.pcan.4500859. [DOI] [PubMed] [Google Scholar]
- 14.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 15.De Bruin ML, Huisbrink J, Hauptmann M, et al. Treatment-related risk factors for premature menopause following Hodgkin lymphoma. Blood. 2008;111:101–108. doi: 10.1182/blood-2007-05-090225. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin PJ, Ennis M, Pritchard KI, et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 17.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skinner R, Mulder RL, Kremer LC, et al. Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol. 2017;18:e75–90. doi: 10.1016/S1470-2045(17)30026-8. [DOI] [PubMed] [Google Scholar]
- 19.Krekow LK, Hellerstedt BA, Collea RP, et al. Incidence and predictive factors for recovery of ovarian function in amenorrheic women in their 40s treated with letrozole. J Clin Oncol. 2016;34:1594–1600. doi: 10.1200/JCO.2015.62.2985. [DOI] [PubMed] [Google Scholar]
- 20.Su HI, Sammel MD, Green J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592–599. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015;22:1155–1172. doi: 10.1097/GME.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 22.Drewe J, Bucher KA, Zahner C. A systematic review of non-hormonal treatments of vasomotor symptoms in climacteric and cancer patients. Springerplus. 2015;4:65. doi: 10.1186/s40064-015-0808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johns C, Seav SM, Dominick SA, et al. Informing hot flash treatment decisions for breast cancer survivors: a systematic review of randomized trials comparing active interventions. Breast Cancer Res Treat. 2016;156:415–426. doi: 10.1007/s10549-016-3765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010:CD004923. doi: 10.1002/14651858.CD004923.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med. 2014;174:1058–1066. doi: 10.1001/jamainternmed.2014.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelsberg G, Maragh L, Safranek S. Clinical inquiry: which nonhormonal treatments are effective for hot flashes? J Fam Pract. 2016;65:E1–3. [PubMed] [Google Scholar]
- 27.Simon JA, Portman DJ, Kaunitz AM, et al. Low-dose paroxetine 7. 5 mg for menopausal vasomotor symptoms: two randomized controlled trials. Menopause. 2013;20:1027–1035. doi: 10.1097/GME.0b013e3182a66aa7. [DOI] [PubMed] [Google Scholar]
- 28.Barton DL, Loprinzi CL, Novotny P, et al. Pilot evaluation of citalopram for the relief of hot flashes. J Support Oncol. 2003;1:47–51. [PubMed] [Google Scholar]
- 29.Capriglione S, Plotti F, Montera R, et al. Role of paroxetine in the management of hot flashes in gynecological cancer survivors: results of the first randomized single-center controlled trial. Gynecol Oncol. 2016;143:584–588. doi: 10.1016/j.ygyno.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter JS, Storniolo AM, Johns S, et al. Randomized, double-blind, placebo-controlled crossover trials of venlafaxine for hot flashes after breast cancer. Oncologist. 2007;12:124–135. doi: 10.1634/theoncologist.12-1-124. [DOI] [PubMed] [Google Scholar]
- 31.Biglia N, Bounous VE, Susini T, et al. Duloxetine and escitalopram for hot flushes: efficacy and compliance in breast cancer survivors. Eur J Cancer Care (Engl) 2016 doi: 10.1111/ecc.12484. [DOI] [PubMed] [Google Scholar]
- 32.Kimmick GG, Lovato J, McQuellon R, et al. Randomized, double-blind, placebo-controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J. 2006;12:114–122. doi: 10.1111/j.1075-122X.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 33.Loprinzi CL, Pisansky TM, Fonseca R, et al. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol. 1998;16:2377–2381. doi: 10.1200/JCO.1998.16.7.2377. [DOI] [PubMed] [Google Scholar]
- 34.Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578–1583. doi: 10.1200/JCO.2002.20.6.1578. [DOI] [PubMed] [Google Scholar]
- 35.Wu MF, Hilsenbeck SG, Tham YL, et al. The efficacy of sertraline for controlling hot flashes in women with or at high risk of developing breast cancer. Breast Cancer Res Treat. 2009;118:369–375. doi: 10.1007/s10549-009-0425-y. [DOI] [PubMed] [Google Scholar]
- 36.Ramaswami R, Villarreal MD, Pitta DM, et al. Venlafaxine in management of hot flashes in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2015;152:231–237. doi: 10.1007/s10549-015-3465-5. [DOI] [PubMed] [Google Scholar]
- 37.Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29:204–213. doi: 10.1007/s11606-013-2535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brauch H, Murdter TE, Eichelbaum M, Schwab M. Pharmacogenomics of tamoxifen therapy. Clin Chem. 2009;55:1770–1782. doi: 10.1373/clinchem.2008.121756. [DOI] [PubMed] [Google Scholar]
- 39.Haque R, Shi J, Schottinger JE, et al. Tamoxifen and antidepressant drug interaction in a cohort of 16,887 breast cancer survivors. J Natl Cancer Inst. 2016;108:djv337. doi: 10.1093/jnci/djv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20142325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butt DA, Lock M, Lewis JE, et al. Gabapentin for the treatment of menopausal hot flashes: a randomized controlled trial. Menopause. 2008;15:310–318. doi: 10.1097/gme.0b013e3180dca175. [DOI] [PubMed] [Google Scholar]
- 42.Yurcheshen ME, Guttuso T, Jr, McDermott M, et al. Effects of gabapentin on sleep in menopausal women with hot flashes as measured by a Pittsburgh Sleep Quality Index factor scoring model. J Womens Health (Larchmt) 2009;18:1355–1360. doi: 10.1089/jwh.2008.1257. [DOI] [PubMed] [Google Scholar]
- 43.Reddy SY, Warner H, Guttuso T, Jr, et al. Gabapentin, estrogen, and placebo for treating hot flushes: a randomized controlled trial. Obstet Gynecol. 2006;108:41–48. doi: 10.1097/01.AOG.0000222383.43913.ed. [DOI] [PubMed] [Google Scholar]
- 44.Loprinzi CL, Qin R, Balcueva EP, et al. Phase III, randomized, double-blind, placebo-controlled evaluation of pregabalin for alleviating hot flashes, N07C1. J Clin Oncol. 2010;28:641–647. doi: 10.1200/JCO.2009.24.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet. 2005;366:818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 2010;28:5147–5152. doi: 10.1200/JCO.2010.29.9230. [DOI] [PubMed] [Google Scholar]
- 47.Laufer LR, Erlik Y, Meldrum DR, Judd HL. Effect of clonidine on hot flashes in postmenopausal women. Obstet Gynecol. 1982;60:583–586. [PubMed] [Google Scholar]
- 48.Nagamani M, Kelver ME, Smith ER. Treatment of menopausal hot flashes with transdermal administration of clonidine. Am J Obstet Gynecol. 1987;156:561–565. doi: 10.1016/0002-9378(87)90050-0. [DOI] [PubMed] [Google Scholar]
- 49.Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med. 2000;132:788–793. doi: 10.7326/0003-4819-132-10-200005160-00004. [DOI] [PubMed] [Google Scholar]
- 50.Goldberg RM, Loprinzi CL, O’ Fallon JR, et al. Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol. 1994;12:155–158. doi: 10.1200/JCO.1994.12.1.155. [DOI] [PubMed] [Google Scholar]
- 51.Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients—a double-blind, randomized study. Ann Oncol. 2007;18:689–693. doi: 10.1093/annonc/mdl478. [DOI] [PubMed] [Google Scholar]
