Abstract
Traumatic brain injury (TBI) impairs autoregulation of cerebral blood flow, which contributes to the development of secondary brain injury, increasing mortality of patients. Impairment of pressure-induced myogenic constriction of cerebral arteries plays a critical role in autoregulatory dysfunction; however, the underlying cellular and molecular mechanisms are not well understood. To determine the role of mitochondria-derived H2O2 and large-conductance calcium-activated potassium channels (BKCa) in myogenic autoregulatory dysfunction, middle cerebral arteries (MCAs) were isolated from rats with severe weight drop–impact acceleration brain injury. We found that 24 h post-TBI MCAs exhibited impaired myogenic constriction, which was restored by treatment with a mitochondria-targeted antioxidant (mitoTEMPO), by scavenging of H2O2 (polyethylene glycol [PEG]-catalase) and by blocking both BKCa channels (paxilline) and transient receptor potential cation channel subfamily V member 4 (TRPV4) channels (HC 067047). Further, exogenous administration of H2O2 elicited significant dilation of MCAs, which was inhibited by blocking either BKCa or TRPV4 channels. Vasodilation induced by the TRPV4 agonist GSK1016790A was inhibited by paxilline. In cultured vascular smooth muscle cells H2O2 activated BKCa currents, which were inhibited by blockade of TRPV4 channels. Collectively, our results suggest that after TBI, excessive mitochondria-derived H2O2 activates BKCa channels via a TRPV4-dependent pathway in the vascular smooth muscle cells, which impairs pressure-induced constriction of cerebral arteries. Future studies should elucidate the therapeutic potential of pharmacological targeting of this pathway in TBI, to restore autoregulatory function in order to prevent secondary brain damage and decrease mortality.
Keywords: : autoregulation, intracranial hypertension, oxidative stress, secondary injury
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability.1 TBI affects ∼1,700,000 patients in the United States1–3 and 2,500,000 patients in the European Union,3 with a mortality rate of 35–40%. Approximately 5,300,000 people live with TBI-related disabilities in the United States,2 as do 7,700,000 million in the European Union.3 Major causes of TBI include falls, vehicle accidents, and violence. In addition to the brain trauma caused by a focal impact to the head, blast waves or a sudden acceleration/deceleration within the cranium at the moment of injury can cause secondary damage, which develops through multiple parallel pathological processes. These processes include dysregulation of cerebral blood flow (CBF), which promotes development of cerebral edema and intracranial hypertension (ICH). The resulting secondary damage exacerbates the damage from the initial injury, and thus determines the outcome of trauma.4 Because secondary brain damage is potentially preventable, better understanding of the underlying mechanisms will identify important targets for therapeutic interventions for prevention.
There is growing experimental and clinical evidence that TBI impairs autoregulation of CBF,5–17 in which impairment of pressure-induced myogenic constriction of cerebral resistance vessels plays a significant role.18–21 On the one hand, TBI-induced myogenic autoregulatory dysfunction of cerebral vessels results in ischemia with relatively small reductions in systemic blood pressure. On the other hand, with modest increases in blood pressure, it permits marked increases in CBF and penetration of high pressure to the vulnerable distal portion of the cerebral microcirculation. Thus, TBI-induced myogenic autoregulatory dysfunction exacerbates ischemic brain damage, contributes to vascular congestion and intracranial hypertension, and promotes blood–brain barrier disruption, vasogenic edema, and cerebromicrovascular injury. (For a recent review, see the study by Toth and colleagues.22) Despite its pathophysiological importance,5–8,21,23 the cellular and molecular mechanisms underlying myogenic autoregulatory dysfunction of cerebral arteries following TBI are not well understood. Early studies suggested that TBI-related oxidative stress exerts vasodilator effects in pial vessels following trauma24; however, the source of increased reactive oxygen species (ROS) and the mechanisms by which ROS contribute to TBI-induced myogenic autoregulatory dysfunction of cerebral arteries remained obscure.
The present study was designed to test the hypothesis that following brain trauma, myogenic response of cerebral arteries is compromised because of the excessive mitochondrial production of vasodilator H2O2 in the vascular smooth muscle cells (VSMCs). To test our hypothesis, we induced diffuse brain trauma in rats by the impact acceleration technique, and compared vascular H2O2 production and pressure-induced myogenic responses of isolated middle cerebral arteries (MCAs) in the presence and absence of scavengers of mitochondria-derived H2O2. We also aimed to elucidate the downstream targets of increased H2O2. Specifically, we tested the hypothesis, developed based on previous findings,25–29 that after TBI, excessive mitochondria-derived H2O2 activates large conductance calcium-activated potassium (BKCa) channels30,31 via a transient receptor potential cation channel subfamily V member 4 (TRPV4)-dependent pathway in the VSMCs, which impairs pressure-induced constriction of cerebral arteries. To achieve this goal, we assessed the effects of specific blockers of TRPV4 and BKCa channels on TBI-induced myogenic autoregulatory dysfunction of cerebral arteries, determined the role of these channels in vasomotor responses elicited by exogenous H2O2, and used a patch clamp to characterize the effects of H2O2 and TRPV4 inhibitors on BKCa ion currents.
Methods
TBI in rats (constrained impact acceleration)
All procedures were approved by the Institutional Animal Use and Care Committee of the University of Pecs Medical School and the National Scientific Ethical Committee on Animal Experimentation, Budapest Hungary in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Wistar–Kyoto (WKY) rats (male, 300–350 g) were purchased from Charles River Laboratories (Wilmington, MA) and were used for all experiments. Severe impact acceleration diffuse brain injury was induced by Marmarou's well-characterized weight drop model.32 In brief, with the animals under isoflurane (2%) anesthesia, the skull was exposed by a midline incision between lambda and bregma, and a steel disc was fixed with dental acrylic on the skull. A 450 g cylindrical weight was dropped from 1.5 m onto the disc, causing severe diffuse TBI to the animals. Mortality rate was ∼15%. MCAs were isolated from animals who survived at 2 and 24 h after trauma for further studies.
Pressure-induced responses of isolated MCAs and pharmacological studies
We assessed myogenic responses of isolated MCAs to stepwise increases of intraluminal pressure using pressure myography in control rats and in rats 2 (n = 5) and 24 h (n = 5) after severe TBI, based on studies by Golding and colleagues18 in a controlled cortical impact model. In brief, rats were anesthetized and decapitated, and the brains were removed. MCA segments were isolated using microsurgical instruments under an operating microscope, as previously described.33,34 The MCA segments were transferred into an organ chamber filled with oxygenated physiological Krebs' solution (21% O2, 5% CO2, 75% N2; 37°C), and mounted onto two glass micropipettes and pressurized to 80 mm Hg. The hydrodynamic resistance of the micropipettes was matched, and the inflow and outflow pressures were controlled and measured by a pressure servo-control system (Living Systems Instrumentation, Burlington, VT). Inner vascular diameter was assessed with a custom-built videomicroscope system and continuously recorded using a computerized data acquisition system, as reported.15,16 All vessels were allowed to stabilize their pressure-induced tone for 60 min. Myogenic responses were obtained by assessing changes in vascular diameter in response to stepwise increases (20 mm Hg steps, for 5 min each) in intraluminal pressure (from 0 to 140 mm Hg). In order to scavenge mitochondrial ROS we administered the mitochondrial antioxidant mitoTEMPO (3 × 10−8 mol/L, n = 5) into the vessel chamber, and reassessed myogenic responses after 30 min.
