Abstract
Background: BRAFV600E is the most common mutation in papillary thyroid carcinoma (PTC) and can be associated with aggressive disease. Previously, a highly sensitive blood RNA-based BRAFV600E assay was reported. The objective of this study was to assess the correlation of BRAFV600E circulating tumor RNA levels with surgical and medical treatment.
Methods: Circulating BRAFV600E levels were assessed in (i) a murine model of undifferentiated (anaplastic) thyroid carcinoma with known BRAFV600E mutation undergoing BRAFV600E-inhibitor (BRAFi) treatment, and (ii) in 111 patients enrolled prior to thyroidectomy (n = 86) or treatment of advanced recurrent or metastatic PTC (n = 25). Blood samples were drawn for BRAFV600E analysis before and after treatment. Testing characteristics were assessed and positivity criteria optimized. Changes in blood BRAFV600E values were assessed and compared to clinical characteristics and response to therapy.
Results: In a murine model of anaplastic thyroid carcinoma with BRAFV600E mutation, blood BRAFV600E RNA correlated with tumor volume in animals treated with BRAFi. In tissue BRAFV600E-positive (n = 36) patients undergoing initial surgery for PTC, blood BRAFV600E levels declined postoperatively (median 370.0–178.5 fg/ng; p = 0.002). In four patients with metastatic or poorly differentiated thyroid carcinoma receiving targeted therapies, blood BRAFV600E declined following therapy and corresponded with radiographic evidence of partial response or stable disease.
Conclusions: This study shows the correlation of blood BRAFV600E levels in response to treatment in both an established animal model of thyroid cancer and in patients with BRAFV600E-positive tumors with all stages of disease. This assay represents an alternative biomarker in patients with positive thyroglobulin antibodies, and tumors, which do not express thyroglobulin.
Keywords: : papillary thyroid cancer, thyroid cancer, BRAFV600E, biomarker, circulation tumor cells
Introduction
Thyroid cancer has been increasing in incidence greater than any other cancer and is expected to be the third most common cancer in women by 2019 (1,2). Long-term disease-specific survival is excellent for most patients in this younger-skewing population, leading to an escalating prevalence of disease. While initially this increase in incidence was entirely attributed to increased diagnosis of small indolent tumors, a recent report shows increases in higher risk tumors as well (3). Acknowledgment of overdiagnosis of indolent disease and report of active surveillance protocols in Japan, among other observations, has led to a general shift in recommendations for less aggressive treatment of papillary thyroid carcinoma (PTC) (4–6). Improved risk stratification is highlighted as an important focus of thyroid cancer research (5).
Most risk-stratification algorithms were designed to predict mortality and do not integrate mutational status. The 2009 American Thyroid Association (ATA) guidelines for the management of differentiated thyroid cancer introduced a three-tiered risk of persistent/recurrent disease following initial treatment, which was validated in a number of countries (7). In the 2015 revision of the ATA guidelines, the authors include BRAFV600E as a potential additional prognostic variable (5). BRAFV600E causes constitutive activation of the MAPK pathway and is present in more than one half of PTCs and in 5–20% of patients with poorly differentiated and anaplastic thyroid carcinomas (ATC) (8,9). The proportion of BRAFV600E-positive PTC is reportedly increasing (10). In thyroid cancer patients, this mutation is highly specific for PTC. Commercial DNA and gene expression profile assays performed on thyroid fine-needle aspirates (FNA) are highly predictive of malignancy when the BRAFV600E DNA mutation is present (11–13). Currently, assessment of the thyroid tumor tissue for this mutation requires obtaining genetic material for mutational profiling through FNA or tissue for immunohistochemical staining with specific anti-BRAFV600E antibodies (14). Traditional tissue assays are considered less sensitive because of the potential for background tissue contamination and sampling error (i.e., testing an adjacent BRAFwt nodule) (5).
Knowing the mutation status of each tumor is important, since the mutation is associated with aggressive pathological features, local tumor recurrence, loss of radioactive iodine avidity (RAI), and increased disease-specific mortality (15–21). Even in papillary thyroid microcarcinomas (<1 cm), aggressive features and a higher risk of recurrence are observed in patients with BRAFV600E tumors (22,23). While prophylactic central cervical lymph node dissection in patients with cytological BRAFV600E tumors is controversial, some experts recommend using BRAFV600E status to guide extent of initial surgery and use of RAI (24–27).
Recently, the development and feasibility of a highly sensitive blood RNA-based BRAFV600E assay in patients with thyroid disease was reported (28). Circulating BRAFV600E was able to be detected in the blood of thyroid cancer patients, and a good correlation was found with conventional tissue methods. The correlation of response to treatment in patients with melanoma undergoing BRAFV600E-inhibitor (BRAFi) therapy has also been reported (5,29–32). In patients with thyroid cancer, a liquid biopsy that can accurately measure circulating BRAFV600E levels would be beneficial at multiple levels: (i) in patients with tumor recurrences, where tissue is not easily accessible; (ii) an alternative biomarker for surveillance of those patients with thyroglobulin antibodies (TgAb); (iii) a biomarker for advanced thyroid cancer patients undergoing BRAFV600E-targeted therapies; and (iv) an alternative to Tg in patients undergoing thyroid lobectomy alone for lower-risk PTC, since the remnant gland is left in situ (5,30–32). Compared to FNA, a routine blood draw is less expensive and less invasive. In addition, the FNA can only be done on one lesion at a time.
This work further characterized the RNA-based blood assay to see if it would allow for serial, quantitative analysis correlating with effects of treatments such as surgery or targeted therapies. In addition, BRAFV600E levels were analyzed in a murine model of undifferentiated (anaplastic) thyroid carcinoma harboring the BRAFV600E mutation during treatment with BRAFV600E inhibitors. It was hypothesized that blood BRAFV600E levels would correlate with response to treatment. Serial blood BRAFV600E levels could provide an inexpensive, safe, and simple mechanism for risk stratification and surveillance in the 50% of patients with PTC with BRAFV600E mutation, offer an alternative biomarker in in patients where Tg levels are not informative, and provide longitudinal assessment of treatment response.