- 52.Buijs C, Mom CH, Willemse PH, et al. Venlafaxine versus clonidine for the treatment of hot flashes in breast cancer patients: a double-blind, randomized cross-over study. Breast Cancer Res Treat. 2009;115:573–580. doi: 10.1007/s10549-008-0138-7. [DOI] [PubMed] [Google Scholar]
- 53.Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2011;29:3862–3868. doi: 10.1200/JCO.2010.33.1298. [DOI] [PubMed] [Google Scholar]
- 54.Cramer H, Rabsilber S, Lauche R, et al. Yoga and meditation for menopausal symptoms in breast cancer survivors—a randomized controlled trial. Cancer. 2015;121:2175–2184. doi: 10.1002/cncr.29330. [DOI] [PubMed] [Google Scholar]
- 55.Elkins G, Marcus J, Stearns V, et al. Randomized trial of a hypnosis intervention for treatment of hot flashes among breast cancer survivors. J Clin Oncol. 2008;26:5022–5026. doi: 10.1200/JCO.2008.16.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kroenke CH, Caan BJ, Stefanick ML, et al. Effects of a dietary intervention and weight change on vasomotor symptoms in the Women’ s Health Initiative. Menopause. 2012;19:980–988. doi: 10.1097/gme.0b013e31824f606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caan BJ, Emond JA, Su HI, et al. Effect of postdiagnosis weight change on hot flash status among early-stage breast cancer survivors. J Clin Oncol. 2012;30:1492–1497. doi: 10.1200/JCO.2011.36.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stefanopoulou E, Grunfeld EA. Mind-body interventions for vasomotor symptoms in healthy menopausal women and breast cancer survivors. A systematic review. J Psychosom Obstet Gynaecol. 2017;38:210–225. doi: 10.1080/0167482X.2016.1235147. [DOI] [PubMed] [Google Scholar]
- 59.Su HI, Sammel MD, Springer E, et al. Weight gain is associated with increased risk of hot flashes in breast cancer survivors on aromatase inhibitors. Breast Cancer Res Treat. 2010;124:205–211. doi: 10.1007/s10549-010-0802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franco OH, Chowdhury R, Troup J, et al. Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis. JAMA. 2016;315:2554–2563. doi: 10.1001/jama.2016.8012. [DOI] [PubMed] [Google Scholar]
- 61.Quella SK, Loprinzi CL, Barton DL, et al. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: a North Central Cancer Treatment Group trial. J Clin Oncol. 2000;18:1068–1074. doi: 10.1200/JCO.2000.18.5.1068. [DOI] [PubMed] [Google Scholar]
- 62.Taku K, Melby MK, Kronenberg F, et al. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: systematic review and meta-analysis of randomized controlled trials. Menopause. 2012;19:776–790. doi: 10.1097/gme.0b013e3182410159. [DOI] [PubMed] [Google Scholar]
- 63.Thomas AJ, Ismail R, Taylor-Swanson L, et al. Effects of isoflavones and amino acid therapies for hot flashes and co-occurring symptoms during the menopausal transition and early postmenopause: a systematic review. Maturitas. 2014;78:263–276. doi: 10.1016/j.maturitas.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Patten CL, Olivotto IA, Chambers GK, et al. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized, controlled clinical trial. J Clin Oncol. 2002;20:1449–1455. doi: 10.1200/JCO.2002.20.6.1449. [DOI] [PubMed] [Google Scholar]
- 65.MacGregor CA, Canney PA, Patterson G, et al. A randomised double-blind controlled trial of oral soy supplements versus placebo for treatment of menopausal symptoms in patients with early breast cancer. Eur J Cancer. 2005;41:708–714. doi: 10.1016/j.ejca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Sharma P, Wisniewski A, Braga-Basaria M, et al. Lack of an effect of high dose isoflavones in men with prostate cancer undergoing androgen deprivation therapy. J Urol. 2009;182:2265–2272. doi: 10.1016/j.juro.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen WY, Giobbie-Hurder A, Gantman K, et al. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat. 2014;145:381–388. doi: 10.1007/s10549-014-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dennehy C, Tsourounis C. A review of select vitamins and minerals used by postmenopausal women. Maturitas. 2010;66:370–380. doi: 10.1016/j.maturitas.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Laakmann E, Grajecki D, Doege K, et al. Efficacy of Cimicifuga racemosa, Hypericum perforatum and Agnus castus in the treatment of climacteric complaints: a systematic review. Gynecol Endocrinol. 2012;28:703–709. doi: 10.3109/09513590.2011.650772. [DOI] [PubMed] [Google Scholar]
- 70.Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;9:CD007244. doi: 10.1002/14651858.CD007244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobson JS, Troxel AB, Evans J, et al. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol. 2001;19:2739–2745. doi: 10.1200/JCO.2001.19.10.2739. [DOI] [PubMed] [Google Scholar]
- 72.Pockaj BA, Gallagher JG, Loprinzi CL, et al. Phase III double-blind, randomized, placebo-controlled crossover trial of black cohosh in the management of hot flashes: NCCTG Trial N01CC1. J Clin Oncol. 2006;24:2836–2841. doi: 10.1200/JCO.2005.05.4296. [DOI] [PubMed] [Google Scholar]
- 73.Cho SH, Whang WW. Acupuncture for vasomotor menopausal symptoms: a systematic review. Menopause. 2009;16:1065–1073. doi: 10.1097/gme.0b013e3181a48abd. [DOI] [PubMed] [Google Scholar]
- 74.Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flushes. Cochrane Database Syst Rev. 2013;7:CD007410. doi: 10.1002/14651858.CD007410.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garland SN, Xie SX, Li Q, et al. Comparative effectiveness of electroacupuncture versus gabapentin for sleep disturbances in breast cancer survivors with hot flashes: a randomized trial. Menopause. 2017;24:517–523. doi: 10.1097/GME.0000000000000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lesi G, Razzini G, Musti MA, et al. Acupuncture as an integrative approach for the treatment of hot flashes in women with breast cancer: a prospective multicenter randomized controlled trial (AcCliMaT) J Clin Oncol. 2016;34:1795–1802. doi: 10.1200/JCO.2015.63.2893. [DOI] [PubMed] [Google Scholar]
- 77.Walker EM, Rodriguez AI, Kohn B, et al. Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor-positive breast cancer: a randomized controlled trial. J Clin Oncol. 2010;28:634–640. doi: 10.1200/JCO.2009.23.5150. [DOI] [PubMed] [Google Scholar]
- 78.Mao JJ, Bowman MA, Xie SX, et al. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: a randomized placebo-controlled trial. J Clin Oncol. 2015;33:3615–3620. doi: 10.1200/JCO.2015.60.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reed SD, Guthrie KA, Newton KM, et al. Menopausal quality of life: RCT of yoga, exercise, and omega-3 supplements. Am J Obstet Gynecol. 2014;210:244e1–11. doi: 10.1016/j.ajog.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21:339–346. doi: 10.1097/GME.0b013e31829e4baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aiello EJ, Yasui Y, Tworoger SS, et al. Effect of a yearlong, moderate-intensity exercise intervention on the occurrence and severity of menopause symptoms in postmenopausal women. Menopause. 2004;11:382–388. doi: 10.1097/01.gme.0000113932.56832.27. [DOI] [PubMed] [Google Scholar]
