Abstract
The objective of the study was to determine whether repetitive hits to the head at a subclinical level are associated with structural and functional brain abnormalities and whether these effects are influenced by high levels of fitness associated with intense physical activity. Seventy-two college students were recruited: 24 nonathletic, 24 athletes practicing a varsity contact sport, and 24 athletes practicing a varsity noncontact sport. They were recruited for a neuropsychological evaluation and a magnetic resonance imaging session that included magnetic resonance spectroscopy of primary motor cortex (M1) and prefrontal cortex and susceptibility-weighted imaging. There was no evidence for reduced cognitive performance or presence of micro bleeds in contact sports athletes. Abnormalities in contact sports athletes were found for myo-inositol concentration (mIns) in M1, where levels were significantly higher compared with noncontact sports athletes (p = 0.016) and nonathletes (p = 0.029). In prefrontal cortex, glutamate + glutamine (Glx) was significantly reduced in contact sports athletes compared with noncontact sports athletes (p = 0.016), and a similar reduction was observed for gamma-aminobutyric acid (GABA) levels (p = 0.005). Varsity contact sports are associated with area-specific alterations in mIns concentration in the primary motor cortex. In the prefrontal cortex, high levels of fitness could modulate the effects of head impact exposure on prefrontal metabolite concentration. Indeed, although athletes in contact and noncontact sports show different neurometabolic profiles, they do not differ from sedentary controls.
Keywords: : brain imaging, concussion, physical activity, sports, subconcussive
Introduction
Sports-related concussions have long been considered minor and completely reversible insults to the brain. This belief is supported by the common spontaneous resolution of symptoms within seven to 10 days1 and failure to observe any overt brain damage as assessed by standard neuroimaging techniques.2 Sports concussions, however, have been the object of intense scrutiny in the last decade, leading to growing concerns of lasting physical, behavioral, and cognitive dysfunction (post-concussion syndrome) and the possible risk of development of neurodegenerative pathology after multiple concussions.1,3
While the relation between clinical and cognitive symptoms and changes in brain structure and function remains uncertain, several studies have reported widespread brain abnormalities thought to be concussion-related.4 At the neurochemical level, studies using magnetic resonance spectroscopy (MRS) most consistently have found lower levels of N-acetylaspartate (NAA), a marker of neuronal integrity, in athletes with a history of concussion.5 These reductions have been found in the acute6 and chronic7 phases of concussion, although others have reported recovery of NAA abnormalities within 30 days post-concussion.8,9 Imbalanced glutamate (Glu) levels and post-acute fluctuations of myo-inositol (mIns) levels have also been identified, further suggesting lasting cellular alterations in the brain of athletes with a history of concussion.5
Significantly, alterations in metabolite concentration have also been reported more than 30 years after the last concussive event. For example, increases in mIns and decreases in choline (Cho) levels were found in the medial temporal lobe of athletes who sustained their last concussion more than 30 years before testing.10 In a younger sample of retired rugby players, however, Gardner and collaborators11 found no evidence for altered NAA, Cho, Glu, and mIns concentrations in the occipitoparietal cortex, although glutathione was significantly lower.
In recent years, the issue of whether alterations in brain anatomy and function are specific and caused by concussions has been raised.12 Indeed, repetitive, subconcussive hits to the head have been associated with distributed structural damage to both white matter (WM)13,14 and gray15 matter (GM). Abnormalities in GM metabolite concentration have also been reported in athletes without a history of clinically diagnosed concussions.16 In a small sample study, retired soccer players who never sustained a concussion were compared with former athletes who competed in noncontact sports (NCS), revealing higher levels of mIns and Cho in the posterior cingulate gyrus.16 Significantly, mIns levels in soccer players were positively correlated with the estimated number of headings performed during the last year and throughout the career of the athlete.16 Along the same lines, a prospective study with American football high-school players reported decreases in creatine (Cr), mIns, Cho, and Glu + glutamine (Glx) throughout a season in the absence of a diagnosed concussion, which were not present in NCS athletes.17
Taken together, MRS findings in athletes practicing a contact sport (CS), with or without a history of concussion, suggest the presence of significant neurometabolic abnormalities that may persist decades after retirement. Most previous studies, however, have compared CS athletes (A-CS) with NCS athletes (A-NCS) or teammates without a history of concussion. This approach does not take into account the fact that elevated levels of fitness are associated with significant alterations in brain structure and function.18
Indeed, the brain of athletes differs from that of sedentary controls in terms of WM,19 GM,20 and neurochemistry.21 Importantly, many of the brain effects associated with physical activity overlap those associated with concussion or repeated subconcussive hits to the head.18 For example, MRS studies have shown that exercise increases brain lactate (Lac) concentration in the brain22,23 and that vigorous physical activity significantly increases cortical Glu and gamma-aminobutyric acid (GABA).24
In addition to the immediate effects of physical exercise, higher levels of fitness have also been associated with changes in metabolite concentration. Gonzalez and coworkers21 have reported elevated levels of NAA and Cho in the frontal and occipitotemporal cortex, respectively, in a group of middle-aged endurance athletes compared with matched sedentary controls. Further, in older persons, high fitness levels have been shown to reduce the effects of age on NAA concentration.25 These studies make it clear that the brain of highly fit athletes differs from that of sedentary individuals, which in turn may modulate its response to repetitive head impact.
In the present study, MRS of the primary motor cortex (M1) and the prefrontal cortex was used to examine whether a history of repeated exposure to subconcussive hits to the head in CS athletes is associated with neurochemical abnormalities compared with NCS athletes. The M1 was selected as a region of interest (ROI) based on previous studies showing this area to be vulnerable to the effects of concussion using MRS,5 diffusion tensor Imaging,26 and transcranial magnetic stimulation,27 whereas the prefrontal cortex was chosen because of its central role in cognitive functioning. In addition, A-CS and A-NCS were compared with sedentary controls. Participants also underwent neuropsychological testing and susceptibility weighted imaging to assess cognitive function and presence of brain micro-hemorrhages.
Methods
Participants
A total of 72 right-handed participants were recruited for this study (see Table 1 for demographic data), which included three groups: (i) 24 athletes (12 women) practicing a CS in a university team (rugby, soccer; A-CS); (ii) 24 athletes (12 women) practicing a NCS in a university team (swimming; A-NCS); and (iii) 24 nonathletes (12 women; NA). Athletes were recruited from local varsity athletics departments and were screened carefully with a semi-structured interview to rule out the presence of any history of concussions. A semi-structured interview was also conducted with NA participants to ensure they had not taken part in organized sports or regular physical activity (more than once per week). Participants had no psychological, psychiatric, or neurological disorder, and no substance abuse history. The groups were matched with regards to gender, education level, and age.
Table 1.
