Abstract
Background
Younger siblings of children with autism spectrum disorder (ASD) are themselves at increased risk for ASD and other developmental concerns. It is unclear if infants who display developmental concerns, but are unaffected by ASD, share similar or dissimilar behavioral and brain phenotypes to infants with ASD. Most individuals with ASD exhibit heterogeneous difficulties with language, and their receptive-expressive language profiles are often atypical. Yet, little is known about the neurobiology that contributes to these language difficulties.
Methods
In this study, we used behavioral assessments and structural magnetic resonance imaging to investigate early brain structure and associations with later language skills. High-risk infants who were later diagnosed with ASD (n = 86) were compared to high-risk infants who showed signs of early language delay (n = 41), and high- and low-risk infants who did not have ASD or language delay (n= 255, n = 143, respectively).
Results
Results indicated that diminished language skills were evident at 12-months in infants with ASD and infants with early language delay. At 24-months of age, only the ASD infants displayed atypical receptive-expressive language profiles. Associations between 12-month subcortical volumes and 24-month language skills were moderated by group status, indicating disordinal brain-behavior associations among ASD infants and language delay infants.
Conclusions
These results suggest that there are different brain mechanisms influencing language development in ASD and language delay infants, and that the two groups likely experience unique sets of genetic and environmental risk factors.
Keywords: infancy, ASD, brain, subcortical structure, language delay, language profile
Introduction
In the first two years of life, the brain undergoes dynamic changes that are influenced by genetic and environmental factors. In infants later diagnosed with autism spectrum disorder (ASD), aberrant brain development is apparent during the first year of life, well before the defining features are exhibited (1–5). Delayed language onset is often the first warning sign for ASD, and the majority of affected individuals exhibit difficulties in speech production, speech comprehension, and/or pragmatic language (6). Patterns of brain development contributing to early language difficulties in ASD have yet to be fully examined during infancy.
Language Development in Children with ASD
The latter half of the first year and the second year of life encompasses a time of rapidly expanding language skills for typically developing children. This peak period of language acquisition is more variable in ASD. Many children with ASD show delays in early milestones such as onset of babbling and first word acquisition (7–9). Delays in language are evident at the group level around 12 months and become more pronounced by 24 months of age (10, 11). Difficulties in semantic and pragmatic language, and atypical receptive-expressive language profiles also emerge as language skills develop (6, 12). Early language deficits persist for a substantial proportion of children with ASD. About 29% of school-age children with ASD display minimal language and another 24% produce words but not sentences (13, 14). Also, unaffected siblings of children with ASD demonstrate higher rates of language delay (7, 15) and lower language scores (16–21) than infants with no familial risk for ASD.
Typically developing children generally understand considerably more language than they can produce (22), a pattern termed a “receptive advantage”. This discrepancy reflects that language comprehension is an important prerequisite for production. Several studies have shown that children with ASD, in contrast to those with other neurodevelopmental disorders, do not consistently display this normative profile (12). Late talkers, children with specific language impairment (SLI), Down syndrome, and general developmental delay all tend to have deficits in language, but their language profiles follow the normative trend (23–25).
The Subcortical Neurobiology of Language
Most previous language neurobiology research has involved participants who are past the period of early language development. The few studies to date involving infants and toddlers have shown that the inferior frontal gyrus and superior temporal gyrus (26, 27), the amygdala (28), and the splenium of the corpus callosum (29) may be integral to language acquisition. The current study investigates an often-overlooked aspect of language neurobiology, the role of subcortical structures.
To date, there are no published data pertaining to subcortical development and language skills in infants at-risk for ASD. However, research at later ages in both typical and atypical development suggests several targets for investigation including the amygdala, thalamus, and caudate nucleus. These structures were selected a priori for analyses in the current research because they had the strongest evidence for a role in the development of early language skills, or had been implicated in language or social cognition more broadly in ASD.
In children with ASD, amygdala volumes have been both positively and negatively associated with language and communication skills (30–32). In typically developing infants the amygdala has been negatively associated with language scores later in life (28). To date, the amygdala's role in language is not clear. For decades, lesion studies have implicated the thalamus in processes that support language (33). This structure is thought to influence language development by acting as a hub for information via ‘specific alerting responses.’ In this model, the thalamus directs certain salient forms of information while inhibiting others, gating information to the cortex and striatum (34). Atypical thalamus volumes have been reported in ASD, and thalamic tracts have been shown to be associated with social affect (35, 36). The caudate nucleus has been proposed to be a major region associated with language control, impacting the selection and inhibition of language through a cortico-subcortical loop that connects the caudate to the prefrontal cortex (37, 38). Atypical caudate nucleus size has been reported in individuals with SLI and their unaffected siblings; however, caudate size was only significantly correlated with phonological processing in the SLI group (39–41). These results suggest that caudate size may be a heritable risk factor for SLI, but additional risk factors are necessary for the disorder to be penetrant. Likewise, determining familial or disorder-specific risk factors in subgroups of infants at high-risk for ASD may improve the specificity of early identification efforts and investigations of causal pathways (42). For a review of neurobiological language disorder studies see (43, 44).
The current study utilizes a high-risk family design where younger siblings of children with ASD are prospectively studied. Using language skills and brain-behavior phenotypes, we aimed to tease apart disorder-specific effects from those attributed to familial risk. The current study had two main objectives: (1) to chart the development of language skills in high-risk infants later diagnosed with ASD and high-risk infants who showed signs of early language delay, and (2), to determine if the subcortical neurobiology of language and brain-behavior associations differed between high-risk infants with ASD and high-risk infants with language delay. Given the previous literature showing brain changes preceding behavioral changes in ASD (1, 3, 45), we focused on 12-month subcortical volumes and 24-month language skills.
Methods and Materials
Participants
This study includes data from n= 382 infants at high familial risk for ASD and n= 143 at low familial risk for ASD collected across four clinical data sites (University of North Carolina, Chapel Hill; University of Washington, Seattle; The Children's Hospital of Philadelphia; and Washington University, St. Louis). Parents provided written informed consent prior to participating in this study. The Institutional Review Boards at each site approved the study procedures. See the Supplement for full inclusion/exclusion criteria.
