Abstract
Mild traumatic brain injury (mTBI) or concussion represents the majority of brain trauma in the United States. The pathophysiology of mTBI is complex and may include both focal and diffuse injury patterns. In addition to altered circuit dysfunction and traumatic axonal injury (TAI), chronic neuroinflammation has also been implicated in the pathophysiology of mTBI. Recently, our laboratory has reported the detrimental effects of mild hyperthermic mTBI in terms of worsening histopathological and behavioral outcomes. To clarify the role of temperature-sensitive neuroinflammatory processes on these consequences, we evaluated the effects of elevated brain temperature (39°C) on altered microglia/macrophage phenotype patterns after mTBI, changes in leukocyte recruitment, and TAI. Sprague-Dawley male rats underwent mild parasagittal fluid-percussion injury under normothermic (37°C) or hyperthermic (39°C) conditions. Cortical and hippocampal regions were analyzed using several cellular and molecular outcome measures. At 24 h, the ratio of iNOS-positive (M1 type phenotype) to arginase-positive (M2 type phenotype) cells after hyperthermic mTBI showed an increase compared with normothermia by flow cytometry. Inflammatory response gene arrays also demonstrated a significant increase in several classes of pro-inflammatory genes with hyperthermia treatment over normothermia. The injury-induced expression of chemokine ligand 2 (Ccl2) and alpha-2-macroglobulin were also increased with hyperthermic mTBI. With western blot analysis, an increase in CD18 and intercellular cell adhesion molecule-1 (ICAM-1) with hyperthermia and a significant increase in Iba1 reactive microglia are reported in the cerebral cortex. Together, these results demonstrate significant differences in the cellular and molecular consequences of raised brain temperature at the time of mTBI. The observed polarization toward a M1-phenotype with mild hyperthermia would be expected to augment chronic inflammatory cascades, sustained functional deficits, and increased vulnerability to secondary insults. Mild elevations in brain temperature may contribute to the more severe and longer lasting consequences of mTBI or concussion reported in some patients.
Keywords: : BBB perturbations, concussion, cytokines, hyperthermia, inflammation, macrophages, microglia, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major clinical problem in the United States with concussion and mild TBI (mTBI) accounting for approximately 75% of all TBIs.1–3 A subset of patients with mTBI or concussion sustain long-term debilitating symptoms.4–9 Indeed, persistent post-concussion syndromes caused by mTBI has become a major cause of morbidity and poor quality of life.8,10,11 Mild TBI is commonly caused by blunt, non-penetrating brain trauma that can lead to the stretching and tearing of axons and subsequent diffuse axonal injury (DAI).7 A variety of imaging approaches including diffuse tensor imaging (DTI) are clinically used as a diagnostic tool and predictor for post-concussion syndrome.12–14 In addition, immunocytochemical approaches have been used to identify patterns of diffuse and local axonal injury and inflammatory cell activation in experimental models and human specimens.15–17 The effects of mTBI or concussion on alterations in local cerebral blood flow, vascular reactivity, cerebral glucose metabolism, and blood–brain barrier (BBB) permeability have also been described, which may participate in the pathophysiology of mTBI and underlie some of the functional consequences or increased vulnerability to secondary insults.18–25
Mild TBI leads to inflammatory responses that compared with more severe levels of injury are usually considered short-lived.23,26–28 However, recent studies have also emphasized that single or repetitive mild head injury may lead to more complex patterns of neural inflammation with related tau pathology associated with neurodegenerative disorders including Alzheimer's disease as well as chronic traumatic encephalopathy (CTE).23,27,29–32 Factors that may promote or alter the acute inflammatory response to mTBI or concussion include injury severity, sex, age, environmental and genetic factors as well as repetitive insults.1,7,17,33
Previous studies have emphasized the importance of brain temperature in the pathophysiology of moderate-to-severe TBI and have identified various temperature-sensitive secondary injury mechanisms.34–37 Whereas reducing brain temperature following experimental brain injury diminishes multiple injury mechanisms and is neuroprotective, mild hyperthermia worsens outcome in multiple models of cerebral ischemia and neurotrauma.38,39 Recently, our laboratory has evaluated the importance of brain temperature in the setting of mTBI. Initially, we tested whether a relatively mild temperature elevation at the time of mTBI would influence histopathological or behavioral outcomes in a model of mild fluid percussion injury (mFPI).40,41 This question was considered important because it is known that physiological strain during exercise or work in hot climates can increase core temperatures under a number of situations and occupations.42–44 Indeed, clinical studies have reported significant elevations in body temperatures as high as 39–40°C in individuals during periods of strenuous activity such as exercise or sports and that this temperature increase may be more long lasting in hot environments.45–47 Thus, it was hypothesized that individuals prone to mTBI or concussion who are engaged in strenuous activities including both civilian or military personnel may experience periods of mild hyperthermia that could potentially alter traumatic consequences.
Elevated brain temperature to 39°C before mTBI significantly increases patterns of gray and white matter damage as well as results in persistent cognitive deficits that are not seen under normothermic mTBI conditions.40,41 Although the underlying mechanisms responsible for this hyperthermia-induced augmentation after mTBI are unknown, a number of inflammatory processes initiated after neurotrauma and cerebral ischemia are known to be temperature sensitive.34,35,48–50 The activation profiles of macrophages and microglial are complex and may promote cytotoxicity as well as beneficial effects such as enhancing wound repair and the synthesis of growth-promoting substances.51–57 These diverse forms of inflammation are thought to involve subsets of microglial and macrophage phenotypes that may have distinctive beneficial and detrimental effects on outcomes.51,56,58,59
Recently, we have reported that mild hypothermia after moderate TBI promotes M1/M2 polarization to a beneficial phenotype.60 However, it is not known whether temperature modifications such as hyperthermia would also lead to specific inflammatory alterations and promote changes in M1/M2 polarization after mTBI. In this study we utilized a mFPI model that is sensitive to elevated brain temperatures in terms of histopathological and behavioral outcome measures.40,41 We evaluated the effects of normothermic or hyperthermic mTBI on several outcome measures including the assessment of functionally distinct subsets of microglial and macrophage phenotypes using flow cytometry. We also examined patterns of inflammatory gene expression and endothelial cerebral vascular disturbances using reverse transcription polymerase chain reaction (RT-PCR) and western blot analysis. Our findings indicate that in contrast to normothermia, mild hyperthermic mTBI leads to an augmentation of inflammatory cell and microvascular responses including an M1-dominated microglia/macrophage phenotype that would be expected to influence multiple secondary injury mechanisms.
Methods
Mild fluid percussion injury surgery
Experimental procedures complied with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Miami Animal Care and Use Committee. Animals were maintained on a 12/12 h light-dark cycle and had free access to food and water. Surgical and temperature controls were prospectively randomized and animals were assessed by individuals who were blinded to the experimental groups. Power analysis was conducted to determine the number of animals needed to detect a 10% difference in biochemical indicators of microglial and macrophage phenotype as well as RT-PCR results.
Twenty-four hours prior to surgery adult male Sprague-Dawley rats (2–4 months old; Charles Rivers Laboratories, MA) were surgically prepared for mFPI as previously described.40,41 Briefly, anesthesia was induced with 3% isoflurane, 70% nitrous oxide, and 30% oxygen, and animals were maintained via nose cone at 0.5–1% isoflurane, 70% nitrous oxide, and 30% oxygen. A 4.8-mm craniotomy (−3.8 mm bregma, 2.5 mm lateral) was prepared over the right parietal cortex and an 18-gauge syringe hub was secured to the craniotomy window with cyanoacrylic adhesive. After recovery for 24 h during which time the animals were fasted and provided water ad libitum, animals were re-anesthetized with 70% nitrous oxide, 1–3% isoflurane, and 30% oxygen; intubated; and mechanically ventilated (Stoelting, Wood Dale, IL). A catheter was placed in the tail artery to monitor arterial blood pressure, and arterial blood samples were used to calculate blood gases (pO2 and pCO2) and blood pH. Animals were stabilized and physiological parameters were monitored prior to temperature manipulation and post-TBI beginning at 20 min then hourly for the surgery duration. After stabilization of physiological parameters, animals received mTBI (1.4–1.6 atm, or sham surgery). During the 4 h post-TBI monitoring period, the intubated animals' blood gas measurements were maintained at normal physiological levels by ventilator adjustments. Buprenorphine 0.03 mg/kg was administered subcutaneously after injury.