Demographic Data
| Group | Gender | Age | Education (years) | BMI (kg/m2) |
|---|---|---|---|---|
| N-A | ♂ (n = 12) | 23.58 (3.29) | 16.50 (2.43) | 22.25 (2.76) |
| ♀ (n = 12) | 22.33 (2.42) | 16.08 (2.30) | 20.97 (2.44) | |
| A-NCS (swimming) | ♂ (n = 12) | 21.83 (2.33) | 15.83 (1,90) | 23.30 (2.16) |
| ♀ (n = 12) | 21.67 (1.92) | 16.08 (1.78) | 21.65 (1.54) | |
| A-CS (rugby & soccer) | ♂ (n = 12) | 22.83 (3.01) | 16.33 (2.77) | 23.26 (2.01) |
| ♀ (n = 12) | 23.83 (2.51) | 17.25 (1.37) | 24.30 (2.52) |
BMI, body mass index; N-A, nonathletes; A-NCS, noncontact-sports athletes; A-CS, contact-sports athletes.
Numbers in parentheses represent standard déviations.
Participants were recruited for a single session that included a neuropsychological assessment followed by magnetic resonance imaging (MRI). The study was conducted at the Unité de Neuroimagerie Fonctionnelle of the Centre de Recherche de l'Institut Universitaire de Gériatrie de Montréal and was approved by the local ethics committee. All subjects gave written informed consent and received financial compensation for their participation.
Training frequency
Athletes were tested during the off-season or summer training camp and were never tested after vigorous physical activity or on a practice day. Frequency of training, number of university-level years of play, and total years of practice are presented in Table 2 (information was available for NA: n = 20; A-NCS: n = 22; A-CS: n = 19). In contact-sports athletes, position data were available for 12 soccer players (six defenders, five midfielders, and one forward), and five rugby players (one prop, one lock, one wing, one scrum half, and one outside center).
Table 2.
Group Characteristics
| Group | Frequency of training (times/week) | University level years | Total years of practice |
|---|---|---|---|
| N-A | 0.80 (0.76) | - | - |
| A-NCS (swimming) | 6.41 (2.92) | 2.07 (1.28) | 11.93 (3.43) |
| A-CS (rugby & soccer) | 4.68 (1.00) | 2.53 (1.18) | 13.00 (6.66) |
N-A, nonathletes; A-NCS, noncontact-sports athletes; A-CS, contact-sports athletes.
Numbers in parentheses represent standard deviations.
Cognitive assessment
Before the imaging session, participants were evaluated by a trained neuropsychologist. Neuropsychological tests were chosen to evaluate cognitive functions that have been reported to be vulnerable to the effects of concussion and head impact exposure.28,29 These were (i) Attention: focused and sustained attention (Ruff 2&7, D2), and processing speed (Symbol Digit Modality Test, Color Trail A, D-KEFS Color-Word Interference Test–Naming and Reading conditions); (ii) Executive Functions: inhibition and mental flexibility (D-KEFS Color Word Interference Test–Inhibition and Inhibition/Switching Conditions, Color Trail B); and (iii) Memory: visual memory (Rey Complex Figure Test).
MRI
MR acquisitions were made using a Siemens Trio 3T whole-body MRI system (Siemens, Erlangen, Germany) with a 32-channel receive-only head coil.
Susceptibility-weighted imaging
Data were acquired using a high-resolution three-dimensional gradient-echo sequence with the following parameters: time to repetition (TR) = 28 msec, time to echo (TE) = 20 msec, flip angle (FA) = 15 degrees, voxel size = 0.8 × 0.7 × 1.2 mm2, BW = 120 Hz/Px. MRI images were reviewed by a board-certified neuroradiologist, blind to group information, to detect the presence of micro-hemorrhages. Micro-hemorrhages were defined as small round lesions with homogeneous low signal intensity, without continuity with surrounding vascular structures.
MRS
Acquisition of neurometabolite signals was performed using a MEGA-PRESS sequence30 with the following parameters: TR = 3 sec, TE = 68 msec, FA = 9 degrees. Double-banded pulses for water suppression and editing of c-CH2 GABA resonance at three ppm were used. Variable power with optimized relaxation delays (VAPOR31) and outer volume suppression were optimized for the human 3T system and used for additional water suppression. Four blocks of 32 EDIT OFF and 32 EDIT ON alternate scans were acquired. Frequency drift correction was applied between each block. Using anatomical landmarks described by Youstry and associates,32 a first voxel-of-interest (VOI) (30 × 30 × 30 mm3) was positioned over the knob-like area representing the hand in left M1. The second VOI (30 × 30 × 30 mm3) was positioned over the left prefrontal cortex.
MRS data were analyzed using LC Model33 for the EDIT DIFF (difference spectra) and the EDIT OFF spectra. Data with Cramer-Rao lower bounds (CRLB) of 35% or more were excluded from further analysis. An experimentally measured metabolite-nulled macromolecular spectrum from the occipital region and metabolite spectra using chemical shifts and J couplings were used as the basis set for editOff spectra. The metabolite-nulled basis spectra used in this study were built from occipital acquisitions on an independent cohort of 11 healthy adults. The occipital region was chosen for its high signal-to-noise ratio, and subjects were selected for having no medical, neurological, or psychiatric conditions and for not receiving médication. LC Model fitting was performed over the spectral range from 0.2 to 4.0 ppm, and LC Model spline model of the baseline was deactivated using the NOBASE = T input parameter.
An experimentally measured metabolite-nulled macromolecular spectrum from the occipital region (average from 11 subjects) and the experimentally measured spectra from 100 mM phantoms of NAA, GABA, Glu, and Gln at 37°C and with pH adjusted to 7.2 were used as the basis set for difference spectra. LC Model spline model of the baseline was also deactivated using the NOBASE = T input parameter. No baseline correction, zero-filling, or apodization functions were applied to the in vivo data before LC Model analysis. tCr (Cr-CH3 + PCr-CH3), mIns, and tNAA (sNAA+NAAG) concentrations were obtained from EDIT OFF spectra, and GABA and Glx concentrations were obtained from difference spectra.
Metabolite quantification was performed using a water reference. Tissue composition was obtained from the anatomical MR images of each subject, which were segmented to GM, WM, and cerebrospinal fluid (CSF) content using the automated FreeSurfer pipeline (V 5.3.0). The T1 and T2 relaxation times of water used in the calculation of attenuation factors were taken from published reports (T1[GM] = 1.29 s, T1[WM] = 0.87 s, T1[CSF] = 4 s, T2[GM] = 110 ms, T2[WM] = 80 msec, and T2[CSF] = 400 msec).34,35 Water attenuation was computed using the fractional volume of each compartment.
Statistical analysis
Statistical analysis was performed with SPSS version 24 (IBM Corporation, Armonk, NY). One way analyses of variance with group (A-CS, A-NCS, NA) as the between-subjects factor were conducted for neuropsychological and neurometabolic data. A Kruskal-Wallis H test was used to compare the number of micro-hemorrhages between groups.