Materials and Methods
Orthotopic murine model of human ATC
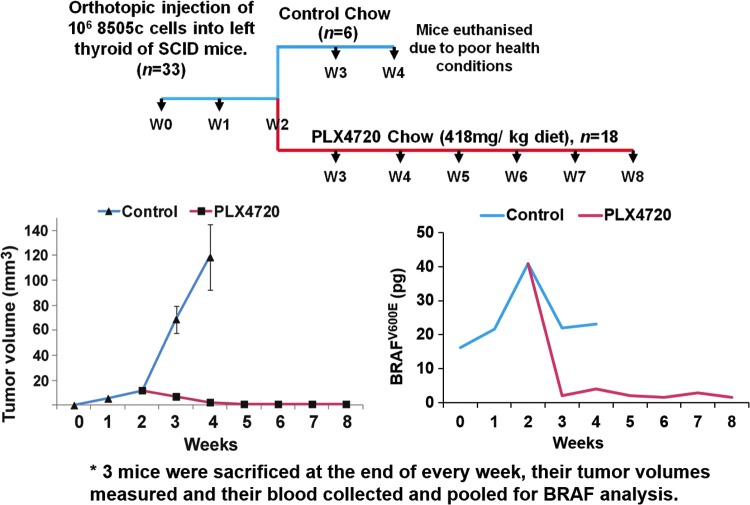
All animal work was done in the animal facility at Massachusetts General Hospital (Boston, MA) in accordance with federal, local, and institutional guidelines. Human ATC cells (8505c; BRAFV600E/–, TP53R248G/–; Deutsche Sammlung von Mikroorganismen und Zellkulturen) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in a 37°C 5% CO2 incubator. An orthotopic murine model of BRAFV600E-mutant ATC was chosen in order to provide proof-of-principle of the feasibility of detecting plasma BRAFV600E RNA and the ability to correlate plasma levels with tumor volume. The 8505c human ATC orthotopic severe combined immunodeficiency (SCID) model progresses at a much faster rate compared to PTC models, which lends to improved ease of use. Human ATC cells were injected into the thyroid of 33 ten-week-old female SCID mice, as previously described (33). Briefly, the mice were anesthetized, the thyroid gland exposed, and 106 8505c cells were injected into the left thyroid lobe with a 27-gauge needle. The right thyroid lobe was not manipulated to serve as an internal control. Mice were randomized to either a control diet (Research Diets, Inc.) or 418 mg/kg BRAFi (PLX4720)-embedded chow (Research Diets, Inc.) ad libitum, starting two weeks after injection (34). Three mice were euthanized weekly for assessment of tumor volume (volume = [length × width × depth]/2), and blood was obtained by cardiopuncture and pooled to yield sufficient quantity. The blood BRAFV600E level was quantified, as previously described and below (29).
Patient selection
Under approval by the Partners Human Research Committee Institutional Review Board at the Massachusetts General Hospital, patients with benign and malignant thyroid disorders undergoing initial curative surgery or treatment of recurrent disease were enrolled between September 2013 and September 2015. After informed consent, a 5 mL sample of peripheral blood was obtained from each patient before and after surgery or, in the cases of iodine-refractory metastatic disease, during treatment with targeted chemotherapies. The post-treatment blood was drawn at the time of the subsequent clinic visit, the vast majority being at the postoperative visit (median 17.5 days; interquartile range (IQR) 13.0 to 30.3 days).
Blood BRAFV600E assay protocol
The assay was previously described in detail (28,29). Briefly, peripheral blood lymphocytes were isolated by Ficoll density centrifugation from each blood sample and stored in freezing medium. RNA was isolated by the Trizol method (Invitrogen, Grand Island, NY) and (50–100 ng) reverse transcribed to cDNA by standard methods, normalized in quantity with 18S RNA, and amplified. After DNA cleanup, wild-type BRAF (BRAFwt) was digested with TSPR1 (restriction site = NNCASTGNN; New England Biolabs, Beverly, MA) to reduce contamination by normal BRAFV600E from surrounding normal tissue in the blood samples. The product was then subjected to a second round of polymerase chain reaction (PCR) and BRAFwt digestion. To favor the mutant further over the wild-type product, a 33-fold excess of the reverse (common sequence in mutant and wild-type) to forward (exact match for mutant and one base mismatch for wild-type sequences) primers were used in the final real-time PCR assay for BRAFV600E. Oligonucleotides were custom synthesized from Invitrogen (Carlsbad, CA) and Sigma–Aldrich (St. Louis, MO). Purified BRAFV600E first-round PCR product with a known concentration was also run through the assay and was used to create a standard curve.
It was previously established that the assay can detect as low as 1 pg of BRAFV600E and has a 1000-fold increased sensitivity compared to the BRAFwt (28). This assay was used for the orthotopic murine model (discussed above), reported in pictograms, with a positivity criterion of 4.8 pg. The 18S reverse transcription PCR assay is now run in the presence of known amounts of purified RNA in order to generate a standard curve (input RNA in nanograms). Going forward, all new data generated by this assay will be reported as femtograms of BRAFV600E/nanogram of RNA.
Tissue-based BRAFV600E analysis
Tissue-based BRAFV600E analysis has been previously described (28). Briefly, patients meeting inclusion criteria and with tissue available had BRAFV600E mutational analysis as part of standard clinical care either via SNaPshot (Massachusetts General Hospital Cancer Center Translational Research Laboratory) or immunohistochemistry was done using a BRAFV600E monoclonal antibody with 97% correlation with SNaPshot molecular testing (98% sensitivity and 97% specificity) (35). For PTC cases in which mutational status was not obtained clinically, BRAFV600E mutation was sequenced from formalin-fixed, paraffin-embedded (FFPE) tissue blocks, as previously described.
Tg assay
A commercial assay (Mayo Medical Laboratories New England, MA) was used to determine Tg levels in all patients, as per standard clinical practice.
Statistical analysis
Clinical variables were chosen based on established demographic and pathological risk factors for decreased thyroid cancer-free survival. The American Joint Committee on Cancer TNM (AJCC TNM) stage was determined for each patient. For comparisons between BRAFV600E and wild-type groups, pathological variables were only considered present if they were specifically described in the final pathology report (as per convention). Univariate comparisons of categorical variables were analyzed using Fisher's exact test, and continuous variables were assessed by Student's t-test or Wilcoxon's rank-sum test for nonparametric data. Correlations of postsurgical blood BRAFV600E expression levels according to mutational status and demographic and pathological characteristics were based on linear regression models. A p-value of <0.05 was considered statistically significant. Using tissue results as the gold standard, likelihood ratios (sensitivity/1 – specificity) were calculated, and a receiver operating characteristic curve was produced. An a priori decision was made to maximize specificity for determining the positivity criterion. Blood BRAFV600E levels before and after surgery were compared, and Wilcoxon's signed rank test was carried out to assess the non-normal blood BRAFV600E level change before and after surgery for tissue BRAFV600E-positive and tissue BRAFwt patients, respectively.
Results
Circulating BRAFV600E levels correlate with tumor response after treatment with BRAFi in an orthotopic murine model of ATC
The previously described immunocompromised orthotopic mouse model of ATC was utilized to study the correlation between BRAFV600E tumor growth and BRAFV600E levels both before and following treatment with the selective BRAFi (PLX4720) (33). This model results in tumor growth with extrathyroidal extension over several weeks and cervical lymph node and pulmonary metastases; control animals have a life expectancy of up to five weeks.