Beginning 15 min prior to the brain injury, the brain and body temperature of the animal was elevated to a mildly hyperthermic level (39°C) or kept at a normothermic level (37°C) for 4 h as previously described.40 This temperature protocol was used to mimic the clinical condition of increased ambient temperatures combined with high levels of physical activity leading to a restricted period of elevated brain temperature at and following the time of brain injury. This experimental protocol would therefore not necessarily replicate a clinical situation where an individual was acclimated or sustained longer periods of mild hyperthermia either prior to or following TBI.61 Rectal temperature was used to monitor core temperatures and a thermistor probe was placed into the temporalis muscle as an indirect measure of brain temperature. Two automatic feedback heating lamps were placed over the animal's head and body to regulate brain and core temperature. Animals were also maintained at normothermia or mild hyperthermia temperatures for 4 h post-injury. Sham-operated animals received identical surgical and temperature manipulations as in mTBI animals but were not injured. Sham animals were maintained for 4 h at normothermia or hyperthermia during the surgery. Naïve animals underwent no surgical manipulation.
Flow cytometry
The methods that were used for the phenotype analysis by flow cytometry have recently been described.60 Flow cytometry was performed to differentiate surveillant microglia from activated microglia and macrophages. At 24 h after TBI, animals were perfused with cold phosphate-buffered saline (PBS), and ipsilateral parietal cortex and hippocampus were dissected for analysis. This early time period was selected based on previous data indicating that the 24 hr period would be appropriate to assess early activation patterns.60 Single cell suspensions were made by passage through a 70-μm cell strainer (Falcon, Madison, WI), lysed with ammonium-chloride-potassium (ACK) buffer (Life Technologies, Grand Island, NY), and stained with live/dead reagent (Life Technologies). Following a non-specific block with CD16/CD32 antibody, cells were incubated with fluorescent antibodies for surface markers CD11b (v450, BD Horizon, San Jose, CA), which binds to neutrophils and myeloid cells but not lymphocytes (BD Horizon), and CD45 (Alexa 647, Biolegend, San Diego, CA) to separate microglia (CD11b+, CD45low) and infiltrating leukocyte (CD11b+, CD45high) populations. CD45 is expressed on all hemotopoietic cells except erythrocytes and platelets (BioLegend). Cells were also incubated with RP1 (PE, BD Horizons) to identify granulocytes, but lymphocytes or monocytes do not express RP1 (BD Pharmingen).
After fixing and permeabilization, cells were incubated with antibodies for iNOS (FITC, eBioscience, San Diego, CA) and arginase (PE, R&D Systems, Minneapolis, MN) to separate those activated cells that display either the M1 (iNOS+) or M2 (Arg+) phenotype as analyzed on a flow cytometer (BD FACSDiva LSRII) using Kaluza® software (Beckman Coulter, Inc.) for analysis. Cell populations were gated to include only live cells, and standard forward and side scatter gating were used in the analysis. Further, CD11b, CD45, iNOS, and arginase populations of cells were gated using the appropriate isotype controls to separate positively labeled cells from the negative population. All samples were also incubated with isotype controls for each antibody. Experimental groups analyzed for iNOS and arginase included naïve, sham normothermic (37°C), sham hyperthermic (4 h, 39°C), TBI normothermic, and TBI hyperthermic with a survival time of 24 h.
Western blot
Western blot analysis was performed to assess the effect of mTBI with hyperthermia on the expression of ICAM-1 (intracellular adhesion molecule-1/CD54) and CD18 (integrin beta2). Specimens from the cerebral cortex were obtained and 15 μg of total protein from normothermia- or hyperthermia-treated animals (n = 5/group) 24 h after mTBI were loaded onto acrylamide gradient gels 4–15% (Bio-Rad) along with a protein ladder (Bio-Rad) and run for 1.5–2 h at 90V. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes and blocked with standard block for 30 min. Incubation with primary anti-rat ICAM-1 (R&D Systems #MAB141011, 1:400 dilution) and CD18 (Abcam #MEM-148, 1:400 dilution) were conducted at 4°C overnight followed by incubation with anti-rat mouse secondary (Cell Signaling #7076, 1:1000) horseradish peroxidase (HRP)-linked antibody. Detection was done using LumiGLO® (Cell Signaling) as per manufacturer's instructions. All blots were normalized to actin control at 1:10,000 (Sigma #A5441).
Immunohistochemistry
At 3 days post-TBI normothermic, hyperthermic, or sham surgery animals (n = 5 per group) were re-anesthetized and transcardially perfused with saline (80 mL) followed by 4% paraformaldehyde (4°C, 350 mL). The brains were placed in 30% sucrose in PBS and sectioned (30 μm) with a sliding microtome (Leica SM2000R) followed by cryoprotection. Serial sections spaced 400 μm apart were immunostained with Iba1 (ionized calcium binding adaptor molecule 1, Waco) or β-APP (amyloid precursor protein, Millipore). After antigen retrieval in citrate buffer in a steamer for 20 min, sections were incubated with primary antibody for Iba1 (1:100) or β-APP (1:100) in PBS with 4% normal goat serum overnight at 4°C. After washing in PBS + Tween, sections were incubated with secondary biotinylated goat anti-mouse or anti-rabbit (Waco) for 2 h room temperature (RT) followed by ABC (avidin/biotin complex, Vectashield) for 1.5 h RT. Sections were stained with DAB (3,3-diaminobenzidine-peroxidase (Sigma) and analyzed on an Olympus microscope (Olympus BX51) using Stereo Investigator® software version 9.10.2 (MicroBrightfield, Inc.).
Five bregma levels (−2.8 to −4.8) were analyzed. Iba1-positive microglia and β-APP profiles were also evaluated by an investigator blinded to the experimental groups to assess patterns of microglial activation including surveillant, reactive, and ameboid using an established classification strategy as well as DAI within the external capsule, respectively.62,63 The parietal cortex overlying the contusion area was contoured at 4 × , and a counting grid of 800 × 800 μm was placed over the contoured parietal cortex. Using a 120 × 120 μm counting frame, Iba1-positive cells were counted in 30–40 randomly placed sampling sites with a 63 × 1.4 numerical aperture objective. Similar procedures were used for the β-APP analysis within the external capsule.
Gene expression arrays
Ribonucleic acid (RNA; 0.5 μg) from normothermia- or hyperthermia-treated mTBI cortical samples 24 h after injury were pooled (n = 4–5), reverse transcribed, and analyzed using TaqMan (Applied Biosystems, #4414212) rat arrays, a 96-well platform that contains primers for inflammatory genes. Those genes that showed more than a 2-fold change in expression were used for further quantitative RT-PCR (qRT-PCR) studies.
RT-PCR
Real-time RT-PCR was performed on ipsilateral cortex and hippocampus from TBI normothermia and TBI hyperthermia (4 h) animals. Contralateral cortex or hippocampus from normothermic animals were used as the control. Four hour and 24 h survival (n = 5/group) animal groups were assessed for several genes associated with M1 or M2 microglia/macrophage phenotypes, as well as genes associated with leukocyte/macrophage recruitment. Tissue was fresh frozen after decapitation and RNA was extracted using the Direct-zol™ (Zymo Research, Irvine, CA) kit. After quantification of RNA concentration, equal amounts (0.5 μg) of RNA were reverse transcribed using the RT2 First Strand Kit (SABioscience, Frederick, MD), which includes a genomic deoxyribonucleic acid (DNA) removal step. Real-time qPCR was conducted using Sybr green dye and gene-specific primers (SA Biosciences) performed on the Applied Biosystems 7300 Real Time platform (Foster City, CA). Primers for markers of M1 phenotype included interleukin (IL)-1β and tumor necrosis factor (TNF)-α and M2 phenotype YM1. RT-PCR was done for ICAM-1 as well as the monocyte chemoattractant chemokine ligand 2 (Ccl2, formally known as monocyte chemoattractant protein 1, MCP1) as well. Ccl2 recruits monocytes, memory T cells, and dendritic cells to the sites of inflammation produced by either tissue injury or infection.64 RT-PCR was also conducted on alpha-2 macroglobulin (A2M), a protease inhibitor and cytokine transporter. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used to normalize samples that were run in triplicate, and relative expression levels were analyzed using the 2-ddCt method.
