Abstract
Preexposure prophylaxis is a highly protective HIV prevention strategy, yet nonadherence can significantly reduce its effectiveness. We conducted a mixed methods evaluation of a mobile health intervention (iText) that utilized weekly bidirectional text or e-mail support messages to encourage preexposure prophylaxis (PrEP) adherence among participants in the multi-site iPrEx open-label extension study. A convenience sample of PrEP users from the San Francisco and Chicago sites participated in a 12-week pilot study. Fifty-six men who have sex with men were enrolled; a quarter of them were less than 30 years of age, 13% were black/African American, 11% were Latino, and most (88%) completed some college. Two-thirds opted for text message delivery. Of the 667 messages sent, only 1 individual requested support; initial nonresponse was observed in 22% and was higher among e-mail compared to text message recipients. Poststudy, a majority of participants would recommend the intervention to others, especially during PrEP initiation. Moreover, younger participants and men of color were more likely to report that they would use the iText strategy if it were available to them. Several participants commented that while they were aware that the messages were automated, they felt supported and encouraged that “someone was always there.” Study staff reported that the intervention is feasible to administer and can be incorporated readily into clinic flow. A pre–post intervention regression discontinuity analysis using clinic-based pill counts showed a 50% reduction in missed doses [95% confidence interval (CI) 16–71; p = 0.008] and 77% (95% CI 33–92; p = 0.007) when comparing pill counts at quarterly visits just before and after iText enrollment. A mobile health intervention using weekly bidirectional messaging was highly acceptable and demonstrated promising effects on PrEP adherence warranting further evaluation for efficacy in a randomized controlled trial.
Keywords: : HIV, preexposure prophylaxis, adherence, mobile health, text messaging
Introduction
Given its significant potential to contribute to population-level reductions in HIV incidence, the recently updated US National HIV/AIDS Strategy endorses preexposure prophylaxis (PrEP) with daily oral co-formulated tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) for those at risk for sexually acquired or injection drug-associated infection.1 This recommendation draws from compelling clinical trial data2,3 that led to Federal Drug Administration approval in 2012 and subsequent Centers for Disease Control and Prevention (CDC)-issued clinical guidelines. These guidelines reinforce that PrEP effectiveness is highly influenced by adherence to medication. For example, the iPrEx trial showed an overall efficacy of 44% among the men who have sex with men (MSM) and transgender women enrolled, but substantially higher levels of protection (92%) were seen in those with detectable blood levels of study drug.2 Thus, to achieve high levels of protection, PrEP must be conceptualized as a biobehavioral strategy that relies on consistent pill taking, likening it to oral birth control for pregnancy prevention.
To date, few studies have evaluated methods to optimize adherence to PrEP.4 Most PrEP trials have incorporated counseling on facilitators and barriers to pill taking that is offered during regularly scheduled follow-up visits.5–7 Recently, a brief client-centered counseling intervention implemented during a PrEP demonstration project showed beneficial, although short-lived, effects on adherence as measured by tenofovir diphosphate levels in dried blood spot testing at 3-month follow-up visits,8 and a pilot randomized controlled trial evaluated a nurse-delivered, multi-session cognitive behavioral therapy-based intervention to improve PrEP adherence among MSM at high risk for infection. While this intervention led to improved adherence by some measures (i.e., plasma tenofovir levels at 6 months), a fully powered study is needed to establish its effectiveness.9
Mobile health (mHealth), which refers to the use of mobile and wireless technologies to improve health, holds significant promise given widespread mobile phone ownership in the United States and worldwide.10 There is growing evidence that short message service (SMS) or text message reminder and other support approaches can improve adherence to prevention and treatment interventions across a wide range of clinical conditions.11–16 Many of these mHealth programs use unidirectional or interactive text message strategies, sometimes sent to patients on a daily basis, to improve diabetes care and asthma self-management.13
Several studies also have evaluated mHealth interventions to optimize antiretroviral therapy (ART) adherence among those being treated for HIV infection.14,17–22 For example, the Weltel Kenya trial used a weekly bidirectional SMS support strategy to encourage adherence among HIV positive persons initiating ART.14 This intervention demonstrated significantly better adherence and viral suppression rates compared to controls and was highly acceptable to staff implementing the system.19 This approach was also found to be well received by HIV-positive patients and by staff at an interdisciplinary clinic in British Columbia.23 Whether such a strategy could be effective among HIV negative persons using PrEP for prevention in a high income country setting is unknown.