Results
Cognitive assessment
There were no significant differences between groups in terms of age and education level (Table 1), and any of the neuropsychological measures (Table 3).
Table 3.
Neuropsychological Assessment
| Raw score | Group effect | ||||||
|---|---|---|---|---|---|---|---|
| Function | Test | Scales | NA | A-NCS | A-CS | f | p |
| Attention | Ruff 2&7 | AD speed | 176.9 | 181.1 | 186.0 | 0.75 | 0.48 |
| AD errors | 5.5 | 5.0 | 4.5 | 0.21 | 0.81 | ||
| AD accuracy | 97.0 | 97.4 | 97.7 | 0.45 | 0.64 | ||
| CS speed | 139.6 | 144.9 | 150.0 | 1.55 | 0.22 | ||
| CS errors | 11.7 | 10.2 | 9.8 | 0.44 | 0.64 | ||
| CS accuracy | 92.4 | 93.5 | 93.8 | 0.69 | 0.51 | ||
| D2 | Total hits | 198.1 | 206.7 | 203.0 | 0.49 | 0.62 | |
| Total errors | 16.7 | 12.1 | 19.1 | 2.57 | 0.09 | ||
| Processing speed | SDMT | Total correct | 60.8 | 64.1 | 58.2 | 2.43 | 0.10 |
| CWIT | Color name time | 25.4 | 24.1 | 25.8 | 1.34 | 0.27 | |
| Color read time | 19.5 | 17.8 | 19.0 | 1.91 | 0.16 | ||
| Color trail | CT-A time | 29.6 | 24.9 | 27.70 | 1.74 | 0.19 | |
| Inhibition/flexibility | CWIT | Inhibition time | 41.9 | 41.8 | 40.7 | 0.26 | 0.77 |
| Inhib/flex time | 49.8 | 47.1 | 50.1 | 0.95 | 0.39 | ||
| Color trail | CT-B time | 58.6 | 54.8 | 52.3 | 1.53 | 0.23 | |
| Visual memory | RCFT | Copy | 33.9 | 33.6 | 33.7 | 0.10 | 0.91 |
| Immediate recall | 23.6 | 22.9 | 21.0 | 1.36 | 0.26 | ||
| Delayed recall | 23.6 | 22.7 | 20.6 | 1.54 | 0.22 | ||
| Recognition | 21.3 | 21.5 | 20.6 | 2.24 | 0.12 | ||
N-A, nonathletes; A-NCS, noncontact-sports athletes; A-CS, contact-sports athlètes; SMDT, Symbol Digit Modality test; CWIT, Color-Word Interference Test; RCFT, Rey Complex Figure Test.
Susceptibility weighted imaging
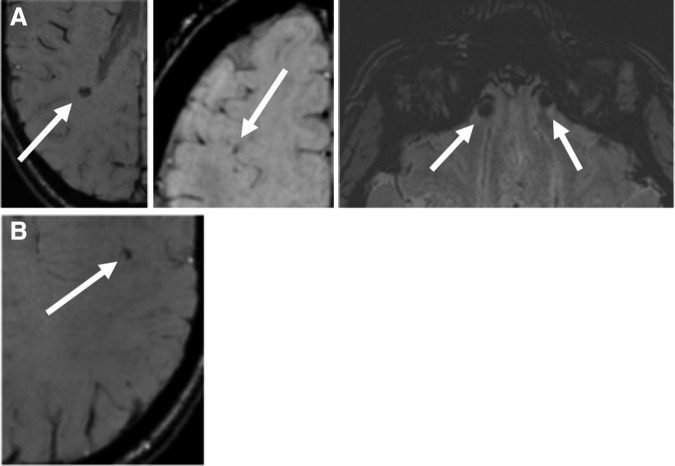
Possible micro-hemorrhages were found in one participant in the NA group in the right temporal, bilateral inferior frontal, and right frontal lobes (Fig. 1A), and one participant in the A-NCS group in the left frontal lobe (Fig. 1B). A Kruskal-Wallis H test showed no difference between the three groups (h = 1.01, p = 0.60).
FIG. 1.
Possible micro-hemorrhages in participants. (A) Nonathlete (right temporal, bilateralinferior frontal, and right frontal lobes). (B) Athlete in a noncontact sport (left frontal lobe).
MRS
Six participants were excluded from MRS GABA analysis. In the M1, one participant was excluded because of CRLB above 35% (A-NCS). In the prefrontal cortex, five participants were excluded because of CRLB above 35% (A-CS: two participants; NA: three participants). Analysis of all MRS spectra in two additional participants (A-CS, A-NCS) in the prefrontal ROI could not be performed because of signal quality issues.
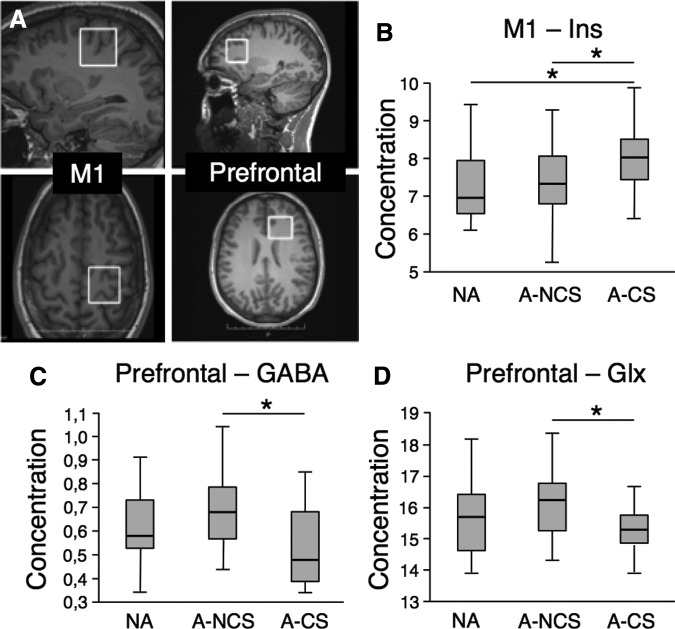
In the M1 (Fig. 2A), a significant group effect was found for mIns (n = 72; f = 3.76, p = 0.029), which was higher in the A-CS group compared with both NA (p = 0.029) and A-NCS (p = 0.016) groups (Fig. 2B). In the prefrontal cortex (Fig 2A), a significant group effect was found for GABA (n = 65; f = 4.32, p = 0.017; Fig. 2C), where concentrations were lower in the A-CS group compared with the A-NCS group (p = 0.005). A significant group effect was also found for Glx (n = 70; f = 3.15, p = 0.049; Fig. 2D), where concentrations were lower in the A-CS group compared with the A-NCS group (p = 0.016).
FIG. 2.