Statistical analysis
Flow cytometry, western blot, immunohistochemistry, and RT-PCR data were analyzed using one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls post hoc comparison. Data are expressed as mean ± standard error of the mean (SEM).
Results
Animal physiology
Physiological measurements of rectal and brain temperature, PCO2, PO2, pH, and mean arterial blood pressure (MAP) were taken prior to TBI to ensure that all parameters were at normal levels and then 20 min, 1, 2, 3, and 4 h after injury. Physiological variables were within normal ranges pre- and post-TBI. The only significant difference between groups was seen in the temporalis muscle temperature where the hyperthermic group had significantly elevated temporalis temperature compared with that of the normothermic mTBI animals. (see Table 1; initial post-monitoring data are presented).
Table 1.
Physiological Variables Post-TBI
| TBI-normothermia | TBI-hyperthermia | |
|---|---|---|
| Weight | 334.9 ± 4.91 | 338.8 ± 6.09 |
| Brain temperature | 37.0 ± 0.14 | 39.0 ± 0.06 |
| pH | 7.42 ± 0.01 | 7.42 ± 0.01 |
| pCO2 | 39.85 ± 0.43 | 39.06 ± 0.66 |
| pO2 | 136.9 ± 6.19 | 133.9 ± 4.93 |
| MAP | 110.38 ± 3.41 | 112.02 ± 2.55 |
MAP, mean arterial pressure; TBI, traumatic brain injury.
Flow cytometry
Flow cytometry was used to separate populations of resident microglia and infiltrating cells including neutrophils and macrophages. Cells were labeled with CD11b, which is a cell surface marker for neutrophils, macrophages, and microglia. Additionally, cortical and hippocampal cells were labeled with CD45, which is a cell surface marker for leukocytes. The cells that were positive for CD11b but not for CD45 included resident microglia, and those that were positive for both CD11b and CD45 indicated infiltrating neutrophils and monocytes, including macrophages. Twenty-four hours after mTBI, the proportion of infiltrating leukocytes and macrophages observed in the cerebral cortex (Fig. 1B,C, upper right quadrant of flow cytometry gated populations in purple) did not differ between temperature groups but was higher than sham operated animals in both the cerebral cortex and hippocampus (Fig. 1D,E). Naïve and sham operated animals treated with normothermia or hyperthermia showed no significance differences (data not shown). For control comparisons, naïve flow cytometry is shown (Fig. 1A). Likewise, the amount of CD45 high-infiltrating cells was elevated in both mTBI temperature groups (Fig. 1D,E, gray bars), a finding that is consistent with cerebral vascular dysfunction and the local accumulation or extravasation of circulating monocytes.
FIG. 1.
Flow cytometry. Cortical cells labeled with CD11b and CD45 in naïve (A), normothermia traumatic brain injury (TBI) (B) and hyperthermia TBI (C) 24 h after injury. CD45 high cells (purple) include infiltrating leukocytes and macrophages and CD45 low cells (blue) indicate resident microglia. There is an increase after injury of infiltrating cells (CD45 high in gray) in all TBI animals in the cortex (D) and hippocampus (E) after TBI versus sham.
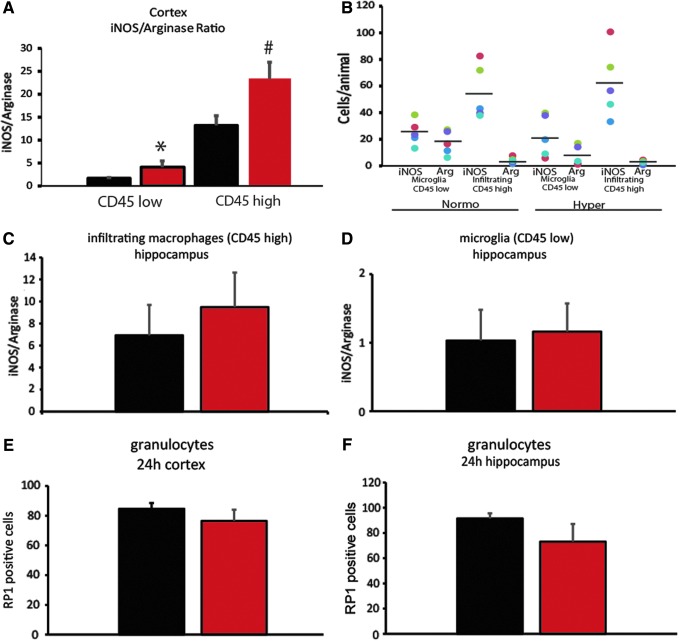
CD11b positive cells were also labeled with intracellular markers for M1 (iNOS) and M2 (arginase) phenotypes. iNOS is expressed in many cell types but in our flow experiments we only assessed CD11b+ cells. Cells that were positive for CD11b but negative for CD45 which include microglia were separated by flow cytometry (Fig. 2) as were cells positive for both CD11b and CD45 which include leukocytes and macrophages (Fig. 2). The ratio of iNOS+ to arginase+ cells showed a relative increase in the M1 phenotype expression in relation to an M2 phenotype in cortical samples subjected to hyperthermia (red bars) among the microglia (CD45 low; Fig. 2A; *p < 0.001) after normothermic mTBI. For the infiltrating macrophages there was also an increase in the ratio of iNOS+ to arginase+ cells (CD45 high; #p < 0.05) in the cortex. A scatter plot of each individual animal number of iNOS+ or arginase+ cells in each group is shown in Figure 2B. There were no significant differences in the hippocampus (Fig. 2C,D). Finally, cortical and hippocampal granulocytes labeled with RP1 and analyzed by flow cytometry at 24 h after TBI (Fig. 2E,F) demonstrated no significant differences between the temperature groups.
FIG. 2.
Flow cytometry of microglia phenotypes. The ratio of iNOS-positive to arginase-positive microglia cells (CD45 low) in the cortex (A) is significantly increased with hyperthermia (*p < 0.01, red bars) as well as the infiltrating leukocytes (CD45 high; #p < 0.05) compared with normothermic mild traumatic brain injury (mTBI; black bars). Scatter plot of individual animals in each group (B). There is no difference in the ratio of iNOS to arginase expressing cells in the infiltrating CD45 high cells (C) or the microglia (D) in the hippocampus. There is no difference with temperature on the number of RP1-positive granulocytes in the cortex (E) or hippocampus (F); n = 5–7 per group. One-way analysis of variance (ANOVA) with Student-Newman-Keuls post hoc.
Western blot analysis
Total protein extracted from ipsilateral cerebral cortex 3days after mTBI was analyzed by western blot for ICAM-1 and CD18. Protein levels in sham (control), normothermic, or hyperthermic mTBI animals (n = 6 per group) were compared (Fig. 3). ICAM-1 levels were significantly increased in both injury groups above sham (*p < 0.001) with an additional increase of ICAM-1 in animals treated with hyperthermia (#p < 0.001) over normothermia. CD18 protein levels (Fig. 3) were also significantly increased over sham levels (#p < 0.05) in the hyperthermia group only. Representative western blots are shown in Figure 3B. These findings emphasize the effects of hyperthermia with mTBI on the cortical cerebral vasculature.
FIG. 3.
Western blot analysis of ICAM-1 and CD18 protein levels 3 days after traumatic brain injury (TBI) in injured cortex. ICAM-1 levels were significantly increased over sham (control) in both TBI groups (*p < 0.001) and additionally, ICAM-1 was higher in hyperthermic animals versus normothermic animals (#p < 0.001). CD18 was increased over sham (control) levels in the hyperthermic group only (#p < 0.05). One-way analysis of variance (ANOVA) with Student-Newman-Keuls post hoc. Representative blots shown in B.