Procedures
Infants and their families participated in clinic visits at ages 6, 12, and 24 months, and were scanned using MRI at 12-months. Assessments at 24 months included the Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Interview- Revised (46, 47). Clinical-best estimate diagnoses were made by experienced, licensed clinicians using DSM-IV-TR criteria for Autistic Disorder (ASD) or Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS). See Estes and colleagues (10) for a full description of the assessment and diagnostic procedures.
Clinical Measures
Infant cognitive development was measured using the Mullen Scales of Early Learning (MSEL, 48) at 6, 12, and 24 months. The MSEL is widely used, and normed for children from birth to 68 months. Verbal developmental quotients (MSEL VDQ) were calculated from the receptive and expressive subscales, and non-verbal developmental quotients (MSEL NVDQ) were calculated from the visual reception and fine motor subscales. MSEL receptive advantage scores were computed by creating a receptive-expressive age equivalent difference score (11). A greater receptive advantage would be reflected in a positive mean difference. These scores are agnostic to overall level of language skills (e.g., it is possible to have a positive receptive advantage score and have language skills in the normative or delayed range). Reports from non-clinical comparison samples using the MSEL in this early age range have consistently shown the trend of above average receptive scores when concurrently compared to expressive scores; hence in this instance, a positive receptive advantage score, and not a score of zero, may reflect the “normative” profile (16, 18, 49, 50).
Infant functional language development was assessed with the Vineland Adaptive Behavior Scales-II (VABS-II, 51) at 6, 12, and 24 months. The VABS-II is a semi-structured parent interview, and the Communication standard score provides an index of a child's expressive and receptive functional language skills. The ADOS is a semi-structured observational play assessment of social interaction, communication, and repetitive behaviors (47). Module 1 or 2 was administered for all participants at 24 months and conventional scoring algorithms were applied (52).
Diagnostic Classification
At 24-months, infants were assessed for ASD and language delay and classified into the following four groups:
ASD (HR-ASD)
Based on clinical best estimate, 86 high-risk infants met criteria for ASD.
Language Delay (HR-LD)
The criteria for this group (n= 41) were 1) high risk; 2) not meeting criteria for ASD; and 3) a t-score < 35 (1.5 SD below the mean) on either the MSEL receptive or expressive language subscale, or both, in accordance with standard measures (16, 53). A general cognitive delay (i.e., MSEL nonverbal developmental quotient ≥ 2 SD below the mean) would have been grounds for exclusion from this group; however, none of the infants met this criterion.
High-Risk Negative and Low-Risk Negative
Infants who were unaffected by ASD and language delay were separated into two groups based on their familial risk status (HR-Neg n= 255; LR-Neg n= 143).
MRI Acquisition and Processing
Pediatric imaging was completed during natural sleep at each clinical site using identical 3-T Siemens TIM Trio scanners. T1 and T2-weighted scans (1mm3 voxels) were acquired. A full description of the MRI acquisition, image preprocessing, and segmentation of subcortical structures can be found in the supplement. See also Hazlett and colleagues for a description of the acquisition and processing procedures (1). Figure S1 shows the results of the segmentation of subcortical structures of interest: bilateral thalamus, amygdala, and caudate.
Results
Participant Characteristics
Data were available for 525 infants who completed at least two behavioral visits and had a 24-month diagnostic evaluation. Full demographic information is available in Table 1. See the Supplement for analyses related to participant characteristics.
Table 1.
Descriptive data for study sample by group.
| Variable | HR-ASD | HR-LD | HR-Neg | LR-Neg |
|---|---|---|---|---|
| Brain × behavior sample (n) | 46 | 29 | 189 | 104 |
| Longitudinal sample (n) | 86 | 41 | 255 | 143 |
| Longitudinal visit complement | ||||
| 6, 12, & 24m visit (n) | 61 | 35 | 192 | 125 |
| 6 & 12m visit (n) | 1 | 0 | 0 | 0 |
| 6 & 24m visit (n) | 8 | 3 | 13 | 11 |
| 12 & 24m visit (n) | 16 | 3 | 50 | 7 |
| 6m visit (n) | 70 | 38 | 205 | 136 |
| 12m visit (n) | 78 | 38 | 242 | 132 |
| 24m visit (n) | 85 | 41 | 255 | 143 |
| Mean age 6m. visit | 6.49 (0.64) | 6.63 (0.83) | 6.57 (0.65) | 6.71 (0.84) |
| Mean age 12m. visit | 12.68 (0.69) | 12.69 (0.62) | 12.56 (0.62) | 12.64 (0.74) |
| Mean age 24m. visit | 24.75 (1.41) | 24.87 (0.80) | 24.73 (1.01) | 24.70 (0.99) |
| % Male | 77 | 65 | 54 | 58 |
| 24m. MSEL ELC | 80.60 (17.67) | 80.90 (9.56) | 106.03 (13.70) | 112.01 (13.80) |
| 24m. MSEL NVDQ | 87.80 (12.95) | 91.72 (9.08) | 103.60 (12.84) | 109.14 (13.13) |
| 24m. ADOS Severity Score | 5.85 (1.82) | 1.70 (0.96) | 1.58 (1.00) | 1.44 (0.95) |
| Child race (%) | ||||
| White | 82 | 80 | 83 | 81 |
| African American | 1 | 5 | 2 | 5 |
| Asian | 0 | 0 | 1 | 1 |
| More than one race | 14 | 10 | 10 | 11 |
| Not answered | 3 | 5 | 4 | 2 |
| Maternal Education (%) | ||||
| High school diploma | 35 | 44 | 27 | 17 |
| College degree | 30 | 29 | 43 | 37 |
| Graduate degree | 22 | 17 | 24 | 40 |
| Missing | 13 | 10 | 6 | 6 |
Notes: MSEL ELC, MSEL Early Learning Composite Standard Score; MSEL NVDQ, MSEL Non-verbal developmental quotients. ADOS Severity Score, Autism Diagnostic Observation Schedule Calibrated Severity Score
Development of Language Skills
The development of language skills was measured using GLMM with maternal education, clinical site, MSEL NVDQ, and sex of the infant as covariates (see the Supplement for full statistical analysis plan and model building strategy). Tables S1 and S2 contain least squares means and fixed effects results for all language models. Figure S3 displays individual trajectories for language measures.