- 82.Daley AJ, Thomas A, Roalfe AK, et al. The effectiveness of exercise as treatment for vasomotor menopausal symptoms: randomised controlled trial. BJOG. 2015;122:565–575. doi: 10.1111/1471-0528.13193. [DOI] [PubMed] [Google Scholar]
- 83.Daley A, Stokes-Lampard H, Thomas A, MacArthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;11:CD006108. doi: 10.1002/14651858.CD006108.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindh-Astrand L, Nedstrand E, Wyon Y, Hammar M. Vasomotor symptoms and quality of life in previously sedentary postmenopausal women randomised to physical activity or estrogen therapy. Maturitas. 2004;48:97–105. doi: 10.1016/S0378-5122(03)00187-7. [DOI] [PubMed] [Google Scholar]
- 85.Sternfeld B, Guthrie KA, Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause. 2014;21:330–338. doi: 10.1097/GME.0b013e31829e4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sternfeld B, Dugan S. Physical activity and health during the menopausal transition. Obstet Gynecol Clin North Am. 2011;38:537–566. doi: 10.1016/j.ogc.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ueda M. A 12-week structured education and exercise program improved climacteric symptoms in middle-aged women. J Physiol Anthropol Appl Human Sci. 2004;23:143–148. doi: 10.2114/jpa.23.143. [DOI] [PubMed] [Google Scholar]
- 88.Duijts SF, van Beurden M, Oldenburg HS, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment-induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. J Clin Oncol. 2012;30:4124–4133. doi: 10.1200/JCO.2012.41.8525. [DOI] [PubMed] [Google Scholar]
- 89.Smith RL, Flaws JA, Gallicchio L. Does quitting smoking decrease the risk of midlife hot flashes? A longitudinal analysis Maturitas. 2015;82:123–127. doi: 10.1016/j.maturitas.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peppone LJ, Mustian KM, Morrow GR, et al. The effect of cigarette smoking on cancer treatment-related side effects. Oncologist. 2011;16:1784–1792. doi: 10.1634/theoncologist.2011-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gallicchio L, Miller SR, Kiefer J, et al. Risk factors for hot flashes among women undergoing the menopausal transition: baseline results from the Midlife Women’s Health Study. Menopause. 2015;22:1098–1107. doi: 10.1097/GME.0000000000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ayers B, Smith M, Hellier J, et al. Effectiveness of group and self-help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): a randomized controlled trial. Menopause. 2012;19:749–759. doi: 10.1097/gme.0b013e31823fe835. [DOI] [PubMed] [Google Scholar]
- 93.Alder J, Eymann Besken K, Armbruster U, et al. Cognitive-behavioural group intervention for climacteric syndrome. Psychother Psychosom. 2006;75:298–303. doi: 10.1159/000093951. [DOI] [PubMed] [Google Scholar]
- 94.Mann E, Smith MJ, Hellier J, et al. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol. 2012;13:309–318. doi: 10.1016/S1470-2045(11)70364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baber RJ, Panay N, Fenton A, Group IMSW. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric. 2016;19:109–150. doi: 10.3109/13697137.2015.1129166. [DOI] [PubMed] [Google Scholar]
- 96.Barnabei VM, Grady D, Stovall DW, et al. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet Gynecol. 2002;100:1209–1218. doi: 10.1016/s0029-7844(02)02369-4. [DOI] [PubMed] [Google Scholar]
- 97.Brunner RL, Aragaki A, Barnabei V, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trial. Menopause. 2010;17:946–954. doi: 10.1097/gme.0b013e3181d76953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Climacteric. 2016;19:313–315. doi: 10.1080/13697137.2016.1196047. [DOI] [PubMed] [Google Scholar]
- 99.Greendale GA, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet Gynecol. 1998;92:982–988. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- 100.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 101.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 102.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 103.Chlebowski RT, Schwartz AG, Wakelee H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374:1243–1251. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simon MS, Chlebowski RT, Wactawski-Wende J, et al. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol. 2012;30:3983–3990. doi: 10.1200/JCO.2012.42.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chlebowski RT, Rohan TE, Manson JE, et al. Breast cancer after use of estrogen plus progestin and estrogen alone: analyses of data from 2 Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1:296–305. doi: 10.1001/jamaoncol.2015.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marjoribanks J, Farquhar C, Roberts H, et al. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143. doi: 10.1002/14651858.CD004143.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:587–592. doi: 10.1200/JCO.2005.02.8464. [DOI] [PubMed] [Google Scholar]
- 109.Chapman JA, DiSaia PJ, Osann K, et al. Estrogen replacement in surgical stage I and II endometrial cancer survivors. Am J Obstet Gynecol. 1996;175:1195–1200. doi: 10.1016/s0002-9378(96)70027-3. [DOI] [PubMed] [Google Scholar]
- 110.Creasman WT, Henderson D, Hinshaw W, Clarke-Pearson DL. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol. 1986;67:326–330. [PubMed] [Google Scholar]
- 111.Lee RB, Burke TW, Park RC. Estrogen replacement therapy following treatment for stage I endometrial carcinoma. Gynecol Oncol. 1990;36:189–191. doi: 10.1016/0090-8258(90)90171-g. [DOI] [PubMed] [Google Scholar]
- 112.Suriano KA, McHale M, McLaren CE, et al. Estrogen replacement therapy in endometrial cancer patients: a matched control study. Obstet Gynecol. 2001;97:555–560. doi: 10.1016/s0029-7844(00)01221-7. [DOI] [PubMed] [Google Scholar]
- 113.Holmberg L, Iversen OE, Rudenstam CM, et al. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst. 2008;100:475–482. doi: 10.1093/jnci/djn058. [DOI] [PubMed] [Google Scholar]
- 114.Fahlen M, Fornander T, Johansson H, et al. Hormone replacement therapy after breast cancer: 10 year follow up of the Stockholm randomised trial. Eur J Cancer. 2013;49:52–59. doi: 10.1016/j.ejca.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 115.Bergendal A, Kieler H, Sundstrom A, et al. Risk of venous thromboembolism associated with local and systemic use of hormone therapy in peri- and postmenopausal women and in relation to type and route of administration. Menopause. 2016;23:593–599. doi: 10.1097/GME.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 116.Kagan R, Williams RS, Pan K, et al. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause. 2010;17:281–289. doi: 10.1097/GME.0b013e3181b7c65f. [DOI] [PubMed] [Google Scholar]
