Abstract
During maturation, pancreatic islets achieve their full capacity to secrete insulin in response to glucose, undergo morphological changes in which alpha-cells decrease and beta-cell mass increases, and they acquire the normal alpha- and beta-cell proportion changes that are important for islet functions later in life. In rodents, the first week of postweaning is critical for islet maturation. Multiple studies have documented the detrimental effects of several conditions on pancreatic maturation; however, few studies have addressed the use of pharmacological agents to enhance islet maturation. Biotin might have a potential action on islet maturation. Pharmacological concentrations of biotin have been found to modify islet morphology and function. In a previous study, we found that mice fed a biotin-supplemented diet for 8 weeks after weaning showed an increase in basal and glucose stimulated insulin secretion, enlarged islet size, and modified islet structure. In the present study, we investigated the effect of biotin on maturation features during the first week postweaning. Female BALB/cAnN Hsd mice were fed a control or a biotin-supplemented diet for 1 week after weaning. Compared with the control, biotin-supplemented mice showed an increase in pancreatic islet number and area in addition to an augmented proportion of beta-cells in the islet. These effects were related to an increase in beta-cell proliferation. No differences were found in insulin secretion, blood glucose concentrations, or serum insulin levels. These results indicate that biotin supplementation is capable of affecting beta-cell proliferation and might be a therapeutic agent for establishing strategies for regenerative medicine.
KEYWORDS: : beta-cell proliferation, biotin, insulin secretion, islet-maturation
Introduction
Pancreatic islet development and maturation are critical for islet function later in life.1 Human epidemiologic and experimental animal studies have shown a link between poor fetal and neonatal growth and increased risk of developing type-2 diabetes.2–5 Altered nutrition such as calorie and protein-restricted diets,4–10 high-carbohydrate and high-fat diets,3,11 or vitamin A-deficient diets12 impair islet development and maturity, thus affecting their capacity to respond to metabolic challenges later in life.
Islet maturation culminates after weaning, a period which represents a metabolic challenge due to the nutritional switch from a milk diet to omnivorous food intake. During this period, islets achieved the full capacity to secrete insulin in response to glucose,5,13–15 decrease alpha-cells and increase beta-cell mass, and acquire the normal alpha- and beta-cell distribution and proportion.13 In rodents, the maturation period takes place during the first week of nutrient shifts at weaning.13–15 Although the detrimental effects of several conditions on pancreatic maturation, such low-protein, low-energy, or high-fat diets, have been documented,6,7,9–11 few investigators have studied pharmacological agents directed at enhancing maturation features.16,17 The vitamin biotin might have potential positive effects on this process.
Biotin participates in intermediary metabolism as a covalently bound coenzyme of carboxylases. At pharmacological concentrations, which are about 30 to 700 times greater than its daily requirement (30 μg), biotin modifies different biological functions such as glucose and lipid metabolism,18,19 cell proliferation,20 tissue morphology,21,22 apoptosis, and development.23
Several studies have found that pharmacological concentrations of biotin increase glucose-induced insulin secretion in vitro24–27 and in vivo.21
Furthermore, eight weeks of biotin supplementation after weaning augmented the pancreatic proportion of beta-cells by enlarging islet size and modifying islet morphology, increasing the percentage of alpha-cells in the islet core.21 In this study, we investigated biotin's effects during the first week postweaning on islet morphology and morphometry, islet-cell proliferation, neogenesis, and apoptosis, in addition to glucose-induced insulin secretion in isolated islets. Glucose tolerance, blood glucose, and serum insulin concentrations were also determined.
Materials and Methods
Animal model and experimental design
Animal handling and procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, Washington, DC, USA, 1996). All procedures were approved by the Ethics Committee for Experimentation of the Biomedical Research Institute of the National Autonomous University of Mexico.
At weaning, 21-day-old female BALBc/ANN Hsd mice were randomly assigned to groups that were fed either a control or biotin-supplemented diet (0.8 versus 100 mg of biotin/kg diet, Teklad Global 18% protein rodent diet [sterilizable] Cat. No. T.20185.15. Harlan, Teklad, Madison, WI, USA). Mice were kept under conditions of 12-h light/dark cycles with water and food ad libitum, except when fasting conditions were required. Mice and diet were weighed daily. After 1 week of diet administration, the mice were deprived of food for 12 h and anesthetized with Sevorane® (Sevoflurane; Abbott Laboratories, Mexico City, Mexico). The pancreas and blood were obtained after mice were sacrificed and processed as described below.