A total of 33 mice had orthotopic injection of 8505c cells (Fig. 1). Three mice were euthanized weekly to evaluate mean tumor volumes, and their blood was pooled to obtain sufficient peripheral blood leukocytes for RNA-based BRAFV600E analysis. Two days after implantation of tumor, mouse blood BRAFV600E levels were 16.17 pg and steadily rose such that at two weeks post implantation, mice had a peak BRAFV600E RNA level of 40.8 pg in the pooled blood and a mean tumor volume of 12.0 ± 0.6 mm3. Subsequently, mice were randomized to either control chow or treatment with BRAFi embedded chow at a standard dose (418 mg/kg BRAFi). Over the next set of measurements, tumor volume rose steadily to a peak of 118.6 ± 26.1 mm3 at four weeks in the control group, consistent with previously reported data. Blood BRAFV600E levels remained elevated at each weekly time point (34). During the third week post implantation, the BRAFV600E blood level dipped slightly to 22.01 pg and remained stable at four weeks. The control mice had to be euthanized at the beginning of week 5 post implantation in order to meet humane endpoints. In the BRAFi-treated group, tumor volume declined to 7.11 ± 1.0 mm3 and 2.53 ± 2.7 mm3 at weeks 3 and 4, respectively. Tumors subsequently became and remained undetectable. Mean blood BRAFV600E RNA declined in tandem with tumor volume to 2.1 pg and 4.0 pg at weeks 3 and 4, respectively, and remained between 1.5 pg and 2.8 pg through week 8 of the study.
FIG. 1.
BRAFV600E-mutated thyroid tumors grow in the first two weeks after orthotopic tumor implantation. Blood BRAFV600E levels also increase. Treatment with BRAFi (PLX4720) prevents tumor progression and dramatically reduces blood BRAFV600E levels.
Patient cohort characteristics
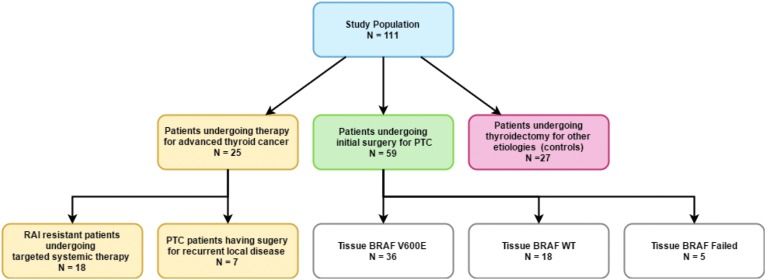
The goal was to analyze this assay in a variety of thyroid cancer patients to understand assay utility fully. The study population included 111 patients who had their blood BRAFV600E level measured before and after surgery or adjuvant therapy (Fig. 2). Of the 111 patients, 25 were undergoing advanced thyroid cancer therapy, including seven PTC patients who had surgery for recurrent local disease and 18 patients with RAI-resistant differentiated, poorly differentiated, or ATC undergoing active surveillance, or systemic therapy with small molecule inhibitors or, in the case of ATC, cytotoxic chemotherapy and radiotherapy. Fifty-nine patients underwent initial surgical treatment with curative intent for PTC, 51 (94.4%) of whom underwent at least total thyroidectomy. In this group, 36 patients had tumor tissue harboring the BRAFV600E mutation, whereas 18 patients had tumor tissue with BRAFwt. In five patients, the assay failed (n = 1) or BRAFV600E testing was not performed. An additional 27 patients were recruited as controls, and their surgical pathology diagnoses were multinodular goiter (n = 23) and follicular adenoma (n = 4). None of the patients had known melanoma or colorectal adenocarcinoma. Patients with cancer had AJCC TNM stages I–IV (Table 1).
FIG. 2.
Patient cohort and associated therapy.
Table 1.
Characteristics of Cohort Patients Who Underwent Initial Surgery for Curative Treatment for PTC
| All (n = 54) | Tissue BRAFV600Epositive (n = 36) | Tissue BRAFwt(n = 18) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Female, n (%) | 38 (70) | 25 (69) | 13 (72) | 0.99 |
| Age at surgery, M (±SD) | 46.9 (15.1) | 44.8 (13.5) | 51.0 (17.5) | 0.16 |
| Primary tumor characteristics | ||||
| Tumor maximum diameter (mm), M (±SD) | 1.9 (1.3) | 1.9 (1.2) | 1.8 (1.3) | 0.92 |
| Extrathyroidal extension, n (%) | 19 (35) | 14 (39) | 5 (28) | 0.55 |
| Lymphovascular invasion, n (%) | 27 (51) | 21 (58) | 6 (35) | 0.15 |
| Cervical lymph node status, n (%) | ||||
| Central lymph node metastases | 23 (42.6) | 18 (50.0) | 5 (27.8) | 0.15 |
| Lateral lymph node metastases | 11 (20.4) | 8 (22.2) | 3 (16.7) | 0.73 |
| AJCC TNM stage, n (%) | 0.35 | |||
| I | 37 (69) | 25 (69) | 12 (67) | |
| II | 4 (7) | 1 (3) | 3 (7) | |
| III | 5 (9) | 4 (1) | 1 (6) | |
| IV | 8 (5) | 6 (7) | 2 (1) | |
| Blood BRAFV600Elevel | ||||
| Preoperative level (fg/ng RNA), median (IQR) | 301.5 (136.3–608.0) | 370.0 (203.3–686.5) | 136.5 (85.3–354.0) | 0.03 |
| Postoperative level (fg/ng RNA), median (IQR) | 177.5 (80.0–254.8) | 178.5 (75.8–305.5) | 158.0 (87.0–223.3) | 0.73 |
PTC, papillary thyroid carcinoma; wt, wild type; SD, standard deviation; AJCC TNM, American Joint Committee of Cancer Tumor, Node, Metastasis; IQR, interquartile range.