We sought to evaluate the feasibility, acceptability, and adherence effects of a weekly, bidirectional SMS- or email-based adherence support system for HIV negative MSM taking PrEP in two urban centers in the United States. In this study, we report the results of a mixed methods evaluation of the intervention, iText, in a sample of iPrEx open label extension (OLE) study participants to inform a randomized controlled trial of the intervention.
Methods
Participants
Formative research and the pilot intervention study were conducted with participants enrolled in the iPrEx OLE Study. The methods and design of iPrEx OLE are described elsewhere.24 Briefly, after the completion of the iPrEx randomized controlled trial,2 1603 HIV-negative participants were enrolled in a 72-week prospective cohort study of open label TDF/FTC; of those, 1225 took PrEP (76%). For the mHealth project described herein, a convenience sample was selected from participants on PrEP at the San Francisco (n = 48) and Chicago (n = 8) iPrEx OLE sites over a 3-month period near the completion of iPrEx OLE study follow-up. Participants were eligible to evaluate the mHealth intervention if they had taken TDF/FTC for at least 12 weeks and were willing to continue taking TDF/FTC for an additional 12 weeks and if they had an SMS-capable phone or active e-mail account they could use to receive and send messages.
All participants provided signed informed consent, and the study was approved by the University of California, San Francisco Committee on Human Research and Chicago Cook County Health & Hospital System Institutional Review Board.
Intervention
Before developing the mHealth intervention, focus groups were conducted with 59 iPrEx OLE participants on PrEP from San Francisco (n = 21), Chicago (n = 22), and Boston (n = 16) to assess interest in and inform the adaptation of an SMS-based support strategy based on the WelTel intervention.14,25 Focus group participants were asked to reflect on the WelTel strategy that involved sending simple text messages to individuals on a weekly basis asking if they were “okay” or “not okay”; those responding they were not okay were provided additional support by phone, tailored to their needs. All iPrEx OLE focus group participants expressed interest in this approach to support PrEP use. However, a significant number stated that they preferred e-mail as the mode of messaging, rather than SMS. In addition, many participants expressed interest in personalizing both the content and timing of the messages (i.e., choosing among different message options, as well as the day of the week or time of day to receive them).
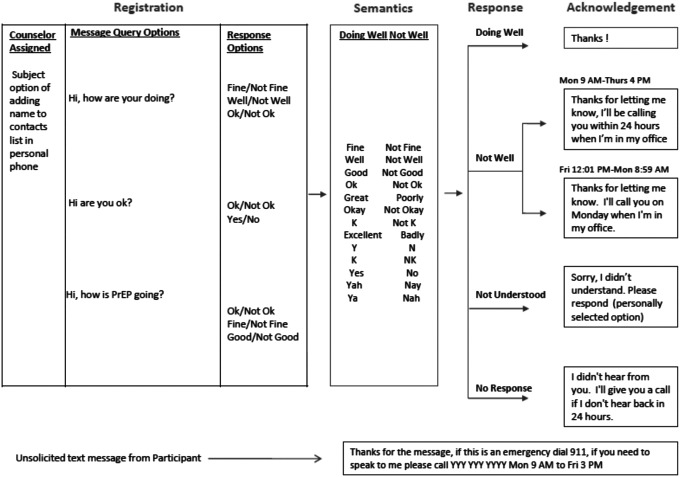
The iText support platform was developed in collaboration with the technology partner Capito Life Technologies, Inc. (San Francisco, CA). The platform used Health Insurance Portability and Accountability Act (HIPAA)-compliant SMS and e-mail check-in messages sent weekly. Based on formative feedback, the platform offered three outgoing message options to choose from: “How are you doing?,” “Are you okay?,” or “How is PrEP going?” Participants were also given the option to choose the responses they could text message or e-mail back (e.g., “Ok” or “not Ok,” “Fine” or “not fine”). For participants that responded “not Ok,” or those that did not respond to the weekly message, even after a reminder message was sent within a 48-h period, study staff would contact them by phone (see Fig. 1 for the messaging algorithm). The iText platform stored all responses securely, which were accessible only to study staff. The platform dashboard offered study staff a snapshot of outgoing message and response status.
FIG. 1.
The iText bidirectional messaging algorithm.
Procedures
During iText enrollment, participants completed a baseline questionnaire about mobile phone ownership and e-mail use and then were registered in iText which allowed them to receive weekly check-in messages over the next 12 weeks. At their next regularly scheduled quarterly iPrEx OLE visit, participants completed a follow-up questionnaire assessing the acceptability of the iText support strategy and were then deactivated from the iText system. Data extracted from the iText platform included participant preference for the days and times they requested messages be sent, as well as the total number, timing, and content of messages sent and received by the system.