Magnetic resonance spectroscopy analysis. (A) Position of the voxels-of-interest in the left M1 and left prefrontal cortex. (B) mIns concentration in the primary motor cortex. (C) GABA concentration in the prefrontal cortex. (D) Glutamate and glutamine (Glx) concentration in prefrontal cortex. N-A, nonathletes; A-NCS, noncontact-sports athletes; A-CS, contact-sports athlètes ; GABA, gamma-aminobutyric acid.
Exploratory correlational analysis was performed to determine whether GABA and Glx concentrations in the prefrontal cortex were associated with neuropsychological test scores. For the whole sample, there was a significant correlation between GABA concentration and D2-hits scores (n = 65; r = 0.26; p = 0.036). For the A-CS group, Glx was positively correlated with RCFT-recognition (n = 23; r = 0.50, p = 0.015; Fig. 3A), RCFT-immediate recall (n = 23; r = 0.43, p = 0.042; Fig. 3B) and RCFT-delayed recall (n = 23; r = 0.50, p = 0.016; Fig. 3C).
FIG. 3.
Correlational analysis in contact sports athletes. (A) Positive correlation between prefrontal glutamate and glutamine (Glx) concentration and performance on RCFT recognition task. (B) Positive correlation between prefrontal Glx concentration and performance on RCFT immediate recall task. (C) Positive correlation between prefrontal Glx concentration and performance on RCFT delayed recall task. RCFT, Rey Complex Figure Test.
Discussion
The present results provide evidence that repeated exposure to subconcussive hits to the head is associated with higher levels of mIns in the M1 of A-CS compared with both A-NCS and sedentary controls. In the prefrontal cortex, GABA and Glx concentrations were lower in A-CS compared with A-NCS only, because sedentary controls and A-CS did not differ significantly on both measures. Further, in A-CS, increased prefrontal Glx levels were associated with superior performance on the RCFT. Finally, groups were equivalent in terms of neuropsychological test scores and presence of micro-hemorrhages.
Elevated mIns levels have been reported after nonsports-related traumatic brain injury (TBI). In children with moderate-to-severe TBI with presumed diffuse axonal injury, mIns concentrations were higher in occipital gray matter compared with controls and higher in patients with poor outcome compared with patients with good outcome.36 In adults, Kierans and colleagues37 reported elevated putaminal mIns in patients with mild TBI at 21 days post-injury. Similar findings have been reported in human38,39 and animal models of TBI.40,41
Myo-inositol is an organic osmolyte that is believed to be a marker of glial cell activation associated with neuroinflammation and gliosis.42 After TBI, reactive astrogliosis occurs leading to what is called glial scarring, which may limit axonal repair and regeneration.42 In athletes with a history of concussion, mIns levels have also been found to be elevated in the M1 six months post-concussion.7 More importantly, the present findings are strikingly similar to those reported by Koerte and associates,16 who found higher levels of mIns in the posterior cingulate cortex of former professional soccer players without a known concussion history, which were positively correlated with an estimate of lifetime headings.16
The present study confirms these results in a larger sample and shows that mIns levels in M1 are elevated in A-CS compared with both A-NCS and sedentary controls. Further, because participants were not tested after vigorous physical activity or on a practice day, and in light of data showing that mIns levels remain unchanged immediately after high-intensity exercise,24 the present results suggest that mIns alterations are independent of physical activity levels and most likely related to repeated exposure to head impacts.
In the prefrontal cortex, GABA and Glx levels were lower in A-CS compared with A-NCS. The A-CS and A-NCS were not significantly different from sedentary controls, however. These data underscore the need to include participants with no sports exposure in studies assessing the effects of repeated subconcussive head impacts. Indeed, a simple comparison between the two athlete groups could lead to the conclusion that practicing a contact sport is associated with lower GABA and Glx concentrations. This is not necessarily the case, however, because we show that GABA and Glx levels in A-CS are not different from those of nonathletes.
Two distinct mechanisms may therefore underlie the observed effect. First, repeated subconcussive hits may be associated with small, nonsignificant reductions in Glx and GABA. Although GABA levels have been shown to be normal in athletes with a history of concussion,43,44 reduced Glx has been found in the acute7 and chronic45 phases of concussion. Second, high fitness levels may increase Glx and GABA and concentrations. Vigorous physical activity has been associated with acute increases in Glu and GABA levels in the visual cortex.24 Further, the same study reported that baseline Glu levels could be predicted by the amount of physical activity performed in the week before testing.24 This suggests that high levels of fitness achieved through repeated physical activity could lead to sustained increases in baseline levels of Glx and GABA.
The present results cannot disentangle the relative contributions of a history of subconcusive hits to the head and sustained physical activity in explaining the differences in prefrontal metabolite concentration between A-CS and A-NCS. They suggest, however, that neurometabolic effects of subconcussive hits to the head in the prefrontal cortex are, if present, modest.
Contrary to other neuroimaging methods such as functional MRI or diffusion imaging, MRS requires a priori selection of ROIs where metabolite concentrations are assessed. This has resulted in important study heterogeneity,4,5 where neurochemical abnormalities in athletes with a history of concussion have been reported in areas such as the corpus callosum,46 thalamus,47 M1,6 and frontal cortex.9 In the study of soccer players with no history of concussion, increases in mIns, similar to those reported here, were found in the posterior cingulate cortex.16 Importantly, as mentioned by Dimou and coworkers,4 these different ROIs can show different patterns of metabolic alterations, even within a single study.
The reason for these discrepancies remains difficult to determine. It has been suggested that cellular differences between brain areas may account for some of the differential effects reported between brain areas.7 Because concussions occur in response to linear and rotational accelerations, primarily through shear deformation, specific cortical areas may be more vulnerable to the biomechanical forces resulting from direct impact with the head.48 Although concussions result from heterogeneous insults, possible gliosis in the present study was limited to the M1.
Region-specific increases in mIns levels have been reported in previous studies of patients with TBI.7,36,37 For example, Kierans and associates37 found higher mIns in the putamen 22 days after mTBI with normal levels in the caudate nucleus, globaus pallidus, and thalamus. Similarly, mIns in M1 was found to be higher in concussed athletes six months post-concussion while it remained unchanged in the dorsolateral prefrontal cortex.7 It has been shown that astrocytes are heterogeneous in terms of gene expression profile, physiology, developmental pattern, and functional specialization.49 Further, reactive astrocytes do not respond to TBI in a spatially homogeneous fashion. Rather, studies in rats have shown that the morphology of reactive astrocytes can be very different across brain areas after brain injury, where the presence of a gliotic scar was found in the hippocampal area but not in the medial geniculate body after lateral fluid percussion.50 In addition, in the same study, the presence of gliosis was found to vary across locations as a function of time since brain injury.50 These data suggest that glial response to repeated hits to the head is not homogeneous and that some brain areas may be more vulnerable than others.