Immunohistochemistry
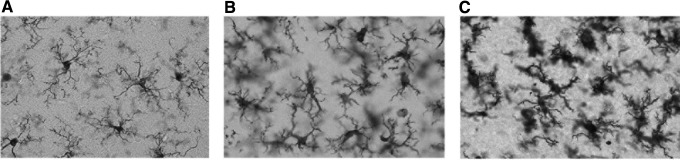
At 24 h after TBI, evidence for different states of microglial activation was demonstrated in the cerebral cortex of mTBI animals compared with sham operated controls. Using anti-Iba1 immunohistochemistry, microglia from sham operated animals displayed surveillant body types with thin and highly branched processes (Fig. 4A). In contrast, cortical specimens from mTBI rats of both temperature groups showed evidence of activation including hypertrophied cell bodies with stubby and swollen short processes (Fig. 4B,C). Also present, especially in the hyperthermic mTBI animals, were cells that were more rounded and also stained with anti-Iba1. These cells were commonly located around blood vessels and appeared to be infiltrating monocytes (data not shown).
FIG. 4.
Iba1 immunohistochemistry demonstrated morphological changes after traumatic brain injury (TBI) in both normothermia (B) and hyperthermia (C) compared with sham animals (A) in the ipsilateral cortex. The morphology of the microglia in sham (A) exhibited a surveillant phenotype, whereas in normothermic TBI animals (B) there was an increase in reactive phenotype and a further activation and ameboid appearance of the microglia in hyperthermic TBI (C) microglia. A, B, C: 60 × magnification.
We next assessed whether significant differences in the degree of microglial activation could be demonstrated between the two mTBI temperature groups. Using non-biased stereological approaches, Iba1 immunoreactive microglia were characterized in the ipsilateral cerebral cortex and placed in one of three categories including surveillant, activated, and ameboid.65,66 Numbers of surveillant, non-activated cells decreased significantly in normothermic (#p < 0.05) and hyperthermic (*p < 0.001) groups compared with sham (Fig. 5A). Counts of reactive and ameboid microglia were significantly increased in mTBI rats compared with sham operated animals (Fig. 5B,C; #p < 0.05). In addition, numbers of reactive and ameboid microglia were significantly increased in the hyperthermic versus normothermic mTBI animals (Fig. 5 B,C; **p < 0.05). These immunohistochemical findings demonstrate that hyperthermic mTBI augments the microglial response to trauma compared with normothermic conditions.
FIG. 5.

Quantification of Iba1-positive microglia in injured cortex. Cells were classified into three major types: surveillant (A), reactive (B), and ameboid (C). There was a significant reduction in surveillant cells after traumatic brain injury (TBI; *p < 0.001, #p < 0.05) in both injury groups compared with sham. There was an increase in reactive and ameboid (#p < 0.05) cells in both TBI groups over sham. In the hyperthermia TBI animals, there was a significant increase in reactive and ameboid phenotype versus normothermic animals (**p < 0.05); n = 5 per group. One-way analysis of variance (ANOVA) with Student-Newman-Keuls post hoc.
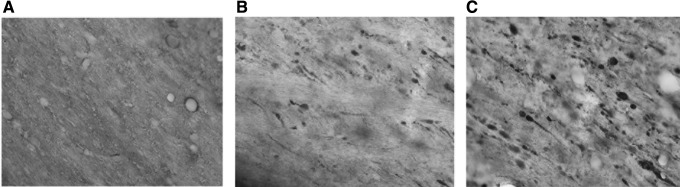
TAI is a common structural consequence of TBI and is seen in some models of mTBI and concussion. Immunohistochemical analysis of β-APP-positive profiles was conducted in the ipsilateral external capsule adjacent to the cortical contusion site (Fig. 6). These abnormal axonal structures were not present in the contralateral external capsule (Fig. 6A) but were evident in the ipsilateral external capsule of normothermic and hyperthermic TBI animals (Fig. 6B,C). Quantitative analysis at three bregma levels rostral to, at the injury site, and caudal to the injury site were assessed. Caudal to the injury site a non-significant increase in β-APP immunoreactive profiles in the hyperthermic versus normothermic mTBI rats (Fig. 7) was demonstrated (p = 0.06; bregma level −4.8). Rostral to the injury site at bregma −2.8 there was a trend for a decrease in β-APP in hyperthermic animals but it was not significant. These results indicate that patterns of TAI seen after mTBI may be augmented under hyperthermic conditions.
FIG. 6.
β-APP immunohistochemistry. There was no β-APP observed in the contralateral external capsule (A). In both normothermic traumatic brain injury (TBI) (B) and hyperthermic TBI (C) there was an increase in β-APP in the external capsule adjacent to the contusion. A, B, C: 60 × magnification. Bregma level −4.8 shown.
FIG. 7.
Quantification of β-APP. There was no significant difference in the two traumatic brain injury (TBI) groups at three bregma levels counted, although there was a strong trend posterior to the injury site (bregma −4.8) for there to be more β-APP in the hyperthermia group (p = 0.06); n = 5 per group. There seemed to be a decrease in the hyperthermia group at bregma −2.8 but it was not significant. One-way analysis of variance (ANOVA) with Student-Newman-Keuls post hoc.
Gene array
Arrays that contain genes associated with inflammation were probed by real-time RT-PCR with cortical samples from 24 h mTBI animals with either normothermia or hyperthermia. Those genes that showed more than a 2-fold difference between groups are listed in Table 2. Included are several members of the MAP kinase family that are known to direct cellular responses to a number of stimuli including pro-inflammatory cytokines and apoptosis. Several members of the phospholipase A2 family were also higher in the hyperthermia animals. These proteins hydrolyze phospholipids and function in a variety of cellular processes including the production of molecules that induce inflammatory responses. A2M, a protease inhibitor and cytokine transporter, was also increased by hyperthermia. We chose to quantify this and other gene expression patterns by RT-PCR as well.
Table 2.
Inflammation Array Results
| Gene | Fold change from normothermia |
|---|---|
| A2m | 3.3 |
| Alox | 3.0 |
| Mapk14 | 2.4 |
| Mapk3 | 2.2 |
| Pla2g1b | 6.3 |
| Pla2g2a | 2.5 |
| Tnf | 3.0 |
Genes that show >2-fold change in hyperthermia mTBI animals versus normothermia mTBI animals. Pooled samples; n = 4 per group.
mTBI, mild traumatic brain injury.
RT-PCR
Real-time RT-PCR was performed on a subset of genes identified by the gene arrays and also genes associated with M1/M2 phenotype differences. As shown in Figure 8, there were significant increases above sham levels in IL1-β (Fig. 8A) at both 4 h and 24 h after TBI (#p < 0.05), as well as Ccl2 (Fig. 8C; *p < 0.001) Additionally, hyperthermia further increased Ccl2 expression at 24 h over normothermic TBI animals (#p < 0.05, Fig. 8C). A2M (Fig. 8D) showed much higher expression at 24 h in the normothermic and hyperthermic group over both sham (*p < 0.001). TBI hyperthermic was significantly higher than normothermic (#p < 0.05) for A2M at 24 h as well. TNF-α and ICAM-1 both had transient increases over sham levels at 4 h that did not persist at 24 h (Fig. 8B,E; *p < 0.001). These variable gene responses to indirect temperature manipulations are consistent with previous findings showing that cytokine responses vary depending on factors including time after injury and specific characteristics of the cytokine.67
FIG. 8.
RT-PCR. Real-time quantitative RT-PCR was done on 4 h and 24 h animals with sham (white), normothermia traumatic brain injury (TBI; black), and hyperthermic TBI (red); n = 5 per group. There were significant increases in both TBI groups compared with sham in all genes tested. (A) IL1-β: #p < 0.05; (B) TNF-α: *p < 0.001; (C) Ccl2: *p < 0.001; (D) A2M: *p < 0.001; (E) ICAM-1: *p < 0.001. TBI plus hyperthermia significantly (#p < 0.05) increased expression of Ccl2 and A2M over TBI normothermia at 24 h.One-way analysis of variance (ANOVA) with Student-Newman-Keuls post hoc.