Immunofluorescence and morphometric analysis
Immunohistochemistry and morphometric analysis on pancreas sections were performed as previously reported.28 In brief, consecutive 5 μm-thick sections were cut and mounted on a glass slide. Sections were deparaffinized, rehydrated, permeabilized with 0.3% Triton X-100 (Sigma, St. Louis, MO, USA), and subsequently incubated overnight with guinea pig anti-porcine insulin antibody (1:150; Thermo Invitrogen, Rockford, IL, USA) and mouse antiglucagon antibody (1:4500; Sigma). Sections were incubated then for 1 h with a secondary donkey anti-guinea pig Alexa fluor 488 conjugate (1:300; Jakson Immuno Research Laboratories, Baltimore Pike, PA, USA) and Cy3 donkey anti-mouse conjugate (1:800; Jakson Immuno Research Laboratories). Nuclei were stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma). After incubation, the sections were washed with phosphate-buffered saline and mounted onto a cover slip with fluorescent mounting medium (DAKO; North America, Inc., Carpinteria, CA, USA). Islets were defined as four or more clusters of insulin-positive cells. Areas of islets, beta- and alpha-cells, and total pancreatic sections were quantified using an Olympus inverted1X70 microscope (Tokyo, Japan) at 40 × with an attached Media Cibernetics Evolution VF (Georgia, MD, USA). Total pancreatic sections were assessed with Olympus BX51-WI DSU at 4 × using an attached Hamamatsu C9100 camera EM-CCD (Hamamatsu City, Japan). Images were taken with identical configurations. All image analyses were done with Image J 1.40 software (Research Services Branch, National Institute of Mental Health, Bethesda, MD, USA).
Islet cell proliferation
Islet-cell proliferation was assessed by immunofluorescence using anti-Ki67 (Abcam, Cambridge, MA, USA) coimmunostained with insulin and DAPI as described above. Quantification of Ki67 and Ki67 insulin positive costaining was assessed. β-cell proliferation was measured by quantifying the number of Ki67-positive nuclei in relationship to the number of total nuclei. A total of 160 and 228 islets were counted for the control and the biotin-supplemented group, respectively.
Islet neogenesis
Islet neogenesis was determined by immunofluorescence using islet neogenesis-associated protein-related protein (INGAPrP) also known as Reg IIIδ, a 98 amino acid mouse protein that belongs to the Reg family.29–31 Pancreatic sections were coimmunostained with insulin, DAPI, and INGAPrP (1:300; Santa Cruz, Biotechnology, Inc., Santa Cruz, CA, USA). The percentage of immunopositive cells to INGAPrP in relationship to the number of insulin-positive cells was calculated. A total of 5730 and 5995 cells were counted for the control and the biotin-supplemented group, respectively.
Islet apoptosis
The number of apoptotic cells was analyzed on the 5 μm pancreas sections slides that had been stained for immunofluorescence. Slides were deparafinnized, rehydrated, and incubated with 20 μg/μL proteinase K (Solon, OH, USA) for 10 min followed by TUNEL (In situ Cell Dead Detection kit, TMR red; Roche Diagnostics, Manheim, Germany) according to the manufacturer's instruction. Pancreatic sections were counterstained with DAPI. Approximately 40 islets were assessed in each group with a total of 4682 and 5008 nuclei counted for the control and the supplemented group, respectively.
Glucose-stimulated insulin secretion
Pancreases were perfused, dissected, and digested by collagenase P (Roche Diagnostics) and diluted in Hanks' Balanced Salt solution (Gibco, Grand Island, NY, USA) at 1 mg/mL. Islets were separated from exocrine tissue by a Histopaque-1077 (Sigma) gradient and handpicked as reported previously.32 Islets were cultured overnight in biotin-free DMEM (11 mM glucose, 400 U/mL penicillin, 200 mg/L streptomycin, and 10% dialyzed FBS; Gibco) at 37°C in a humidified atmosphere of 5% CO2. Glucose-induced insulin secretion analysis from 20 to 30 pair-sized islets was performed as previously reported.21 Insulin in the different media was measured using an ultrasensitive rat insulin EIA ELISA kit (ALPCO Diagnostics, Windham, NH, USA).
Blood glucose concentration measurements
Blood glucose concentrations were determined from tail vein blood samples using a portable glucose meter (Precision QID; MediSense, Inc., Abbott Laboratories).
Glucose tolerance tests
Intraperitoneal glucose tolerance tests were performed as previously described.21 To calculate the area under the curves, we used the software GraphPad (La Jolla, CA, USA).
Serum insulin concentration measurements
Blood samples were collected and treated, and serum was obtained as described previously.21 Insulin concentrations were measured with the ultrasensitive rat insulin EIA ELISA kit (ALPCO Diagnostics). All measurements were performed in triplicate.
Statistical analysis
All data are presented as the mean ± SEM; n denotes the number of evaluated subjects. Statistical analysis was performed using GraphPad. The data were analyzed by Student's t-test, Mann–Whitney U test, or two-way analysis of variance (ANOVA). P ≤ .05 was considered statistically significant. *P ≤ .05; **P ≤ .005.