Similarly, results from the present study show that the possible effects of sports practice on metabolite levels are also not distributed homogeneously across brain areas. In a study exploring the association between aerobic fitness and neurometabolite concentration, Gonzales and colleagues21 reported that higher cardiorespiratory fitness was associated with higher NAA in the frontal cortex and higher Cho in the occipitoparietal cortex. It was suggested that regional differences could be explained by the fact that the cognitive benefits of increased fitness are particularly important for executive functions, which are located primarily in frontal areas.21 It is therefore possible that the frontal-specific modulation of GABA and Glx reported here are associated with cognitive functioning, despite the absence of group differences in neuropsychological testing. More studies are needed to identify the cause of regional heterogeneity in the metabolic response to head impacts and physical activity and determine whether the spatial pattern of abormalities, in the case of concussion and subconcussive hits, has clinical significance.
Although the three groups were equivalent in terms of cognitive performance, Glx levels were positively correlated with scores on the recognition, immediate recall, and delayed recall tasks of the RCFT in A-CS, where a higher concentration of Glx in the prefrontal cortex was associated with better performance. Whereas some studies have reported that subconcussive hits to the head, such as those associated with heading in soccer, can lead to long-term cognitive impairments,51 recent data suggest that the impact of repeated blows to the head may disappear or have limited impact in later life.52–55 Studies have also shown equivalent cognitive performance between A-CS and A-NCS.16,56–58
Despite this evidence, recent data suggest that traditional neuropsychological tests may lack sensitivity and specificity in detecting subtle cognitive alterations associated with repetitive head impacts, especially in the absence of concussion.59 Koerte and coworkers60 recently addressed this issue by using pointing tasks to assess sensorimotor and cognitive control in adolescent soccer players with no history of concussion. Whereas cognitive control improved over time with practice in table tennis players and swimmers, performance remained stable in soccer players. Further, improvement in cognitive performance was negatively correlated with the number of long headers performed by soccer players during the season.60
Taken together, the available data suggest that subtle impairments in cognitive function in A-CS may have been missed in the present study. Indeed, traditional neuropsychological tests in a university-level, healthy population may lack the necessary sensitivity to detect impairments related to a history of subconcussive hits, although the present data also suggest that practicing CS does not lead to clinically relevant alterations in general cognitive functioning in young athletes.
Another important issue regarding neuropsychological results in the present study is the absence of significantly better cognitive performance in athletes, especially those practicing a NCS, compared with nonathletes. Although contradictory findings have been reported when assessing the link between sports practice and cognitive performance,61 some studies have shown that higher fitness levels may be associated with better cognition. For example, Zhu and colleagues62 found that cardiorespiratory fitness at 25 years of age was associated with cognitive performance 25 years later on tests of memory (Rey Auditory Vernal Learning Test) and psychomotor speed (Digit Symbol Substitution Test). Further, in a meta-analysis comprising mainly professional and varsity college athletes, Voss and collaborators61 reported an overall positive and significant effect of sports practice on cognitive function. The most robust and straightforward result was found for processing speed, with a medium effect size of 0.67.61
The absence of significant group differences in the present study could be because of a ceiling effect, where neuropsychological tests lack sensitivity to detect subtle cognitive differences in young, healthy, university-level students. Nevertheless, in a measure of attention (D2), a statistical trend was found for a reduced number of errors in A-NCS compared with non-athletes and A-CS. Further studies using sensitive laboratory tasks are needed therefore to better understand the cognitive effects of sports and their interaction with repeated hits to the head.
Finally, the present study found no evidence for the presence of cerebral micro-hemorrhages in the brain of A-CS without a history of concussions, which is in line with previous studies. Indeed, Toth and associates63 found no evidence for micro-hemorrhages in a sample of 14 patients with uncomplicated mTBI patients compared with healthy controls, and Hasiloglu and coworkers64 reported no significant difference in the number of micro-hemorrhages between amateur boxers and healthy controls. In another study conducted with patients with a mTBI unrelated to sports practice, however, it was found that patients presented up to four times more micro-hemorrhages in subcortical and cortical areas compared with healthy controls.65 This discrepancy may be because susceptibility-weighted angiography was used in the study of Huang and collaborators65 and that TBI in a nonsporting context may be associated with different outcomes than sports concussions. Taken together, these data suggest that athletes without a history of concussion that practice a CS are not at a higher risk of presenting cerebral micro-hemorrhages than the general population.
Limitations to the present study include the absence of objective data detailing exposure to subconcussive hits. Although a detailed description of lifetime exposure was not possible, quantitative assessment can be achieved prospectively by using helmets fitted with systems that measure biomechanical impact66 or patch accelerometers.67 Although head impact telemetry systems would have provided valuable data regarding recent exposure, the bulk of lifetime subconcussive hits would still have been missed, however. Nevertheless, in a study in soccer players, Koerte and colleagues16 reported an average of 32.7 self-reported headers per week in the year preceding testing in a sample of former professional soccer players. Also, in a recent study using a mastoid patch accelerometer, Reynolds and coworkers67 showed an estimated average of 2133 head impacts per year in male soccer players. Soccer is also one of the sports with the highest incidence of concussion at the collegiate level.68
While epidemiological and head impact exposure data are more scarce for rugby, a recent prospective epidemiological study found that concussion rates were significantly higher in college rugby than in college football. Taken together, these data strongly suggest that athletes in the CS group were exposed repeatedly to head contacts compared with sedentary and NCS controls. It should be mentioned, however, that exposure to subconcussive hits and concussion rates are not necessarily correlated. Soccer is probably associated with higher numbers of sub-concussive hits because of frequent headings while high-velocity contact in rugby could lead to more concussive events. Nevertheless, these data strongly suggest that in the present study, head impacts were frequent and sustained in A-CS.
A second limitation to the present study is the absence of an objective measure of fitness levels, which is usually represented as maximal oxygen uptake (VO2max). Although VO2max was not measured in the present study, there is overwhelming evidence that elite athletes present higher overall aerobic fitness compared with healthy, sedentary individuals. Maximal oxygen uptake has been shown to be around 40 mL · kg1 · min−1 in young69 and adult70 persons within the general population. Values upward of 55 to 60 mL · kg1 · min−1, on the other hand, have been reported in elite athletes practicing soccer,71 rugby,72 and swimming.73 This suggests that athletes in the present study presented superior fitness levels than nonathletes, because inclusion in a varsity-level sports club such as soccer, rugby, and swimming requires intense and repeated physical training. In addition, it can be assumed reasonably that fitness levels were comparable for the three sports, as similar VO2max values have been reported.71–73
Conclusion
The present study suggests the existence of a specific neurochemical marker of repeated exposure to subconcussive hits in the M1 of athletes practicing a CS at the varsity level. The clinical significance of the present results, however, remains to be determined, because cognitive performance and vascular integrity were found to be normal in A-CS. Other neurometabolic group differences were observed in the prefrontal cortex, which may be related to the beneficial effects of high levels of fitness in athletes. These findings are important in light of the recent surge in scientific and media interest in the issue of possible brain damage associated with sports concussion and hits to the head in general.74 As suggested elsewhere, data supporting the notion of detrimental effects of CS on brain health should be weighted against the known beneficial effects of sports practice.74
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research, the Football Players Health Study at Harvard University, and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758).