Discussion
mTBI or concussion is a worldwide problem that may produce acute as well as long-term consequences.11 Recent studies have emphasized that sport-related concussion is a significant public health concern with the rate of diagnosed concussions increasing in college athletes.68,69 Although most concussions lead to only transient periods of behavioral abnormalities, a significant percentage of patients after a single or repetitive insult are left with more long-term problems associated with cognitive function and post-concussive syndromes such as post-traumatic stress disorder.9,70,71 The continued investigation into what specific factors contribute to the long-term functional consequences of concussion and underlying pathomechanisms is therefore an area of active investigation. In the present study we report for the first time that a restricted period of induced mild hyperthermia at the time of a mTBI significantly augments the inflammatory and microvascular consequences of the primary insult while polarizing microglial/macrophage responses to a pro-inflammatory state. Hyperthermic mTBI also significantly altered patterns of microglial and macrophage activation that included structural and biochemical signatures. In addition, gene and protein analysis showed increased levels of pro-inflammatory mediators including ICAM-1, CD-18, and Ccl2 that are key regulators of vascular endothelial-leukocyte interactions including adhesion and infiltration after TBI.64,72,73 Together these studies emphasize the importance of brain temperature at the time of mTBI on altering cellular and molecular events associated with augmentation of post-traumatic inflammatory cascades involving both resident and circulating inflammatory cells.
Recent studies have shown that inflammatory activation after different types of brain injury can have both detrimental and reparative consequences.51,53,56,74,75 In most TBI studies, trauma has been reported to lead to polarization of microglia/microphages to a pro-inflammatory state compared with sham operated animals involving M1 phenotype expression patterns.76–78 The realization that environmental or targeted treatment strategies may alter this polarization pattern to a more reparative M2 state emphasizes that the divergent states of inflammatory activation after injury may represent an important therapeutic target for specific treatment modalities.52,57,79,80 In this regard, recent studies have reported that post-traumatic therapeutic hypothermia following trauma or cerebral ischemia can promote M2 polarization of inflammatory cell responses compared with normothermic injury.60,81 In the current study we now report that hyperthermic mTBI produces the opposite effect in terms of aggravating inflammatory and microvascular responses including enhancing M1 polarization phenotype expression.
In addition to flow cytometry data indicating phenotype expression patterns with elevations in temperature, we also evaluated other cellular and molecular responses to mTBI that were sensitive to this temperature modification. We found that with hyperthermia, morphological patterns of microglia activation as indicated by Iba1 immunostaining were more robust than normothermic mTBI. Recent studies have reported different patterns of microglia activation in models of mild and diffuse TBI using small and large animal models.15,51,54,56 Because microglia are an important source of pro-inflammatory cytokines and chemokines, this resident brain cell may represent an important target for therapeutic strategies to limit the detrimental consequences of mTBI or concussion.52,54,62,82–84 For example, recent findings have emphasized the potentially significant effects of microglial activation and infiltrating monocytes/macrophages on cognition, learning, and behavior.72,85,86 Evidence for activated microglial cells altering neural networks by phagocytosis or the synthesis and release of various factors has also been reported.83 It is therefore intriguing to speculate that augmented microvascular dysfunction and microglia/macrophage polarization to a more M1 phenotype with hyperthermic mTBI may participate in the emergence of post-concussion syndrome as previously described.41
White matter pathology is commonly reported after moderate and severe TBI.78,87–90 Damage to white matter tracts can lead to a variety of consequences including circuit dysfunction, wallerian degeneration, and altered cortical and subcortical communication. Previous studies have reported that the degree of axonal pathology is sensitive to temperature manipulations after moderate or severe injuries.63,91 In animal models and clinical studies of mTBI and concussion, evidence for axonal damage including oligodendrocyte cell death and myelin disruption are also reported in some but not all cases.30,88–90,92–94 In the current study we used β-APP as an indicator of white matter axonal pathology following normothermic and hyperthermic mTBI. We showed that with hyperthermia, there is a non-significant (p = 0.06) increase in the frequency of β-APP immunoreactive axonal profiles in the most vulnerable region of the external capsule. These studies emphasize that in addition to the inflammatory consequences reported predominantly in gray matter regions, white matter pathology commonly associated with diffuse brain injury may also be augmented with this temperature modification.17
Alterations in cerebral vasculature function including altered BBB, vascular reactivity, and cerebral blood flow (CBF) are commonly detected in models of moderate-to-severe TBI.20,50,91,95–97 In models of mTBI or concussion, evidence for altered BBB has also been reported.98–100 In one study that assessed BBB permeability in a model of blast injury, BBB breakdown, oxidative stress, and microglia activation were reported 1 and 3 days after injury.99 In a more conventional model of blunt head injury, Li and colleagues reported increased BBB permeability after mild, controlled, cortical impact injury mainly restricted to the superficial layers of the cerebral cortex.100 Previous studies have emphasized the importance of brain temperature in the vulnerability of the cerebral vasculature to different types of insults and stress.50,101 In the current mTBI study, evidence for enhanced microvascular compromise with hyperthermia included increased expression of ICAM-1 and CD18 signals compared with normothermic mTBI; both participate in the extravasation of leukocytes from the blood.73,102 Also, Ccl2, a key regulator of the brain infiltration by monocytes was increased with hyperthermia compared with normothermic conditions.103 Hyperthermic mTBI may lead to increased synthesis of cytokines and chemokines that participate in secondary injury mechanisms including the accumulation and infiltration of activated CD45-positive monocytes into the brain parenchyma.
Current translational research has concentrated on clarifying potential therapeutic interventions to target the long-term consequences of mTBI or concussion.1,8,52,104–106 At present, cognitive rehabilitation programs and the use of hyperbaric oxygen are utilized to treat post-concussion syndromes.8 A previous study reported that an induced reduction in brain temperature to normothermic levels after hyperthermic mTBI reduced long-term cognitive abnormalities.41 Therefore, targeted temperature management in mTBI or concussion may be a strategy for improving long-term outcomes. In this regard, non-invasive approaches are being developed and tested to acutely normalize elevated levels of brain temperature to enhance outcome.47 Also, due to the importance of post-traumatic inflammatory processes, anti-inflammatory treatments previously targeted for more severe TBI could be repurposed for patients with concussion.52 In this regard, safe anti-inflammatory treatments including those that have been FDA approved for other indications may be considered for future investigations targeting mTBI.80,82,107
In summary, our current study emphasizes the importance of brain temperature in the pathophysiology of mTBI and concussion. We report that a relatively mild increase in brain temperature at the time of injury that can occur in people during periods of exercise or high activity significantly augments inflammatory and cerebral vascular events that are associated with the emergence of behavioral abnormalities in this experimental paradigm. Hyperthermic mTBI enhanced the polarization of the M1 microglia/macrophage phenotype as compared with normothermic mTBI leading to patterns of increased microglia activation, the accumulation and extravasation of circulating monocytes as well as increased axonal injury within the underlying white matter. Together these studies suggest that monitoring of core temperature in situations of mTBI or concussion may be warranted with targeted temperature management being one strategy for reducing the potential for lingering or long-term consequences.36,42
Acknowledgments
The authors wish to thank Ofelia Furones-Alonso and Juliana Sanchez for performing the animal surgeries and David Sequeira and Yoandy Ferrer for the immunofluorescence experiments. Support: NIH/NINDS R01 NS089443 and R01 NS042133.
WDD and HMB conceived of and designed the research project, analyzed and interpreted the data, and helped write the manuscript. JST conducted the analysis of the flow cytometry, western blot, immunofluorescence, gene arrays, and RT-PCR and analyzed the data and helped write the Methods and Results sections. JST and HMB also worked on the figures and performed statistical analyses. All authors critically reviewed and approved the manuscript.