Results
Effect of biotin supplementation on body weight and food consumption
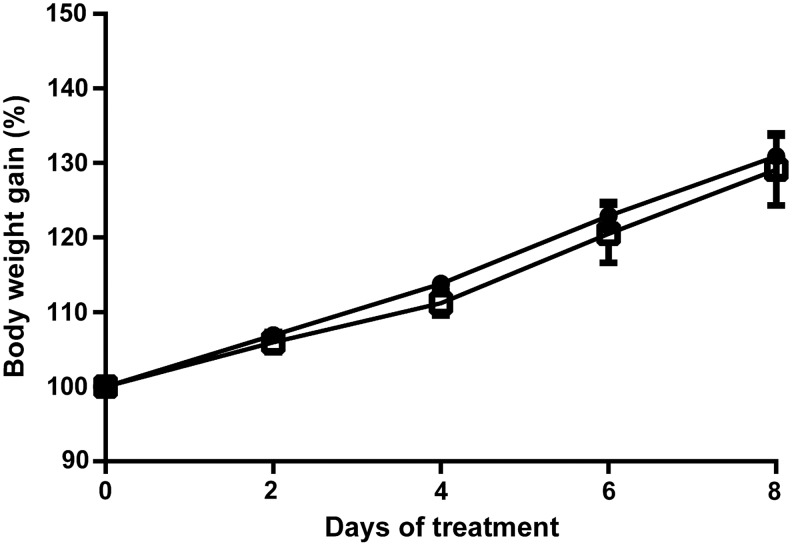
During the experimental period, we determined daily mouse body weight (Fig. 1). Mice in the biotin-control group and biotin-supplemented group showed a steady weight gain during the experimental period, which was not significantly different between the groups. The amount of food intake/body weight over the period was not significantly different between the groups (average food intake: control = 0.229 ± 0.014; biotin-supplemented = 0.223 ± 0.015 g of food/g of body weight/day).
FIG. 1.
Effect of biotin supplementation on body weight. Three-week-old mice were fed a control or a biotin-supplemented diet for 1 week. Body weight was measured every day. Values are the mean ± SEM. n: Control = 8; supplemented = 9 mice. Circles: control group; squares: biotin-supplemented. Significance was assessed by the two-factor repeated measurements ANOVA (diet, time). SEM, standard error of the mean.
Effect of biotin supplementation on islet morphometry and morphology
Beta- and alpha-cell islet composition
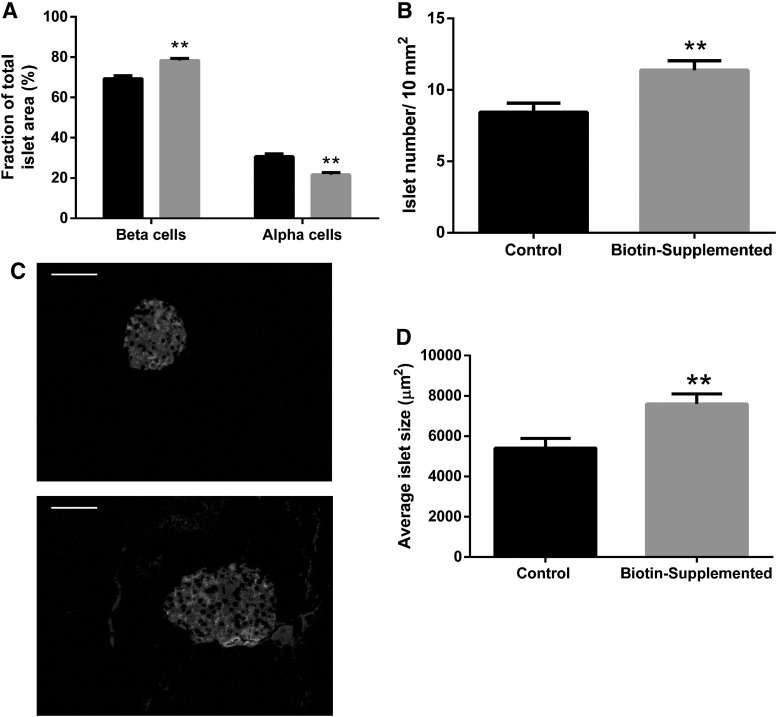
As it has been documented after weaning, islet cell composition is modified,13 and we quantified the effects of 1 week of biotin supplementation postweaning on the islet proportion of beta- and alpha-cell areas (Fig. 2A). Biotin supplementation significantly increased beta-cell proportion (control = 69.4 ± 1.49; biotin-supplemented = 78.3% ± 1.04%; P < .005) and decreased alpha-cell (control = 30.7 ± 1; biotin-supplemented = 21.7% ± 1.04%, P < .05).
FIG. 2.
Effect of biotin supplementation on islet morphometry and morphology. Morphometry from pancreatic sections of control and biotin-supplemented mice. (A) Mean proportions of alpha- and beta-area for islet area. Significance was assessed by Mann–Whitney U test. **P ≤ .005 compared with the control group. (B) Islet number per pancreatic area (10 mm2). Significance was assessed by Student's t-test. **P ≤ .005 compared with the control group. (C) Immunofluorescence images of pancreatic islets from control (C, upper panel) and biotin-supplemented (C, bottom panel) mice pancreas sections stained for insulin and glucagon. Scale bar represents 50 μm. (D) Average islet size (μm2) ± SEM. Significance was assessed by Mann–Whitney U test. Values are mean ± SEM. n = 4 mice per group. **P ≤ .005 compared with the control group.