Longitudinal trajectories from 6 to 24 months showed significant group differences in MSEL VDQ, F(3,399) = 52.38, q < .0001 (Fig. 1A). At 6 months, the groups did not differ on MSEL VDQ, F(3,409) = 1.56, q = .332. At 12-months the groups did differ significantly, F(3,451) = 12.21, q < .0001. Follow-up pair-wise comparisons revealed the HR-ASD group scored lower than the HR-Neg and LR-Neg groups, t(451) = -4.84, q <.0001, and t(451) = -5.74, q < .0001, respectively. The HR-LD group also scored lower than the HR-Neg and LR-Neg groups, t(451) = -2.62, q = .009, and t(451) = -3.62, q = .0003, respectively. Finally, the HR-Neg group scored lower than the LR-Neg group, t(451) = -2.14, q = .032. Remaining pair-wise comparisons were not significant.
Fig. 1.
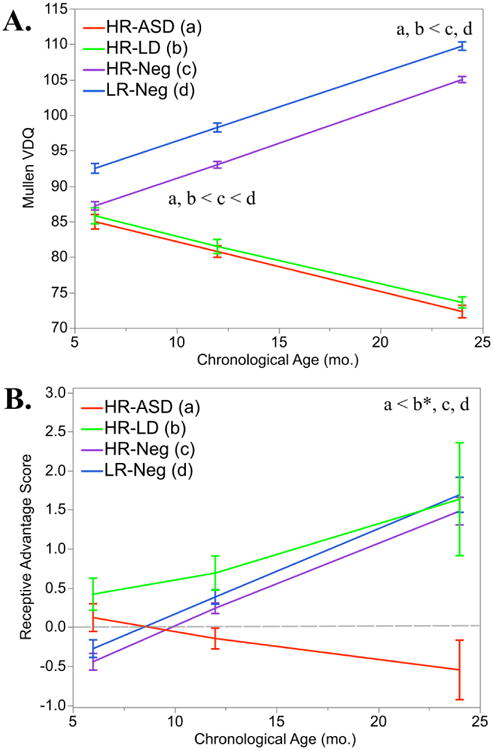
Language skills are delayed at 12-months in HR-ASD and HR-LD infants and delays were more evident at 24-months. Receptive-expressive language profiles differ at 24-months.
Panel A, MSEL VDQ from 6-24 months. Panel B, Receptive Advantage scores from 6-24 months. Dotted gray line represents a receptive advantage score of zero. Note: Contrast legend is as follows: HR-ASD (a), HR-LD (b), HR-Neg (c), and LR-Neg (d). Lines represent LS means which are adjusted for covariates in model (maternal education, clinical site, MSEL NVDQ, and sex of the infant). Error bars = ±1 SEM.
At 24-months the group differences expanded, F(3,473) = 57.67, q < .0001. Pair-wise comparisons showed the HR-ASD groups scoring lower than the HR-Neg and LR-Neg groups, t(473) = -10.60, q < .0001, and t(473) = -9.82, q < .0001, respectively. The HR-LD group also scored lower than the HR-Neg and LR-Neg groups, t(473) = -9.43, q < .0001, and t(473) = -9.04, q < .0001, respectively. Longitudinal results for MSEL Expressive t-score and MSEL Receptive t-score are available in Table S1 and Table S2.
To corroborate the MSEL findings (an examiner-based assessment), we conducted follow-up analyses by examining Communication standard scores from the VABS-II (a parent-report). Results followed the same pattern as the MSEL VDQ: at 6-months the VABS communication scores were not significantly different across groups, at 12-months the HR-ASD and HR-LD groups scored significantly lower than the HR-Neg and LR-Neg groups, and at 24-months these group differences were more pronounced (Table S1 and Table S2, Figure S2).
Development of Language Profiles
To examine the development of language profiles we utilized receptive advantage scores (receptive advantage scores = MSEL receptive age equivalent - MSEL expressive age equivalent). Longitudinal trajectories from 6 to 24 months showed significant group differences in language profiles, F(3,397) = 4.25, q = .005 (Fig. 1B). At 6- and 12-months the groups did not significantly differ, F(3,407) = 1.32, q = .332 and F(3,450) = 1.91, q = .127, respectively. At 24-months the groups differed significantly, F(3,473) = 4.31, q = .005. The HR-ASD group had lower receptive advantage scores (indicating an atypical language profile) than HR-Neg and LR-Neg groups, t(473) = -3.41, q = .0007 and t(473) = -3.28, q = .001, respectively. The HR-ASD also scored lower than the HR-LD group, however, this result did not survive multiple comparison corrections, t(473) = -2.13, p = .033, q = .067. The HR-LD group did not differ from HR-Neg and LR-Neg infants on receptive advantage scores, t(473) = -0.40, q = .689, and t(473) = -0.57, q = .568.
Subcortical Associations with Later Language Skills
MRI data at 12-months was available for 368 infants (70% of the larger behavioral dataset). Group n's were as follows: HR-ASD n= 46, HR-LD n= 29; HR-Neg n= 189, LR-Neg n= 104. Using cross-sectional GLMM, we aimed to investigate how the size of the amygdala, thalamus, and caudate are related to the later language outcomes of high-risk infants (covariates included clinical site, MSEL NVDQ, sex of the infant, and total cerebral volume, see Supplement for full analysis plan and model building strategy). Given a lack of laterality (Table S3), the left and right substructure volumes were summed to create a total volume of each structure.
The main aim for these analyses was to determine if the HR-ASD and HR-LD group have similar or dissimilar brain-behavior phenotypes by testing the difference in the effect of one specific planned contrast, HR-ASD vs. HR-LD. Full fixed effects and tests of simple slopes can be found in Table 2.