After iText study completion, 14 participants were purposefully sampled from both study sites to engage in focus groups or in-depth interviews. We selected participants based on a range of responsiveness with the iText system; interviews assessed attitudes about the support strategy and solicited recommendations for future strategy development. In addition, focus groups with iText staff from both study sites were conducted to explore the platform's ease of use, utility in managing their participant panel, and integration into clinic flow.
Data analysis
Quantitative
For the iText pilot study, basic frequencies and percentages were computed for categorical variables and means and standard deviations for continuous variables. Response time was calculated from data extracted from the iText platform. Unadjusted logistic models were used to compare acceptability outcomes by demographic characteristics. We used negative binomial models to estimate the effect of the intervention on adherence, as measured by the self-reported number of days doses were missed in the previous 30 days, as well as by the number of doses missed in each reporting period as measured by clinic-based pill counts, treating the number of days in the reporting period as an “offset.” A linear model was used to assess changes in the log-transformed medication possession ratio, defined as the number of doses dispensed, divided by the number of days in the reporting period. All models flexibly controlled for underlying secular trend using a 3-knot restricted cubic spline in days since entry into iPrEx OLE and accounted for within-subject correlation of the repeated outcomes using generalized estimating equations with robust standard errors. We repeated these analyses restricting the adherence measures to the last pre-iText enrollment visit and the first post-iText visit, minimizing the vulnerability of this regression-discontinuity analysis to confounding by inadequately modeled secular effects.26
Qualitative
The post-iText focus groups and in-depth interviews were analyzed using framework analysis with two investigators (KS and HG) coding the transcripts to ensure consistency and agreement over coded themes. This process involved five stages of analysis: noting key content areas, laying out a thematic framework, indexing or coding the data, charting salient quotes, and finally, interpreting the results.
Results
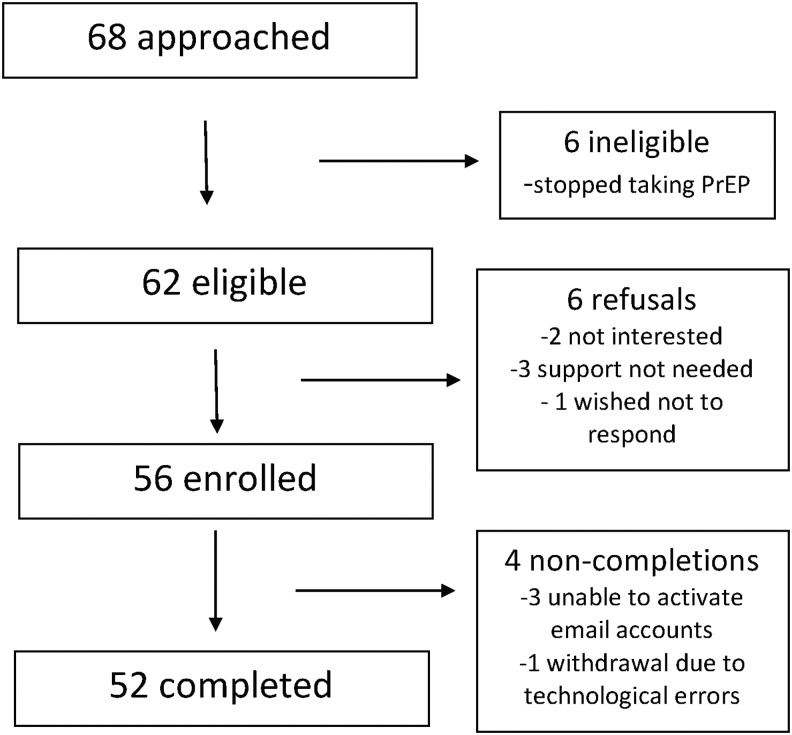
A total of 68 participants were approached for the iText pilot; 6 were deemed ineligible, due to going off study drug within the next 12 weeks, and 6 refused, stating they were unwilling to receive messages or not needing support, leaving a total of 56 enrolled for a 90% participation rate (Fig. 2). Of the participants that enrolled, three that chose e-mail messaging were unable to activate their e-mail accounts, and one participant who elected to receive e-mail messaging was inadvertently registered for the SMS and subsequently dropped out. All of the remaining 52 participants completed the study.