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.McGinn M.J., and Povlishock J.T. (2016). Pathophysiology of traumatic brain injury. Neurosurg. Clin. North Am. 27, 397–407 [DOI] [PubMed] [Google Scholar]
- 2.Faul M.X.L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 3.Gardner A.J., and Zafonte R. (2016). Neuroepidemiology of traumatic brain injury. Handb. Clin. Neurol. 138, 207–223 [DOI] [PubMed] [Google Scholar]
- 4.McMahon P., Hricik A., Yue J.K., Puccio A.M., Inoue T., Lingsma H.F., Beers S.R., Gordon W.A., Valadka A.B., Manley G.T., Okonkwo D.O., and Investigators T.-T. (2014). Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. J. Neurotrauma 31, 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins M.W., Grindel S.H., Lovell M.R., Dede D.E., Moser D.J., Phalin B.R., Nogle S., Wasik M., Cordry D., Daugherty K.M., Sears S.F., Nicolette G., Indelicato P., and McKeag D.B. (1999). Relationship between concussion and neuropsychological performance in college football players. JAMA 282, 964–970 [DOI] [PubMed] [Google Scholar]
- 6.Riggio S., and Jagoda A. (2016). Concussion and its neurobehavioural sequelae. Intl. Rev. Psychiatry 28, 579–586 [DOI] [PubMed] [Google Scholar]
- 7.Blennow K., Brody D.L., Kochanek P.M., Levin H., McKee A., Ribbers G.M., Yaffe K., and Zetterberg H. (2016). Traumatic brain injuries. Nat. Rev. Dis. Primers 2, 16084. [DOI] [PubMed] [Google Scholar]
- 8.Hadanny A., and Efrati S. (2016). Treatment of persistent post-concussion syndrome due to mild traumatic brain injury: current status and future directions. Expert Rev. Neurothera. 16, 875–887 [DOI] [PubMed] [Google Scholar]
- 9.Balaban C., Hoffer M.E., Szczupak M., Snapp H., Crawford J., Murphy S., Marshall K., Pelusso C., Knowles S., and Kiderman A. (2016). Oculomotor, vestibular, and reaction time tests in mild traumatic brain injury. PLoS One 11, e0162168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkhoudarian G., Hovda D.A., and Giza C.C. (2016). The molecular pathophysiology of concussive brain injury - an update. Phys. Med. Rehabil. Clin. North Am. 27, 373–393 [DOI] [PubMed] [Google Scholar]
- 11.Hiploylee C., Dufort P.A., Davis H.S., Wennberg R.A., Tartaglia M.C., Mikulis D., Hazrati L.N., and Tator C.H. (2017). Longitudinal study of postconcussion syndrome: not everyone recovers. J. Neurotrauma 34, 1511–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oehr L., and Anderson J. (2017). Diffusion-tensor imaging findings and cognitive function following hospitalized mixed-mechanism mild traumatic brain injury: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 98, 2308–2319 [DOI] [PubMed] [Google Scholar]
- 13.Churchill N.W., Hutchison M.G., Richards D., Leung G., Graham S.J., and Schweizer T.A. (2017). The first week after concussion: blood flow, brain function and white matter microstructure. Neuroimage Clin. 14, 480–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naess-Schmidt E.T., Blicher J.U., Eskildsen S.F., Tietze A., Hansen B., Stubbs P.W., Jespersen S., Ostergaard L., and Nielsen J.F. (2017). Microstructural changes in the thalamus after mild traumatic brain injury: a longitudinal diffusion and mean kurtosis tensor MRI study. Brain Inj. 31, 230–236 [DOI] [PubMed] [Google Scholar]
- 15.Lafrenaye A.D., Todani M., Walker S.A., and Povlishock J.T. (2015). Microglia processes associate with diffusely injured axons following mild traumatic brain injury in the micro pig. J. Neuroinflammation 12, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller D.R., Hayes J.P., Lafleche G., Salat D.H., and Verfaellie M. (2016). White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum. Brain Mapp. 37, 220–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L., Nguyen J.V., Lehar M., Menon A., Rha E., Arena J., Ryu J., Marsh-Armstrong N., Marmarou C.R., and Koliatsos V.E. (2016). Repetitive mild traumatic brain injury with impact acceleration in the mouse: multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp. Neurol. 275, Pt. 3, 436–449 [DOI] [PubMed] [Google Scholar]
- 18.Vallez Garcia D., Otte A., Dierckx R.A., and Doorduin J. (2016). Three month follow-up of rat mild traumatic brain injury: a combined [18F]FDG and [11C]PK11195 positron emission study. J. Neurotrauma 33, 1855–1865 [DOI] [PubMed] [Google Scholar]
- 19.Meier T.B., Bellgowan P.S., Singh R., Kuplicki R., Polanski D.W., and Mayer A.R. (2015). Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. 72, 530–538 [DOI] [PubMed] [Google Scholar]
- 20.Stahel P.F., Shohami E., Younis F.M., Kariya K., Otto V.I., Lenzlinger P.M., Grosjean M.B., Eugster H.P., Trentz O., Kossmann T., and Morganti-Kossmann M.C. (2000). Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 20, 369–380 [DOI] [PubMed] [Google Scholar]
- 21.Bonne O., Gilboa A., Louzoun Y., Kempf-Sherf O., Katz M., Fishman Y., Ben-Nahum Z., Krausz Y., Bocher M., Lester H., Chisin R., and Lerer B. (2003). Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Res. 124, 141–152 [DOI] [PubMed] [Google Scholar]
- 22.Long J.A., Watts L.T., Li W., Shen Q., Muir E.R., Huang S., Boggs R.C., Suri A., and Duong T.Q. (2015). The effects of perturbed cerebral blood flow and cerebrovascular reactivity on structural MRI and behavioral readouts in mild traumatic brain injury. J. Cereb. Blood Flow Metab. 35, 1852–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherry J.D., Tripodis Y., Alvarez V.E., Huber B., Kiernan P.T., Daneshvar D.H., Mez J., Montenigro P.H., Solomon T.M., Alosco M.L., Stern R.A., McKee A.C., and Stein T.D. (2016). Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 4, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safinia C., Bershad E.M., Clark H.B., SantaCruz K., Alakbarova N., Suarez J.I., and Divani A.A. (2016). Chronic traumatic encephalopathy in athletes involved with high-impact sports. J. Vasc. Intervent. Neurol. 9, 34–48 [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow K.M., Marcil L.D., Dewey D., Carlson H.L., MacMaster F.P., Brooks B.L., and Lebel R.M. (2017). Cerebral perfusion changes in post-concussion syndrome: a prospective controlled cohort study. J. Neurotrauma 34, 996–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith C., Gentleman S.M., Leclercq P.D., Murray L.S., Griffin W.S., Graham D.I., and Nicoll J.A. (2013). The neuroinflammatory response in humans after traumatic brain injury. Neuropathol. Appl. Neurobiol. 39, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maphis N., Xu G., Kokiko-Cochran O.N., Jiang S., Cardona A., Ransohoff R.M., Lamb B.T., and Bhaskar K. (2015). Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 138, 1738–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Febinger H.Y., Thomasy H.E., Pavlova M.N., Ringgold K.M., Barf P.R., George A.M., Grillo J.N., Bachstetter A.D., Garcia J.A., Cardona A.E., Opp M.R., and Gemma C. (2015). Time-dependent effects of CX3CR1 in a mouse model of mild traumatic brain injury. J. Neuroinflamm. 12, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKee A.C., and Daneshvar D.H. (2015). The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 127, 45–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coughlin J.M., Wang Y., Minn I., Bienko N., Ambinder E.B., Xu X., Peters M.E., Dougherty J.W., Vranesic M., Koo S.M., Ahn H.H., Lee M., Cottrell C., Sair H.I., Sawa A., Munro C.A., Nowinski C.J., Dannals R.F., Lyketsos C.G., Kassiou M., Smith G., Caffo B., Mori S., Guilarte T.R., and Pomper M.G. (2017). Imaging of glial cell activation and white matter integrity in brains of active and recently retired National Football League players. JAMA Neurol. 74, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins-Praino L.E., and Corrigan F. (2017). Does neuroinflammation drive the relationship between tau hyperphosphorylation and dementia development following traumatic brain injury? Brain Behav. Immun. 60, 369–382 [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S., Wu M.D., Shaftel S.S., Kyrkanides S., LaFerla F.M., Olschowka J.A., and O'Banion M.K. (2013). Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J. Neurosci. 33, 5053–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villapol S., Loane D.J., and Burns M.P. (2017). Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 65, 1423–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatzipanteli K., Alonso O.F., Kraydieh S., and Dietrich W.D. (2000). Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J. Cereb. Blood Flow Metab. 20, 531–542 [DOI] [PubMed] [Google Scholar]