- 117.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; American Society for Reproductive Medicine Practice Committee. Compounded bioidentical menopausal hormone therapy. Fertil Steril. 2012;98:308–312. doi: 10.1016/j.fertnstert.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 118.Whelan AM, Jurgens TM, Trinacty M. Bioidentical progesterone cream for menopause-related vasomotor symptoms: is it effective? Ann Pharmacother. 2013;47:112–116. doi: 10.1345/aph.1R362. [DOI] [PubMed] [Google Scholar]
- 119.Biglia N, Peano E, Sgandurra P, et al. Low-dose vaginal estrogens or vaginal moisturizer in breast cancer survivors with urogenital atrophy: a preliminary study. Gynecol Endocrinol. 2010;26:404–412. doi: 10.3109/09513591003632258. [DOI] [PubMed] [Google Scholar]
- 120.Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240–250. doi: 10.1111/j.1743-6109.2011.02543.x. [DOI] [PubMed] [Google Scholar]
- 121.Loprinzi CL, Abu-Ghazaleh S, Sloan JA, et al. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol. 1997;15:969–973. doi: 10.1200/JCO.1997.15.3.969. [DOI] [PubMed] [Google Scholar]
- 122.Nachtigall LE. Comparative study: replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178–180. doi: 10.1016/s0015-0282(16)56474-7. [DOI] [PubMed] [Google Scholar]
- 123.Ayton RA, Darling GM, Murkies AL, et al. A comparative study of safety and efficacy of continuous low dose oestradiol released from a vaginal ring compared with conjugated equine oestrogen vaginal cream in the treatment of postmenopausal urogenital atrophy. Br J Obstet Gynaecol. 1996;103:351–358. doi: 10.1111/j.1471-0528.1996.tb09741.x. [DOI] [PubMed] [Google Scholar]
- 124.Fooladi E, Davis SR. An update on the pharmacological management of female sexual dysfunction. Expert Opin Pharmacother. 2012;13:2131–2142. doi: 10.1517/14656566.2012.725046. [DOI] [PubMed] [Google Scholar]
- 125.Krychman ML. Vaginal estrogens for the treatment of dyspareunia. J Sex Med. 2011;8:666–674. doi: 10.1111/j.1743-6109.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 126.Raghunandan C, Agrawal S, Dubey P, et al. A comparative study of the effects of local estrogen with or without local testosterone on vulvovaginal and sexual dysfunction in postmenopausal women. J Sex Med. 2010;7:1284–1290. doi: 10.1111/j.1743-6109.2009.01667.x. [DOI] [PubMed] [Google Scholar]
- 127.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006:CD001500. doi: 10.1002/14651858.CD001500.pub2. [DOI] [PubMed] [Google Scholar]
- 128.Committee Opinion No. 659 summary: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol. 2016;127:618–619. doi: 10.1097/AOG.0000000000001349. [DOI] [PubMed] [Google Scholar]
- 129.Le Ray I, Dell’Aniello S, Bonnetain F, et al. Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study. Breast Cancer Res Treat. 2012;135:603–609. doi: 10.1007/s10549-012-2198-y. [DOI] [PubMed] [Google Scholar]
- 130.Trinkaus M, Chin S, Wolfman W, et al. Should urogenital atrophy in breast cancer survivors be treated with topical estrogens? Oncologist. 2008;13:222–231. doi: 10.1634/theoncologist.2007-0234. [DOI] [PubMed] [Google Scholar]
- 131.Wills S, Ravipati A, Venuturumilli P, et al. Effects of vaginal estrogens on serum estradiol levels in postmenopausal breast cancer survivors and women at risk of breast cancer taking an aromatase inhibitor or a selective estrogen receptor modulator. J Oncol Pract. 2012;8:144–148. doi: 10.1200/JOP.2011.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barton DL, Sloan JA, Shuster LT, et al. Impact of vaginal dehydroepiandosterone (DHEA) on vaginal symptoms in female cancer survivors: Trial N10C1 (Alliance) [abstract] J Clin Oncol. 2014;32(Suppl) Abstract 9507. [Google Scholar]
- 133.Mazzarello S, Hutton B, Ibrahim MF, et al. Management of urogenital atrophy in breast cancer patients: a systematic review of available evidence from randomized trials. Breast Cancer Res Treat. 2015;152:1–8. doi: 10.1007/s10549-015-3434-z. [DOI] [PubMed] [Google Scholar]
- 134.Frisk J. Managing hot flushes in men after prostate cancer—a systematic review. Maturitas. 2010;65:15–22. doi: 10.1016/j.maturitas.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 135.Gerber GS, Zagaja GP, Ray PS, Rukstalis DB. Transdermal estrogen in the treatment of hot flushes in men with prostate cancer. Urology. 2000;55:97–101. doi: 10.1016/s0090-4295(99)00370-2. [DOI] [PubMed] [Google Scholar]
- 136.Irani J, Salomon L, Oba R, et al. Efficacy of venlafaxine, medroxyprogesterone acetate, and cyproterone acetate for the treatment of vasomotor hot flushes in men taking gonadotropin-releasing hormone analogues for prostate cancer: a double-blind, randomised trial. Lancet Oncol. 2010;11:147–154. doi: 10.1016/S1470-2045(09)70338-9. [DOI] [PubMed] [Google Scholar]
- 137.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;331:347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- 138.Ahmadi H, Daneshmand S. Androgen deprivation therapy: evidence-based management of side effects. BJU Int. 2013;111:543–548. doi: 10.1111/j.1464-410X.2012.11774.x. [DOI] [PubMed] [Google Scholar]
- 139.Moraska AR, Atherton PJ, Szydlo DW, et al. Gabapentin for the management of hot flashes in prostate cancer survivors: a longitudinal continuation Study-NCCTG Trial N00CB. J Support Oncol. 2010;8:128–132. [PMC free article] [PubMed] [Google Scholar]
- 140.Loprinzi CL, Dueck AC, Khoyratty BS, et al. A phase III randomized, double-blind, placebo-controlled trial of gabapentin in the management of hot flashes in men (N00CB) Ann Oncol. 2009;20:542–549. doi: 10.1093/annonc/mdn644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quella SK, Loprinzi CL, Sloan J, et al. Pilot evaluation of venlafaxine for the treatment of hot flashes in men undergoing androgen ablation therapy for prostate cancer. J Urol. 1999;162:98–102. doi: 10.1097/00005392-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 142.Ashamalla H, Jiang ML, Guirguis A, et al. Acupuncture for the alleviation of hot flashes in men treated with androgen ablation therapy. Int J Radiat Oncol Biol Phys. 2011;79:1358–1363. doi: 10.1016/j.ijrobp.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 143.Frisk J, Spetz AC, Hjertberg H, et al. Two modes of acupuncture as a treatment for hot flushes in men with prostate cancer—a prospective multicenter study with long-term follow-up. Eur Urol. 2009;55:156–163. doi: 10.1016/j.eururo.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 144.Stefanopoulou E, Yousaf O, Grunfeld EA, Hunter MS. A randomised controlled trial of a brief cognitive behavioural intervention for men who have hot flushes following prostate cancer treatment (MANCAN) Psychooncology. 2015;24:1159–1166. doi: 10.1002/pon.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dueregger A, Heidegger I, Ofer P, et al. The use of dietary supplements to alleviate androgen deprivation therapy side effects during prostate cancer treatment. Nutrients. 2014;6:4491–4519. doi: 10.3390/nu6104491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Klein EA, Thompson IM, Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peters U, Littman AJ, Kristal AR, et al. Vitamin E and selenium supplementation and risk of prostate cancer in the Vitamins and Lifestyle (VITAL) study cohort. Cancer Causes Control. 2008;19:75–87. doi: 10.1007/s10552-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 148.Bautista-Vidal C, Barnoiu O, Garcia-Galisteo E, et al. Treatment of gynecomastia in patients with prostate cancer and androgen deprivation. Actas Urol Esp. 2014;38:34–40. doi: 10.1016/j.acuro.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 149.Viani GA, Bernardes da Silva LG, Stefano EJ. Prevention of gynecomastia and breast pain caused by androgen deprivation therapy in prostate cancer: tamoxifen or radiotherapy? Int J Radiat Oncol Biol Phys. 2012;83:e519–524. doi: 10.1016/j.ijrobp.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 150.Bober SL, Varela VS. Sexuality in adult cancer survivors: challenges and intervention. J Clin Oncol. 2012;30:3712–3719. doi: 10.1200/JCO.2012.41.7915. [DOI] [PubMed] [Google Scholar]