Table 2.
Tests of fixed effects and tests of simple slopes for brain-behavior analyses.
| Response Variable | MSEL VDQ | MSEL VDQ | MSEL VDQ | Receptive Adv. | Receptive Adv. | Receptive Adv. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Predictor Variable | Thalamus | Amygdala | Caudate Nucleus | Thalamus | Amygdala | Caudate Nucleus | ||||||
|
| ||||||||||||
| F | p/q a | F | p/q a | F | p/q a | F | p/q a | F | p/q a | F | p/q a | |
|
|
||||||||||||
| Sex of Infant | 2.21 | 0.138 | 1.55 | 0.214 | 1.64 | 0.200 | 0.03 | 0.870 | 0.07 | 0.794 | 0.01 | 0.926 |
| Mullen NVDQ | 120.64 | <.0001 | 123.42 | <.0001 | 122.72 | <.0001 | 0.14 | 0.706 | 0.07 | 0.792 | 0.07 | 0.790 |
| Site | 1.64 | 0.179 | 1.62 | 0.184 | 1.71 | 0.164 | 7.45 | <.0001 | 6.52 | 0.0003 | 7.40 | <.0001 |
| Age | 0.06 | 0.809 | 0.03 | 0.871 | 0.12 | 0.734 | 0.21 | 0.644 | 0.10 | 0.752 | 0.19 | 0.662 |
| TCV | 1.06 | 0.303 | 2.41 | 0.121 | 1.20 | 0.274 | 0.31 | 0.575 | 0.80 | 0.371 | 0.05 | 0.816 |
| Subcortical Volume | 0.10 | 0.748 | 0.13 | 0.718 | 0.31 | 0.577 | 0.38 | 0.539 | 0.04 | 0.840 | 2.38 | 0.123 |
| Group | 3.61 | 0.013 | 4.51 | 0.004 | 3.72 | 0.011 | 5.54 | 0.001 | 3.00 | 0.030 | 2.71 | 0.044 |
| Subcortical × Group | 2.21 | 0.087 | 2.79 | 0.080+ | 1.70 | 0.166 | 4.90 | 0.004* | 2.43 | 0.064 | 2.21 | 0.086 |
|
| ||||||||||||
| Tests of Group Effect | t | q | ||||||||||
|
| ||||||||||||
| HR-ASD | 1.58 | 0.230 | ||||||||||
| HR-LD | -3.07 | 0.009* | ||||||||||
| HR-Neg | 0.17 | 0.865 | ||||||||||
| LR-Neg | 0.61 | 0.726 | ||||||||||
Notes: MSEL VDQ, MSEL Verbal Developmental Quotient; Receptive Adv, Receptive Advantage Score
p-values are reported for tests of fixed effects; q-values are reported for interaction terms.
q-value < .05
uncorrected p-value < .05
First, we examined brain-behavior associations between 12-month subcortical volumes and 24-month language skills (MSEL VDQ) in HR-ASD and HR-LD infants. The HR-ASD and HR-LD groups significantly differed in their associations between MSEL VDQ and thalamus volume, t(350) = -2.11, p = .035 (Figure S4A); and amygdala volume, t(350) = -2.50, p = .012 (Figure S4B). The two groups did not differ in their association between caudate volume and MSEL VDQ, t(350) = -1.85, p = .065 (Figure S4C).
Subcortical Associations with Later Language Profiles
Next, we examined brain-behavior associations between subcortical volumes and later receptive advantage scores. The HR-ASD and HR-LD groups significantly differed in their associations between receptive advantage scores and thalamus volume, t(350) = -3.66, p = .0003 (Fig. 2A); amygdala volume, t(350) = -2.57, p = .010 (Fig. 2B); and caudate volume, t(350) = -2.26, p = .024 (Fig. 2C).
Fig. 2. HR-ASD and HR-LD groups have distinct brain-behavior associations.
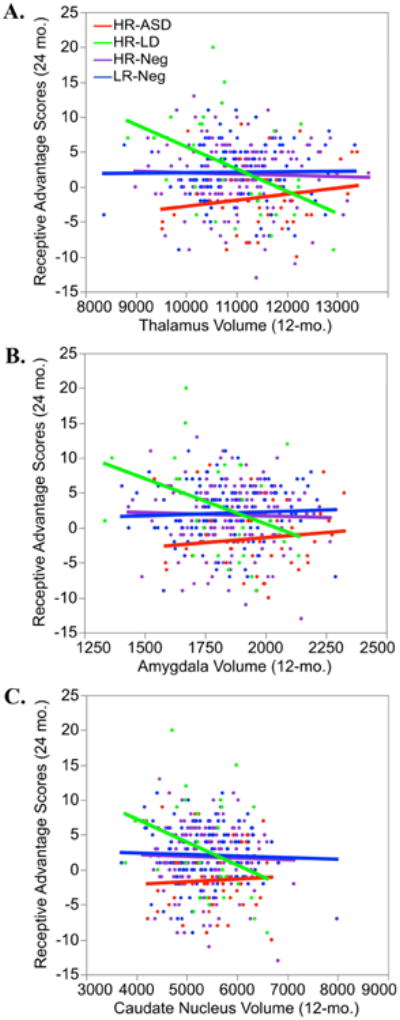
Panel A, association between thalamus volume (mm3) and receptive advantage score (n data points = 365), Panel B, association between amygdala volume (mm3) and receptive advantage score (n data points = 365), Panel C, association between caudate nucleus volume (mm3) and receptive advantage score (n data points = 365). Note: Lines represent LS means which are adjusted for covariates in model (TCV, age at scan, clinical site, MSEL NVDQ, and sex of the infant).
Follow-up Analyses Comparing ASD Infants with and without Language Delay to the Language Delay Group
Finally, we examined whether language delay infants without ASD (HR-LD, n = 29) had different brain-behavior associations than HR-ASD infants who also met criteria for language delay (ASD-LD+, n = 28) or ASD peers without language delay (ASD-LD-, n= 16). Based on our previous results, this exploratory analysis focused on the association between thalamus volume at 12-months and receptive advantage score at 24-months, since these measures provided the strongest support for distinct phenotypes for the HR-ASD and HR-LD groups.