Number of islets
We compared the number of islets per pancreatic area (10 mm2), and our results (Fig. 2B) revealed that the number of islets increased in the biotin-supplemented group when compared with the control group (control = 8.44 ± 0.64; biotin-supplemented = 11.4 ± 0.66 islets/10 mm2 pancreatic area; P < .005). This effect is not related to changes in total pancreatic area since no significant differences were observed between the groups (control = 16,053 ± 1016; biotin-supplemented = 15,832 ± 2070 mm2).
Islet area
The islets' areas were assessed in pancreatic sections from the control and supplemented mice (Fig. 2C, D). Compared with the control mice, a significant increase in 40% was observed in the supplemented mice (control = 5415 ± 474; biotin-supplemented = 7593 ± 499 μm2; P < .005).
Effect of biotin supplementation on islet cell proliferation, neogenesis, and apoptosis
Islet proliferation
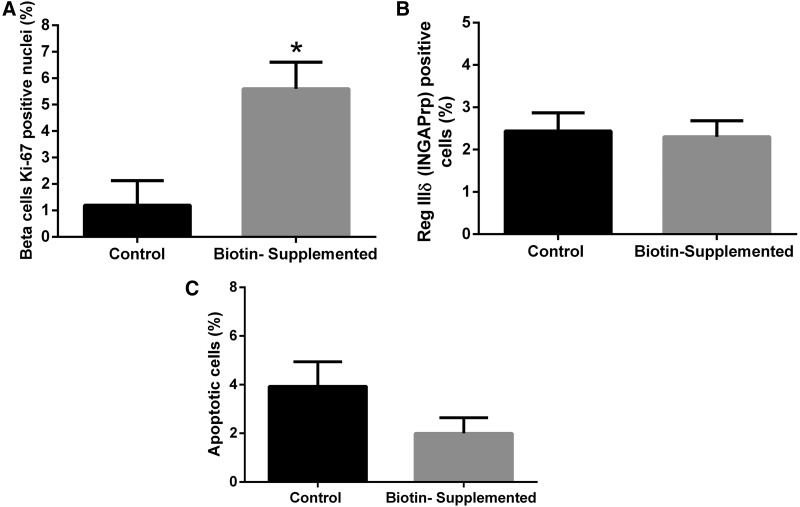
To evaluate whether the increased beta-cell and islet areas produced by biotin supplementation were due to increased proliferation, we used immunofluorescence to assess the proportion of cells stained for the nuclear antigen Ki67 (Fig. 3A). Our results revealed that 1 week of biotin supplementation caused a significant increase in Ki67 positive beta-cells (control = 1.20 ± 0.93; biotin-supplemented = 5.59 ± 1.01; P < .05).
FIG. 3.
Effect of biotin supplementation on islet cell proliferation, neogenesis, and apoptosis. (A) Percentage of beta-cells staining for antigen Ki67 in the nuclei. Values are mean percentages ± SEM. n = 4 mice per group. Significance was assessed by Student's t-test. *P ≤ .05 compared with the control group. (B) Percentage of immunopositive cells to INGAPrP, in relationship to the number of insulin-positive cells. Values are mean percentages ± SEM. n = 4 mice per group. Significance was assessed by Student's t-test. (C) Percent of TUNEL-positive nuclei per total nuclei number. Values are mean percentages ± SEM. n = 4 mice per group. Significance was assessed by Student's t-test. INGAPrP, islet neogenesis-associated protein-related protein.
Islet neogenesis
We determined the percentage of immunopositive cells to islet neogenesis-associated protein29 in relationship to the number of insulin-positive cells (Fig. 3B). No significant differences were observed between the control mice and the biotin-supplemented group (2.44% ± 0.43% and 2.30% ± 0.38%; respectively, P > .05).
Effect of biotin supplementation on islet cell apoptosis
Next, experiments were carried out to determine the effects of biotin on cell apoptosis (Fig. 3C). The percentage of TUNEL-positive nuclei per total islet nuclei was not significantly different between the groups (control 3.93% ± 1.01%; biotin-supplemented 2.00% ± 0.64%; P > .05).