- 151.Donovan KA, Thompson LM, Hoffe SE. Sexual function in colorectal cancer survivors. Cancer Control. 2010;17:44–51. doi: 10.1177/107327481001700106. [DOI] [PubMed] [Google Scholar]
- 152.Jackson SE, Wardle J, Steptoe A, Fisher A. Sexuality after a cancer diagnosis: a population-based study. Cancer. 2016;122:3883–3891. doi: 10.1002/cncr.30263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 154.Morreale MK. The impact of cancer on sexual function. Adv Psychosom Med. 2011;31:72–82. doi: 10.1159/000328809. [DOI] [PubMed] [Google Scholar]
- 155.Vomvas D, Iconomou G, Soubasi E, et al. Assessment of sexual function in patients with cancer undergoing radiotherapy—a single centre prospective study. Anticancer Res. 2012;32:657–664. [PubMed] [Google Scholar]
- 156.Bober SL, Carter J, Falk S. Addressing female sexual function after cancer by internists and primary care providers. J Sex Med. 2013;10(Suppl 1):112–119. doi: 10.1111/jsm.12027. [DOI] [PubMed] [Google Scholar]
- 157.Forbat L, White I, Marshall-Lucette S, Kelly D. Discussing the sexual consequences of treatment in radiotherapy and urology consultations with couples affected by prostate cancer. BJU Int. 2012;109:98–103. doi: 10.1111/j.1464-410X.2011.10257.x. [DOI] [PubMed] [Google Scholar]
- 158.Reese JB, Sorice K, Beach MC, et al. Patient-provider communication about sexual concerns in cancer: a systematic review. J Cancer Surviv. 2017;11:175–188. doi: 10.1007/s11764-016-0577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sporn NJ, Smith KB, Pirl WF, et al. Sexual health communication between cancer survivors and providers: how frequently does it occur and which providers are preferred? Psychooncology. 2015;24:1167–1173. doi: 10.1002/pon.3736. [DOI] [PubMed] [Google Scholar]
- 160.White ID, Allan H, Faithfull S. Assessment of treatment-induced female sexual morbidity in oncology: is this a part of routine medical follow-up after radical pelvic radiotherapy? Br J Cancer. 2011;105:903–910. doi: 10.1038/bjc.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.ACOG Practice Bulletin No. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996–1007. doi: 10.1097/AOG.0b013e31821921ce. [DOI] [PubMed] [Google Scholar]
- 162.Gilbert E, Ussher JM, Perz J. Sexuality after breast cancer: a review. Maturitas. 2010;66:397–407. doi: 10.1016/j.maturitas.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 163.Krychman M, Millheiser LS. Sexual health issues in women with cancer. J Sex Med. 2013;10(Suppl 1):5–15. doi: 10.1111/jsm.12034. [DOI] [PubMed] [Google Scholar]
- 164.Barni S, Mondin R. Sexual dysfunction in treated breast cancer patients. Ann Oncol. 1997;8:149–153. doi: 10.1023/a:1008298615272. [DOI] [PubMed] [Google Scholar]
- 165.Frumovitz M, Sun CC, Schover LR, et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol. 2005;23:7428–7436. doi: 10.1200/JCO.2004.00.3996. [DOI] [PubMed] [Google Scholar]
- 166.Ganz PA, Desmond KA, Belin TR, et al. Predictors of sexual health in women after a breast cancer diagnosis. J Clin Oncol. 1999;17:2371–2380. doi: 10.1200/JCO.1999.17.8.2371. [DOI] [PubMed] [Google Scholar]
- 167.Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 168.Lindau ST, Gavrilova N, Anderson D. Sexual morbidity in very long term survivors of vaginal and cervical cancer: a comparison to national norms. Gynecol Oncol. 2007;106:413–418. doi: 10.1016/j.ygyno.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Park SY, Bae DS, Nam JH, et al. Quality of life and sexual problems in disease-free survivors of cervical cancer compared with the general population. Cancer. 2007;110:2716–2725. doi: 10.1002/cncr.23094. [DOI] [PubMed] [Google Scholar]
- 170.Rodrigues AC, Teixeira R, Teixeira T, et al. Impact of pelvic radiotherapy on female sexuality. Arch Gynecol Obstet. 2012;285:505–514. doi: 10.1007/s00404-011-1988-5. [DOI] [PubMed] [Google Scholar]
- 171.Gershenson DM, Miller AM, Champion VL, et al. Reproductive and sexual function after platinum-based chemotherapy in long-term ovarian germ cell tumor survivors: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25:2792–2797. doi: 10.1200/JCO.2006.08.4590. [DOI] [PubMed] [Google Scholar]
- 172.Lammerink EA, de Bock GH, Pras E, et al. Sexual functioning of cervical cancer survivors: a review with a female perspective. Maturitas. 2012;72:296–304. doi: 10.1016/j.maturitas.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 173.Fobair P, Stewart SL, Chang S, et al. Body image and sexual problems in young women with breast cancer. Psychooncology. 2006;15:579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- 174.Syrjala KL, Kurland BF, Abrams JR, et al. Sexual function changes during the 5 years after high-dose treatment and hematopoietic cell transplantation for malignancy, with case-matched controls at 5 years. Blood. 2008;111:989–996. doi: 10.1182/blood-2007-06-096594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thygesen KH, Schjodt I, Jarden M. The impact of hematopoietic stem cell transplantation on sexuality: a systematic review of the literature. Bone Marrow Transplant. 2012;47:716–724. doi: 10.1038/bmt.2011.169. [DOI] [PubMed] [Google Scholar]
- 176.Watson M, Wheatley K, Harrison GA, et al. Severe adverse impact on sexual functioning and fertility of bone marrow transplantation, either allogeneic or autologous, compared with consolidation chemotherapy alone: analysis of the MRC AML 10 trial. Cancer. 1999;86:1231–1239. doi: 10.1002/(sici)1097-0142(19991001)86:7<1231::aid-cncr18>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 177.Zantomio D, Grigg AP, MacGregor L, et al. Female genital tract graft-versus-host disease: incidence, risk factors and recommendations for management. Bone Marrow Transplant. 2006;38:567–572. doi: 10.1038/sj.bmt.1705487. [DOI] [PubMed] [Google Scholar]
- 178.NIH consensus conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 179.Montague DK, Jarow JP, Broderick GA, et al. Erectile Dysfunction. American Urological Association; 2005. [Accessed May 3, 2017]. Available at: http://www.auanet.org/education/guidelines/erectile-dysfunction.cfm. [Google Scholar]