- 35.Tomura S., de Rivero Vaccari J.P., Keane R.W., Bramlett H.M., and Dietrich W.D. (2012). Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J. Cereb. Blood Flow Metab. 32, 1939–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietrich W.D., and Bramlett H.M. (2016). Therapeutic hypothermia and targeted temperature management in traumatic brain injury: clinical challenges for successful translation. Brain Res. 1640, 94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yenari M.A., and Han H.S. (2012). Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 13, 267–278 [DOI] [PubMed] [Google Scholar]
- 38.Dietrich W.D., Atkins C.M., and Bramlett H.M. (2009). Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J. Neurotrauma 26, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietrich W.D., and Bramlett H.M. (2007). Hyperthermia and central nervous system injury. Prog. Brain Res. 162, 201–217 [DOI] [PubMed] [Google Scholar]
- 40.Sakurai A., Atkins C.M., Alonso O.F., Bramlett H.M., and Dietrich W.D. (2012). Mild hyperthermia worsens the neuropathological damage associated with mild traumatic brain injury in rats. J. Neurotrauma 29, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Titus D.J., Furones C., Atkins C.M., and Dietrich W.D. (2015). Emergence of cognitive deficits after mild traumatic brain injury due to hyperthermia. Exp. Neurol. 263, 254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochanek P.M., and Jackson T.C. (2015). It might be time to let cooler heads prevail after mild traumatic brain injury or concussion. Exp. Neurol. 267, 13–17 [DOI] [PubMed] [Google Scholar]
- 43.Cadarette B.S., Levine L., Staab J.E., Kolka M.A., Correa M., Whipple M., and Sawka M.N. (2001). Heat strain imposed by toxic agent protective systems. Aviat. Space Environ. Med. 72, 32–37 [PubMed] [Google Scholar]
- 44.Army Medical Surveillance Activity. (1997). Case reports: hyponatremia associated with heat stress and excessive water consumption: Fort Benning, GA; Fort Leonard Wood, MO; Fort Jackson, SC, June - August 1997. Medical Surveillance Monthly Report (MSMR) 3, 2–3, 8 [Google Scholar]
- 45.Nybo L. (2012). Brain temperature and exercise performance. Exp. Physiol. 97, 333–339 [DOI] [PubMed] [Google Scholar]
- 46.Ozgunen K.T., Kurdak S.S., Maughan R.J., Zeren C., Korkmaz S., Yazici Z., Ersoz G., Shirreffs S.M., Binnet M.S., and Dvorak J. (2010). Effect of hot environmental conditions on physical activity patterns and temperature response of football players. Scand. J. Med. Sci. Sports 20, Suppl. 3, 140–147 [DOI] [PubMed] [Google Scholar]
- 47.Goosey-Tolfrey V., Swainson M., Boyd C., Atkinson G., Tolfrey K. (2008). The effectiveness of hand cooling at reducing exercise-induced hyperthermia and improving distance-race performance in wheelchair and able-bodied athletes. J. Appl. Physiol. 105, 37–43 [DOI] [PubMed] [Google Scholar]
- 48.Whalen M.J., Carlos T.M., Clark R.S., Marion D.W., DeKosky S.T., Heineman S., Schiding J.K., Memarzadeh F., and Kochanek P.M. (1997). The effect of brain temperature on acute inflammation after traumatic brain injury in rats. J. Neurotrauma 14, 561–572 [DOI] [PubMed] [Google Scholar]
- 49.Kinoshita K., Chatzipanteli K., Vitarbo E., Truettner J.S., Alonso O.F., and Dietrich W.D. (2002). Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurg. 51, 195–203; discussion 203 [DOI] [PubMed] [Google Scholar]
- 50.Lotocki G., de Rivero Vaccari J.P., Perez E.R., Sanchez-Molano J., Furones-Alonso O., Bramlett H.M., and Dietrich W.D. (2009). Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: effects of post-traumatic hypothermia. J. Neurotrauma 26, 1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loane D.J., and Kumar A. (2016). Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp. Neurol. 275, Pt. 3, 316–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar A., and Loane D.J. (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 26, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 53.Ziebell J.M., and Morganti-Kossmann M.C. (2010). Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loane D.J., Kumar A., Stoica B.A., Cabatbat R., and Faden A.I. (2014). Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 73, 14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., and Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsieh C.L., Kim C.C., Ryba B.E., Niemi E.C., Bando J.K., Locksley R.M., Liu J., Nakamura M.C., and Seaman W.E. (2013). Traumatic brain injury induces macrophage subsets in the brain. Eur. J. Immunol. 43, 2010–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du L., Zhang Y., Chen Y., Zhu J., Yang Y., and Zhang H.L. (2017). Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol. Neurobiol. 54, 7567–7584 [DOI] [PubMed] [Google Scholar]
- 58.Sica A., and Mantovani A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nahrendorf M., and Swirski F.K. (2016). Abandoning M1/M2 for a network model of macrophage function. Circ. Res. 119, 414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Truettner J.S., Bramlett H.M., and Dietrich W.D. (2017). Posttraumatic therapeutic hypothermia alters microglial and macrophage polarization toward a beneficial phenotype. J. Cereb. Blood Flow Metab. 37, 2952–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shohami E., Novikov M., and Horowitz M. (1994). Long term exposure to heat reduces edema formation after closed head injury in the rat. Acta Neurochir. Suppl. (Wien) 60, 443–445 [DOI] [PubMed] [Google Scholar]
- 62.Kettenmann H., Hanisch U.K., Noda M., and Verkhratsky A. (2011). Physiology of microglia. Physiol. Rev. 91, 461–553 [DOI] [PubMed] [Google Scholar]
- 63.Suzuki T., Bramlett H.M., Ruenes G., and Dietrich W.D. (2004). The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J. Neurotrauma 21, 842–853 [DOI] [PubMed] [Google Scholar]
- 64.Szmydynger-Chodobska J., Shan R., Thomasian N., and Chodobski A. (2016). The involvement of pial microvessels in leukocyte invasion after mild traumatic brain injury. PLoS One 11, e0167677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torres-Platas S.G., Comeau S., Rachalski A., Bo G.D., Cruceanu C., Turecki G., Giros B., and Mechawar N. (2014). Morphometric characterization of microglial phenotypes in human cerebral cortex. J. Neuroinflamm. 11, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karperien A., Ahammer H., and Jelinek H.F. (2013). Quantitating the subtleties of microglial morphology with fractal analysis. Front. Cell. Neurosci. 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truettner J.S., Suzuki T., and Dietrich W.D. (2005). The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res. Mol. Brain Res. 138, 124–134 [DOI] [PubMed] [Google Scholar]
- 68.Asken B.M., McCrea M.A., Clugston J.R., Snyder A.R., Houck Z.M., and Bauer R.M. (2016). “Playing Through It”: Delayed reporting and removal from athletic activity after concussion predicts prolonged recovery. J. Athl. Train. 51, 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kilcoyne K.G., Dickens J.F., Svoboda S.J., Owens B.D., Cameron K.L., Sullivan R.T., and Rue J.P. (2014). Reported concussion rates for three Division I football programs: an evaluation of the New NCAA concussion policy. Sports Health 6, 402–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meehan W.P., 3rd, Zhang J., Mannix R., and Whalen M.J. (2012). Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurg. 71, 885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCrory P., Davis G., and Makdissi M. (2012). Second impact syndrome or cerebral swelling after sporting head injury. Curr. Sports Med. Rep. 11, 21–23 [DOI] [PubMed] [Google Scholar]
- 72.Minogue A.M. (2017). Role of infiltrating monocytes/macrophages in acute and chronic neuroinflammation: effects on cognition, learning and affective behaviour. Prog. Neuro-psychopharmacol. Biol. Psychiatry 79, 15–18 [DOI] [PubMed] [Google Scholar]
- 73.Lutton E.M., Razmpour R., Andrews A.M., Cannella L.A., Son Y.J., Shuvaev V.V., Muzykantov V.R., and Ramirez S.H. (2017). Acute administration of catalase targeted to ICAM-1 attenuates neuropathology in experimental traumatic brain injury. Sci. Rep. 7, 3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelley B.J., Lifshitz J., and Povlishock J.T. (2007). Neuroinflammatory responses after experimental diffuse traumatic brain injury. J. Neuropathol. Exp. Neurol. 66, 989–1001 [DOI] [PubMed] [Google Scholar]