In a different series of experiments, the decomposition of vascular hydrogen peroxide (H2O2) into water and oxygen was catalyzed by the administration of polyethylene glycol (PEG)-catalase (CAT) (120 U/mL, n = 5) onto the vessels for 30 min, and myogenic responses of MCAs were repeated. To test the role BKCa and TRPV4 channels in the attenuated myogenic constriction of MCAs after TBI, pressure-induced responses of MCAs were obtained in the presence of paxilline (a specific blocker of BKCa channels, 10−6 mol/L for 20 min, n = 5) and the specific TRPV4 blocker HC 067047 (0.5 × 10−6 mol/L for 20 min, n = 5). To test whether H2O2 activates BKCa and TRPV4 channels, we induced dose-dependent dilation of MCAs in response to H2O2 in the presence of paxilline (Pax) and HC 067047. To examine whether TRPV4 channels initiate dilation of MCAs through BKCa, we obtained dose-dependent dilation of vessels by the TRPV4-agonist GSK1016790A in the absence and presence of Pax. All drugs were purchased from SigmaAldrich Hungary (Budapest, Hungary). At the end of each experiment, the pressure-passive diameter curves were obtained in maximally dilated MCAs in the presence of nifedipine. Diameter responses at each pressure step are shown as the percentage of the corresponding passive diameter value at 80 mm Hg. In a separate series of experiments, basilar arteries (BA) were isolated from the same control and TBI rats from which the MCAs were used, and constrictor responses to the thromboxane analogue U46619 were determined in a wire myograph (Danish Myo Technology, Aarhus, Denmark), as previously described.35 In brief, rings were cut out of the BA and were mounted on 40 μm stainless steel wires in myograph chambers for measurement of isometric tension. The vessels were superfused with Krebs buffer solution (118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose; at 37°C; gassed with 95% air and 5% CO2). Optimal passive tension (as determined from the vascular length–tension relationship) was applied for 1 h (equilibration period) and then contraction to U46619 (from 10−8 to 10−5 mol/L, n = 5) was obtained. Contraction is expressed as the percent of the maximally relaxed rings in the presence of nimodipine.
Measurement of vascular H2O2 production: CM-H2DCFDA staining and confocal microscopy
In order to detect cerebrovascular H2O2 production after TBI, we used the cell-permeant oxidative fluorescent indicator dye CM-H2DCFDA (5 [and 6]- chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester, SigmaAldrich Hungary, Budapest, Hungary) and confocal microscopy, as previously reported. In brief, MCAs of control rats and rats 24 h after TBI were freshly isolated with microsurgical instruments, placed in wells filled with oxygenated Krebs' buffer and incubated at 37°C with CM-H2DCFDA (5 μM, at 37°C for 10 min).36 Then, the vessel samples were washed five times in warm oxygenated Krebs' buffer, and placed on slides covered by glass covers. A laser scanning confocal microscope (Olympus Fluoview FV1000) was used to visualize CM-H2DCFDA fluorescence of the vessels. Fluorescence intensity is expressed as fold change compared with control vessels. In a different series of experiments, we repeated the above protocols in the presence of CAT (120 U/mL for 20 min, n = 5 for each group).
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR)
A quantitative real-time RT-PCR technique was used to analyze the mRNA expression of KCNMA1 and KCNMB1 (α and β subunits of BKCa channels, respectively) and TRPV4 in isolated cerebral arteries of control and TBI rats (n = 5 in each group). Briefly, total RNA was isolated with the Pure Link™ RNA Mini Kit (Life Sciences, Carlsbad CA). Vascular samples were homogenized, and RNA was purified by ethanol treatment and eluted from the membrane. The total amount of RNA was determined by using NanoDrop (Thermo Scientific, Waltham MA). High Capacity cDNA kit was applied (Applied Biosystems, Foster City CA) to perform cDNA synthesis. For gene expression analysis, quantitative RT-PCR (qRT-PCR) was performed using SensiFast SYBR Green reagent (BioLine, Luckenwalde, Germany). Amplifications were run on ABI StepOnePlus system (Applied Biosystems, Foster City CA). StepOne software was used to analyze gene expressions, which was normalized to peptidylprolyl isomerase A (PPiA) as a reference gene. The primer sequences are shown in Table 1. The amplification of PCR products was calculated according to the 2-ΔΔCtmethod.37
Table 1.
| Primers | Forward | Reverse |
|---|---|---|
| PPiA | GCAGACAAAGTTCCAAAGACAG | CCATTATGGCGTGTGAAGTC |
| KCNMA1 | CTTGCGGTTTATTGCAGCCA | ACAGACACAAACACGGGAGG |
| KCNMB1 | GTGGAGAGAAACCATCTGCCA | CCATCACCAGCTTCTTCCCC |
| TRPV4 | AAGCCGATATGAGGCGACAG | TGGTGTTCTCTCGGGTGTTG |
Isolation of cerebral VSMCs
Experiments were performed on WKY rats housed in the Animal Care Facility at the University of Mississippi Medical Center (UMMC), which is approved by the American Association for the Accreditation of Laboratory Animal Care. They had free access to food and water throughout the study. All protocols were approved by the Animal Care and Use Committee of UMMC.
The rats were euthanized using 4% isoflurane. MCAs were microdissected and digested in a low calcium dissociation solution containing (in mM): 145 NaCl, 4 KCl, 1 MgCl2, 10 HEPES, 0.05 CaCl2 and 10 glucose (pH 7.4). Vessels were cut into small pieces, spun down at 1000 rpm for 1 min, and incubated in the dissociation solution containing papain (50 U or 2 mg/mL; Sigma, St. Louis, MO) and dithiothreitol (2 mg/mL) for 10–15 min 37°C in a water bath. The partially digested vessels were spun down at 1000 rpm for 1 min, and the pellet was washed and resuspended in fresh dissociation solution containing albumin (1 mg/mL) collagenase (250 units/mL or 2 mg/mL), and incubated for 10–15 min at 37°C in a water bath. VSMCs were released into the media by gentle pipetting of the digested tissue. The supernatant was collected, and the cells were pelleted by centrifugation at 1000 rpm for 1 min. The cells were resuspended in a low Ca2+ dissociation solution and maintained at 4°C. Patch-clamp experiments were completed within 4 h after isolation of the cells (two to three smooth muscle cells from four rats were studied in each group [8–12 cells/group]).
Whole cell patch clamp on VSMCs
BK channel currents were recorded from VSMCs using a whole cell patch-clamp mode at room temperature (22–23°C). The bath solution contained (in mM): 130 NaCl, 5 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.4). The pipettes were filled with a solution containing: 130 K gluconate, 30 KCl, 10 NaCl, 1 MgCl2, and 10 HEPES (pH 7.2). The concentrations of EGTA and Ca2+ in the pipette solution were adjusted to obtain cytosolic free Ca2+ concentrations of 100 nM as determined using WinMAXC software written by C. Patton (Stanford University Pacific Grove, CA). The pipettes were pulled from 1.5 mm borosilicate glass capillaries using a two stage micropipette puller (model P-97; Sutter Instrument, San Rafael, CA) and heat polished using a microforge. The pipettes had tip resistances of 2–8 MΩ. After the tip of a pipette was positioned on a cell, a 5–20 GΩ seal was formed, and the membrane was ruptured by gentle suction using a glass syringe. An Axopatch 200B amplifier (Axon Instruments, Foster City, CA) was used to clamp the pipette potential and to record whole cell currents. Outward membrane K+ currents were elicited by a series of 20 mV voltage steps (from −60 to +120 mV) from a holding potential of −40 mV. The amplifier output signal was filtered at 2 kHz using an eight-pole Bessel filter. The currents were acquired using p-CLAMP software (version 10, Axon Instruments) at a rate of 10 kHz, and stored on the hard disk of a computer for off-line analysis. Data analysis was performed using Clampfit software (version 10.0, Axon Instruments). Peak current amplitudes were determined from the average of 5–10 trials. Membrane capacitance (in pF) was determined from the average of 30 currents measured in response to a 5 mV pulse. Peak currents (in pA) were expressed as current density (pA/pF) to normalize for differences in the size of the VSMCs. H2O2 (10μM), Pax 100 nM), and 4-aminopyridine (4-AP) (1 mM) were applied to the bath solution to activate and inhibit BK and TRPV4 channels, respectively.