- 180.Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307–319. doi: 10.1038/sj.ijir.3901327. [DOI] [PubMed] [Google Scholar]
- 181.Monga M, Bettencourt R, Barrett-Connor E. Community-based study of erectile dysfunction and sildenafil use: the Rancho Bernardo study. Urology. 2002;59:753–757. doi: 10.1016/s0090-4295(02)01503-0. [DOI] [PubMed] [Google Scholar]
- 182.Ellis R, Smith A, Wilson S, et al. The prevalence of erectile dysfunction in post-treatment colorectal cancer patients and their interests in seeking treatment: a cross-sectional survey in the west-midlands. J Sex Med. 2010;7:1488–1496. doi: 10.1111/j.1743-6109.2009.01461.x. [DOI] [PubMed] [Google Scholar]
- 183.Hendren SK, O’Connor BI, Liu M, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242:212–223. doi: 10.1097/01.sla.0000171299.43954.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 185.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schover LR, Fouladi RT, Warneke CL, et al. Defining sexual outcomes after treatment for localized prostate carcinoma. Cancer. 2002;95:1773–1785. doi: 10.1002/cncr.10848. [DOI] [PubMed] [Google Scholar]
- 187.Siegel T, Moul JW, Spevak M, et al. The development of erectile dysfunction in men treated for prostate cancer. J Urol. 2001;165:430–435. doi: 10.1097/00005392-200102000-00019. [DOI] [PubMed] [Google Scholar]
- 188.Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354–360. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 189.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Armuand GM, Wettergren L, Rodriguez-Wallberg KA, Lampic C. Desire for children, difficulties achieving a pregnancy, and infertility distress 3 to 7 years after cancer diagnosis. Support Care Cancer. 2014;22:2805–2812. doi: 10.1007/s00520-014-2279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kort JD, Eisenberg ML, Millheiser LS, Westphal LM. Fertility issues in cancer survivorship. CA Cancer J Clin. 2014;64:118–134. doi: 10.3322/caac.21205. [DOI] [PubMed] [Google Scholar]
- 192.Murphy D, Orgel E, Termuhlen A, et al. Why healthcare providers should focus on the fertility of AYA cancer survivors: it’s not too late! Front Oncol. 2013;3:248. doi: 10.3389/fonc.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Quinn MM, Letourneau JM, Rosen MP. Contraception after cancer treatment: describing methods, counseling, and unintended pregnancy risk. Contraception. 2014;89:466–471. doi: 10.1016/j.contraception.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 194.Bartula I, Sherman KA. Development and validation of the Female Sexual Function Index adaptation for breast cancer patients (FSFI-BC) Breast Cancer Res Treat. 2015;152:477–488. doi: 10.1007/s10549-015-3499-8. [DOI] [PubMed] [Google Scholar]
- 195.Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337–348. doi: 10.1111/j.1743-6109.2009.01619.x. [DOI] [PubMed] [Google Scholar]
- 196.McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26:25–40. doi: 10.1080/009262300278623. [DOI] [PubMed] [Google Scholar]
- 197.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 198.Abraham L, Symonds T, Morris MF. Psychometric validation of a sexual quality of life questionnaire for use in men with premature ejaculation or erectile dysfunction. J Sex Med. 2008;5:595–601. doi: 10.1111/j.1743-6109.2007.00749.x. [DOI] [PubMed] [Google Scholar]
- 199.Assessment Center. [Accessed May 3, 2017]; Available at: http://www.assessmentcenter.net/
- 200.Baser RE, Li Y, Carter J. Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors. Cancer. 2012;118:4606–4618. doi: 10.1002/cncr.26739. [DOI] [PubMed] [Google Scholar]
- 201.Jeffery DD, Tzeng JP, Keefe FJ, et al. Initial report of the cancer Patient-Reported Outcomes Measurement Information System (PROMIS) sexual function committee: review of sexual function measures and domains used in oncology. Cancer. 2009;115:1142–1153. doi: 10.1002/cncr.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bartula I, Sherman KA. Screening for sexual dysfunction in women diagnosed with breast cancer: systematic review and recommendations. Breast Cancer Res Treat. 2013;141:173–185. doi: 10.1007/s10549-013-2685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Flynn P, Kew F, Kisely SR. Interventions for psychosexual dysfunction in women treated for gynaecological malignancy. Cochrane Database Syst Rev. 2009:CD004708. doi: 10.1002/14651858.CD004708.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Katz A. Interventions for sexuality after pelvic radiation therapy and gynecological cancer. Cancer J. 2009;15:45–47. doi: 10.1097/PPO.0b013e31819585cf. [DOI] [PubMed] [Google Scholar]
- 205.Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus waitlist control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320–325. doi: 10.1016/j.ygyno.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hummel SB, van Lankveld JJ, Oldenburg HS, et al. Efficacy of internet-based cognitive behavioral therapy in improving sexual functioning of breast cancer survivors: results of a randomized controlled trial. J Clin Oncol. 2017;35:1328–1340. doi: 10.1200/JCO.2016.69.6021. [DOI] [PubMed] [Google Scholar]
- 207.Hickey M, Marino JL, Braat S, Wong S. A randomized, double-blind, crossover trial comparing a silicone-versus water-based lubricant for sexual discomfort after breast cancer. Breast Cancer Res Treat. 2016 doi: 10.1007/s10549-016-3865-1. [DOI] [PubMed] [Google Scholar]
- 208.Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394–3400. doi: 10.1200/JCO.2014.60.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang EJ, Lim JY, Rah UW, Kim YB. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol. 2012;125:705–711. doi: 10.1016/j.ygyno.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 210.Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2010:CD007291. doi: 10.1002/14651858.CD007291.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Melisko ME, Goldman ME, Hwang J, et al. Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer: a randomized clinical trial. JAMA Oncol. 2017;3:313–319. doi: 10.1001/jamaoncol.2016.3904. [DOI] [PubMed] [Google Scholar]
- 212.Archer DF, Labrie F, Bouchard C, et al. Treatment of pain at sexual activity (dyspareunia) with intravaginal dehydroepiandrosterone (prasterone) Menopause. 2015;22:950–963. doi: 10.1097/GME.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 213.Archer DF, Labrie F, Montesino M, Martel C. Comparison of intravaginal 6.5mg (0.50%) prasterone, 0.3mg conjugated estrogens and 10mcg estradiol on symptoms of vulvovaginal atrophy. J Steroid Biochem Mol Biol. 2017 doi: 10.1016/j.jsbmb.2017.03.014. pii: S0960-0760(17)30079-1. [DOI] [PubMed] [Google Scholar]
- 214.Labrie F, Archer DF, Bouchard C, et al. Intravaginal dehydroepiandrosterone (prasterone), a highly efficient treatment of dyspareunia. Climacteric. 2011;14:282–288. doi: 10.3109/13697137.2010.535226. [DOI] [PubMed] [Google Scholar]