Author Disclosure Statement
Dr. Pascual- Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab, Neuroelectrics, Constant Therapy, and Neosync and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). For the remaining authors, no competing financial interests exist.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, or its affiliated academic health carecenters.
References
- 1.McCrory P., Meeuwisse W.H., Aubry M., Cantu B., Dvorak J., Echemendia R.J., Engebretsen L., Johnston K., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R.G., Guskiewicz K., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H. and Turner M. (2013). Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br. J. Sports Med. 47, 250–258 [DOI] [PubMed] [Google Scholar]
- 2.Johnston K.M., Ptito A., Chankowsky J., and Chen J.K. (2001). New frontiers in diagnostic imaging in concussive head injury. Clin. J. Sport Med. 11, 166–175 [DOI] [PubMed] [Google Scholar]
- 3.McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.S., Wojtowicz S.M., Hall G., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W. and Cantu R.C. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimou S. and Lagopoulos J. (2014). Toward objective markers of concussion in sport: a review of white matter and neurometabolic changes in the brain after sports-related concussion. J. Neurotrauma 31, 413–424 [DOI] [PubMed] [Google Scholar]
- 5.Gardner A., Iverson G.L., and Stanwell P. (2014). A systematic review of proton magnetic resonance spectroscopy findings in sport-related concussion. J. Neurotrauma 31, 1–18 [DOI] [PubMed] [Google Scholar]
- 6.Henry L.C., Tremblay S., Boulanger Y., Ellemberg D., and Lassonde M. (2010). Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. J. Neurotrauma 27, 65–76 [DOI] [PubMed] [Google Scholar]
- 7.Henry L.C., Tremblay S., Leclerc S., Khiat A., Boulanger Y., Ellemberg D., and Lassonde M. (2011). Metabolic changes in concussed American football players during the acute and chronic post-injury phases. BMC Neurol. 11, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vagnozzi R., Signoretti S., Tavazzi B., Floris R., Ludovici A., Marziali S., Tarascio G., Amorini A.M., Di Pietro V., Delfini R., and Lazzarino G. (2008). Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athlètes—part III. Neurosurgery 62, 1286–1295 [DOI] [PubMed] [Google Scholar]
- 9.Vagnozzi R., Signoretti S., Cristofori L., Alessandrini F., Floris R., Isgro E., Ria A., Marziali S., Zoccatelli G., Tavazzi B., Del Bolgia F., Sorge R., Broglio S.P., McIntosh T.K., and Lazzarino G. (2010). Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain 133, 3232–3242 [DOI] [PubMed] [Google Scholar]
- 10.Tremblay S., De Beaumont L., Henry L.C., Boulanger Y., Evans A.C., Bourgouin P., Poirier J., Théoret H., and Lassonde M. (2013). Sports concussions and aging: a neuroimaging investigation. Cereb. Cortex 23, 1159–1166 [DOI] [PubMed] [Google Scholar]
- 11.Gardner A.J., Iverson G.L., Wojtowicz M., Levi C.R., Kay-Lambkin F., Schofield P.W., Zafonte R., Shultz S.R., Lin A.P., and Stanwell P. (2017). MR Spectroscopy findings in retired professional rugby league players. Int. J. Sports Med. 38, 241–252 [DOI] [PubMed] [Google Scholar]
- 12.Bailes J.E., Petraglia A.L., Omalu B.I., Nauman E., and Talavage T. (2013). Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg 119, 1235–1245 [DOI] [PubMed] [Google Scholar]
- 13.Lipton M.L., Kim N., Zimmerman M.E., Kim M., Stewart W.F., Branch C.A., and Lipton R.B. (2013). Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology 268, 850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koerte I.K., Ertl-Wagner B., Reiser M., Zafonte R., and Shenton M.E. (2012). White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 308, 1859-1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koerte I.K., Mayinger M., Muehlmann M., Kaufmann D., Lin A.P., Steffinger D., Fisch B., Rauchmann B.S., Immler S., Karch S., Heinen F.R., Ertl-Wagner B., Reiser M., Stern R.A., Zafonte R., and Shenton M.E. (2016). Cortical thinning in former professional soccer players. Brain Imaging Behav. 10, 792–798 [DOI] [PubMed] [Google Scholar]
- 16.Koerte I.K., Lin A.P., Muehlmann M., Merugumala S., Liao H., Starr T., Kaufmann D., Mayinger M., Steffinger D., Fisch B., Karch S., Heinen F., Ertl-Wagner B., Reiser M., Stern R.A., Zafonte R., and Shenton M.E. (2015). Altered neurochemistry in former professional soccer players without a history of concussion. J. Neurotrauma 32, 1287–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole V.N., Abbas K., Shenk T.E., Breedlove E.L., Breedlove K.M., Robinson M.E., Leverenz L.J., Nauman E.A., Talavage T.M., and Dydak U. (2014). MR spectroscopic evidence of brain injury in the non-diagnosed collision sport athlete. Dev. Neuropsychol. 39, 459–473 [DOI] [PubMed] [Google Scholar]
- 18.Nakata H., Yoshie M., Miura A., and Kudo K. (2010). Characteristics of the athletes' brain: evidence from neurophysiology and neuroimaging. Brain Res. Rev. 62, 197–211 [DOI] [PubMed] [Google Scholar]
- 19.Wang B., Fan Y., Lu M., Li S., Song Z., Peng X., Zhang R., Lin Q., He Y., Wang J., and Huang R. (2013). Brain anatomical networks in world class gymnasts: a DTI tractography study. Neuroimage 65, 476–487 [DOI] [PubMed] [Google Scholar]
- 20.Huang R., Lu M., Song Z., and Wang J. (2015). Long-term intensive training induced brain structural changes in world class gymnasts. Brain Struct. Funct. 220, 625–644 [DOI] [PubMed] [Google Scholar]
- 21.Gonzales M.M., Tarumi T., Kaur S., Nualnim N., Fallow B.A., Pyron M., Tanaka H., and Haley A.P. (2013). Aerobic fitness and the brain: increased N-acetyl-aspartate and choline concentrations in endurance-trained middle-aged adults. Brain Topogr. 26, 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddock R.J., Casazza G.A., Buonocore M.H., and Tanase C. (2011). Vigorous exercise increases brain lactate and Glx (glutamate + glutamine): a dynamic 1H-MRS study. Neuroimage 57, 1324–1330 [DOI] [PubMed] [Google Scholar]