FIG. 2.
Study enrollment.
As seen in Table 1, a quarter of enrollees (n = 14) were 30 years of age or younger, with a majority of these participants enrolling at the Chicago site (n = 8). Overall the median age was 49 years (range, 21–66). Most San Francisco site participants were white (68%), while 100% of the Chicago site participants were men of color, and overall, most (88%) had completed some college. The demographics of our sample were reflective of the parent study at each site (data not shown). Only one participant, from San Francisco, did not own a mobile phone. A majority (81%) of mobile phone owners had unlimited text messaging plans and almost all (93%) used smartphones with e-mail capabilities. Over one-third of participants opted to receive weekly e-mails rather than SMS, with most (94%) of the e-mail recipients enrolled at the San Francisco site.
Table 1.
iText Participant Demographic Characteristics, Cell Phone Ownership and Use, and Mode of Messaging Selected
| Total (n = 56) | San Francisco (n = 48) | Chicago (n = 8) | ||||
|---|---|---|---|---|---|---|
| Variables | N | % | N | % | N | % |
| Age | ||||||
| < = 30 years | 14 | 25.0 | 6 | 12.5 | 8 | 100.0 |
| >30 years | 42 | 75.0 | 42 | 87.5 | 0 | 0.0 |
| Race/ethnicity | ||||||
| Latino/Hispanic | 6 | 10.7 | 4 | 8.3 | 2 | 25.0 |
| White | 38 | 67.9 | 38 | 79.2 | 0 | 0.0 |
| Black | 7 | 12.5 | 3 | 6.3 | 4 | 50.0 |
| Other | 5 | 8.9 | 3 | 6.3 | 2 | 25.0 |
| Education | ||||||
| Completed some college | 49 | 87.5 | 45 | 93.8 | 4 | 50.0 |
| Living situation | ||||||
| Alone | 15 | 26.8 | 13 | 27.1 | 2 | 25.0 |
| W/male sexual partner | 21 | 37.5 | 20 | 41.7 | 1 | 12.5 |
| Family/friends | 9 | 16.1 | 4 | 8.3 | 5 | 62.5 |
| Other/roommates | 11 | 19.6 | 11 | 22.9 | 0 | 0.0 |
| Mobile phone and plans | ||||||
| Mobile phone ownership | 55 | 98.2 | 47 | 97.9 | 8 | 100.0 |
| Have unlimited SMS plana | 43 | 81.1 | 35 | 77.8 | 8 | 100.0 |
| Smartphone ownershipa | 51 | 92.7 | 45 | 95.7 | 6 | 75.0 |
| Mode of messaging selected | ||||||
| 18 | 32.1 | 17 | 35.4 | 1 | 12.5 | |
| SMS | 38 | 67.9 | 31 | 64.6 | 7 | 87.5 |
Of mobile phone owners.
SMS, short message service.
With regard to message content, a majority of the participants (60%) selected a PrEP-specific outgoing message (i.e., “How is PrEP going?”). Overall, participants preferred receiving weekly messages in the morning (63%), followed by the afternoon (30%), with few electing to receive evening messages. Most (80%) preferred weekly messages that were sent at the beginning of the calendar week (i.e., Sunday or Monday). Of the total 667 messages sent, 77% of responses were “Ok,” 0.4% (n = 3) were “not Ok,” and initial nonresponse was observed in 22%. One of the three “not Ok” responses was associated with a desire to consult with a clinician regarding a 1-week history of sore throat, cough, and body aches that prompted self-discontinuation of PrEP. The other two responses were not associated with requests for assistance, but those participants reported that they were curious if a “not Ok” response would indeed trigger clinic follow-up.
We found that those who opted for e-mail messages were less likely to respond. Of e-mail users, 14% did not respond to the message at all, compared to 9% of SMS users (p < 0.009). Only 53 messages (4%) were not delivered, due to an early technical issue with the platform and not due to mobile phone loss. In addition, the response time to SMS was faster compared to the e-mail messaging [mean, 4.4 h (range, 0.01–46.7) vs. 6.1 h (0.02–43.8); p = 0.03].