Statistical analysis
Data were analyzed by Student's t test for paired observations or ANOVA followed by Tukey's posth-oc test for multiple comparisons, as appropriate. A p value <0.05 was considered statistically significant. Data are expressed as mean ± S.E.M.
Results
Role of mitochondria-derived H2O2 in impaired myogenic constriction of cerebral arteries after TBI
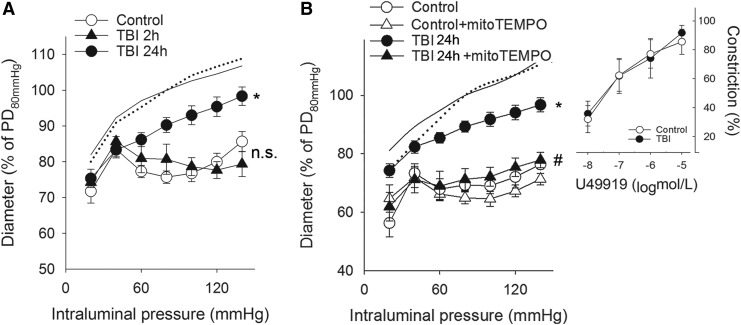
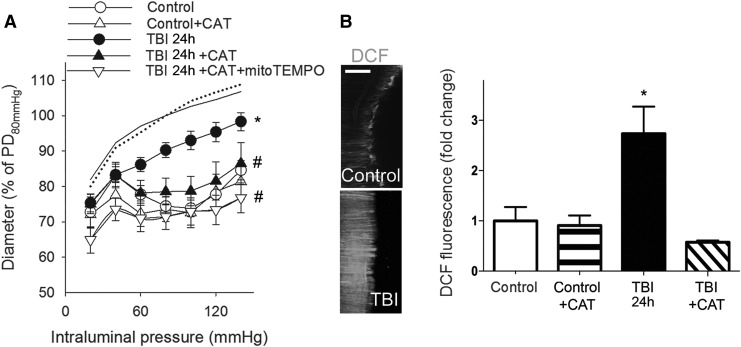
We found that myogenic constrictions of MCAs isolated from rats 2 h after TBI were intact, whereas myogenic responses of MCAs 24 h after TBI were significantly decreased compared with control MCAs in the autoregulated pressure range (between 60 and 140 mm Hg) (Fig. 1A, B) These results confirm the findings of Golding and colleagues18 (obtained in a controlled cortical impact model) for the first time after impact acceleration diffuse brain injury. We continued our studies with MCAs isolated from rat brains 24 h after TBI. The thromboxane A2 analog U46619 agonist-induced constrictions of basilar arteries from the same animals were intact after TBI and did not differ from control responses (Fig. 1B inlet). We demonstrate here that administration of the mitochondrial antioxidant mitoTEMPO restored myogenic constriction of MCAs of TBI rats to the level of control MCAs (p < 0.05 vs. TBI) suggesting a key role of mitochondria-derived ROS in the TBI-induced impairment of cerebral myogenic responses (Fig 1B). Our results that administration of CAT restores TBI-induced impaired myogenic responses of MCAs, as well (p < 0.05 vs. TBI), and co-administration of mitoTEMPO did not have any additional effects, suggest that mitochondria-derived H2O2 is the primary factor that attenuates myogenic constriction of MCAs after TBI (Fig. 2A). This is supported by our further findings that TBI enhances cerebrovascular H2O2 production significantly (p < 0.05), as shown by the CAT-sensitive increased CM-H2DCFDA fluorescence in isolated MCAs (Fig 2B) in TBI vessels compared with controls.
FIG. 1.
Traumatic brain injury (TBI) impairs myogenic constriction of cerebral arteries: role of mitochondrial reactive oxygen species (ROS) production. (A) Diameter responses (as percent of passive diameter [PD] at 80 mm Hg intraluminal pressure) of isolated middle cerebral arteries (MCA) are shown as a function of intraluminal pressure (myogenic response) in control rats and in rats 2 (TBI 2 h) and 24 (TBI 24 h) h after severe TBI. Note that the pressure-induced constrictor response is intact 2 h after the impact, and it is significantly attenuated 24 h post-injury. Data are mean ± S.E.M. (n = 5 for each group) *p < 0.05 versus control (lines without symbols show passive pressure-diameter curves of MCAs). (B) Myogenic responses of MCAs are depicted in control and TBI 24 h rats in the absence and presence of the mitochondrial antioxidant mitoTEMPO. Inlet depicts the constriction of basilar arteries of control and TBI 24 h rats in response to the thromboxane analogue U46619. Data are mean ± S.E.M. (n = 5 for each group) *p < 0.05 versus control; #p < 0.05 versus TBI 24 h.
FIG. 2.
Traumatic brain injury (TBI) impairs myogenic constriction of cerebral arteries: role of mitochondrial H2O2. (A) Diameter responses (as percent of passive diameter [PD] at 80 mm Hg intraluminal pressure) of isolated middle cerebral arteries (MCA) are shown as a function of intraluminal pressure (myogenic response) in control and TBI 24 h (24 h after the impact) rats after the administration of catalase (CAT). Note that additional administration of mitoTEMPO does not augment the effect of CAT on the diameter responses. Data are mean ± S.E.M. (n = 5 for each group) *p < 0.05 versus control; #p < 0.05 versus TBI 24 h. (B) Summary data and representative images of cerebrovascular H2O2 production in endothelium-denuded MCAs of control rats, TBI 24 h rats and control and TBI 24 h rats after incubation of the vessels in CAT shown by the fluorescence of the cell-permeant oxidative fluorescent indicator dye DCF (5 [and 6]- chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester). Scale bar is 50 μm. Data are mean ± S.E.M. (n = 5 for each group). *p < 0.05 versus control.