The association between thalamus volume and receptive advantage score differed across the three groups, Group × Thalamus F(2, 60) = 5.50, p = .006. Fixed effects for TCV, site, and thalamus volume were not significant, p > .409. However, the fixed effects were significant for group, F(2, 60) = 6.37, p = .003, sex of the infant, F(2, 60) = 5.44, p = .023, MSEL NVDQ, F(2, 60) = 6.35, p = .014, and age at MRI, F(2, 60) = 4.36, p = .041. The contrast between ASD-LD+ and ASD-LD- indicated that the two ASD groups did not significantly differ in their brain-behavior association, t(60) = -0.47, p = .637. Both ASD groups differed significantly in their brain-behavior association when compared to the HR-LD group (ASD-LD+ vs. HR-LD, t(60) = -3.18, p = .002; ASD-LD- vs. HR-LD, t(60) = -2.10, p = .040). Tests of effects within each group revealed a negative association between thalamus volume and receptive advantage score for the HR-LD group, t(60) = -2.33, p = .023, q = .069, that did not survive FDR correction. The association between thalamus volume and receptive advantage score was not significant for the ASD-LD+ nor the ASD-LD- group, t(60) = 1.19, q = .357, t(60) = 0.35, q = .724, respectively. Together, these contrasts suggest ASD infants with language delay have brain-behavior phenotypes that more closely resemble ASD infants without language delay than infants with language delay only.
Discussion
The overarching goal of the current study was to determine if examining infant language development and brain-behavior associations could detect distinct phenotypes in subgroups of infants at high-familial risk for ASD. We examined language profiles in infants who were later diagnosed with ASD, infants who went on to show signs of early language delay, and infants who were at low- and high-familial risk for ASD without ASD or language delay. Lastly, we explored whether associations between brain development in the first year of life and later language skills were similar or distinct in high-risk infants with ASD and high-risk infants with signs of early language delay.
Our results supported three conclusions. First, trajectories of language development diverged over time across groups, such that groups did not differ in language skills at 6-months, at 12-months the ASD and language delay (LD) groups were scoring lower than their low and high-risk peers, with further divergence by 24-months. The ASD and LD groups did not differ from one another at any time point on available measures. These results are aligned with previous reports showing differences in language skills emerging around 12-months in infants who go to have ASD (10). Using a larger sample, we also confirmed previous results showing infants who present with language delay at 24 months first display delayed skills at, or soon after, their first birthday (54). More generally, our results highlight the increased vulnerabilities in families with a history of ASD. In this sample, 17% of high risk infants went on to have ASD themselves, and an additional 11% demonstrated signs of early language delay but not ASD. Recent community sample studies have suggested that early language skills are correlated with school-age vocabulary and literacy; however, the relationship is insufficiently strong to predict individual outcomes from infant data (55). School-age children with a family history of ASD have higher than expected rates of impairment, including difficulties in speech and language (56), thus early language delays in high-risk infants may herald these school-age difficulties, but this link remains to be determined.
Our second conclusion is that at 24-months the ASD group displayed language profiles that were either balanced or represented an expressive-advantage (e.g., better expressive than receptive skills). All other groups showed a profile of receptive-advantage (e.g., better receptive than expressive skills), consistent with previous reports for typically developing children in this age range tested with the same instrument (16, 18, 49). The current LD group showed delayed language skills but their language profiles, while varied, did not differ from their low and high-risk peers. This pattern of results for the LD group is also similar to previous work in SLI, general developmental delay, and Down syndrome (23, 24), where language is delayed but language profiles are intact. When contrasting the language profiles of the ASD and LD groups, results suggest that atypical language profiles are not a familial effect, but are more reflective of a disorder-specific effect for ASD.
Our last conclusion is that high-risk infants who go on to have ASD show distinct brain-behavior associations when compared to high-risk infants with early language delay. Specifically, associations between subcortical structures at 12-months and 24-month language skills differed in ASD and LD infants. For example, ASD and LD infants differed in their association between the thalamus and language profile, such that for LD infants the smaller the volume of the thalamus the larger the receptive advantage. Similar patterns were found for the caudate nucleus and amygdala. The negative association between caudate nucleus volume and language profile is in line with previous research on SLI showing a smaller caudate was associated with better language skills (40). Amygdala volume has been both positively and negatively associated with language and communications skills (30–32); here we find that HR-LD infants with smaller amygdala volumes had more normative language profiles. We recently reported that early brain overgrowth was associated with later ASD diagnosis and social deficits (1). It is possible that for infants with a genetic liability for ASD inhibited overgrowth is protective for later ASD, and the negative association between language and subcortical volume in HR-LD reflects this subgroups susceptibility to alterations in brain development. In the current study, we reported similar patterns of association across multiple structures, which could be a result of examining the brain as the behavior (e.g., language) is emerging. The theory of interactive specialization predicts that “developmental change in cognitive skills or behaviour will be accompanied by widespread changes across multiple regions”(57 page 11). If our findings are situated within this framework we would expect that interconnected brain structures show similar brain-behavior patterns.
We found that high-risk infants showing signs of language delay are distinct from those who develop ASD in both their behavioral trajectories and brain-behavior associations. With respect to brain-behavior results, the overall pattern of association when comparing ASD and LD groups was disordinal in nature, suggesting that the two groups display distinct brain-behavior associations across selected subcortical structures. The behavioral phenotype of these two groups was also distinct. The ASD group showed delayed language skills and atypical language profiles, whereas the LD group showed delayed language skills but language profiles that did not differ from their typically developing peers. In our exploratory analyses, we found that ASD infants with LD displayed brain-behavior phenotypes that were indistinguishable from ASD infants without LD, and all ASD infants (with and without LD), differed from LD infants (without ASD). These results suggest that a negative association between subcortical volume and language profile is a disorder-specific effect for LD.