While more than half of enrollees (56%) reported that the messaging strategy was helpful, a substantially higher proportion of Chicago compared to San Francisco participants noted this (100% vs. 48%, p < 0.01). Similarly, all of the Chicago participants stated that the strategy should be offered to those initiating PrEP, whereas 87% of those in San Francisco agreed it should be offered. Unadjusted analysis showed that younger participants (less than 30 years of age) were more likely to say that they would use the iText support strategy if available to them [odds ratio (OR) = 14.8, 95% confidence interval (CI) [1.66–131.4]; p = 0.004]. Compared to white participants, non-white participants were more likely to state that the iText support strategy was helpful (OR = 7.3, 95% CI [1.4–37.5]; p = 0.017) and were more likely to use the strategy if offered to them (OR = 4.7, 95% CI [1.3–17.7]; p = 0.024). Neither selection of messaging strategy type (SMS vs. e-mail) nor educational attainment was associated with acceptability.
The regression discontinuity analysis provided clear evidence for increases in adherence as measured by pill counts (Table 2). Specifically, we found that the mean number of days when medication was not taken was reduced by 50% (95% CI 16–71; p = 0.008), and when we restricted the analysis to include the two visits just before and after entering iText, we observed a reduction in the proportion of missed doses by 77% (95% CI 33–92; p = 0.007). We found a trend toward reductions in the self-reported number of days missed in the month before each visit (p = 0.11), as well as increases of ∼28% in the medication possession ratio (p = 0.05).
Table 2.
Changes in Adherence After Entry into iText
| All visits | Visits just before and after entering iText | |||||||
|---|---|---|---|---|---|---|---|---|
| Adherence measure | N | RR | CI | p | N | RR | CI | p |
| Pill counta | 355 | 0.50 | 0.29–0.84 | 0.008 | 95 | 0.23 | 0.08–0.67 | 0.007 |
| Self-reportb | 359 | 0.52 | 0.23–1.17 | 0.11 | 91 | 0.45 | 0.19–1.06 | 0.07 |
| N | % increase | CI (%) | p | N | % increase | CI (%) | p | |
|---|---|---|---|---|---|---|---|---|
| Medication possession ratioc | 357 | 27.8 | −0.2–63.7 | 0.052 | 96 | 28.4 | 0.2–64.6 | 0.048 |
N refers to the number of included observations. RR refers to the relative risk reduction in missed doses before and after intervention. All estimates are adjusted for age, race, iPrEx OLE entry date, days since entry, and study site.
Days missed in period, as measured by clinic-based pill counts, with length of period as offset.
Self-reported missed days in reporting period.
Sum of days supply for all pills in period/Number of days in period; treatment effects are summarized as percent increases.
CI, confidence interval; OLE, open label extension.
Postfocus group discussion and in-depth interviews indicated a few important themes regarding the strategy and recommendations. Several participants reported that iText provided additional support: “So like just getting those messages made me feel like there was always somebody there just in case something went wrong…it's kind of like I was on my own before iText.”—Chicago participant. Early during pilot implementation, some participants had experienced technical glitches, “That was when it first started I had problems receiving text messages. I don't know. But it went back into play…”—Chicago participant.
There also seemed to be differences between study sites in the perceived utility of the mHealth approach, with a substantial proportion of the Chicago participants noting that they felt a sense of greater security from the messages, “He's right. That sense of security; that makes a huge difference. A big difference because you get the support you need when you come to the clinic but when you're going back to your daily routine, you have to take the pill, you're not telling every single body about it.”—Chicago participant, while in San Francisco, a majority of participants felt their pill taking routine was in place, “And I mean I take the pill every morning religiously. It doesn't…it didn't help me. I mean it just didn't help me.”—San Francisco participant.
Participants had several suggestions for improvement. For example, several San Francisco participants stated that the strategy may be most useful to people when they first start taking PrEP and less helpful for those who have been taking it for a while, “So if you're starting out [on PrEP] and maybe you would have some symptoms or something you wanted to talk to somebody about, maybe that makes sense.” A number of Chicago participants suggested that it may be helpful to integrate the strategy with social networking sites they already use, “…give me the option to say, would you like a profile to contact you on your hookup sites or something like that, or would you like for there to be some type of communication with the different social networking sites? If I had that option, it would be kind of better.”