- 215.Labrie F, Archer DF, Koltun W, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23:243–256. doi: 10.1097/GME.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 216.Labrie F, Archer DF, Bouchard C, et al. Prasterone has parallel beneficial effects on the main symptoms of vulvovaginal atrophy: 52-week open-label study. Maturitas. 2015;81:46–56. doi: 10.1016/j.maturitas.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 217.Scheffers CS, Armstrong S, Cantineau AE, et al. Dehydroepiandrosterone for women in the peri- or postmenopausal phase. Cochrane Database Syst Rev. 2015;1:CD011066. doi: 10.1002/14651858.CD011066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.INTRAROSA (prasterone) vaginal inserts. Shionogi Inc; 2016. [Accessed April 28, 2017]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208470s000lbl.pdf. [Google Scholar]
- 219.OSPHENA (ospemifene) Shionogi Inc; 2015. [Accessed May 3, 2017]. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/203505s005lbl.pdf. [Google Scholar]
- 220.Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480–486. doi: 10.1097/gme.0b013e3181c1ac01. [DOI] [PubMed] [Google Scholar]
- 221.Goldstein SR, Bachmann GA, Koninckx PR, et al. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric. 2014;17:173–182. doi: 10.3109/13697137.2013.834493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623–630. doi: 10.1097/gme.0b013e318279ba64. [DOI] [PubMed] [Google Scholar]
- 223.ADDYI (flibanserin) Sprout Pharmaceuticals, Inc; 2015. [Accessed May 3, 2017]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022526lbl.pdf. [Google Scholar]
- 224.Jaspers L, Feys F, Bramer WM, et al. Efficacy and safety of flibanserin for the treatment of hypoactive sexual desire disorder in women: a systematic review and meta-analysis. JAMA Intern Med. 2016;176:453–462. doi: 10.1001/jamainternmed.2015.8565. [DOI] [PubMed] [Google Scholar]
- 225.Gao Z, Yang D, Yu L, Cui Y. Efficacy and safety of flibanserin in women with hypoactive sexual desire disorder: a systematic review and metaanalysis. J Sex Med. 2015;12:2095–2104. doi: 10.1111/jsm.13037. [DOI] [PubMed] [Google Scholar]
- 226.Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114–128. doi: 10.1016/j.mayocp.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 227.Segraves RT, Croft H, Kavoussi R, et al. Bupropion sustained release (SR) for the treatment of hypoactive sexual desire disorder (HSDD) in nondepressed women. J Sex Marital Ther. 2001;27:303–316. doi: 10.1080/009262301750257155. [DOI] [PubMed] [Google Scholar]
- 228.Segraves RT, Clayton A, Croft H, et al. Bupropion sustained release for the treatment of hypoactive sexual desire disorder in premenopausal women. J Clin Psychopharmacol. 2004;24:339–342. doi: 10.1097/01.jcp.0000125686.20338.c1. [DOI] [PubMed] [Google Scholar]
- 229.Landen M, Eriksson E, Agren H, Fahlen T. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19:268–271. doi: 10.1097/00004714-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 230.Cavalcanti AL, Bagnoli VR, Fonseca AM, et al. Effect of sildenafil on clitoral blood flow and sexual response in postmenopausal women with orgasmic dysfunction. Int J Gynaecol Obstet. 2008;102:115–119. doi: 10.1016/j.ijgo.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 231.Yang CC, Cao YY, Guan QY, et al. Influence of PDE5 inhibitor on MRI measurement of clitoral volume response in women with FSAD: a feasibility study of a potential technique for evaluating drug response. Int J Impot Res. 2008;20:105–110. doi: 10.1038/sj.ijir.3901625. [DOI] [PubMed] [Google Scholar]
- 232.Alexander MS, Rosen RC, Steinberg S, et al. Sildenafil in women with sexual arousal disorder following spinal cord injury. Spinal Cord. 2011;49:273–279. doi: 10.1038/sc.2010.107. [DOI] [PubMed] [Google Scholar]
- 233.Basson R, McInnes R, Smith MD, et al. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with female sexual arousal disorder. J Womens Health Gend Based Med. 2002;11:367–377. doi: 10.1089/152460902317586001. [DOI] [PubMed] [Google Scholar]
- 234.Basson R, Brotto LA. Sexual psychophysiology and effects of sildenafil citrate in oestrogenised women with acquired genital arousal disorder and impaired orgasm: a randomised controlled trial. BJOG. 2003;110:1014–1024. [PubMed] [Google Scholar]
- 235.Berman JR, Berman LA, Toler SM, et al. Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: a double-blind, placebo controlled study. J Urol. 2003;170:2333–2338. doi: 10.1097/01.ju.0000090966.74607.34. [DOI] [PubMed] [Google Scholar]
- 236.Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG. 2001;108:623–628. doi: 10.1111/j.1471-0528.2001.00143.x. [DOI] [PubMed] [Google Scholar]
- 237.Caruso S, Rugolo S, Agnello C, et al. Sildenafil improves sexual functioning in premenopausal women with type 1 diabetes who are affected by sexual arousal disorder: a double-blind, crossover, placebo-controlled pilot study. Fertil Steril. 2006;85:1496–1501. doi: 10.1016/j.fertnstert.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 238.Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 239.Lamina S, Okoye CG, Dagogo TT. Therapeutic effect of an interval exercise training program in the management of erectile dysfunction in hypertensive patients. J Clin Hypertens (Greenwich) 2009;11:125–129. doi: 10.1111/j.1751-7176.2009.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Khoo J, Piantadosi C, Duncan R, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868–2875. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- 241.Khoo J, Piantadosi C, Worthley S, Wittert GA. Effects of a low-energy diet on sexual function and lower urinary tract symptoms in obese men. Int J Obes (Lond) 2010;34:1396–1403. doi: 10.1038/ijo.2010.76. [DOI] [PubMed] [Google Scholar]
- 242.Maio G, Saraeb S, Marchiori A. Physical activity and PDE5 inhibitors in the treatment of erectile dysfunction: results of a randomized controlled study. J Sex Med. 2010;7:2201–2208. doi: 10.1111/j.1743-6109.2010.01783.x. [DOI] [PubMed] [Google Scholar]
- 243.Andersson E, Walen C, Hallberg J, et al. A randomized controlled trial of guided Internet-delivered cognitive behavioral therapy for erectile dysfunction. J Sex Med. 2011;8:2800–2809. doi: 10.1111/j.1743-6109.2011.02391.x. [DOI] [PubMed] [Google Scholar]
- 244.Aubin S, Heiman JR, Berger RE, et al. Comparing sildenafil alone vs. sildenafil plus brief couple sex therapy on erectile dysfunction and couples’ sexual and marital quality of life: a pilot study. J Sex Marital Ther. 2009;35:122–143. doi: 10.1080/00926230802712319. [DOI] [PubMed] [Google Scholar]
- 245.Banner LL, Anderson RU. Integrated sildenafil and cognitive-behavior sex therapy for psychogenic erectile dysfunction: a pilot study. J Sex Med. 2007;4:1117–1125. doi: 10.1111/j.1743-6109.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 246.Boddi V, Castellini G, Casale H, et al. An integrated approach with vardenafil orodispersible tablet and cognitive behavioral sex therapy for treatment of erectile dysfunction: a randomized controlled pilot study. Andrology. 2015;3:909–918. doi: 10.1111/andr.12079. [DOI] [PubMed] [Google Scholar]