Role of BKCa channels in impaired myogenic constriction of cerebral arteries after TBI
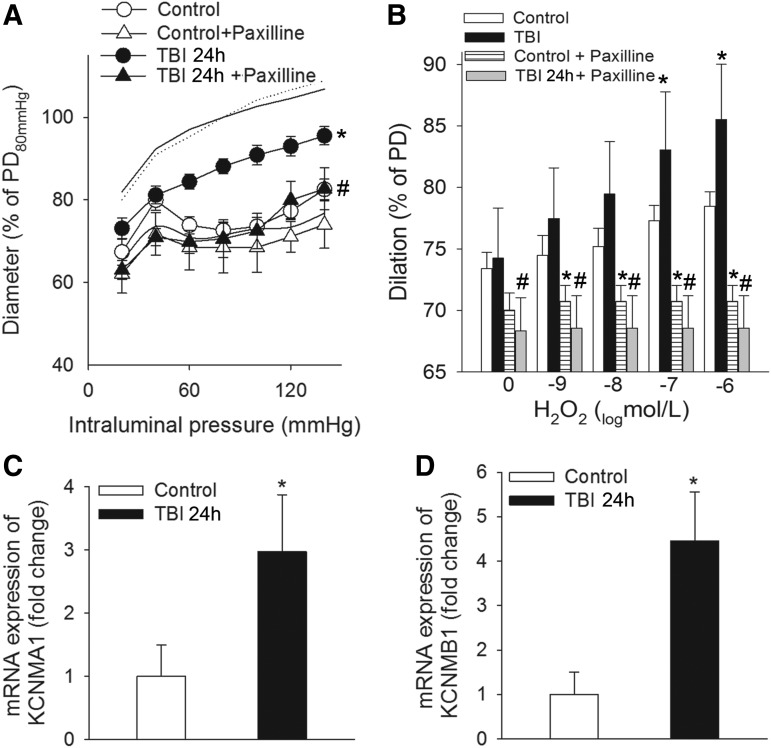
Activation of BKCa channels with the consequent hyperpolarization of vascular smooth muscle cell membrane is a negative feedback regulator of myogenic constriction,26–28 and inhibition of BKCa channel was shown to constrict cerebral arteries after TBI.20 These previous findings are supported by our present results (Fig. 3A) that specific inhibition of BKCa channels on isolated MCAs by Pax restores myogenic constriction of MCAs of TBI rats to the control level. We found that H2O2-induced dilations of MCAs of TBI rats are (1) significantly larger than dilator responses of MCAs from control animals and (2) inhibited by blocking BKCa channels (Fig. 3B). These results (Fig. 3A and B) suggest that TBI-related increased production of cerebrovascular H2O2 (Fig 2B) impairs myogenic constriction of MCAs via activation of BKCa channels. TBI upregulates the cerebrovascular mRNA expression of BKCa channels, which is likely to explain the augmented dilator effect of the channels after brain trauma as well (Fig. 3C and D).
FIG. 3.
Traumatic brain injury (TBI) impairs myogenic constriction of cerebral arteries: role of Ca2+-activated K+ (BK) channels. (A) The effect of paxilline, a specific blocker of calcium-activated potassium (BKCa) channels on pressure-induced myogenic constriction of middle cerebral arteries (MCAs) of control rats and rats 24 h after severe TBI (TBI 24 h). Data are mean ± S.E.M. (n = 5 for each group) *p < 0.05 versus control; #p < 0.05 versus TBI 24 h. (B) Blocking BKCa channels by paxilline inhibits H2O2-induced dose-dependent dilations of MCAs of control and TBI 24 h rats. Note that H2O2-induced dilations are significantly augmented in MCAs isolated from TBI 24 h rats. Data are mean ± S.E.M. (n = 5 for each group); *p < 0.05 versus control, #p < 0.05 versus TBI 24 h. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) data of mRNA expression of the α (panel C) and β (panel D) subunits of BKCa channels (KCNMA1 and KCNMB1, respectively) in MCAs of control and TBI-rats. Data are mean ± S.E.M. (n = 5 for each group) *p < 0.05 versus control.
Role of TRPV4 channel activation in impaired myogenic constriction of cerebral arteries after TBI
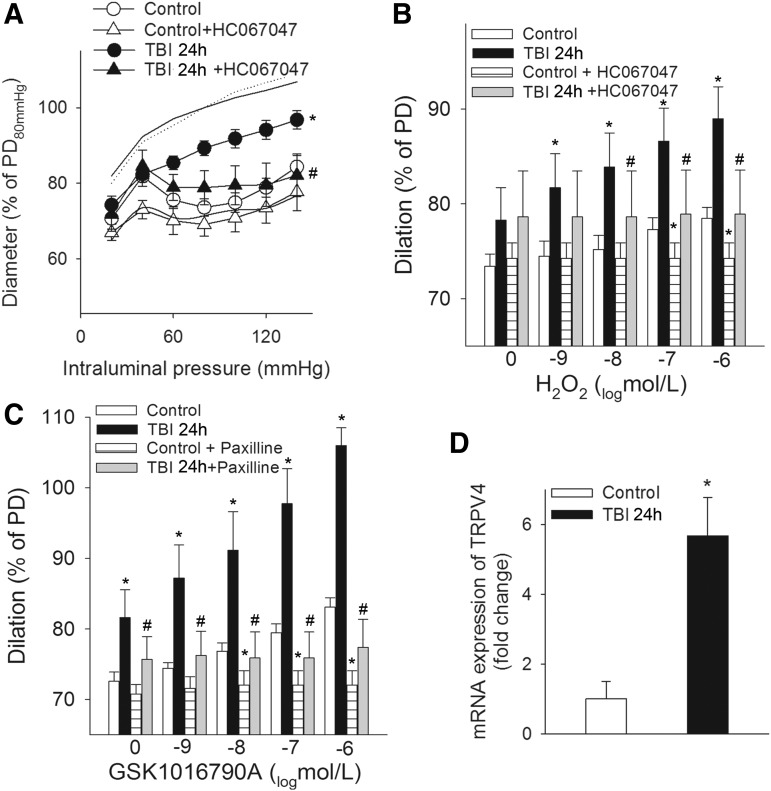
TRPV4 channels have been suggested to be redox sensitive38 and capable of activating BKCa channels.39–42 Therefore, we tested the hypothesis that H2O activates BKCa channels via TRPV4 channels. Here we show for the first time that TBI-induced impaired myogenic response of MCAs is improved and restored to the control level in the presence of HC 067047, a specific blocker of TRPV4 channels (Fig. 4A). Establishing the link between H2O2, TRPV4, and BKCa channels, we demonstrate that H2O2-evoked dilations of MCAs are diminished in the presence of HC 067047 (10−6 mol/L) in both control and TBI MCAs, and that the TRPV4 agonist GSK1016790A-induced dose-dependent dilations of MCAs are (1) are significantly greater in MCAs after TBI than in control vessels and (2) blocked by the specific BKCa channel blocker Pax (Fig. 4 B, C). TBI significantly enhances the cerebrovascular mRNA expression of the TRPV4 gene, which is likely to contribute to the demonstrated effect of TRPV4 channels in the impaired myogenic constriction of MCAs after TBI (Fig. 4D) and explains the attenuated dilator responses to the TRPV4 agonist GSK1016790A.
FIG. 4.
Traumatic brain injury (TBI) impairs myogenic constriction of cerebral arteries: role of transient receptor potential cation channel subfamily V member 4 (TRPV4) channels. (A) Myogenic constriction of middle cerebral arteries (MCAs) of control rats and rats 24 h after TBI (TBI 24 h) in the absence and presence of the specific TRPV4 channel blocker HC 067047. Data are mean ± S.E.M. (n = 5 for each group) *p < 0.05 versus control; #p < 0.05 versus TBI 24 h. Panel B depicts the effect of the TRPV4 channel blocker HC 067047 on H2O2-induced dilations of MCAs of control and TBI rats, and C shows the effect of blocking BKCa channels on dilations of MCAs evoked by the TRPV4 agonist GSK1016790A in the same groups of animals. Note that both H2O2-induced and GSK1016790A-induced dilations of MCAs are significantly higher after TBI. Data are mean ± S.E.M. (n = 5 for each group) *p < 0.05 versus control; #p < 0.05 versus TBI 24 h. (D) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) data of mRNA expression of TRPV4 channels in MCAs of control and TBI 24h rats. Data are mean ± S.E.M. (n = 5 for each group) *p < 0.05 versus control.