Testing characteristics
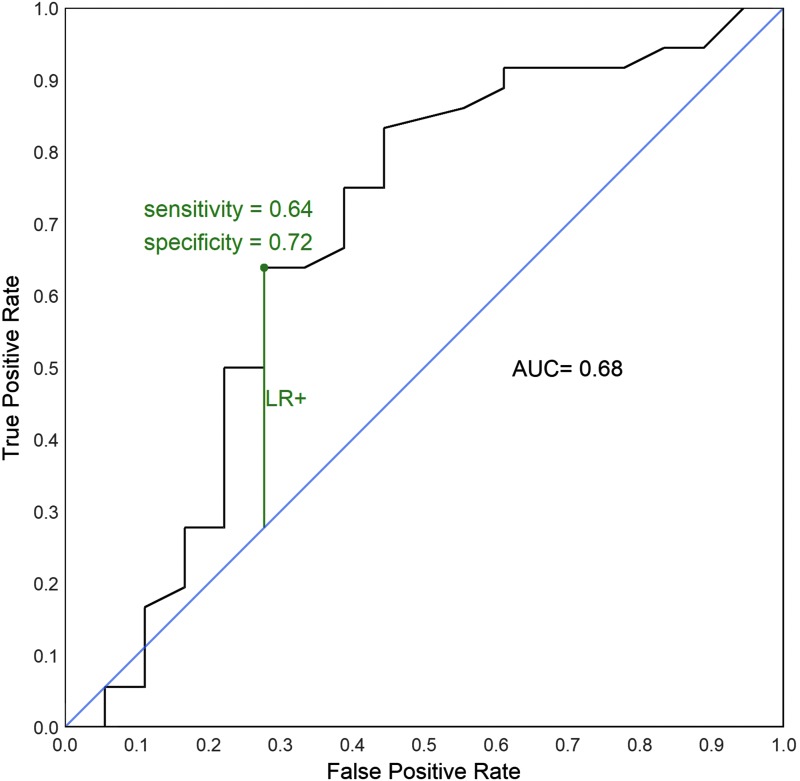
To compare the blood BRAFV600E assay results with the conventional tissue testing, the results of the optimized blood assay were compared to conventional tissue mutational testing using the group of patients undergoing initial surgery for curative intent in which tissue genotyping was available. Using the tissue BRAFV600E result as the gold standard, likelihood ratios were calculated and a receiver operating characteristic curve for the blood assay predicting tissue BRAFV600E mutational status was constructed (Fig. 3). The area under the curve (AUC) was 0.68, indicating moderate correlation of tests assuming the tissue results are true. Using maximum likelihood ratio for a positive test (LR+) as the optimal cutoff, the threshold for blood BRAFV600E level is 140–145 fg/ng RNA.
FIG. 3.
Receiver operating characteristic curve based on 54 patients receiving initial papillary thyroid carcinoma therapy who have tissue BRAFV600E status.
Blood BRAFV600E levels decline following initial surgical treatment for PTC
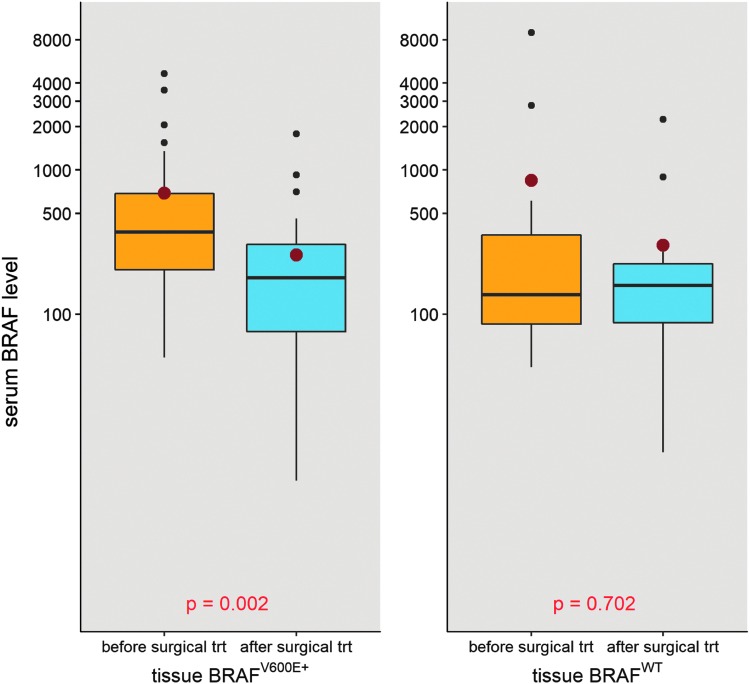
Preoperative blood BRAFV600E levels were assessed for association with clinical and pathological characteristics. On multivariable linear regression, adjusting for sex, age, and tumor volume, only extrathyroidal extension was correlated with the preoperative blood BRAFV600E level (p = 0.03). The blood BRAFV600E levels were compared before and after initial surgical treatment for PTC patients stratified by their tissue BRAFV600E status (Fig. 4). For tissue BRAFV600E-positive patients (n = 36), the median blood BRAFV600E level dropped from 370.0 fg/ng RNA to 178.5 fg/ng RNA (p = 0.002). For tissue BRAFwt (n = 18) and for non-PTC controls with tissue BRAFwt (n = 15), the median blood BRAFV600E level did not change significantly with surgery (p = 0.70 and p = 0.69, respectively). Three of these patients for whom blood BRAFV600E levels were available before and after initial surgery for curative intent had a structural recurrence requiring surgery during the study period, all of whom were tissue BRAFV600E positive. Blood BRAFV600E levels correlated with clinical disease status in the two patients with high preoperative levels. In one patient, the preoperative BRAFV600E level of 3567 fg/ng RNA fell to139 fg/ng (Tg 8.8 ng/mL), below the established threshold, at six months postoperatively. With the development of recurrent clinical nodal disease six months later, the BRAFV600E level had increased to 316 fg/ng RNA and correlated with an increase in the Tg level, indicating recurrence (Tg level 54 ng/mL). To see whether serum thyrotropin (TSH) levels influenced blood BRAFV600E levels, the length of the time lapse between collection of the blood BRAFV600E levels, tissue BRAFV600E levels, and the recorded TSH were adjusted, and no significant association was found between blood BRAFV600E levels and TSH levels following surgery (data not shown). The effect between postoperational serum BRAFV600E level and the time between surgery and measurement was also tested (median 17.5 days; IQR 13.0 to 30.3 days), adjusting for preoperative blood BRAFV600E levels and tissue BRAF status in a linear regression model, and there was no significant association (p = 0.328).
FIG. 4.
Comparison of blood BRAFV600E level (log10 scale) before and after initial surgical treatment among tissue BRAFV600E-positive and BRAFwt patients. The box plots measure 1st quartile −1.5 interquartile range (IQR), 1st quartile, median, 3rd quartile, 3rd quartile +1.5 IQR from bottom to top.
Blood BRAFV600E levels before and after surgical treatment for locally recurrent PTC
Seven patients in the cohort had remote thyroidectomy with or without cervical lymph node dissection. Five of the patients had tumor tissue positive for BRAFV600E, one in the index tumor and four in the recurrent tumors. Blood BRAFV600E was obtained before and after surgery to characterize recurrent local disease. Median blood BRAFV600E before and after salvage surgery was 319.0 fg/ng RNA (IQR 192.5–562.6 fg/ng) and 260 fg/ng RNA (IQR 109.0–491.0fg/ng). In one patient, clinical recurrence occurred one year following initial total thyroidectomy with central lymph node dissection for a tall-cell variant PTC with a small focus of ATC. The preoperative BRAFV600E level was 1193 fg/ng. While two weeks following salvage surgery this level failed to decline (2163 fg/ng), after external beam radiation, the level decreased to 598 fg/ng RNA. Six months following this, the level returned to 2104 fg/ng RNA, which was concerning for worsening disease. The patient has a new thyroid bed mass that is being observed (based on the patient's age and choice), which is consistent with the observed elevation in BRAFV600E levels. Throughout the clinical course, Tg levels have been very low to undetectable (0.1–0.3 ng/mL). Two other patients in this group had elevated TgAb.