Effect of biotin supplementation on insulin secretion from isolated islets
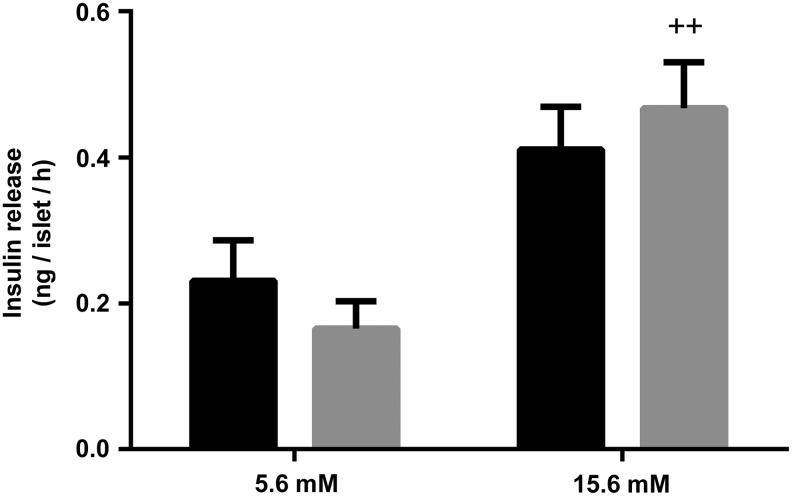
We determined the effects of 1 week of a biotin-supplemented diet on glucose-stimulated insulin release. As depicted in Figure 4, insulin secretion levels did not differ significantly at both the basal and high glucose concentrations (5.5 and 15.6 mM, respectively) in islets isolated from control and biotin-supplemented mice (control = 0.23 ± 0.05 and 0.41 ± 0.05; biotin-supplemented = 0.16 ± 0.03 and 0.46 ± 0.06 ng of insulin/islet/h for high and low glucose concentrations, respectively).
FIG. 4.
Effect of biotin supplementation on insulin secretion. Glucose-induced insulin release from cultured pancreatic islets isolated from control (black) or biotin-supplemented mice (gray). Data represent mean ± SEM. Results are the mean of three independent experiments. Significance was assessed by two-way ANOVA. ++P ≤ .005 compared with basal secretion (5.6 mM glucose). ANOVA, analysis of variance.
Effect of biotin supplementation on blood glucose concentrations and glucose tolerance test
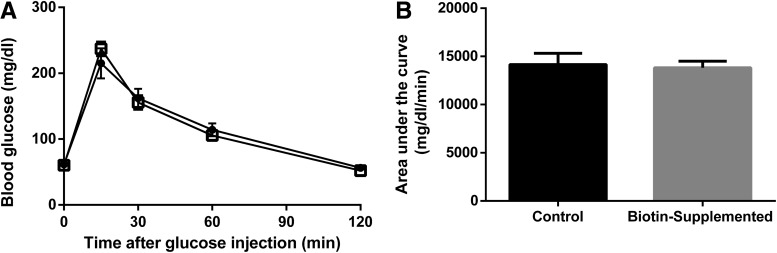
One week of biotin supplementation in the diet did not modify fasting blood glucose levels (control = 61.6 ± 3.07; biotin-supplemented = 60.3 ± 3.09 mg/dL). Additional experiments were carried out to determine glucose tolerance. No significant differences were found between the biotin-supplemented and the control group (Fig. 5). The mean total area under the curve was 14,159 ± 1171 for the control and 13,819 ± 682 for the biotin-supplemented mice.
FIG. 5.
Effect of biotin supplementation on glucose tolerance test. (A) Blood glucose concentrations during glucose tolerance test (i.p. injection of 2 g/kg of glucose). Data represent mean ± SEM. Results are the mean ± SEM of n = 8 mice per group. Significance of glucose tolerance test was assessed by the two-factor repeated measurements ANOVA. (B) Significance of the area under the curve was determined by Student's t-test.
Effect of biotin supplementation on serum insulin concentrations
The effects of biotin supplementation on fasting serum insulin levels were analyzed. No differences were observed between the groups (control = 0.22 ± 0.09; biotin-supplemented = 0.24 ± 0.07 ng/mL; P > .05).
Discussion
Pancreatic islet maturation is critical for its function later in life.6,9–11 In rodents, islet maturation is attained the week after weaning, a period in which they acquire the normal alpha- and beta-cell proportion13 and increase in beta-cell mass.7,14 In this study using mice, we have shown that biotin supplementation during this period increased islet area, islet number, and the proportion of beta-cells in the islet, and that these changes were related to increased beta-cell proliferation.
After weaning, normal islets expand their mass in proportion to an individual's body weight.33 Our results demonstrated that biotin-supplementation 1 week after weaning increased islet area and that this effect was not related to mouse growth since weight gain was not different between the control and the supplemented groups; this finding was constant throughout the present and all of our previous studies.21,34
The 3.6-fold increase in cell proliferation observed in our studies in conjunction with no significant changes in apoptosis or islet neogenesis indicate that the enhanced islet area and number and proportion of beta-cells in response to biotin supplementation is induced by cell proliferation. Some studies have indicated that pharmacological biotin concentrations are capable of increasing proliferation; this information, however, remains controversial. In JAr choriocarcinoma cells, biotin supply increased proliferation rates.20 Jurkat-cells cultured in medium containing a pharmacological concentration of biotin showed a transient increase in proliferation rates during the course of the 4 week study.35 In contrast, healthy humans receiving oral biotin (3.1 μmol) for 14 days showed a decrease in mitogen-stimulated peripheral blood mononuclear cell proliferation.36 Studies in early embryonic chick eyes cultured with biotin (eye local concentration 1 × 10–5 M) between Hamburger and Hamilton stages 14–17 affected retinal and lens structures development; these effects were not related to proliferation but to apoptosis.23 The difference in cell types, concentrations, and length of treatments might account for the observed differences.