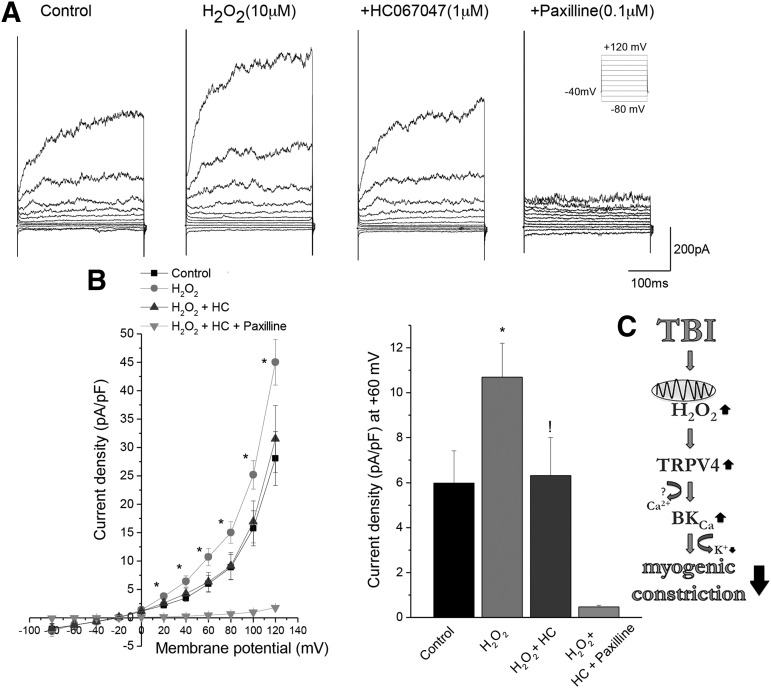
H2O2-induced activation of BKCa channel currents in VSMCs is mediated by TRPV4 channels
We measured BKCa channel currents from VSMCs that are isolated from MCAs of Wistar–Kyoto rats using the patch-clamp method. Supporting our findings in isolated MCAs, we found that H2O2 significantly increased BKCa currents on VSCMs and that inhibition of TRPV4 channels (10−6 mol/L MHC 067047 for 5 min) returned BKCa channel activity to the control level. H2O2 has no effect in the presence of the BKCa channel inhibitor Pax (100nM) (Fig. 5).
FIG. 5.
H2O2-mediated increase in calcium-activated potassium (BKCa) channel activity requires a transient receptor potential cation channel subfamily V member 4 (TRPV4) channel. Whole cell BKCa currents were recorded with 100 nM free cytosolic calcium in the presence and absence of H2O2, TRPV4 channel inhibitor (1 μM HC067047), and BKCa channel inhibitor (paxilline 100 nM). BKCa currents were elicited by 20 ms pulses from −60 to +120 mV from a Vh of −40 mV (Inset). Two to three smooth muscle cells from four Wistar–Kyoto rats were studied in each group (8–12 cells/group). Panel A represents whole cell BKCa currents before and after 10 μM H2O2, in the presence of 1μM HC 067047 and/or 100 nM paxilline. Panel B represents current voltage curves and the current density at +60 mV membrane potential. *p < 0.05 before and after application of H2O2. !p < 0.05 before and after application of 1μM HC 067047 in the presence of H2O2. Data are mean ± S.E.M. Number in the parenthesis is the number of cells studied. (C) Scheme depicting the mechanisms of impaired myogenic constriction of cerebral arteries after TBI. TBI leads to excessive cerebrovascular production of H2O2 mainly of mitochondrial origin, which activates TRPV4 on vascular smooth muscle cells. TRPV4 then activates BKCa channels leading to hyperpolarization of vascular smooth muscle cell (VSMC) membranes and subsequent dilation of cerebral vessels, which attenuates pressure-induced myogenic constriction.
Discussion
TBI is a major health problem worldwide because of its high mortality and the life-long remaining disabilities in the survivors.1–3 After the primary impact and injury of cerebral tissue, TBI initiates a variety of pathophysiological processes leading to secondary injury.43,44 Secondary brain injury and, therefore, outcome of severe TBI is determined by the formation of cytotoxic edema of neuroglial tissue and the consequent development of intracranial hypertension. Pressure-induced myogenic constriction of cerebral resistance vessels (arteries and arterioles) adjusts cerebrovascular resistance (CVR) to increases in perfusion pressure, thus playing a central role in maintaining approximately constant blood flow in the brain despite variations in blood pressure (autoregulation of CBF).18–21
Here, we demonstrate that myogenic constriction of cerebral arteries is intact 2 h after the impact, but compromised 24 h after trauma in the constrained impact acceleration model of TBI (Fig. 1), extending earlier findings in different models of TBI (Golding and colleagues used the controlled cortical impact model, and Villalba and colleagues studied the fluid percussion injury model).18,20 Our results and the findings of the mentioned studies suggest that impairment of myogenic mechanisms is a consequent result of TBI regardless of animal models used, and that it develops subacutely after the impact, most likely being involved in the development of secondary injury of cerebral tissue. The consequences of TBI-induced impairment of myogenic constriction are likely multifaceted. First, it is likely to contribute to increased blood volume in the closed cranium. Second, when blood pressure increases, lack of myogenic protection likely allows high pressure to penetrate the cerebral microcirculation promoting blood–brain barrier disruption and microvascular injury, which exacerbate vasogenic edema. Both increased cerebral blood volume (CBV) and vasogenic edema contributes to a rise in intracranial pressure (ICP), especially when intracranial compliance (to compensate increases in ICP) is attenuated by cytotoxic edema.12,13,22,45,46
This is the first study to demonstrate that mitochondrial ROS production plays a central role in impaired myogenic constriction of cerebral arteries after diffuse TBI (Figs. 1–2). Our studies provide direct evidence that following TBI, the production of ROS is increased in the vascular smooth muscle cells, extending previous findings.24,47 The mechanisms by which TBI promotes mitochondrial oxidative stress in smooth muscle cells may involve changes in the hemodynamic environment/mechanosensitive mtROS production,48,49 factors released from the damaged brain parenchyma (including glutamate neurotoxicity),50–52 and/or humoral factors.53,54 These possibilities should be tested in future studies. Complexes I and III of the electron transport chain are possible major sites of premature electron leakage to oxygen, generating superoxide in the mitochondria.55,56 Future studies using specific inhibitors should elucidate how ROS generation is affected by TBI at these sites in the smooth muscle mitochondria.
Mitochondrial superoxide is readily dismutated to H2O2 by manganese superoxide dismutase (MnSOD), which is abundantly expressed in VSMCs.24,57–60 Whereas superoxide is not membrane permeable, H2O2 can readily penetrate the mitochondrial membranes, increasing cytosolic H2O2 levels. Importantly, H2O2 is a potent vasodilator in the cerebral circulation.25 Therefore, it is significant that H2O2 levels are substantially increased in VSMCs of cerebral arteries after TBI (Fig. 2). The findings that administration of CAT restores myogenic responses of MCAs derived from rats with TBI provide experimental evidence that increased mitochondria-derived H2O2 production plays the key role in dysregulation of arterial myogenic constriction after diffuse brain trauma. Recent studies raise the possibility that activation of nitric oxide synthesis may also contribute to the decreased myogenic constriction after TBI.20 As there are data showing that crosstalk exists between NO and mitochondria-derived H2O2 production,61,62 the possibility that such interaction is also present after TBI and the role of TBI-related endothelial impairment in the decreased myogenic tone should be also tested in future studies.