- 23.Dennis A., Thomas A.G., Rawlings N.B., Near J., Nichols T.E., Clare S., Johansen-Berg H., and Stagg C.J. (2015). An ultra-high field magnetic resonance spectroscopy study of post exercise lactate, glutamate and glutamine change in the human brain. Front. Physiol. 6, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddock R.J., Casazza G.A., Fernandez D.H., and Maddock M.I. (2016). Acute modulation of cortical glutamate and GABA content by physical activity. J. Neurosci. 36, 2449–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erickson K.I., Weinstein A.M., Sutton B.P., Prakash R.S., Voss M.W., Chaddock L., Szabo A.N., Mailey E.L., White S.M., Wojcicki T.R., McAuley E., and Kramer A.F. (2012). Beyond vascularization: aerobic fitness is associated with N-acetylaspartate and working memory. Brain Behav. 2, 32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eierud C., Craddock R.C., Fletcher S., Aulakh M., King-Casas B., Kuehl D., and LaConte S.M. (2014). Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 4, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Major B.P., Rogers M.A., and Pearce A.J. (2015). Using transcranial magnetic stimulation to quantify electrophysiological changes following concussive brain injury: a systematic review. Clin. Exp. Pharmacol. Physiol. 42, 394–405 [DOI] [PubMed] [Google Scholar]
- 28.Belanger H.G. and Vanderploeg R.D. (2005). The neuropsychological impact of sports-related concussion: a meta-analysis. J. Int. Neuropsychol. Soc. 11, 345–357 [DOI] [PubMed] [Google Scholar]
- 29.Belanger H.G., Spiegel E. and Vanderploeg R.D. (2010). Neuropsychological performance following a history of multiple self-reported concussions: a meta-analysis. J. Int. Neuropsychol. Soc. 16, 262–267 [DOI] [PubMed] [Google Scholar]
- 30.Mescher M., Merkle H., Kirsch J., Garwood M., and Gruetter R. (1998). Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 11, 266–272 [DOI] [PubMed] [Google Scholar]
- 31.Tkac I., Starcuk Z., Choi I.Y., and Gruetter R. (1999). In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 41, 649–656 [DOI] [PubMed] [Google Scholar]
- 32.Yousry T.A., Schmid U.D., Alkadhi H., Schmidt D., Peraud A., Buettner A., and Winkler P. (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120, 141–157 [DOI] [PubMed] [Google Scholar]
- 33.Provencher S.W. (1993). Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 30, 672–679 [DOI] [PubMed] [Google Scholar]
- 34.Wansapura J.P., Holland S.K., Dunn R.S., and Ball W.S., Jr. (1999). NMR relaxation times in the human brain at 3.0 tesla. J. Magn. Reson. Imaging 9, 531–538 [DOI] [PubMed] [Google Scholar]
- 35.Rooney W.D., Johnson G., Li X., Cohen E.R., Kim S.G., Ugurbil K., and Springer C.S., Jr. (2007). Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn. Reson. Med. 57, 308–318 [DOI] [PubMed] [Google Scholar]
- 36.Ashwal S., Holshouser B., Tong K., Serna T., Osterdock R., Gross M., and Kido D. (2004). Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr. Res. 56, 630–638 [DOI] [PubMed] [Google Scholar]
- 37.Kierans A.S., Kirov II, Gonen O., Haemer G., Nisenbaum E., Babb J.S., Grossman R.I., and Lui Y.W. (2014). Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology 82, 521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garnett M.R., Blamire A.M., Corkill R.G., Cadoux-Hudson T.A., Rajagopalan B., and Styles P. (2000). Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain 123, 2046–2054 [DOI] [PubMed] [Google Scholar]
- 39.Brooks W.M., Stidley C.A., Petropoulos H., Jung R.E., Weers D.C., Friedman S.D., Barlow M.A., Sibbitt W.L., Jr., and Yeo R.A. (2000). Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J. Neurotrauma 17, 629–640 [DOI] [PubMed] [Google Scholar]
- 40.Pascual J.M., Solivera J., Prieto R., Barrios L., Lopez-Larrubia P., Cerdan S., and Roda J.M. (2007). Time course of early metabolic changes following diffuse traumatic brain injury in rats as detected by (1)H NMR spectroscopy. J. Neurotrauma 24, 944–959 [DOI] [PubMed] [Google Scholar]
- 41.Harris J.L., Yeh H.W., Choi I.Y., Lee P., Berman N.E., Swerdlow R.H., Craciunas S.C., and Brooks W.M. (2012). Altered neurochemical profile after traumatic brain injury: (1)H-MRS biomarkers of pathological mechanisms. J. Cereb. Blood Flow Metab. 32, 2122–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGraw J., Hiebert G.W., and Steeves J.D. (2001). Modulating astrogliosis after neurotrauma. J. Neurosci. Res. 63, 109–115 [DOI] [PubMed] [Google Scholar]
- 43.Tremblay S., Beaulé V., Proulx S., Tremblay S., Marjańska M., Doyon J., Lassonde M., and Théoret H. (2014). Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin. Neurophysiol. 125, 1371–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilke S., List J., Mekle R., Lindenberg R., Bukowski M., Ott S., Schubert F., Ittermann B., and Flöel A. (2017). No effect of anodal transcranial direct current stimulation on gamma-aminobutyric acid levels in patients with recurrent mild traumatic brain injury. J. Neurotrauma 34, 281–290 [DOI] [PubMed] [Google Scholar]
- 45.De Beaumont L., Tremblay S., Henry L.C., Poirier J., Lassonde M., and Théoret H. (2013). Motor system alterations in retired former athletes: the role of aging and concussion history. BMC Neurol. 13, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chamard E., Théoret H., Skopelja E.N., Forwell L.A., Johnson A.M., and Echlin P.S. (2012). A prospective study of physician-observed concussion during a varsity university hockey season: metabolic changes in ice hockey players. Part 4 of 4. Neurosurg. Focus 33, E4 : 1–7 [DOI] [PubMed] [Google Scholar]
- 47.Maugans T.A., Farley C., Altaye M., Leach J., and Cecil K.M. (2012). Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 129, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bayly P.V., Cohen T.S., Leister E.P., Ajo D., Leuthardt E.C., and Genin G.M. (2005). Deformation of the human brain induced by mild acceleration. J. Neurotrauma 22, 845–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y. and Barres B.A. (2010). Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 20, 588–594 [DOI] [PubMed] [Google Scholar]
- 50.Hill S.J., Barbarese E., and McIntosh T.K. (1996). Regional heterogeneity in the response of astrocytes following traumatic brain injury in the adult rat. J. Neuropathol. Exp. Neurol. 55, 1221–1229 [DOI] [PubMed] [Google Scholar]