- 75.Rathbone A.T., Tharmaradinam S., Jiang S., Rathbone M.P., and Kumbhare D.A. (2015). A review of the neuro- and systemic inflammatory responses in post concussion symptoms: introduction of the “post-inflammatory brain syndrome” PIBS. Brain Behav. Immun. 46, 1–16 [DOI] [PubMed] [Google Scholar]
- 76.Ansari M.A. (2015). Temporal profile of M1 and M2 responses in the hippocampus following early 24h of neurotrauma. J. Neurol. Sci. 357, 41–49 [DOI] [PubMed] [Google Scholar]
- 77.Perez-Polo J.R., Rea H.C., Johnson K.M., Parsley M.A., Unabia G.C., Xu G., Infante S.K., Dewitt D.S., and Hulsebosch C.E. (2013). Inflammatory consequences in a rodent model of mild traumatic brain injury. J. Neurotrauma 30, 727–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang G., Zhang J., Hu X., Zhang L., Mao L., Jiang X., Liou A.K., Leak R.K., Gao Y., and Chen J. (2013). Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 1864–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang B., Bailey W.M., Kopper T.J., Orr M.B., Feola D.J., and Gensel J.C. (2015). Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J. Neuroinflamm. 12, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu H., Wang Z., Li J., Wu H., Peng Y., Fan L., Chen J., Gu C., Yan F., Wang L., and Chen G. (2017). The polarization states of microglia in TBI: a new paradigm for pharmacological intervention. Neural. Plast. 2017, 5405104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee J.H., Wei Z.Z., Cao W., Won S., Gu X., Winter M., Dix T.A., Wei L., and Yu S.P. (2016). Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol. Dis. 96, 248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Polo J.R., Rea H.C., Johnson K.M., Parsley M.A., Unabia G.C., Xu G.Y., Prough D., DeWitt D.S., Paulucci-Holthauzen A.A., Werrbach-Perez K., and Hulsebosch C.E. (2016). Inflammatory cytokine receptor blockade in a rodent model of mild traumatic brain injury. J. Neurosci. Res. 94, 27–38 [DOI] [PubMed] [Google Scholar]
- 83.Kettenmann H., Kirchhoff F., and Verkhratsky A. (2013). Microglia: new roles for the synaptic stripper. Neuron 77, 10–18 [DOI] [PubMed] [Google Scholar]
- 84.Abdel Baki S.G., Schwab B., Haber M., Fenton A.A., and Bergold P.J. (2010). Minocycline synergizes with N-acetylcysteine and improves cognition and memory following traumatic brain injury in rats. PLoS One 5, e12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wohleb E.S., McKim D.B., Sheridan J.F., and Godbout J.P. (2014). Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 8, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hsieh C.L., Niemi E.C., Wang S.H., Lee C.C., Bingham D., Zhang J., Cozen M.L., Charo I., Huang E.J., Liu J., and Nakamura M.C. (2014). CCR2 deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J. Neurotrauma 31, 1677–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Armstrong R.C., Mierzwa A.J., Marion C.M., and Sullivan G.M. (2016). White matter involvement after TBI: clues to axon and myelin repair capacity. Exp. Neurol. 275, Pt. 3, 328–333 [DOI] [PubMed] [Google Scholar]
- 88.Mierzwa A.J., Marion C.M., Sullivan G.M., McDaniel D.P., and Armstrong R.C. (2015). Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J. Neuropathol. Exp. Neurol. 74, 218–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin S.S., Pathak S., Presson N., Bird W., Wagener L., Schneider W., Okonkwo D.O., and Fernandez-Miranda J.C. (2014). Detection of white matter injury in concussion using high-definition fiber tractography. Prog. Neurol. Surg. 28, 86–93 [DOI] [PubMed] [Google Scholar]
- 90.Khong E., Odenwald N., Hashim E., and Cusimano M.D. (2016). Diffusion tensor imaging findings in post-concussion syndrome patients after mild traumatic brain injury: a systematic review. Front. Neurol. 7, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chodobski A., Zink B.J., and Szmydynger-Chodobska J. (2011). Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2, 492–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ilvesmaki T., Luoto T.M., Hakulinen U., Brander A., Ryymin P., Eskola H., Iverson G.L., and Ohman J. (2014). Acute mild traumatic brain injury is not associated with white matter change on diffusion tensor imaging. Brain 137, 1876–1882 [DOI] [PubMed] [Google Scholar]
- 93.Wright A.D., Jarrett M., Vavasour I., Shahinfard E., Kolind S., van Donkelaar P., Taunton J., Li D., and Rauscher A. (2016). Myelin water fraction is transiently reduced after a single mild traumatic brain injury - a prospective cohort study in collegiate hockey players. PLoS One 11, e0150215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dennis E.L., Ellis M.U., Marion S.D., Jin Y., Moran L., Olsen A., Kernan C., Babikian T., Mink R., Babbitt C., Johnson J., Giza C.C., Thompson P.M., and Asarnow R.F. (2015). Callosal function in pediatric traumatic brain injury linked to disrupted white matter integrity. J. Neurosci. 35, 10202–10211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miyauchi T., Wei E.P., and Povlishock J.T. (2013). Therapeutic targeting of the axonal and microvascular change associated with repetitive mild traumatic brain injury. J. Neurotrauma 30, 1664–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dietrich W.D., Alonso O., and Halley M. (1994). Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J. Neurotrauma 11, 289–301 [DOI] [PubMed] [Google Scholar]
- 97.Salehi A., Zhang J.H., and Obenaus A. (2017). Response of the cerebral vasculature following traumatic brain injury. J. Cereb. Blood Flow Metab. 37, 2320–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shetty A.K., Mishra V., Kodali M., and Hattiangady B. (2014). Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Front. Cell. Neurosci. 8, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Readnower R.D., Chavko M., Adeeb S., Conroy M.D., Pauly J.R., McCarron R.M., and Sullivan P.G. (2010). Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 88, 3530–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li W., Watts L., Long J., Zhou W., Shen Q., Jiang Z., Li Y., and Duong T.Q. (2016). Spatiotemporal changes in blood-brain barrier permeability, cerebral blood flow, T2 and diffusion following mild traumatic brain injury. Brain Res. 1646, 53–61 [DOI] [PubMed] [Google Scholar]
- 101.Jiang J.Y., Lyeth B.G., Kapasi M.Z., Jenkins L.W., and Povlishock J.T. (1992). Moderate hypothermia reduces blood-brain barrier disruption following traumatic brain injury in the rat. Acta Neuropathol. 84, 495–500 [DOI] [PubMed] [Google Scholar]
- 102.Lyck R., and Enzmann G. (2015). The physiological roles of ICAM-1 and ICAM-2 in neutrophil migration into tissues. Curr. Opin. Hematol. 22, 53–59 [DOI] [PubMed] [Google Scholar]
- 103.Semple B.D., Bye N., Rancan M., Ziebell J.M., and Morganti-Kossmann M.C. (2010). Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J. Cereb. Blood Flow Metab. 30, 769–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miyauchi T., Wei E.P., and Povlishock J.T. (2014). Evidence for the therapeutic efficacy of either mild hypothermia or oxygen radical scavengers after repetitive mild traumatic brain injury. J. Neurotrauma 31, 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hanlon L.A., Raghupathi R., and Huh J.W. (2017). Differential effects of minocycline on microglial activation and neurodegeneration following closed head injury in the neonate rat. Exp. Neurol. 290, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collins M.W., Kontos A.P., Okonkwo D.O., Almquist J., Bailes J., Barisa M., Bazarian J., Bloom O.J., Brody D.L., Cantu R., Cardenas J., Clugston J., Cohen R., Echemendia R., Elbin R.J., Ellenbogen R., Fonseca J., Gioia G., Guskiewicz K., Heyer R., Hotz G., Iverson G.L., Jordan B., Manley G., Maroon J., McAllister T., McCrea M., Mucha A., Pieroth E., Podell K., Pombo M., Shetty T., Sills A., Solomon G., Thomas D.G., Valovich McLeod T.C., Yates T., and Zafonte R. (2016). Statements of agreement from the Targeted Evaluation and Active Management (TEAM) Approaches to Treating Concussion Meeting held in Pittsburgh, October 15–16, 2015. Neurosurg. 79, 912–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.d'Avila J.C., Lam T.I., Bingham D., Shi J., Won S.J., Kauppinen T.M., Massa S., Liu J., and Swanson R.A. (2012). Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J. Neuroinflamm. 9, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]