Blood BRAFV600E levels before and after initiation of targeted therapies
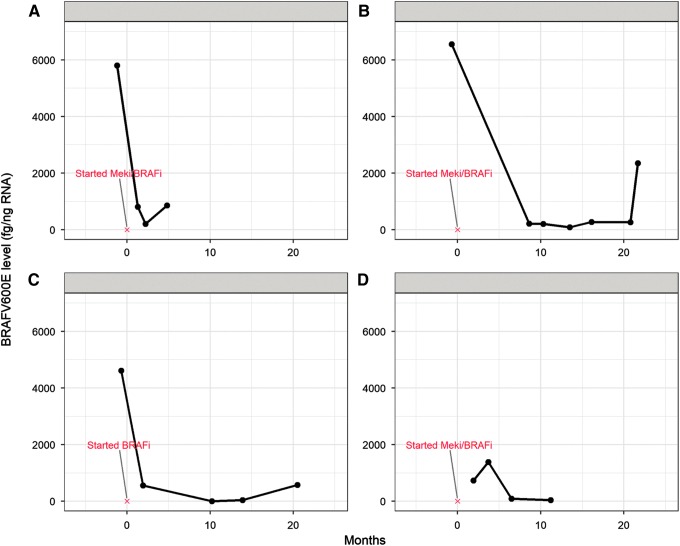
Blood BRAFV600E samples were obtained from 18 patients with metastatic RAI-resistant papillary (n = 11) poorly-differentiated/anaplastic (n = 5), and Hürthle cell (n = 2) thyroid carcinomas undergoing active surveillance, or receiving chemotherapy, radiotherapy, multikinase inhibitors, and/or MAP-kinase pathway-specific inhibitors. Fourteen of the patients had BRAFV600E-mutant tumors, and all 18 had detectable levels of BRAFV600E in their blood (median 615 fg/ng; range 41–6552 fg/ng). Six patients (five BRAFV600E-positive PTC and one with poorly differentiated thyroid carcinoma [BRAFV600E positive]) did not have an alternative means of biochemical monitoring due to lack of Tg production (Tg <1 ng/mL; n = 4) or because of elevated TgAb (n = 2). Five of these six patients without other means of biochemical monitoring had gross elevations in pretreatment blood BRAFV600E levels (range 552–6552 fg/ng) that dropped following systemic therapy initiation (range of log2 [fold change] of 0.76–4.91). Correlation between blood BRAFV600E and treatment response per RECIST v 1.1 was most notable in patients receiving BRAFV600E inhibitors alone or in combination with MEK inhibitors (Fig. 5). In this subset of four patients, blood BRAFV600E levels declined dramatically following initiation of therapy and corresponded with radiographic evidence of partial response or stable disease. In three of these patients, blood BRAFV600E increased from the nadir, one of whom developed progressive disease 13 months after this increase, while the two other patients continued to have stable disease.
FIG. 5.
Circulating levels of BRAFV600E in four patients with radioiodine-refractory metastatic BRAFV600E-positive thyroid cancer undergoing targeted therapy. BRAFi, dabrafenib or vemurafenib; MEKi, trametinib.
Discussion
Thyroid cancer has been increasing in incidence more than any other cancer in the past few decades. While the majority of the increase is attributed to small, seemingly indolent cancers, a recent report also shows an increase in higher-staged tumors and disease-specific mortality (3). With recent changes in guidelines toward less aggressive treatment, improved risk stratification for thyroid cancer patients is essential (5). Additionally, the standard circulating biomarker, Tg, is unreliable in the subset of patients who progress to aggressive disease and whose tumors no longer make Tg, as well as in patients with TgAb that interfere with measurement of Tg. Thus, alternative biomarkers are needed.
Approximately half of patients with PTC harbor a BRAFV600E mutation, a proportion that may be growing, at least in North America, with the reclassification of RAS-mutant (BRAFwt) noninvasive encapsulated follicular-variant PTC to noninvasive follicular thyroid neoplasm with papillary-like nuclear features (36). Large controlled studies have shown a correlation between this mutation and aggressive tumor characteristics, tumor recurrence, and overall survival particularly in the presence of other mutations such as TERT (15,17,18,37). Knowledge of BRAFV600E status may be helpful in diagnosis (nearly 100% positive predictive value for PTC); determining extent of surgery (lobectomy vs. total thyroidectomy ± level VI cervical lymph node dissection), use of RAI, and frequency and intensity of follow-up; as an alternative to Tg in Tg-negative or TgAb-positive patients; and to assess response to BRAFi therapy in patients with advanced disease. Conventionally, cytology or surgical pathology specimens have been utilized to ascertain BRAFV600E mutational status. The feasibility of a sensitive RNA-based blood assay to detect and serially monitor BRAFV600E levels in patients with PTC has been published previously (28).
A key potential advantage of the assay is to serve as an alternative biomarker in patients with anti-TgAb, present in a significant proportion of PTC patients, and in the subset of patients whose tumors stop producing Tg because of disease dedifferentiation (38). Indeed, 12/59 (20%) patients undergoing initial surgery for PTC in this study had TgAb noted postoperatively, making Tg levels useless. Of these 12, nine were positive for BRAFV600E on tissue testing. Indeed, in 6/18 patients with advanced thyroid cancer, the BRAFV600E levels were elevated in the setting of very low or absent Tg <1.0 ng/mL and/or anti-TgAb (n = 2). Moreover, in the small but substantial group of patients destined to do poorly, there is an enrichment of BRAFV600E mutations. Thus, the ability to detect and follow BRAFV600E levels accurately over time as a biomarker for recurrence and response to BRAFi would be of great clinical value.
This work shows the correlation of blood BRAFV600E levels in response to treatment both in an established animal model of thyroid cancer and in BRAFV600E patients with all stages of disease undergoing treatment. In both the orthotopic murine model and in the patients, blood BRAFV600E levels were not associated with tumor size, TSH level, Tg levels, or time of blood draw. In fact, the only clinical or pathological factor correlating with BRAFV600E levels in the patient cohort was extrathyroidal extension. It was found in general that blood BRAFV600E levels were detectable in all stages of disease and responded to both surgical therapy and treatment with systemic targeted therapy.