- 51.Matser E.J., Kessels A.G., Lezak M.D., Jordan B.D., and Troost J. (1999). Neuropsychological impairment in amateur soccer players. JAMA 282, 971–373 [DOI] [PubMed] [Google Scholar]
- 52.Straume-Naesheim T.M., Andersen T.E., Dvorak J,. and Bahr R. (2005). Effects of heading exposure and previous concussions on neuropsychological performance among Norwegian elite footballers. Br. J. Sports Med. 39, Suppl 1, i70–i77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vann Jones S.A., Breakey R.W., and Evans P.J. (2014). Heading in football, long-term cognitive decline and dementia: evidence from screening retired professional footballers. Br. J. Sports Med. 48, 159–161 [DOI] [PubMed] [Google Scholar]
- 54.Kemp S., Duff A., and Hampson N. (2016). The neurological, neuroimaging and neuropsychological effects of playing professional football: results of the UK five-year follow-up study. Brain Inj. 30, 1068–1074 [DOI] [PubMed] [Google Scholar]
- 55.Kontos A.P., Braithwaite R., Chrisman S.P., McAllister-Deitrick J., Symington L., Reeves V.L., and Collins M.W. (2017). Meta-analytical review of the effects of football heading. Br. J. Sports Med. 51, 1118–1124 [DOI] [PubMed] [Google Scholar]
- 56.Stephens R., Rutherford A., Potter D., and Fernie G. (2010). Neuropsychological consequence of soccer play in adolescent U.K. School team soccer players. J. Neuropsychiatry Clin. Neurosci. 22, 295–303 [DOI] [PubMed] [Google Scholar]
- 57.McAllister T.W., Ford J.C., Flashman L.A., Maerlender A., Greenwald R.M., Beckwith J.G., Bolander R.P., Tosteson T.D., Turco J.H., Raman R., and Jain S. (2014). Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology 82, 63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsushima W.T., Geling O., Arnold M., and Oshiro R. (2016). Are there subconcussive neuropsychological effects in youth sports? an exploratory study of high- and low-contact sports. Appl. Neuropsychol. Child 5, 149–155 [DOI] [PubMed] [Google Scholar]
- 59.Register-Mihalik J.K., Guskiewicz K.M., Mihalik J.P., Schmidt J.D., Kerr Z.Y., and McCrea M.A. (2013). Reliable change, sensitivity, and specificity of a multidimensional concussion assessment battery: implications for caution in clinical practice. J. Head Trauma Rehabil. 28, 274–283 [DOI] [PubMed] [Google Scholar]
- 60.Koerte I.K., Nichols E., Tripodis Y., Schultz V., Lehner S., Igbinoba R., Chuang A.Z., Mayinger M., Klier E.M., Muehlmann M., Kaufmann D., Lepage C., Heinen F., Schulte-Körne G., Zafonte R., Shenton M.E., and Sereno A.B. (2017). Impaired cognitive performance in youth athletes exposed to repetitive head impacts. J. Neurotrauma 34, 2389–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voss M.W., Kramer A.F., Basak C., Shaurya Prakash R., and Roberts B. (2010). Are expert athletes ‘expert’ in the cognitive laboratory? a meta-analytic review of cognition and sport expertise. Appl. Cognit. Psychol. 24, 812–826 [Google Scholar]
- 62.Zhu N., Jacobs D.R., Schreiner P.J., Yaffe K., Bryan N., Launer L.J., Whitmer R.A., Sidney S., Demerath E., Thomas W., Bouchard C., He K., Reis J., and Sternfeld B. (2014). Cardiorespiratory fitness and cognitive function in middle age: the CARDIA study. Neurology 82, 1339–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toth A., Kovacs N., Perlaki G., Orsi G., Aradi M., Komaromy H., Ezer E., Bukovics P., Farkas O., Janszky J., Doczi T., Buki A., and Schwarcz A. (2013). Multi-modal magnetic resonance imaging in the acute and sub-acute phase of mild traumatic brain injury: can we see the difference? J. Neurotrauma 30, 2–10 [DOI] [PubMed] [Google Scholar]
- 64.Hasiloglu Z.I., Albayram S., Selcuk H., Ceyhan E., Delil S., Arkan B., and Baskoy L. (2011). Cerebral microhemorrhages detected by susceptibility-weighted imaging in amateur boxers. AJNR Am. J. Neuroradiol. 32, 99–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Y.L., Kuo Y.S., Tseng Y.C., Chen D.Y., Chiu W.T., and Chen C.J. (2015). Susceptibility-weighted MRI in mild traumatic brain injury. Neurology 84, 580–585 [DOI] [PubMed] [Google Scholar]
- 66.Broglio S.P., Williams R.M., O’ Connor K.L., and Goldstick J. (2016). Football players’ head-impact exposure after limiting of full-contact practices. J. Athl. Train. 51, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reynolds B.B., Patrie J., Henry E.J., Goodkin H.P., Broshek D.K., Wintermark M., and Druzgal T.J. (2017). Effects of sex and event type on head impact in collegiate soccer. Orthop. J. Sports Med. 5, 2325967117701708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zuckerman S.L., Kerr Z.Y., Yengo-Kahn A., Wasserman E., Covassin T., and Solomon G.S. (2015). Epidemiology of sports-related concussion in NCAA athletes from 2009–2010 to 2013–2014: incidence, recurrence, and mechanisms. Am. J. Sports Med. 43, 2654–2662 [DOI] [PubMed] [Google Scholar]
- 69.Pate R.R., Wang C.Y., Dowda M., Farrell S.W., and O'Neill J.R. (2006). Cardiorespiratory fitness levels among US youth 12 to 19 years of age: findings from the 1999–2002 National Health and Nutrition Examination Survey. Arch. Pediatr. Adolesc. Med. 160, 1005–1012 [DOI] [PubMed] [Google Scholar]
- 70.Ring-Dimitriou S., von Duvillard S.P., Stadlmann M., Kinnunen H., Drachta O., Müller E., Laukkanen R., Hamra J., Weeks S., and Peak K. (2008). Changes in physical fitness in moderately fit adults with and without the use of exercise telemetry monitors. Eur. J. Appl. Physiol. 102, 505–513 [DOI] [PubMed] [Google Scholar]
- 71.Da Silva C.D., Bloomfield J., and Marins J.C. (2008). A review of stature, body mass and maximal oxygen uptake profiles of u17, u20 and first division players in brazilian soccer. J. Sports Sci. Med. 7, 309–319 [PMC free article] [PubMed] [Google Scholar]
- 72.Brewer J. and Davis J. (1995). Applied physiology of rugby league. Sports Med. 20, 129–135 [DOI] [PubMed] [Google Scholar]
- 73.Fernandes R.J., Keskinen K.L., Colaço P., Querido A.J., Machado L.J., Morais P.A., Novais D.Q., Marinho D.A., and Vilas Boas J.P. (2008). Time limit at VO2max velocity in elite crawl swimmers. Int. J. Sports Med. 29, 145–150 [DOI] [PubMed] [Google Scholar]
- 74.Mannix R., Meehan W.P., 3rd, and Pascual-Leone A. (2016). Sports-related concussions—media, science and policy. Nat. Rev. Neurol. 12, 486–490 [DOI] [PMC free article] [PubMed] [Google Scholar]