The mechanisms by which H2O2 induced vasodilation in the cerebral circulation likely involve activation of large conductance BKCa channels.30,31 In support of this concept, we demonstrate that selective blockade of BKCa channels restored myogenic constriction of MCAs derived from rats with TBI, and that H2O2-induced dilations of MCAs were inhibited in the presence of the BKCa channel blocker Pax (Fig. 3). Further, H2O2 induced a significant increase of BK channel currents on vascular smooth muscle cells (Fig. 5). There is strong evidence that activation of BKCa channels readily hyperpolarizes smooth muscle cells, which inhibits pressure-induced activation of voltage-sensitive calcium channels and thereby myogenic constriction of cerebral arteries.26–28 It has to be noted here that Armstead and colleagues demonstrated that TBI impairs the function/activation of BKCa channels, which mechanism might be involved in the processes that lead to decreased dilation (thus cerebral hypoperfusion) in response to hypotension after brain trauma.63,64 Although these results cannot be directly compared with our present studies because the authors used an in vivo approach to measure dilation of pial arterioles to hypotension in newborn piglets, location- and vessel-dependent changes in function/activation/expression of BKCa channels after TBI should be established by future studies.
Previous studies reported that the mechanisms by which H2O2 activates BKCa channels in different cell types are multifaceted, and may involve the synthesis of eicosanoid mediators,65 the protein kinase G pathway,66 and/or protein kinase C.67 Importantly, the activity of BKCa is regulated by Ca2+-sparks, the frequency/amplitude of which can also be modulated by H2O2.68 TRPV4 are mechanosensitive, nonselective cation channels, which regulate Ca2+-sparks in vascular smooth muscle cells,39 and there are data extant linking activation of TRPV4 channels to regulation of vasomotor tone.29 Our findings demonstrate that selective blocking of TRPV4 channels inhibits H2O2-induced vasodilation (Fig. 4) and restores myogenic responses of cerebral arteries isolated from rats with TBI (Fig. 4). Further, dilations of cerebral arteries evoked by a TRPV4 agonist are abolished by a BKCa channel blocker (Fig. 4). These results support the concept that in TBI, increased H2O2 levels activate BKCa channels via a pathway that involves activation of TRPV4 channels in the smooth muscle cells. Direct experimental support for this concept is offered by our findings that H2O2-induced increases in BKCa currents in VSMCs are diminished by the TRPV4 blocker HC 067047 (Fig. 5).
Limitations and perspectives of the study
There are important limitations of our study, including the limited end-points tested. We have explored how TBI affects myogenic response of cerebral vessels, but we did not study autoregulation per se, which means the changes of CBF as a function of blood pressure. Although myogenic response of cerebral vessels is a central mechanism of CBF autoregulation and is considered to be a reliable surrogate of cerebral autoregulatory function,22 the results of isolated vessel studies can only be extrapolated to in vivo conditions with caution, because of the lack of other factors determining cerebral perfusion (metabolic effects, innervation, glial effects). Therefore, future studies should determine the role of H2O2 and BKCa activation in impaired autoregulation of CBF after severe, as well as after mild, repetitive trauma in vivo. Also, the mechanisms by which TRPV4 channels activate BKCa and the possible interaction between TRPV4 channels and mitochondrial H2O2 production remain to be determined. Gender differences might affect the TBI-related changes of cerebrovascular responses; therefore, vascular responses of cerebral arteries after TBI from female rats should be studied by follow-up studies. Last but not least, we did not investigate the role of the endothelium in the observed attenuated myogenic tone of cerebral vessels after TBI. Although endothelial function is not directly involved in the pressure-sensitive mechanisms of cerebral vessels,69 it plays a central role in maintaining CBF, and has a modulatory role in the development and maintenance of myogenic tone.70,71
Conclusion
In conclusion, we demonstrate here that diffuse brain trauma leads to excessive production of mitochondria-derived H2O2, which dampens myogenic constriction of cerebral arteries by a mechanism that involves TRPV4-dependent activation of BKCa channels. We propose that this pathway may contribute to autoregulatory dysfunction in TBI patients, and could be targeted pharmacologically in order to restore autoregulation of CBF and prevent the development of secondary brain injury.
Acknowledgments
This work was supported by grants from the Marie Curie Actions SMARTER 7th Framework Program of the European Union 606998 to N.S. and A.K.; the Hungarian Academy of Sciences Bolyai Research Scholarship BO/00634/15 to P.T.; the PTE AOK-KA 3/2016 04.01/F to P.T.; grants from the National Research, Development and Innovation Office to P.T. (NKFI-FK123798) and A.K. (K108444); the UNKP ÚNKP-17-4-I-PTE-7 Scholarship to P.T.; GINOP-2.3, 2–15-2016-00048 to P.T., E.C., A.B.; AOK-Post-Doc 3/2012 07.25/C to G.B.; the Hungarian National Brain Research Program Grant No. KTIA_13_NAP-A-II/8 to P.T., E.C., and A.B.; Program B KTIA_NAP_13-2014-0022, Research site ID number: 888819 to Z.H.; the American Heart Association to M.R.P. (13SDG14000005), P.T., and Z.U.; the National Institutes of Health R01-AT006526, R01-AG047879, R01-AG038747, 3P30AG050911-02S1, and R01-NS056218 to Z.U.; and the Oklahoma Center for the Advancement of Science and Technology to Z.U. This work is dedicated to the 650th anniversary of the University of Pécs.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Roozenbeek B., Maas A.I., and Menon D.K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236 [DOI] [PubMed] [Google Scholar]
- 2.Langlois J.A., and Sattin R.W. (2005). Traumatic brain injury in the United States: research and programs of the Centers for Disease Control and Prevention (CDC). J. Head Trauma Rehabil. 20, 187–188 [DOI] [PubMed] [Google Scholar]
- 3.Tagliaferri F., Compagnone C., Korsic M., Servadei F., and Kraus J. (2006). A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 148, 255–268 [DOI] [PubMed] [Google Scholar]
- 4.Sahuquillo J., Poca M.A., and Amoros S. (2001). Current aspects of pathophysiology and cell dysfunction after severe head injury. Curr. Pharm. Des. 7, 1475–1503 [DOI] [PubMed] [Google Scholar]
- 5.Overgaard J., and Tweed W.A. (1974). Cerebral circulation after head injury. 1. Cerebral blood flow and its regulation after closed head injury with emphasis on clinical correlations. J. Neurosurg. 41, 531–541 [DOI] [PubMed] [Google Scholar]
- 6.Enevoldsen E.M., and Jensen F.T. (1977). “False” autoregulation of cerebral blood flow in patients with acute severe head injury. Acta Neurol. Scand. Suppl. 64, 514–515 [PubMed] [Google Scholar]
- 7.Enevoldsen E.M., and Jensen F.T. (1978). Autoregulation and CO2 responses of cerebral blood flow in patients with acute severe head injury. J. Neurosurg. 48, 689–703 [DOI] [PubMed] [Google Scholar]