It was observed that plasma BRAFV600E RNA levels spontaneously dropped in control mice that did not receive PLX4720 at week 3, albeit to a much lesser extent compared to mice that received treatment. The reasons for this decline (and subsequent rise) are unclear but may be due to the complex dynamic relationship between circulating tumor cells/nucleic acids and the originating tumor volume. In the 8505C orthotopic SCID ATC model, weeks 2–3 coincide with the exponential growth of thyroid tumor volume (in this experiment, mean tumor volume increased from 12.0 mm3 to 68.9 mm3) and the emergence of pulmonary micrometastases. In a review of the primary data, the subsequent rise in BRAF-mutant RNA at week 4 is largely driven by higher levels of the fourth mouse with a small tumor whose blood was analyzed separately. This was included at the four-week time point by taking a mean with a weighted mean of the pooled mice (n = 3). If this mouse is excluded from the analysis, the decline continues to occur. The outlier was included for completeness. Although not specifically shown for circulating tumor RNA, a spontaneous decrease in circulating tumor cells has been reported in the literature, especially during a period of rapid tumor growth following a period of slower growth (39–41). A spontaneous decline and then subsequent rise has also been reported, especially for larger tumors. There are several postulated mechanisms for this phenomenon that remain unproven. One such mechanism includes the activation of host immunity by increasing single or clustered circulating tumor cells and subsequent enhanced clearance, although this immune activation is not enough to penetrate and/or clear the primary solid tumor or metastases, which are associated with an immunosuppressive microenvironment. While one might expect this phenomenon to be dampened in SCID mice, they do retain functional phagocytes such as neutrophils and monocytes/macrophages, which may be contributory to clearance. Additionally, rapid tumor growth may be associated with changes in tumor vessel morphology and function and tumor cell apoptosis/necroptosis; it is conceivable that these variables might decrease the efflux of circulating tumor cells.
Limitations of our study are acknowledged. There is some baseline signal in both BRAFwt patients and patients undergoing thyroidectomy for benign disease. Using tissue BRAFV600E status of the dominant tumor as the reference may be a source of potential error, given that PTC is commonly multifocal, with some carcinomas harboring BRAFV600E mutations and others that do not. It is possible that the larger, dominant nodule undergoing tissue testing may be BRAFwt while other, potentially smaller, carcinomas (which may shed BRAFV600E into the blood) may not have been tested. Another possible explanation is that there is a small volume of BRAF-mutant cells in the thyroid neoplasm that was not identified, since it was below the level of detection of the BRAF tissue testing. The blood test may be more sensitive than tissue testing. However, this is impossible to prove without testing at the tissue level (although testing at the tissue level may be limited by heterogeneity between different lesions, as discussed above). Additionally, the possibility cannot be entirely excluded that detectable circulating levels of BRAFV600E are from an undiagnosed BRAFV600E mutant malignancy (e.g., melanoma or colorectal adenocarcinoma), although there was no clinical documentation of such in the cohort. None of these patients has declared themselves with a secondary malignancy thus far, although small PTC can be indolent. Lastly, it is also possible that there is a false-positivity rate or noise with the assay at low or absent BRAFV600E concentrations that will not be overcome until technical and analytical validation is formally completed, which is the subject of ongoing work. As with other biochemical assays, a functional sensitivity threshold will need to be established. The change from before to after treatment will likely be the most useful metric to follow.
Recently, several commercial BRAFV600E assays, originally designed for and validated on FFPE samples, have been investigated for use with circulating tumor DNA isolated from the blood of patients with melanoma, including the PrimePCR™ ddPCR™ BRAFV600E pV600E Mutation Assay (Bio-Rad, Hercules, CA), the therascreen CRGQ PCR Kit (Qiagen, Hilden, Germany), the Idylla™ BRAFV600E mutation test (Biocartis, Mechelen, Belgium), the PNA Clamp™ BRAFV600E Mutation Detection Kit (Panagene, Daejeon, Korea), and the ctBRAFV600E Mutation Detection Kit (Entrogen, Woodland Hills, CA) (42–48). Kim et al. (45) were the first to adopt the use of the TaqMan® (castPCR™) BRAFV600E Mutation Detection Assay (Life Technologies, Carlsbad, CA) in the plasma of patients with PTC. The present assay detects BRAFV600E amid excess wild-type BRAF with high sensitivity (28,29). The use of RNA with exponentially higher copy numbers of BRAFV600E mRNA as well as digestion of BRAFwt enhances the sensitivity of the assay, which is a potential advantage over the DNA-based techniques described above.
Blood-based BRAFV600E detection has several clinical applications. Unlike tissue mutational testing, in which results are qualitative and binary, this assay allows quantitative, serial measurements of circulating BRAFV600E, and levels are responsive to treatment. The ability to test circulating BRAFV600E levels during treatment with BRAFi and other targeted therapies to assess response to treatment and prediction of re-differentiation would be of great value in guiding therapy. Recent work has shown the BRAFi (dabrafenib) treatment in RAI-refractory patients can lead to re-differentiation and subsequent RAI uptake in some tumor lesions (32). Partial response was also seen in a Phase II trial with the BRAFi vemurafenib in 10/26 patients with metastatic or unresectable RAI-refractory PTC (49). More recent work in two other Phase II trials using combinations of BRAFi and MEKi has shown high response rates in BRAFV600E RAI refractory PTC and BRAFV600E ATC patients (50,51), and one Phase I trial of dabrafenib and LAP was well tolerated in BRAFV600E thyroid cancer patients (52). The ability to test circulating BRAFV600E levels during treatment with BRAFi to assess response to treatment (surgical, RAI, and systemic therapy) and as an early marker of recurrence could be of great value.
In summary, this study reports the correlation of RNA blood BRAFV600E levels with surgical and medical management of patients with PTC. This builds on prior work showing that the assay has good correlation (AUC 0.71) with tissue BRAFV600E testing (28). In a murine model of BRAFV600E-mutant ATC, blood BRAFV600E levels correlate with tumor volume in animals and correlate with clinical response to BRAFi. The study also reports a significant decline in levels following initial surgery for curative intent. While it is challenging to make conclusions in the heterogeneous population of patients with metastatic or poorly differentiated thyroid carcinomas receiving targeted therapies, a subset of patients was found who showed a decline in blood BRAFV600E levels following therapy that corresponded with radiographic evidence of partial response or stable disease. It is possible that this blood assay may work best for patients with BRAFV600E-mutated tumors who have high baseline levels and are undergoing specific targeted therapies that work on reducing MAPK signaling. This assay represents an alternative biomarker in patients with anti-TgAb, and tumors, which do not express Tg. This is important, since no other biomarker exists for this group of patients and standard radiologic means of measuring tumor responses such as RECIST are less reliable in thyroid cancer. Finding a reliable assay that can provide early information into the therapeutic effect at the tumor cell level by measuring the RNA expression of the tumor cells in the blood can be an important tool that the medical oncologists can use to gauge a response to a targeted therapy.