There are several islet morphology and morphometric differences between our prior studies and the present study concerning the effects of biotin-supplementation.21 Eight weeks of a biotin-supplemented diet augmented islet size by increasing the proportion of alpha- and beta-cells, but not islet or beta-cell numbers as observed with 1 week of biotin supplementation. In addition, the increased alpha-cell distribution in the islet core produced by 8 weeks of biotin supplementation was not observed in the present report (data not shown). Time-course studies analyzing islet morphology changes at different periods may unravel the reasons for these differences.
One week of biotin supplementation did not affect basal and glucose-induced insulin secretion; these results contrast with our previous studies, in which we found that 8 weeks of biotin supplemented diet increased basal and glucose-induced insulin secretion.21 Recent studies have demonstrated that beta-cells can be divided into two populations: (1) a proliferation-competent population and (2) mature beta-cells, which attain glucose-induced insulin secretion.37 It might be possible that biotin supplementation during the first week after weaning stimulate actions involved in proliferation and that later may produce its effects on mechanisms involved in insulin secretion. In support of this view, other studies have found that pharmacological concentrations of biotin administrated to adult rats increased plasma insulin levels,38,39 and insulin secretion.24–27 Further studies will be required to test this hypothesis.
In agreement with the lack of effect of biotin supplementation on insulin secretion in vitro observed in the present study, the biotin-supplemented mice did not show significant differences in blood glucose levels, glucose tolerance test, or serum insulin concentrations.
Diabetes is a major worldwide health problem that affects millions of people.40,41 Decreased beta-cell mass is present in both type-1 and type-2 diabetes.42 Agents capable of increasing the beta-cell proliferation without negatively affecting glucose-induced insulin secretion represent an important area for the development of therapeutic strategies for diabetes treatment. Several agents such as gamma-aminobutyric acid, thyroid hormone, the agonist for GPR119 receptor, and glucokinase activators16,17 have been proposed as different methods to increase pancreatic beta-cell expansion. Our present studies showing that biotin supplementation is capable of increasing beta-cell proliferation in conjunction with the lack of adverse effects of biotin administration observed in animals,18,19 normal individuals,43 or patients with diabetes44,45 indicate that pharmacological concentrations of biotin might be considered as an agent to expand beta-cell population in strategies to fight diabetes.
In conclusion, our data show for the first time that biotin supplementation during islet maturation increased pancreatic islet number and area and also augmented the proportion of islet beta-cells. These effects were related to an increase in beta-cell proliferation. These results might lead to new strategies for increasing the mass of functional beta-cells to treat diabetes.
Acknowledgments
The authors are grateful to: Dr. Miguel Tapia from the Unidad de Microscopía, and Dr. Georgina Díaz from the Unidad de Modelos Biológicos, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México (UNAM); Dr. Rodolfo Rodríguez-Jurado from Departamento de Patología, Dr. Rosa María Vigueras-Villaseñor of Laboratorio Biología de la Reproducción, and M en C Gerardo Barragán-Mejía of Laboratorio Neurociencias, for histology facilities; and Everardo Ruiz-Mora, Karina Pastén Hidalgo, Stephany Romero Suárez, Laura Victoria Osorio Marín, Gustavo Adolfo Rojas Olave, and Karen Méndez Coronado for technical support. This work was supported by funds from Dirección General de Asuntos del Personal Académico (PAPIIT IN210714) and UC-Mexus (EBC/433/CFM) and the National Institutes of Health Grant P30 DK63720 (to M.S.G.). Wilma Tixi-Verdugo is a PhD student from the Doctorado en Ciencias Bioquímicas at Universidad Nacional Autónoma de México UNAM, and has a scholarship from Consejo Nacional de Ciencia y Tecnología CONACYT (CVU/scholar: 378371). Juan Contreras-Ramos was a Biology student at the Benemérita Universidad Autonoma de Puebla and has a scholarship from from PAPIIT (IN210714), Dirección General de Asuntos del Personal Académico.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hellerstrom C, Swenne I: Functional maturation and proliferation of fetal pancreatic beta-cells. Diabetes 1991;40 Suppl 2:89–93 [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ: The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc Biol Sci 1995;262:37–43 [DOI] [PubMed] [Google Scholar]