- 247.Wylie KR. Treatment outcome of brief couple therapy in psychogenic male erectile disorder. Arch Sex Behav. 1997;26:527–545. doi: 10.1023/a:1024559906540. [DOI] [PubMed] [Google Scholar]
- 248.Canada AL, Neese LE, Sui D, Schover LR. Pilot intervention to enhance sexual rehabilitation for couples after treatment for localized prostate carcinoma. Cancer. 2005;104:2689–2700. doi: 10.1002/cncr.21537. [DOI] [PubMed] [Google Scholar]
- 249.Schover LR, Canada AL, Yuan Y, et al. A randomized trial of internet-based versus traditional sexual counseling for couples after localized prostate cancer treatment. Cancer. 2012;118:500–509. doi: 10.1002/cncr.26308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fink HA, Mac Donald R, Rutks IR, et al. Sildenafil for male erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med. 2002;162:1349–1360. doi: 10.1001/archinte.162.12.1349. [DOI] [PubMed] [Google Scholar]
- 251.Nehra A. Erectile dysfunction and cardiovascular disease: efficacy and safety of phosphodiesterase type 5 inhibitors in men with both conditions. Mayo Clin Proc. 2009;84:139–148. doi: 10.4065/84.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hubanks JM, Umbreit EC, Karnes RJ, Myers RP. Open radical retropubic prostatectomy using high anterior release of the levator fascia and constant haptic feedback in bilateral neurovascular bundle preservation plus early postoperative phosphodiesterase type 5 inhibition: a contemporary series. Eur Urol. 2012;61:878–884. doi: 10.1016/j.eururo.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 253.Yang L, Qian S, Liu L, et al. Phosphodiesterase-5 inhibitors could be efficacious in the treatment of erectile dysfunction after radiotherapy for prostate cancer: a systematic review and meta-analysis. Urol Int. 2012;90:339–347. doi: 10.1159/000343730. [DOI] [PubMed] [Google Scholar]
- 254.Kloner RA, Hutter AM, Emmick JT, et al. Time course of the interaction between tadalafil and nitrates. J Am Coll Cardiol. 2003;42:1855–1860. doi: 10.1016/j.jacc.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 255.Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol. 1999;83:21C–28C. doi: 10.1016/s0002-9149(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 256.Zhao C, Kim SW, Yang DY, et al. Efficacy and safety of once-daily dosing of udenafil in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60:380–387. doi: 10.1016/j.eururo.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 257.Rajfer J, Aliotta PJ, Steidle CP, et al. Tadalafil dosed once a day in men with erectile dysfunction: a randomized, double-blind, placebo-controlled study in the US. Int J Impot Res. 2007;19:95–103. doi: 10.1038/sj.ijir.3901496. [DOI] [PubMed] [Google Scholar]
- 258.Porst H, Giuliano F, Glina S, et al. Evaluation of the efficacy and safety of once-a-day dosing of tadalafil 5mg and 10mg in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2006;50:351–359. doi: 10.1016/j.eururo.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 259.Shim YS, Pae CU, Cho KJ, et al. Effects of daily low-dose treatment with phosphodiesterase type 5 inhibitor on cognition, depression, somatization and erectile function in patients with erectile dysfunction: a double-blind, placebo-controlled study. Int J Impot Res. 2014;26:76–80. doi: 10.1038/ijir.2013.38. [DOI] [PubMed] [Google Scholar]
- 260.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 261.Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cunningham GR, Stephens-Shields AJ, Rosen RC, et al. Testosterone treatment and sexual function in older men with low testosterone levels. J Clin Endocrinol Metab. 2016;101:3096–3104. doi: 10.1210/jc.2016-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study) J Sex Med. 2011;8:284–293. doi: 10.1111/j.1743-6109.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- 264.Corona G, Vignozzi L, Sforza A, Maggi M. Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health. 2013;31:103–125. doi: 10.5534/wjmh.2013.31.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Khera M, Bhattacharya RK, Blick G, et al. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS) J Sex Med. 2011;8:3204–3213. doi: 10.1111/j.1743-6109.2011.02436.x. [DOI] [PubMed] [Google Scholar]
- 266.Rosenthal BD, May NR, Metro MJ, et al. Adjunctive use of AndroGel (testosterone gel) with sildenafil to treat erectile dysfunction in men with acquired androgen deficiency syndrome after failure using sildenafil alone. Urology. 2006;67:571–574. doi: 10.1016/j.urology.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 267.Hwang TI, Chen HE, Tsai TF, Lin YC. Combined use of androgen and sildenafil for hypogonadal patients unresponsive to sildenafil alone. Int J Impot Res. 2006;18:400–404. doi: 10.1038/sj.ijir.3901446. [DOI] [PubMed] [Google Scholar]
- 268.Zitzmann M, Mattern A, Hanisch J, et al. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1,438 men. J Sex Med. 2013;10:579–588. doi: 10.1111/j.1743-6109.2012.02853.x. [DOI] [PubMed] [Google Scholar]
- 269.Campbell SE, Glazener CM, Hunter KF, et al. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev. 2012;1:CD001843. doi: 10.1002/14651858.CD001843.pub4. [DOI] [PubMed] [Google Scholar]
- 270.Geraerts I, Van Poppel H, Devoogdt N, et al. Pelvic floor muscle training for erectile dysfunction and climacturia 1 year after nerve sparing radical prostatectomy: a randomized controlled trial. Int J Impot Res. 2016;28:9–13. doi: 10.1038/ijir.2015.24. [DOI] [PubMed] [Google Scholar]
- 271.Prota C, Gomes CM, Ribeiro LH, et al. Early postoperative pelvicfloor biofeedback improves erectile function in men undergoing radical prostatectomy: a prospective, randomized, controlled trial. Int J Impot Res. 2012;24:174–178. doi: 10.1038/ijir.2012.11. [DOI] [PubMed] [Google Scholar]
- 272.Nelson CJ, Ahmed A, Valenzuela R, et al. Assessment of penile vibratory stimulation as a management strategy in men with secondary retarded orgasm. Urology. 2007;69:552–555. doi: 10.1016/j.urology.2006.02.048. discussion 555–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cooper K, Martyn-St James M, Kaltenthaler E, et al. Interventions to treat premature ejaculation: a systematic review short report. Health Technol Assess. 2015;19:1–180. doi: 10.3310/hta19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Giuliano F, Clement P. Pharmacology for the treatment of premature ejaculation. Pharmacol Rev. 2012;64:621–644. doi: 10.1124/pr.111.004952. [DOI] [PubMed] [Google Scholar]
- 275.Kim SC, Seo KK. Efficacy and safety of fluoxetine, sertraline and clomipramine in patients with premature ejaculation: a double-blind, placebo controlled study. J Urol. 1998;159:425–427. doi: 10.1016/s0022-5347(01)63940-5. [DOI] [PubMed] [Google Scholar]
- 276.Waldinger MD, Zwinderman AH, Olivier B. On-demand treatment of premature ejaculation with clomipramine and paroxetine: a randomized, double-blind fixed-dose study with stopwatch assessment. Eur Urol. 2004;46:510–515. doi: 10.1016/j.eururo.2004.05.005. discussion 516. [DOI] [PubMed] [Google Scholar]