- 8.Cold G.E., and Jensen F.T. (1978). Cerebral autoregulation in unconscious patients with brain injury. Acta Anaesthesiol. Scand. 22, 270–280 [DOI] [PubMed] [Google Scholar]
- 9.Muizelaar J.P., Lutz H.A., 3rd, and Becker D.P. (1984). Effect of mannitol on ICP and CBF and correlation with pressure autoregulation in severely head-injured patients. J. Neurosurg. 61, 700–706 [DOI] [PubMed] [Google Scholar]
- 10.Muizelaar J.P., Ward J.D., Marmarou A., Newlon P.G., and Wachi A. (1989). Cerebral blood flow and metabolism in severely head-injured children. Part 2: Autoregulation. J. Neurosurg. 71, 72–76 [DOI] [PubMed] [Google Scholar]
- 11.Newell D.W., Aaslid R., Stooss R., Seiler R.W., and Reulen H.J. (1997). Evaluation of hemodynamic responses in head injury patients with transcranial Doppler monitoring. Acta Neurochir. 139, 804–817 [DOI] [PubMed] [Google Scholar]
- 12.Czosnyka M., Smielewski P., Piechnik S., Steiner L.A., and Pickard J.D. (2001). Cerebral autoregulation following head injury. J. Neurosurg. 95, 756–763 [DOI] [PubMed] [Google Scholar]
- 13.Czosnyka M., Smielewski P., Kirkpatrick P., Menon D.K., and Pickard J.D. (1996). Monitoring of cerebral autoregulation in head-injured patients. Stroke 27, 1829–1834 [DOI] [PubMed] [Google Scholar]
- 14.Junger E.C., Newell D.W., Grant G.A., Avellino A.M., Ghatan S., Douville C.M., Lam A.M., Aaslid R., and Winn H.R. (1997). Cerebral autoregulation following minor head injury. J. Neurosurg. 86, 425–432 [DOI] [PubMed] [Google Scholar]
- 15.Engelborghs K., Haseldonckx M., Van Reempts J., Van Rossem K., Wouters L., Borgers M., and Verlooy J. (2000). Impaired autoregulation of cerebral blood flow in an experimental model of traumatic brain injury. J. Neurotrauma 17, 667–677 [DOI] [PubMed] [Google Scholar]
- 16.DeWitt D.S., Prough D.S., Taylor C.L., Whitley J.M., Deal D.D., and Vines S.M. (1992). Regional cerebrovascular responses to progressive hypotension after traumatic brain injury in cats. Am. J. Physiol. 263, H1276–1284 [DOI] [PubMed] [Google Scholar]
- 17.Lewelt W., Jenkins L.W., and Miller J.D. (1980). Autoregulation of cerebral blood flow after experimental fluid percussion injury of the brain. J. Neurosurg. 53, 500–511 [DOI] [PubMed] [Google Scholar]
- 18.Golding E.M., Contant C.F., Jr., Robertson C.S., and Bryan R.M., Jr (1998). Temporal effect of severe controlled cortical impact injury in the rat on the myogenic response of the middle cerebral artery. J. Neurotrauma 15, 973–984 [DOI] [PubMed] [Google Scholar]
- 19.Mathew B.P., DeWitt D.S., Bryan R.M., Jr, Bukoski R.D., and Prough D.S. (1999). Traumatic brain injury reduces myogenic responses in pressurized rodent middle cerebral arteries. J. Neurotrauma 16, 1177–1186 [DOI] [PubMed] [Google Scholar]
- 20.Villalba N., Sonkusare S.K., Longden T.A., Tran T.L., Sackheim A.M., Nelson M.T., Wellman G.C., and Freeman K. (2014). Traumatic brain injury disrupts cerebrovascular tone through endothelial inducible nitric oxide synthase expression and nitric oxide gain of function. J. Am. Heart Assoc. 3, e001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budohoski K.P., Czosnyka M., de Riva N., Smielewski P., Pickard J.D., Menon D.K., Kirkpatrick P.J., and Lavinio A. (2012). The relationship between cerebral blood flow autoregulation and cerebrovascular pressure reactivity after traumatic brain injury. Neurosurgery 71, 652–660 [DOI] [PubMed] [Google Scholar]
- 22.Toth P., Tucsek Z., Sosnowska D., Gautam T., Mitschelen M., Tarantini S., Deak F., Koller A., Sonntag W.E., Csiszar A., and Ungvari Z. (2013). Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J. Cereb. Blood Flow Metab. 33, 1732–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budohoski K.P., Reinhard M., Aries M.J., Czosnyka Z., Smielewski P., Pickard J.D., Kirkpatrick P.J., and Czosnyka M. (2012). Monitoring cerebral autoregulation after head injury. Which component of transcranial Doppler flow velocity is optimal? Neurocrit. Care 17, 211–218 [DOI] [PubMed] [Google Scholar]
- 24.Kontos H.A., and Wei E.P. (1986). Superoxide production in experimental brain injury. J. Neurosurg. 64, 803–807 [DOI] [PubMed] [Google Scholar]
- 25.Modrick M.L., Didion S.P., Lynch C.M., Dayal S., Lentz S.R., and Faraci F.M. (2009). Role of hydrogen peroxide and the impact of glutathione peroxidase-1 in regulation of cerebral vascular tone. J. Cereb. Blood Flow Metab. 29, 1130–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knot H.J., and Nelson M.T. (1995). Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am. J. Physiol. 269, H348–355 [DOI] [PubMed] [Google Scholar]
- 27.Brayden J.E., and Nelson M.T. (1992). Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256, 532–535 [DOI] [PubMed] [Google Scholar]
- 28.Paterno R., Heistad D.D., and Faraci F.M. (2000). Potassium channels modulate cerebral autoregulation during acute hypertension. Am. J. Physiol. Heart Circ. Physiol. 278, H2003–2007 [DOI] [PubMed] [Google Scholar]
- 29.Randhawa P.K., and Jaggi A.S. (2015). TRPV4 channels: physiological and pathological role in cardiovascular system. Basic Res. Cardiol. 110, 54. [DOI] [PubMed] [Google Scholar]
- 30.Hayabuchi Y., Nakaya Y., Matsuoka S., and Kuroda Y. (1998). Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. Heart Vessels 13, 9–17 [DOI] [PubMed] [Google Scholar]
- 31.Iida Y., and Katusic Z.S. (2000). Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke 31, 2224–2230 [DOI] [PubMed] [Google Scholar]
- 32.Marmarou A., Foda M.A., van den Brink W., Campbell J., Kita H., and Demetriadou K. (1994). A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J. Neurosurg. 80, 291–300 [DOI] [PubMed] [Google Scholar]
- 33.Toth P., Rozsa B., Springo Z., Doczi T., and Koller A. (2011). Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J. Cereb. Blood Flow Metab. 31, 2096–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungvari Z., Pacher P., Kecskemeti V., and Koller A. (1999). Fluoxetine dilates isolated small cerebral arteries of rats and attenuates constrictions to serotonin, norepinephrine, and a voltage-dependent Ca(2+) channel opener. Stroke 30, 1949–1954 [DOI] [PubMed] [Google Scholar]
- 35.Bailey-Downs L.C., Sosnowska D., Toth P., Mitschelen M., Gautam T., Henthorn J.C., Ballabh P., Koller A., Farley J.A., Sonntag W.E., Csiszar A., and Ungvari Z. (2012). Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese lewis dwarf rats: implications for vascular aging. J. Gerontol. A Biol. Sci. Med. Sci. 67 A, 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matoba T., Shimokawa H., Nakashima M., Hirakawa Y., Mukai Y., Hirano K., Kanaide H., and Takeshita A. (2000). Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Invest. 106, 1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaffl M.W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suresh K., Servinsky L., Reyes J., Baksh S., Undem C., Caterina M., Pearse D.B., and Shimoda L.A. (2015). Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves TRPV4. Am. J. Physiol. Lung Cell Mol. Physiol. 309, L1467–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Earley S., Heppner T.J., Nelson M.T., and Brayden J.E. (2005). TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ. Res. 97, 1270–1279 [DOI] [PubMed] [Google Scholar]