In both Chicago and San Francisco, study staff members found the iText support strategy easy to use; however, they had several recommendations for improvement. For example, staff reported that interacting with iText outside of their existing visit scheduling software required more time; staff from Chicago mitigated this, in part, by designating a staff member to engage with the iText system: “What we did was have one person be the point person to login to the portal every day and make sure participants were responding, and if they weren't, following up with them. Overall it was a pretty good setup because it didn't get too overwhelming.” San Francisco staff members recommended that if feasible, future versions of the messaging system should be integrated into existing retention strategies, “If the system was more incorporated in our existing retention strategies, it actually could have really helped with retention as well.”—San Francisco staff. Across both sites, staff echoed participant feedback that had the support strategy been implemented at the start of the iPrEx randomized controlled trial; it would have really benefited those participants who were struggling with adherence. “I really think this could have helped people if we started it at the beginning of the participant's pill taking”—Chicago staff and in San Francisco, “I feel like most of my guys already had a habit of taking a pill on a daily basis, but they could see that it would be helpful for people that were having challenges with pill taking”—San Francisco staff.
Discussion
Our 12-week pilot study of a novel mHealth adherence intervention that delivered weekly SMS or e-mail support messages to individuals on PrEP found iText to be feasible and acceptable, particularly among younger participants and participants of color. A majority preferred receiving messages by SMS over e-mail and while some participants chose receiving generic messages that inquired whether they were okay or not, most selected PrEP-specific language in those messages. Using a pre–post evaluation design, we demonstrated that even among this group of experienced PrEP users, the intervention was associated with a 50% reduction in missed doses as measured by pill count. Several participants commented that while they were aware that the messages were automated, they felt supported and encouraged that “someone was always there.” Moreover, study staff found the system easy to navigate and they created ways to integrate this technology-based strategy into regular clinic flow.
Our finding that this pilot intervention was most acceptable among those under 30 years of age and among persons of color is important for several reasons. While HIV diagnoses across the United States are declining in most groups, diagnoses in younger persons between the ages of 25 and 29 continue to rise27 highlighting the urgent need to increase PrEP uptake and, ultimately, its effectiveness for this group. In addition, recent PrEP trials conducted by the Adolescent Trials Network in 18- to 22-year-olds28 and 15- to 17-year olds29 show a drop-off in pill taking when the visit interval changed from monthly to quarterly visits. As CDC clinical guidelines currently recommend quarterly visits for regular follow-up, strategies to support PrEP use between visits may be particularly helpful for youth. Overall adherence was high among MSM and transgender women participating in a three-city PrEP demonstration project. However, adherence in African Americans as measured by tenofovir diphosphate levels in dried blood spots was appreciably lower than whites.30 mHealth adherence strategies can capitalize on burgeoning mobile phone ownership and interest by youth and communities of color in these interventions31 as a way to encourage PrEP use over time. However, the success of any mHealth strategy can only occur if PrEP providers acknowledge the prevailing attitudes and beliefs that may affect ongoing engagement and retention in PrEP care such as understanding medication effectiveness and side effects, mistrust of healthcare providers, and stigma.32,33
Our study has several limitations. The pre–post study evaluation is limited in its ability to demonstrate true intervention effects. The iText pilot involved a relatively short follow-up period, and the timing of implementation near the conclusion of iPrEx OLE limited our ability to measure the persistence of those effects. Further, the study lacked a control group, only used self-report and pill-count data to assess adherence, and was nested within an existing PrEP OLE study where many of those enrolled had been on PrEP for years. It is notable, however, that with weekly bidirectional messages we were still able to detect a modest benefit in pill taking, even in this group of experienced PrEP users. Given our findings, a modified version of this mHealth intervention is being evaluated in the EPIC study—a randomized controlled trial among young MSM and transgender women aged 18–29 from Chicago who initiate PrEP in a safety-net clinic (NCT02371525). This study is exploring the impact of weekly text messages over a 9-month period and incorporates objective measures of adherence using tenofovir diphosphate levels in dried blood spots in addition to self-reported pill taking.34
Another limitation of our study was the finding that over 20% of individuals did not respond to the initial SMS or e-mail communication, which then required study staff to follow-up and contact them by phone. Following a similar protocol in a much larger group of patients in PrEP could entail greater clinic staff burden. We did find that our nonresponse rate mirrored that of the Vancouver interdisciplinary HIV clinic using the WelTel weekly messaging strategy.23 Interviews with those clinic staff confirmed that the workload was augmented initially, but they felt that the benefits to the patient including, but not limited to, viral suppression far exceeded the additional burden.23 Finally, our pilot engaged a small sample of participants, a majority of whom were college educated, and providers at two urban research clinics which may limit the generalizability of these findings.