Acknowledgments
This work was supported by the following: National Institutes of Health/National Cancer Institute Grant CA177900, The Claflin Foundation at Massachusetts General Hospital, and American Thyroid Association/ThyCa Research Grant (C.L.); The NIH National Cancer Institute (R01CA149738-01A1), Ruane Fund for Thyroid Cancer Research (S.P.); Conquer Cancer Foundation and the Clinical Investigator Training Program, Harvard Medical School and Massachusetts Institute of Technology (R.S). National Institutes of Health—Training Program in Endocrinology (5T32DK00702842; B.G.).
Author Disclosure Statement
The authors declare no potential conflicts of interest.
References
- 1.Surveillance, Epidemiology, and End Results. Available at: http://seer.cancer.gov/ (accessed December1, 2016)
- 2.Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, Ogilvie JB, Terris DJ, Netterville J, Wong RJ, Randolph G; AACE Endocrine Surgery Scientific Committee 2015. American Association of Clinical Endocrinologists and American College of Endocrinology Disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract 21:686–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. 2017. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317:1338–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haser GC, Tuttle RM, Su HK, Alon EE, Bergman D, Bernet V, Brett E, Cobin R, Dewey EH, Doherty G, Dos Reis LL, Harris J, Klopper J, Lee S, Levine RA, Lepore SJ, Likhterov I, Lupo MA, Machac J, Mandel SJ, Mechanick JI, Mehra S, Milas M, Orloff L, Randolph G, Revenson TA, Roberts KJ, Ross DS, Rowe ME, Smallridge R, Terris D, Tufano RP, Urken ML. 2016. Active surveillance for papillary thyroid microcarcinoma: new challenges and opportunities for the health care system. Endocr Pract 22:602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito Y, Oda H, Miyauchi A. 2016. Insights and clinical questions about the active surveillance of low-risk papillary thyroid microcarcinomas [Review]. Endocr J 63:323–328 [DOI] [PubMed] [Google Scholar]
- 7.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 8.Eloy C, Ferreira L, Salgado C, Soares P, Sobrinho-Simoes M. 2015. Poorly differentiated and undifferentiated thyroid carcinomas. Turk Patoloji Derg 31:48–59 [DOI] [PubMed] [Google Scholar]
- 9.Penna GC, Vaisman F, Vaisman M, Sobrinho-Simoes M, Soares P. 2016. Molecular markers involved in tumorigenesis of thyroid carcinoma: focus on aggressive histotypes. Cytogenet Genome Res 150:194–207 [DOI] [PubMed] [Google Scholar]
- 10.Kowalska A, Walczyk A, Kowalik A, Palyga I, Trybek T, Kopczynski J, Kajor M, Chrapek M, Pieciak L, Chlopek M, Gozdz S, Kaminski G. 2016. Increase in papillary thyroid cancer incidence is accompanied by changes in the frequency of the BRAF V600E mutation: a single-institution study. Thyroid 26:543–551 [DOI] [PubMed] [Google Scholar]
- 11.Alexander EK, Schorr M, Klopper J, Kim C, Sipos J, Nabhan F, Parker C, Steward DL, Mandel SJ, Haugen BR. 2014. Multicenter clinical experience with the Afirma gene expression classifier. J Clin Endocrinol Metab 99:119–125 [DOI] [PubMed] [Google Scholar]
- 12.Nam SY, Han BK, Ko EY, Kang SS, Hahn SY, Hwang JY, Nam MY, Kim JW, Chung JH, Oh YL, Shin JH. 2010. BRAF V600E mutation analysis of thyroid nodules needle aspirates in relation to their ultrasongraphic classification: a potential guide for selection of samples for molecular analysis. Thyroid 20:273–279 [DOI] [PubMed] [Google Scholar]
- 13.Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN. 2009. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 94:2092–2098 [DOI] [PubMed] [Google Scholar]
- 14.Dvorak K, Aggeler B, Palting J, McKelvie P, Ruszkiewicz A, Waring P. 2014. Immunohistochemistry with the anti-BRAF V600E (VE1) antibody: impact of pre-analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology 46:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elisei R, Viola D, Torregrossa L, Giannini R, Romei C, Ugolini C, Molinaro E, Agate L, Biagini A, Lupi C, Valerio L, Materazzi G, Miccoli P, Piaggi P, Pinchera A, Vitti P, Basolo F. 2012. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab 97:4390–4398 [DOI] [PubMed] [Google Scholar]
- 16.Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, Duh QY, Shibru D, Bastian B, Griffin A. 2007. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 246:466–470; discussion 470–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ, Kim KW, Hahn SK, Youn YK, Kim KH, Cho BY, Park do J. 2012. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118:1764–1773 [DOI] [PubMed] [Google Scholar]
- 18.Prescott JD, Sadow PM, Hodin RA, Le LP, Gaz RD, Randolph GW, Stephen AE, Parangi S, Daniels GH, Lubitz CC. 2012. BRAF V600E status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence. Surgery 152:984–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P. 2006. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I– targeting to the membrane. Endocr Relat Cancer 13:257–269 [DOI] [PubMed] [Google Scholar]
- 20.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V. 2013. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yip L, Nikiforova MN, Carty SE, Yim JH, Stang MT, Tublin MJ, Lebeau SO, Hodak SP, Ogilvie JB, Nikiforov YE. 2009. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery 146:1215–1223 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Sadow PM, Suh H, Lee KE, Choi JY, Suh YJ, Wang TS, Lubitz CC. 2016. BRAF(V600E) is correlated with recurrence of papillary thyroid microcarcinoma: a systematic review, multi-institutional primary data analysis, and meta-analysis. Thyroid 26:248–255 [DOI] [PubMed] [Google Scholar]
- 23.Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ, Qu JM. 2010. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol 17:3294–3300 [DOI] [PubMed] [Google Scholar]
- 24.Barbaro D, Incensati RM, Materazzi G, Boni G, Grosso M, Panicucci E, Lapi P, Pasquini C, Miccoli P. 2014. The BRAF V600E mutation in papillary thyroid cancer with positive or suspected pre-surgical cytological finding is not associated with advanced stages or worse prognosis. Endocrine 45:462–468 [DOI] [PubMed] [Google Scholar]
- 25.Dutenhefner SE, Marui S, Santos AB, de Lima EU, Inoue M, Neto JS, Shiang C, Fukushima JT, Cernea CR, Friguglietti CU. 2013. BRAF: a tool in the decision to perform elective neck dissection? Thyroid 23:1541–1546 [DOI] [PubMed] [Google Scholar]
- 26.Jia Y, Yu Y, Li X, Wei S, Zheng X, Yang X, Zhao J, Xia T, Gao M. 2014. Diagnostic value of B-RAF(V600E) in difficult-to-diagnose thyroid nodules using fine-needle aspiration: systematic review and meta-analysis. Diagn Cytopathol 42:94–101 [DOI] [PubMed] [Google Scholar]