This study highlights the brain and behavioral heterogeneity among those with increased familial liability to ASD. Our ASD and LD groups shared comorbid language delay and familial liability for ASD; however, the two groups displayed qualitatively different brain and behavioral profiles, suggesting that the LD infants exhibit a distinct phenotype, and not merely an intermediate ASD phenotype. These results suggest that different brain mechanisms influence behavioral development in ASD and LD infants, and that the two groups likely experience unique sets of genetic and environmental risk factors. To take steps towards understanding the causal developmental pathways to pathophysiology we must, in part, utilize family studies to outline disorder-specific effects and familial effects (42). Such an approach has the potential to move forward efforts to identify subgroups based on biological and behavioral phenotypes agnostic to diagnostic criteria, and take important steps towards a personalized medicine approach to ASD treatment.
Given that that there is variability in predicting later outcome from early language skills (58), and that some infants will first meet criteria for ASD at or after three years of age (59, 60), future efforts should include follow-up assessment at school age. Such follow-up assessments should include children with LD in the absence of familial risk of ASD, as the generalizability of findings to this group is unknown. Studies examining the genetic overlap between ASD and SLI have been mixed, and the two groups have been shown to have distinct behavioral phenotypes, suggesting that distinct genetic and environmental factors contribute to the developmental course of LD depending on whether there is associated ASD risk (61). It is possible that subcortical structures not examined in the current study are relevant for early language neurobiology, for example, enlargement of the putamen and nucleus accumbens has been reported in adults with developmental language impairment (62), hence future efforts may also benefit from taking a more comprehensive approach to regional analyses.
Supplementary Material
Figure S1. Example segmentation of subcortical brain structures. Coronal slice on left and 3D presentation on the right, showing amygdala in red, thalamus in blue, and caudate nucleus in green.
Figure S2. Adaptive language skills are delayed at 12-months in HR-ASD and HR-LD infants and delays were more evident at 24-months. VABS-II Communication standard scores from 6-24 months, n data points = 1330. Note: Contrast legend is as follows: HR-ASD (a), HR-LD (b), HR-Neg (c), and LR-Neg (d). Lines represent LS means which are adjusted for covariates in model (maternal education, clinical site, MSEL NVDQ, and sex of the infant). Error bars = ±1 SEM.
Figure S3. Spaghetti plots show individual trajectories of language development. Panel A, MSEL VDQ from 6-24 months, n data points = 1369. Panel B, VABS Communication scores from 6-24 months, n data points = 1330. Panel C, receptive advantage scores from 6-24 months, n data points = 1366. Note: Bold lines represent LS means which are adjusted for covariates in model (maternal education, clinical site, MSEL NVDQ, and sex of the infant). Error bars = ±1 SEM.
Figure S4. Associations between 12-month bi-lateral subcortical volume and 24-month MSEL VDQ show different brain-behavior associations in the HR-ASD and HR-LD groups. Panel A, association between total thalamus volume (mm3) and MSEL VDQ (n data points = 365), Panel B, association between total amygdala volume (mm3) and MSEL VDQ (n data points = 365), Panel C, association between total caudate nucleus volume (mm3) and MSEL VDQ (n data points = 365). Note: Bold lines represent LS means which are adjusted for covariates in model (TCV, age at scan, clinical site, MSEL NVDQ, and sex of the infant).
Table S1. Least square means for development of language skills.
Table S2. Tests of fixed effects for longitudinal language analyses.
Table S3. Laterality Index (LI) by subcortical structure and diagnostic group.
Acknowledgments
The authors thank the children and their families for their ongoing participation in this longitudinal study, as well as the numerous research assistant and volunteers who have worked on this project. The authors graciously thank Sun Hyung Kim, Ph.D., Michael M. Graves, Rachel G. Smith, Kirsten N. Z. Consing, Vladimir S. Fonov, Ph.D., D. Louis Collins, Ph.D., and Alan C. Evans, Ph.D., for their work in developing the imaging processing pipelines.
This work was supported by grants through the National Institutes of Health (R01-HD055741 PI Piven, R01-HD055741-S1 PI Piven, P30-HD003110 PI Piven, U54-EB005149 PI Kikinis) and the Simons Foundation (SFARI Grant 140209). Dr. Swanson was supported by a Pathway to Independence Award (K99-MH108700) from NIMH and a National Research Service Award (T32-HD40127) from NICHD. Dr. Wolff was supported by a grant from the National Institute of Mental Health (K01-MH101653). The funders had no role in study design, data collection, analysis, data interpretation, or the writing of the report.
The Infant Brain Imaging Study (IBIS) Network is an NIH funded Autism Center of Excellence project and consists of a consortium of eight universities in the U.S. and Canada. Clinical Sites: University of North Carolina: J. Piven (IBIS Network PI), H.C. Hazlett, C. Chappell; University of Washington: S. Dager, A. Estes, D. Shaw; Washington University: K. Botteron, R. McKinstry, J. Constantino, J. Pruett; The Children's Hospital of Philadelphia: R. Schultz, S. Paterson; University of Alberta: L. Zwaigenbaum; University of Minnesota: J. Elison, J. J. Wolff; Data Coordinating Center: Montreal Neurological Institute: A.C. Evans, D.L. Collins, G.B. Pike, V. Fonov, P. Kostopoulos; S. Das; Image Processing Core: New York University: G. Gerig; University of North Carolina: M. Styner; Statistical Analysis Core: University of North Carolina: H. Gu.
Footnotes
This study was presented, in part, as an oral presentation at the International Meeting for Autism Research, May 2017, San Francisco, California.