- 3.Jones RH, Ozanne SE: Fetal programming of glucose-insulin metabolism. Mol Cell Endocrinol 2009;297:4–9 [DOI] [PubMed] [Google Scholar]
- 4.Schwitzgebel VM, Somm E, Klee P: Modeling intrauterine growth retardation in rodents: Impact on pancreas development and glucose homeostasis. Mol Cell Endocrinol 2009;304:78–83 [DOI] [PubMed] [Google Scholar]
- 5.Garofano A, Czernichow P, Breant B: In utero undernutrition impairs rat beta-cell development. Diabetologia 1997;40:1231–1234 [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Trejo A, Ortiz-Lopez MG, Zambrano E, et al. : Developmental programming of neonatal pancreatic beta-cells by a maternal low-protein diet in rats involves a switch from proliferation to differentiation. Am J Physiol Endocrinol Metab 2012;302:E1431–E1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garofano A, Czernichow P, Breant B: Effect of ageing on beta-cell mass and function in rats malnourished during the perinatal period. Diabetologia 1999;42:711–718 [DOI] [PubMed] [Google Scholar]
- 8.Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ: Islet function in offspring of mothers on low-protein diet during gestation. Diabetes 1991;40 Suppl 2:115–120 [DOI] [PubMed] [Google Scholar]
- 9.Petrik J, Reusens B, Arany E, et al. : A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology 1999;140:4861–4873 [DOI] [PubMed] [Google Scholar]
- 10.Dumortier O, Blondeau B, Duvillie B, Reusens B, Breant B, Remacle C: Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia 2007;50:2495–2503 [DOI] [PubMed] [Google Scholar]
- 11.Jacovetti C, Matkovich SJ, Rodriguez-Trejo A, Guay C, Regazzi R: Postnatal beta-cell maturation is associated with islet-specific microRNA changes induced by nutrient shifts at weaning. Nat Commun 2015;6:8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews KA, Rhoten WB, Driscoll HK, Chertow BS: Vitamin A deficiency impairs fetal islet development and causes subsequent glucose intolerance in adult rats. J Nutr 2004;134:1958–1963 [DOI] [PubMed] [Google Scholar]
- 13.Aguayo-Mazzucato C, Sanchez-Soto C, Godinez-Puig V, Gutierrez-Ospina G, Hiriart M: Restructuring of pancreatic islets and insulin secretion in a postnatal critical window. PLoS One 2006;1:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinze E, Steinke J: Glucose metabolism of isolated pancreatic islets: Difference between fetal, newborn and adult rats. Endocrinology 1971;88:1259–1263 [DOI] [PubMed] [Google Scholar]
- 15.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S: Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 1997;138:1736–1741 [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Lai ZC: Recent progress in studies of factors that elicit pancreatic beta-cell expansion. Protein Cell 2015;6:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox AR, Lam CJ, Rankin MM, et al. : Incretin therapies do not expand beta-cell mass or alter pancreatic histology in young male mice. Endocrinology 2017;158:1701–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez-Mejia C, Lazo-de-la-Vega-Monroy M-L: Biological effects of pharmacological concentrations of biotin. J Evid Based Complementary Altern Med 2011;16:40–48 [Google Scholar]
- 19.Riveron-Negrete L, Fernandez-Mejia C: Pharmacological effects of biotin in animals. Mini Rev Med Chem 2017;17:529–540 [DOI] [PubMed] [Google Scholar]
- 20.Crisp SE, Griffin JB, White BR, et al. : Biotin supply affects rates of cell proliferation, biotinylation of carboxylases and histones, and expression of the gene encoding the sodium-dependent multivitamin transporter in JAr choriocarcinoma cells. Eur J Nutr 2004;43:23–31 [DOI] [PubMed] [Google Scholar]
- 21.Lazo de la Vega-Monroy ML, Larrieta E, German MS, Baez-Saldana A, Fernandez-Mejia C: Effects of biotin supplementation in the diet on insulin secretion, islet gene expression, glucose homeostasis and beta-cell proportion. J Nutr Biochem 2013;24:169–177 [DOI] [PubMed] [Google Scholar]
- 22.Riveron-Negrete L, Sicilia-Argumedo G, Alvarez-Delgado C, Coballase-Urrutia E, Alcantar-Fernandez J, Fernandez-Mejia C: Dietary biotin supplementation modifies hepatic morphology without changes in liver toxicity markers. Biomed Res Int 2016;2016:7276463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenciano AI, Mayordomo R, de La Rosa EJ, Hallbook F: Biotin decreases retinal apoptosis and induces eye malformations in the early chick embryo. Neuroreport 2002;13:297–299 [DOI] [PubMed] [Google Scholar]