- 27.Kim SK, Lee JH, Woo JW, Park I, Choe JH, Kim JH, Kim JS. 2016. BRAF V600E mutation: differential impact on central lymph node metastasis by tumor size in papillary thyroid carcinoma. Head Neck 38:E1203–1209 [DOI] [PubMed] [Google Scholar]
- 28.Lubitz CC, Parangi S, Holm TM, Bernasconi MJ, Schalck AP, Suh H, Economopoulos KP, Gunda V, Donovan SE, Sadow PM, Wirth LJ, Sullivan RJ, Panka DJ. 2016. Detection of circulating BRAF(V600E) in patients with papillary thyroid carcinoma. J Mol Diagn 18:100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panka DJ, Buchbinder E, Giobbie-Hurder A, Schalck AP, Montaser-Kouhsari L, Sepehr A, Lawrence DP, McDermott DF, Cohen R, Carlson A, Wargo JA, Merritt R, Seery VJ, Hodi FS, Gunturi A, Fredrick D, Atkins MB, Iafrate AJ, Flaherty KT, Mier JW, Sullivan RJ. 2014. Clinical utility of a blood-based BRAF(V600E) mutation assay in melanoma. Mol Cancer Ther 13:3210–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. 2015. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res 21:1028–1035 [DOI] [PubMed] [Google Scholar]
- 31.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. 2015. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New Engl J Med 372:621–630 [DOI] [PubMed] [Google Scholar]
- 32.Shah JP, Clayman GL, Wirth LJ. 2015. New and emerging therapeutic options for thyroid carcinoma. Clin Adv Hematol Oncol 13:3–17, 11; quiz 12 p following 18. [PubMed] [Google Scholar]
- 33.Nucera C, Nehs MA, Mekel M, Zhang X, Hodin R, Lawler J, Nose V, Parangi S. 2009. A novel orthotopic mouse model of human anaplastic thyroid carcinoma. Thyroid 19:1077–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nucera C, Nehs MA, Nagarkatti SS, Sadow PM, Mekel M, Fischer AH, Lin PS, Bollag GE, Lawler J, Hodin RA, Parangi S. 2011. Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. Oncologist 16:296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Routhier CA, Mochel MC, Lynch K, Dias-Santagata D, Louis DN, Hoang MP. 2013. Comparison of 2 monoclonal antibodies for immunohistochemical detection of BRAF V600E mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Hum Pathol 44:2563–2570 [DOI] [PubMed] [Google Scholar]
- 36.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nose V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA. 2016. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2:1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Clifton-Bligh R, Tallini G, Holt EH, Sykorova V. 2015. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 33:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupoli GA, Okosieme OE, Evans C, Clark PM, Pickett AJ, Premawardhana LD, Lupoli G, Lazarus JH. 2015. Prognostic significance of thyroglobulin antibody epitopes in differentiated thyroid cancer. J Clin Endocrinol Metab 100:100–108 [DOI] [PubMed] [Google Scholar]
- 39.Juratli MA, Sarimollaoglu M, Nedosekin DA, Melerzanov AV, Zharov VP, Galanzha EI. 2014. Dynamic fluctuation of circulating tumor cells during cancer progression. Cancers (Basel) 6:128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasportas LS, Hori SS, Pratx G, Gambhir SS. 2014. Detection and quantitation of circulating tumor cell dynamics by bioluminescence imaging in an orthotopic mammary carcinoma model. PLoS One 9:e105079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu W, Wu B, Fu L, Chen J, Wang Z, Huang F, Chen J, Zhang M, Zhang Z, Lin J, Lan R, Chen R, Chen W, Chen L, Hong J, Zhang W, Ding Y, Okunieff P, Lin J, Zhang L. 2017. Comparison of three different methods for the detection of circulating tumor cells in mice with lung metastasis. Oncol Rep 37:3219–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashida A, Sakaizawa K, Mikoshiba A, Uhara H, Okuyama R. 2016. Quantitative analysis of the BRAF V600E mutation in circulating tumor-derived DNA in melanoma patients using competitive allele-specific TaqMan PCR. Int J Clin Oncol 21:981–988 [DOI] [PubMed] [Google Scholar]
- 43.Chang GA, Tadepalli JS, Shao Y, Zhang Y, Weiss S, Robinson E, Spittle C, Furtado M, Shelton DN, Karlin-Neumann G, Pavlick A, Osman I, Polsky D. 2016. Sensitivity of plasma BRAFmutant and NRASmutant cell-free DNA assays to detect metastatic melanoma in patients with low RECIST scores and non-RECIST disease progression. Mol Oncol 10:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denis MG, Knol A-C, Vallee A, Theoleyre S, Herbreteau G, Khammari A, Dréno B. 2016. Cross-platform comparison of techniques to detect BRAF mutations in circulating tumor DNA of melanoma patients. J Clin Oncol 34:e21026–e21026 [Google Scholar]
- 45.Kim BH, Kim IJ, Lee BJ, Lee JC, Kim IS, Kim SJ, Kim WJ, Jeon YK, Kim SS, Kim YK. 2015. Detection of plasma BRAF(V600E) mutation is associated with lung metastasis in papillary thyroid carcinomas. Yonsei Med J 56:634–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reid AL, Freeman JB, Millward M, Ziman M, Gray ES. 2015. Detection of BRAF-V600E and V600K in melanoma circulating tumour cells by droplet digital PCR. Clin Biochem 48:999–1002 [DOI] [PubMed] [Google Scholar]
- 47.Schreuer M, Meersseman G, Van Den Herrewegen S, Jansen Y, Chevolet I, Bott A, Wilgenhof S, Seremet T, Jacobs B, Buyl R, Maertens G, Neyns B. 2016. Quantitative assessment of BRAF V600 mutant circulating cell-free tumor DNA as a tool for therapeutic monitoring in metastatic melanoma patients treated with BRAF/MEK inhibitors. J Transl Med 14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsao SC, Weiss J, Hudson C, Christophi C, Cebon J, Behren A, Dobrovic A. 2015. Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations. Sci Rep 5:11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, Sherman SI, Sherman EJ. 2016. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, Phase 2 trial. Lancet Oncol 17:1272–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah MH, Wei L, Wirth LJ, Daniels GA, Souza JA, Timmers CD. 2017. Results of randomized Phase II trial of dabrafenib versus dabrafenib plus trametinib in BRAF-mutated papillary thyroid carcinoma. J Clin Oncol 35:6022 [Google Scholar]
- 51.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC. 2017. Efficacy of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E–mutated anaplastic thyroid cancer (ATC). J Clin Oncol 35:6023 [Google Scholar]
- 52.Sherman EJ, Ho AL, Baxi SS, Dunn L, Korte SH, Haque S. 2017. Combination of dabrafenib (DAB). J Clin Oncol 35:6085 [Google Scholar]