As additional data on the efficacy of mHealth-focused PrEP adherence interventions emerge,35 focusing on how and for whom these approaches may be beneficial is a key research priority and will guide future refinement. Saberi and Johnson reported in a recent systematic review of self-care technology-based approaches to improve antiretroviral adherence that individually tailored methods that enable greater communication with providers are more effective than electronic pill reminders alone.36 In fact, having ready access to doctors and care teams is of particular importance to youth living with HIV.37 As someone initiates PrEP, a daily reminder may be helpful to establish a pill taking routine, but persistence in PrEP, as for ART, may require efforts to encourage ongoing engagement in care. As suggested by Murray et al., creating opportunities for regular check-ins that go beyond medical management invite patients to seek support around key social determinants of health such as housing, food security, and employment.23 We found that participants preferred messages delivered earlier, as opposed to later in the week, giving staff sufficient time to respond to issues that may arise.
Another benefit of the weekly check-in strategy is that persons using antiretrovirals for prevention may wish to stop them due to perceived changes in their risk for HIV. Bidirectional messaging between quarterly visits can prompt a person to share his/her intention to stop PrEP and, with guidance, safely restart it after repeat HIV testing. Larger studies of these technologies in PrEP users with longer follow-up periods will reveal the relative benefits of different messaging strategies, including message frequency, content, and staff support to respond to issues faced by individuals at different stages in their PrEP journeys.
In summary, we have demonstrated the feasibility and acceptability of a bidirectional messaging platform that uses weekly support messages to PrEP users as a way to improve pill taking and create greater opportunity for ongoing engagement with clinic staff. Lessons from both participants and frontline workers have informed the next iteration of our intervention to optimize PrEP adherence.
Acknowledgments
The authors thank the iPrEX OLE participants and study staff at the San Francisco and Chicago iPrEx OLE sites. This study was funded by the National Institute of Mental Health (grant RO1MH095628) and by the National Institute of Allergy and Infectious Diseases (UO1AI4002 and RO1AI062333 to Dr. Grant) in support of the iPrEx OLE study within which iText was nested. The study drug was provided by Gilead Sciences.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.National HIV/AIDS strategy for the United States. Washington, DC: The White House Office of National AIDS Policy, 2015 [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: From clinical trials to routine practice. Curr Opin HIV AIDS 2016;11:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amico KR. Adherence to preexposure chemoprophylaxis: The behavioral bridge from efficacy to effectiveness. Curr Opin in HIV AIDS 2012;7:542–548 [DOI] [PubMed] [Google Scholar]
- 6.Amico KR, Mansoor LE, Corneli A, et al. Adherence support approaches in biomedical HIV prevention trials: Experiences, insights and future directions from four multisite prevention trials. AIDS Behav 2013;17:2143–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amico KR, McMahan V, Goicochea P, et al. Supporting study product use and accuracy in self-report in the iPrEx study: Next step counseling and neutral assessment. AIDS Behav 2012;16:1243–1259 [DOI] [PubMed] [Google Scholar]
- 8.Golub SA, Pena S, Hilley A, et al. Brief Behavioral Intervention Increases PrEP Drug Levels in a Real-World Setting. Seattle, Washington: Paper presented at: Conference on Retroviruses and Opportunistic Infections, 2017 [Google Scholar]
- 9.Mayer KH, Safren SA, Elsesser SA, et al. Optimizing pre-exposure antiretroviral prophylaxis adherence in men who have sex with men: Results of a pilot randomized controlled trial of “Life-Steps for PrEP”. AIDS Behav 2017;21:1350–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Telecommunications Union. Measuring the Information Society Report 2016. Available at: https://www.itu.int/en/ITU-D/statistics/Documents/Publications/MISr2016/MISR2016-W4.pdf (Last accessed September4, 2017)
- 11.Ainsworth J, Palmier-Claus JE, Machin M, et al. A comparison of two delivery modalities of a mobile phone-based assessment for serious mental illness: Native smartphone application vs text-messaging only implementations. J Med Internet Res 2013;15:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith C, Gold J, Ngo TD, et al. Mobile phone-based interventions for improving contraception use. Cochrane Database Syst Rev 2015:CD011159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: A systematic review. Telemed J and E Health 2009;15:231–240 [DOI] [PubMed] [Google Scholar]