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, et al. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542:348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, et al. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain. 2015;138:2046–2058. doi: 10.1093/brain/awv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain. 2013;136:2825–2835. doi: 10.1093/brain/awt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerson RW, Adams C, Nishino T, Hazlett HC, Wolff JJ, Zwaigenbaum L, et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aag2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapin I, Dunn M. Update on the language disorders of individuals on the autistic spectrum. Brain Dev. 2003;25:166–172. doi: 10.1016/s0387-7604(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 7.Iverson JM, Wozniak RH. Variation in vocal-motor development in infant siblings of children with autism. J Autism Dev Disord. 2007;37:158–70. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patten E, Belardi K, Baranek GT, Watson LR, Labban JD, Oller DK. Vocal patterns in infants with autism spectrum disorder: canonical babbling status and vocalization frequency. J Autism Dev Disord. 2014;44:2413–28. doi: 10.1007/s10803-014-2047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo J, Chlebowski C, Fein DA, Eigsti IM. Age of first words predicts cognitive ability and adaptive skills in children with ASD. J Autism Dev Disord. 2013;43:253–264. doi: 10.1007/s10803-012-1558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes AM, Zwaigenbaum L, Gu H, St John T, Paterson S, Elison JT, et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord. 2015;7:24. doi: 10.1186/s11689-015-9117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudry K, Chandler S, Bedford R, Pasco G, Gliga T, Elsabbagh M, et al. Early language profiles in infants at high-risk for autism spectrum disorders. J Autism Dev Disord. 2014;44:154–67. doi: 10.1007/s10803-013-1861-4. [DOI] [PubMed] [Google Scholar]
- 12.Kwok EYL, Brown HM, Smyth RE, Oram Cardy J. Meta-analysis of receptive and expressive language skills in autism spectrum disorder. Res Autism Spectr Disord. 2015;9:202–222. [Google Scholar]
- 13.Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, et al. Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol. 2007;75:594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- 14.Tager-Flusberg H, Kasari C. Minimally Verbal School-Aged Children with Autism Spectrum Disorder: The Neglected End of the Spectrum. Autism Res. 2013;6:468–478. doi: 10.1002/aur.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamliel I, Yirmiya N, Sigman M. The development of young siblings of children with autism from 4 to 54 months. J Autism Dev Disord. 2007;37:171–183. doi: 10.1007/s10803-006-0341-5. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, Bryson S. Early language and communication development of infants later diagnosed with autism spectrum disorder. J Dev Behav Pediatr. 2006;27:S69–78. doi: 10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- 17.Miller M, Young GS, Hutman T, Johnson S, Schwichtenberg AJ, Ozonoff S. Early pragmatic language difficulties in siblings of children with autism: implications for DSM-5 social communication disorder? J Child Psychol Psychiatry. 2015;56:774–781. doi: 10.1111/jcpp.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toth K, Dawson G, Meltzoff AN, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. J Autism Dev Disord. 2007;37:145–57. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: vocal production in infant siblings of children with ASD. J Child Psychol Psychiatry. 2011;52:588–98. doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gangi DN, Ibañez LV, Messinger DS. Joint attention initiation with and without positive affect: Risk group differences and associations with ASD symptoms. J Autism Dev Disord. 2014;44:1414–1424. doi: 10.1007/s10803-013-2002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seery A, Tager-Flusberg H, Nelson CA. Event-related potentials to repeated speech in 9-month-old infants at risk for autism spectrum disorder. J Neurodev Disord. 2014;6:43. doi: 10.1186/1866-1955-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benedict H. Early lexical development: comprehension and production. J Child Lang. 1979;6:183–200. doi: 10.1017/s0305000900002245. [DOI] [PubMed] [Google Scholar]
- 23.Ellis Weismer S, Lord C, Esler A. Early language patterns of toddlers on the autism spectrum compared to toddlers with developmental delay. J Autism Dev Disord. 2010;40:1259–1273. doi: 10.1007/s10803-010-0983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laws G, Bishop DVM. A comparison of language abilities in adolescents with Down syndrome and children with specific language impairment. J Speech Lang Hear Res. 2003;46:1324–1339. doi: 10.1044/1092-4388(2003/103). [DOI] [PubMed] [Google Scholar]
- 25.Davidson MM, Ellis Weismer S. A Discrepancy in Comprehension and Production in Early Language Development in ASD: Is it Clinically Relevant? J Autism Dev Disord. 2017:1–13. doi: 10.1007/s10803-017-3135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emerson RW, Gao W, Lin W. Longitudinal study of the emerging functional connectivity asymmetry of primary language regions during infancy. J Neurosci. 2016;36:10883–10892. doi: 10.1523/JNEUROSCI.3980-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aeby A, De Tiège X, Creuzil M, David P, Balériaux D, Van Overmeire B, et al. Language development at 2 years is correlated to brain microstructure in the left superior temporal gyrus at term equivalent age: a diffusion tensor imaging study. Neuroimage. 2013;78:145–151. doi: 10.1016/j.neuroimage.2013.03.076. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz-Mantilla S, Choe M, Flax J, Grant PE, Benasich AA. Associations between the size of the amygdala in infancy and language abilities during the preschool years in normally developing children. Neuroimage. 2010;49:2791–2799. doi: 10.1016/j.neuroimage.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 29.Swanson MR, Wolff JJ, Elison JT, Gu H, Hazlett HC, Botteron K, et al. Splenium development and early spoken language in human infants. Dev Sci. 2015 doi: 10.1111/desc.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, et al. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry. 2006;63:686–93. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- 31.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–9. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimpel-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2-to 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crosson B. Subcortical Functions in Language and Memory. New York: The Guilford Press; 1992. [Google Scholar]