- 24.Sone H, Ito M, Sugiyama K, Ohneda M, Maebashi M, Furukawa Y: Biotin enhances glucose-stimulated insulin secretion in the isolated perfused pancreas of the rat. J Nutr Biochem 1999;10:237–243 [DOI] [PubMed] [Google Scholar]
- 25.Sone H, Ito M, Shimizu M, Sasaki Y, Komai M, Furukawa Y: Characteristics of the biotin enhancement of glucose-induced insulin release in pancreatic islets of the rat. Biosci Biotechnol Biochem 2000;64:550–554 [DOI] [PubMed] [Google Scholar]
- 26.Romero-Navarro G, Cabrera-Valladares G, German MS, et al. : Biotin regulation of pancreatic glucokinase and insulin in primary cultured rat islets and in biotin-deficient rats. Endocrinology 1999;140:4595–4600 [DOI] [PubMed] [Google Scholar]
- 27.Vilches-Flores A, Tovar AR, Marin-Hernandez A, Rojas-Ochoa A, Fernandez-Mejia C: Biotin increases glucokinase expression via soluble guanylate cyclase/protein kinase G, adenosine triphosphate production and autocrine action of insulin in pancreatic rat islets. J Nutr Biochem 2010;21:606–612 [DOI] [PubMed] [Google Scholar]
- 28.Larrieta E, Vega-Monroy ML, Vital P, et al. : Effects of biotin deficiency on pancreatic islet morphology, insulin sensitivity and glucose homeostasis. J Nutr Biochem 2012;23:392–399 [DOI] [PubMed] [Google Scholar]
- 29.Koya V, Lu S, Sun YP, et al. : Reversal of streptozotocin-induced diabetes in mice by cellular transduction with recombinant pancreatic transcription factor pancreatic duodenal homeobox-1: A novel protein transduction domain-based therapy. Diabetes 2008;57:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittenger GL, Taylor-Fishwick DA, Johns RH, Burcus N, Kosuri S, Vinik AI: Intramuscular injection of islet neogenesis-associated protein peptide stimulates pancreatic islet neogenesis in healthy dogs. Pancreas 2007;34:103–111 [DOI] [PubMed] [Google Scholar]
- 31.Gagliardino JJ, Del Zotto H, Massa L, Flores LE, Borelli MI: Pancreatic duodenal homeobox-1 and islet neogenesis-associated protein: A possible combined marker of activateable pancreatic cell precursors. J Endocrinol 2003;177:249–259 [DOI] [PubMed] [Google Scholar]
- 32.Papadimitriou A, King AJ, Jones PM, Persaud SJ: Anti-apoptotic effects of arachidonic acid and prostaglandin E2 in pancreatic beta-cells. Cell Physiol Biochem 2007;20:607–616 [DOI] [PubMed] [Google Scholar]
- 33.Montanya E, Nacher V, Biarnes M, Soler J: Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: Role of beta-cell hyperplasia and hypertrophy. Diabetes 2000;49:1341–1346 [DOI] [PubMed] [Google Scholar]
- 34.Baez-Saldana A, Camacho-Arroyo I, Espinosa-Aguirre JJ, et al. : Biotin deficiency and biotin excess: Effects on the female reproductive system. Steroids 2009;74:863–869 [DOI] [PubMed] [Google Scholar]
- 35.Manthey KC, Griffin JB, Zempleni J: Biotin supply affects expression of biotin transporters, biotinylation of carboxylases and metabolism of interleukin-2 in Jurkat cells. J Nutr 2002;132:887–892 [DOI] [PubMed] [Google Scholar]
- 36.Zempleni J, Helm RM, Mock DM: In vivo biotin supplementation at a pharmacologic dose decreases proliferation rates of human peripheral blood mononuclear cells and cytokine release. J Nutr 2001;131:1479–1484 [DOI] [PubMed] [Google Scholar]
- 37.Bader E, Migliorini A, Gegg M, et al. : Identification of proliferative and mature beta-cells in the islets of Langerhans. Nature 2016;535:430–434 [DOI] [PubMed] [Google Scholar]
- 38.Sugita Y, Shirakawa H, Sugimoto R, Furukawa Y, Komai M: Effect of biotin treatment on hepatic gene expression in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 2008;72:1290–1298 [DOI] [PubMed] [Google Scholar]
- 39.Sahin K, Tuzcu M, Orhan C, et al. : Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br J Nutr 2013;110:197–205 [DOI] [PubMed] [Google Scholar]
- 40.Whiting DR, Guariguata L, Weil C, Shaw J: IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–321 [DOI] [PubMed] [Google Scholar]
- 41.Shomali M: Diabetes treatment in 2025: Can scientific advances keep pace with prevalence? Ther Adv Endocrinol Metab 2012;3:163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ackermann AM, Gannon M: Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol 2007;38:193–206 [DOI] [PubMed] [Google Scholar]
- 43.Fiume MZ: Final report on the safety assessment of biotin. Int J Toxicol 2001;20 Suppl 4:1–12 [PubMed] [Google Scholar]
- 44.Maebashi M, Makino Y, Furukawa Y, Ohinata K, Kimura S, Sato T: Therapeutic evaluation of the effect of biotin on hyperglycemia in patients with non-insulin dependent diabetes mellitus. J Clin Biochem Nutr 1993;14:211–218 [Google Scholar]
- 45.Koutsikos D, Fourtounas C, Kapetanaki A, et al. : Oral glucose tolerance test after high-dose i.v. biotin administration in normoglucemic hemodialysis patients. Ren Fail 1996;18:131–137 [DOI] [PubMed] [Google Scholar]