- 14.Lester RT, Mills EJ, Kariri A, et al. The HAART cell phone adherence trial (WelTel Kenya1): A randomized controlled trial protocol. Trials 2009;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strandbygaard U, Thomsen SF, Backer V. A daily SMS reminder increases adherence to asthma treatment: A three-month follow-up study. Respir Med 2010;104:166–171 [DOI] [PubMed] [Google Scholar]
- 16.Younes N, Chollet A, Menard E, et al. E-mental health care among young adults and help-seeking behaviors: A transversal study in a community sample. J Med Internet Res 2015;17:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier AC, Ribaudo H, Mukherjee AL, et al. A randomized study of serial telephone call support to increase adherence and thereby improve virologic outcome in persons initiating antiretroviral therapy. J Infect Dis 2005;192:1398–1406 [DOI] [PubMed] [Google Scholar]
- 18.Rana AI, van den Berg JJ, Lamy E, et al. Using a mobile health intervention to support HIV treatment adherence and retention among patients at risk for disengaging with care. AIDS Patient Care STDs 2016;30:178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): A randomised trial. Lancet 2010;376:1838–1845 [DOI] [PubMed] [Google Scholar]
- 20.Ingersoll KS, Dillingham RA, Hettema JE, et al. Pilot RCT of bidirectional text messaging for ART adherence among nonurban substance users with HIV. Health Psychol 2015;(34S):1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pop-Eleches C, Thirumurthy H, Habyarimana JP, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: A randomized controlled trial of text message reminders. AIDS 2011;25:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds NR, Testa MA, Su M, et al. Telephone support to improve antiretroviral medication adherence: A multisite, randomized controlled trial. J Acquir Immune Def Syndr 2008;47:62–68 [DOI] [PubMed] [Google Scholar]
- 23.Murray MC, O'Shaughnessy S, Smillie K, et al. Health care providers' perspectives on a weekly text-messaging intervention to engage HIV-positive persons in care (WelTel BC1). AIDS Behav 2015;19:1875–1887 [DOI] [PubMed] [Google Scholar]
- 24.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: A cohort study. Lancet Infect Dis 2014;14:820–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu A, Stojanovski K, Lester R, et al. Developing and implementing a mobile health (mHealth) adherence support system for HIV-uninfected men who have sex with men (MSM) taking pre-exposure prophylaxis (PrEP): The iText Study. Paper presented at: 8th International Conference on HIV Treatment and Prevention Adherence, Miami, FL, 2013 [Google Scholar]
- 26.Imbens GW, Lemieux T. Regression discontinuity designs: A guide to practice. J Econom 2008;142:615–635 [Google Scholar]
- 27.CDC. Diagnosis of HIV infection in the United States and dependent areas, 2016. HIV Surveillance Report 2017;28 [Google Scholar]
- 28.Hosek SG, Rudy B, Landovitz R, et al. An HIV preexposure prophylaxis demonstration project and safety study for young MSM. J Acquir Immune Def Syndr 2017;74:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosek SG, Landovitz RJ, Kapogiannis B, et al. Safety and feasibility of antiretroviral preexposure prophylaxis for adolescent men who have sex with men aged 15 to 17 years in the United States. JAMA Pediatr 2017;171:1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal-and community-based sexual health services. JAMA Intern Med 2016;176:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedele DA, Cushing CC, Fritz A, et al. Mobile health interventions for improving health outcomes in youth: A meta-analysis. JAMA Pediatr 2017;171:461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mimiaga MJ, Closson EF, Battle S, et al. Reactions and receptivity to framing HIV prevention message concepts about pre-exposure prophylaxis for Black and Latino men who have sex with men in three urban US cities. AIDS Patient Care STDs 2016;30:484–489 [DOI] [PubMed] [Google Scholar]
- 33.Philbin MM, Parker CM, Parker RG, et al. The promise of pre-exposure prophylaxis for Black men who have sex with men: An ecologic approach to attitudes, beliefs and barriers. AIDS Patient Care STDs 2016;30:282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu A, Vittinghoff E, von Felten P, et al. PrEP Initiation and Early Adherence Among Young MSM and Transgender Women in Chicago in the Enhancing PrEP in Community (EPIC) Study. Chicago: Paper presented at: HIV Research for Prevention (R4P), 2016 [Google Scholar]
- 35.Moore DJ, Jain S, Dubé MP, et al. Randomized Controlled Trial of Daily Text Messages to Support Adherence to PrEP in At-Risk for HIV Individuals: The Tapir Study. Clin Infect Dis 2017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saberi P, Johnson MO. Technology-based self-care methods of improving antiretroviral adherence: A systematic review. PLoS One 2011;6:e27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saberi P, Siedle-Khan R, Sheon N, et al. The use of mobile health applications among youth and young adults living with HIV: Focus group findings. AIDS Patient Care STDs 2016;30:254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]