- 34.Hebb AO, Ojemann GA. The thalamus and language revisited. Brain Lang. 2013;126:99–108. doi: 10.1016/j.bandl.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Nair A, Treiber JM, Shukla DK, Shih P, Müller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAlonan GM, Suckling J, Wong N, Cheung V, Lienenkaemper N, Cheung C, Chua SE. Distinct patterns of grey matter abnormality in high-functioning autism and Asperger's syndrome. J Child Psychol Psychiatry. 2008;49:1287–1295. doi: 10.1111/j.1469-7610.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 37.Robles SG. The role of dominant striatum in language: a study using intraoperative electrical stimulations. J Neurol Neurosurg Psychiatry. 2005;76:940–946. doi: 10.1136/jnnp.2004.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffau H. The anatomo-functional connectivity of language revisited: New insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46:927–934. doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 39.Badcock NA, Bishop DVM, Hardiman MJ, Barry JG, Watkins KE. Co-localisation of abnormal brain structure and function in specific language impairment. Brain Lang. 2012;120:310–320. doi: 10.1016/j.bandl.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watkins KE, Vargha-Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, et al. MRI analysis of an inherited speech and language disorder: structural brain abnormalities. Brain. 2002;125:465–478. doi: 10.1093/brain/awf057. [DOI] [PubMed] [Google Scholar]
- 41.Soriano-Mas C, Pujol J, Ortiz H, Deus J, López-Sala A, Sans A. Age-related brain structural alterations in children with specific language impairment. Hum Brain Mapp. 2009;30:1626–1636. doi: 10.1002/hbm.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones EJH, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci Biobehav Rev. 2013;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan S, Watkins KE, Bishop DVM. Neurobiological Basis of Language Learning Difficulties. Trends Cogn Sci. 2016 doi: 10.1016/j.tics.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayes AK, Reilly S, Morgan AT. Neural correlates of childhood language disorder: a systematic review. Dev Med Child Neurol. 2015;57:706–717. doi: 10.1111/dmcn.12714. [DOI] [PubMed] [Google Scholar]
- 45.Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, Nordahl CW, et al. Increased Extra-axial Cerebrospinal Fluid in High-Risk Infants Who Later Develop Autism. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.02.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 47.Lord C, Risi S, Lambrecht L, Cook EHJ, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Schedule – Generic: A standard measures of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 48.Mullen EME. Mullen scales of early learning. Circle Pines, MN: AGS; 1995. [Google Scholar]
- 49.Ozonoff S, Iosif A, Baguio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49:256-66–2. [PMC free article] [PubMed] [Google Scholar]
- 50.Akshoomoff N. Use of the Mullen Scales of Early Learning for the assessment of young children with Autism Spectrum Disorders. Child Neuropsychol. 2006;12:269–77. doi: 10.1080/09297040500473714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales (VABS-II) Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- 52.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37:613–27. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 53.Northrup JB, Iverson JM. Vocal coordination during early parent–infant interactions predicts language outcome in infant siblings of children with autism spectrum disorder. Infancy. 2015;20:523–547. doi: 10.1111/infa.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- 55.Duff FJ, Reen G, Plunkett K, Nation K. Do infant vocabulary skills predict school-age language and literacy outcomes? J Child Psychol Psychiatry. 2015;56:848–856. doi: 10.1111/jcpp.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller M, Iosif AM, Young GS, Hill M, Phelps Hanzel E, Hutman T, et al. School-age outcomes of infants at risk for autism spectrum disorder. Autism Res. 2016;9:632–642. doi: 10.1002/aur.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson MH. Interactive specialization: a domain-general framework for human functional brain development? Dev Cogn Neurosci. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bishop DVM, Snowling MJ, Thompson PA, Greenhalgh T. Phase 2 of CATALISE: a multinational and multidisciplinary Delphi consensus study of problems with language development: Terminology. J Child Psychol Psychiatry. 2017 doi: 10.1111/jcpp.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozonoff S, Young GS, Landa RJ, Brian J, Bryson SE, Charman T, et al. Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study. J Child Psychol Psychiatry. 2015;56:988–998. doi: 10.1111/jcpp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brian J, Bryson SE, Smith IM, Roberts W, Roncadin C, Szatmari P, Zwaigenbaum L. Stability and change in autism spectrum disorder diagnosis from age 3 to middle childhood in a high-risk sibling cohort. Autism. 2015 doi: 10.1177/1362361315614979. 1362361315614979. [DOI] [PubMed] [Google Scholar]
- 61.Williams D, Botting N, Boucher J. Language in autism and specific language impairment: Where are the links? Psychol Bull. 2008;134:944–963. doi: 10.1037/a0013743. [DOI] [PubMed] [Google Scholar]
- 62.Lee JC, Nopoulos PC, Bruce Tomblin J. Abnormal subcortical components of the corticostriatal system in young adults with DLI: A combined structural MRI and DTI study. Neuropsychologia. 2013;51:2154–2161. doi: 10.1016/j.neuropsychologia.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Example segmentation of subcortical brain structures. Coronal slice on left and 3D presentation on the right, showing amygdala in red, thalamus in blue, and caudate nucleus in green.
Figure S2. Adaptive language skills are delayed at 12-months in HR-ASD and HR-LD infants and delays were more evident at 24-months. VABS-II Communication standard scores from 6-24 months, n data points = 1330. Note: Contrast legend is as follows: HR-ASD (a), HR-LD (b), HR-Neg (c), and LR-Neg (d). Lines represent LS means which are adjusted for covariates in model (maternal education, clinical site, MSEL NVDQ, and sex of the infant). Error bars = ±1 SEM.
Figure S3. Spaghetti plots show individual trajectories of language development. Panel A, MSEL VDQ from 6-24 months, n data points = 1369. Panel B, VABS Communication scores from 6-24 months, n data points = 1330. Panel C, receptive advantage scores from 6-24 months, n data points = 1366. Note: Bold lines represent LS means which are adjusted for covariates in model (maternal education, clinical site, MSEL NVDQ, and sex of the infant). Error bars = ±1 SEM.
Figure S4. Associations between 12-month bi-lateral subcortical volume and 24-month MSEL VDQ show different brain-behavior associations in the HR-ASD and HR-LD groups. Panel A, association between total thalamus volume (mm3) and MSEL VDQ (n data points = 365), Panel B, association between total amygdala volume (mm3) and MSEL VDQ (n data points = 365), Panel C, association between total caudate nucleus volume (mm3) and MSEL VDQ (n data points = 365). Note: Bold lines represent LS means which are adjusted for covariates in model (TCV, age at scan, clinical site, MSEL NVDQ, and sex of the infant).
Table S1. Least square means for development of language skills.
Table S2. Tests of fixed effects for longitudinal language analyses.
Table S3. Laterality Index (LI) by subcortical structure and diagnostic group.