- 40.Bubolz A.H., Mendoza S.A., Zheng X., Zinkevich N.S., Li R., Gutterman D.D., and Zhang D.X. (2012). Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am. J. Physiol. Heart Circ. Physiol. 302, H634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earley S., Pauyo T., Drapp R., Tavares M.J., Liedtke W., and Brayden J.E. (2009). TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am. J. Physiol. Heart Circ. Physiol. 297, H1096–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y., Zhao H., Li H., Kalyanaraman B., Nicolosi A.C., and Gutterman D.D. (2003). Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ. Res. 93, 573–580 [DOI] [PubMed] [Google Scholar]
- 43.Kenney K., Amyot F., Haber M., Pronger A., Bogoslovsky T., Moore C., and Diaz-Arrastia R. (2016). Cerebral Vascular Injury in Traumatic Brain Injury. Exp. Neurol. 275 Pt 3, 353–366 [DOI] [PubMed] [Google Scholar]
- 44.Jullienne A., Obenaus A., Ichkova A., Savona-Baron C., Pearce W.J., and Badaut J. (2016). Chronic cerebrovascular dysfunction after traumatic brain injury. J. Neurosci. Res. 94, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price R.S. and Kasner S.E. (2014). Hypertension and hypertensive encephalopathy. Handb. Clin. Neurol. 119, 161–167 [DOI] [PubMed] [Google Scholar]
- 46.Toth P., Tucsek Z., Tarantini S., Sosnowska D., Gautam T., Mitschelen M., Koller A., Sonntag W.E., Csiszar A., and Ungvari Z. (2014). IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J. Cereb. Blood Flow Metab. 34, 1887–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasemsri T., and Armstead W.M. (1997). Endothelin production links superoxide generation to altered opioid-induced pial artery vasodilation after brain injury in pigs. Stroke 28, 190–197 [DOI] [PubMed] [Google Scholar]
- 48.Springo Z., Tarantini S., Toth P., Tucsek Z., Koller A., Sonntag W.E., Csiszar A., and Ungvari Z. (2015). Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1355–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang F., Franco R., Skotak M., Hu G., and Chandra N. (2014). Mechanical stretch exacerbates the cell death in SH-SY5Y cells exposed to paraquat: mitochondrial dysfunction and oxidative stress. Neurotoxicology 41, 54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J.J., Kang Y.J., Shin S.A., Bak D.H., Lee J.W., Lee K.B., Yoo Y.C., Kim D.K., Lee B.H., Kim D.W., Lee J., Jo E.K., and Yuk J.M. (2016). Phlorofucofuroeckol improves glutamate-induced neurotoxicity through modulation of oxidative stress-mediated mitochondrial dysfunction in PC12 cells. PLoS One 11, e0163433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manucha W. (2017). Mitochondrial dysfunction associated with nitric oxide pathways in glutamate neurotoxicity. Clin. Investig. Arterioscler 29, 92–97 [DOI] [PubMed] [Google Scholar]
- 52.Cao Y., Gao Y., Xu S., Bao J., Lin Y., Luo X., Wang Y., Luo Q., Jiang J., Neale J.H., and Zhong C. (2016). Glutamate carboxypeptidase II gene knockout attenuates oxidative stress and cortical apoptosis after traumatic brain injury. BMC Neurosci. 17, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Case A.J., Li S., Basu U., Tian J., and Zimmerman M.C. (2013). Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am. J. Physiol. Heart Circ. Physiol. 305, H19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmerman M.C., Lazartigues E., Lang J.A., Sinnayah P., Ahmad I.M., Spitz D.R., and Davisson R.L. (2002). Superoxide mediates the actions of angiotensin II in the central nervous system. Circ. Res. 91, 1038–1045 [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Fabuel I., Le Douce J., Logan A., James A.M., Bonvento G., Murphy M.P., Almeida A., and Bolanos J.P. (2016). Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc. Natl. Acad. Sci. U. S. A. 113, 13,063–13,068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drose S., Stepanova A., and Galkin A. (2016). Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim. Biophys. Acta 1857, 946–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gidday J.M., Beetsch J.W., and Park T.S. (1999). Endogenous glutathione protects cerebral endothelial cells from traumatic injury. J. Neurotrauma 16, 27–36 [DOI] [PubMed] [Google Scholar]
- 58.Lifshitz J., Sullivan P.G., Hovda D.A., Wieloch T., and McIntosh T.K. (2004). Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 4, 705–713 [DOI] [PubMed] [Google Scholar]
- 59.Mendes Arent A., de Souza L.F., Walz R., and Dafre A.L. (2014). Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed. Res. Int. 2014, 723060(PMID ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J.N., Shi N., and Chen S.Y. (2012). Manganese superoxide dismutase inhibits neointima formation through attenuation of migration and proliferation of vascular smooth muscle cells. Free Radic. Biol. Med. 52, 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guikema B.J., Ginnan R., Singer H.A., and Jourd'heuil D. (2005). Catalase potentiates interleukin-1beta-induced expression of nitric oxide synthase in rat vascular smooth muscle cells. Free Radic. Biol. Med. 38, 597–605 [DOI] [PubMed] [Google Scholar]
- 62.Boveris A., and Cadenas E. (2000). Mitochondrial production of hydrogen peroxide regulation by nitric oxide and the role of ubisemiquinone. IUBMB Life 50, 245–250 [DOI] [PubMed] [Google Scholar]
- 63.Armstead W.M., Kiessling J.W., Riley J., Kofke W.A., and Vavilala M.S. (2011). Phenylephrine infusion prevents impairment of ATP- and calcium-sensitive potassium channel-mediated cerebrovasodilation after brain injury in female, but aggravates impairment in male, piglets through modulation of ERK MAPK upregulation. J. Neurotrauma 28, 105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salvucci A., and Armstead W.M. (2000). Vasopressin impairs K(ATP) and K(ca) channel function after brain injury. Brain Res. 887, 406–412 [DOI] [PubMed] [Google Scholar]
- 65.Barlow R.S., El-Mowafy A.M., and White R.E. (2000). H(2)O(2) opens BK(Ca) channels via the PLA(2)-arachidonic acid signaling cascade in coronary artery smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 279, H475–483 [DOI] [PubMed] [Google Scholar]
- 66.Zhang D.X., Borbouse L., Gebremedhin D., Mendoza S.A., Zinkevich N.S., Li R., and Gutterman D.D. (2012). H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ. Res. 110, 471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chaplin N.L., and Amberg G.C. (2012). Stimulation of arterial smooth muscle L-type calcium channels by hydrogen peroxide requires protein kinase C. Channels (Austin) 6, 385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greensmith D.J., Eisner D.A., and Nirmalan M. (2010). The effects of hydrogen peroxide on intracellular calcium handling and contractility in the rat ventricular myocyte. Cell calcium 48, 341–351 [DOI] [PubMed] [Google Scholar]
- 69.Kisler K., Nelson A.R., Montagne A., and Zlokovic B.V. (2017). Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18, 419–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toth P., Tarantini S., Csiszar A., and Ungvari Z. (2017). Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 312, H1–H20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ungvari Z., and Koller A. (2001). Selected contribution: NO released to flow reduces myogenic tone of skeletal muscle arterioles by decreasing smooth muscle Ca(2+) sensitivity. J. Appl. Physiol. 91, 522–527 [DOI] [PubMed] [Google Scholar]