Abstract
Background
Cancer-specific mortality (CSM) is known to be higher among blacks and lower among Hispanics compared to whites. Private insurance confers CSM benefit, but few studies have examined the relationship between insurance status and racial disparities. We sought to determine differences in CSM between races within insurance subgroups.
Methods
A population-based cohort of 577,716 patients age 18–64 years diagnosed with one of the 10 solid malignancies causing the greatest mortality over 2007–2012 were obtained from SEER. A Cox proportional hazards model for CSM was constructed to adjust for known prognostic factors and interaction analysis between race and insurance was performed to generate stratum-specific hazard ratios (HRs).
Results
Blacks had similar CSM to whites among the uninsured (HR=1.01, 95% CI=0.96–1.05), but higher CSM among the Medicaid (HR=1.04, 95% CI=1.01–1.07) and non-Medicaid (HR=1.14, 95% CI=1.12–1.16) strata. Hispanics had lower CSM compared to whites among uninsured (HR=0.80; 95% CI=0.76–0.85) and Medicaid (HR=0.88, 95% CI=0.85–0.91) patients, but there was no difference among non-Medicaid patients (HR=0.99, 95% CI=0.97–1.01). Asians had lower CSM compared to whites among all insurance types: uninsured (HR=0.80, 95% CI=0.76–0.85), Medicaid (HR=0.81, 95% CI=0.77–0.85), and non-Medicaid (HR=0.85, 95% CI=0.83–0.87).
Conclusions
The disparity between blacks and whites was largest and the advantage of Hispanic race was absent within the non-Medicaid subgroup.
Impact
These findings suggest that whites derive greater benefit from private insurance than blacks and Hispanics. Further research is necessary to determine why this differential exists and how disparities can be improved.
Introduction
Since the landmark Institute of Medicine study assessed the extent of racial and ethnic disparities in health outcomes in the United States(1), numerous initiatives have sought to reduce these differences (2). Despite these efforts, population-based studies of cancer patients have reported continued racial disparities in health care delivery and outcomes that disproportionately affect non-whites by measures including extent of disease at presentation, likelihood of receiving guideline-concordant care, and survival(3–8).
Additionally, population-based studies have shown that patients without private insurance are also more likely to present with advanced disease, less likely to receive treatment, and more likely to experience worse survival(9, 10). Reducing the uninsured population was the primary motivating factor in the passage of the Affordable Care Act (ACA) in 2010, which has led to the lowering of the overall uninsured rate in the United States to an all-time low of 9% in 2015(11, 12).
Given these findings, a pertinent question is whether expanding insurance coverage would be expected to be sufficient to eliminate racial disparities. Previous studies have suggested that these disparities persist despite adjustment for insurance status(13–15), but have been limited in scope. Estimates of the impact of insurance status on racial disparities in cancer survival on a larger scale are lacking. The purpose of this study was to determine the association of insurance status with racial disparities in cancer survival using the population-based Surveillance, Epidemiology, and End Results (SEER) program registry(16).
Materials and Methods
Study Cohort
The study cohort was assembled using SEER*Stat software (version 8.3.2). The SEER 18 dataset represents 28% of the US population based on 2010 census data and captures approximately 97% of incident cases within the registry area(17). The registry collects data on patient demographics, area-level socioeconomic status (SES), primary tumor site, stage at diagnosis, initial course of local treatment, follow up time, and survival. SEER began collecting patient insurance status in 2007, but is unreliable for patients age ≥65 years due to Medicare eligibility. Inclusion criteria for the study therefore included patients aged 18–64 years at the time of diagnosis of an initial malignancy that is one of the 10 solid cancers that cause the most deaths (i.e. lung, colorectal, pancreas, breast, liver, prostate, bladder, central nervous system, esophagus, and renal)(18) over the years 2007–2012. These disease sites account for approximately 75% of annual cancer-related mortality(18). A total of 610,215 patients met the inclusion criteria with a median follow up of 2.8 years. Additionally, 675,826 patients were excluded based on age ≥65 years. The study was deemed institutional review board exempt as the analyzed dataset is in the public domain.
Key Covariates
Patient race and origin, insurance status, age, sex, marital status, rural/urban residence, area poverty level, disease site, and extent of disease at presentation were analyzed. Race was categorized as Hispanic and non-Hispanic white, black, Asian, and Native American. Insurance status was defined as non-Medicaid insurance (insured or insured/no specifics), Medicaid coverage (any Medicaid, including Indian Health Service), or uninsured. The SEER definition for insured includes those with private insurance, Medicare, or military coverage at the time of diagnosis. Age was analyzed as a continuous variable. Married status as defined in SEER includes common law marriage. Residence was classified as urban for counties in metropolitan areas or rural for other counties. SEER linked county-level data regarding the percent of residents below federal poverty level was divided into quartiles. The extent of disease was categorized as local (no nodal or metastatic disease), regional (nodal disease), or distant (any metastatic disease).
Statistical Methods
Patient and clinical characteristics were compared using the Chi-square test. The Kaplan-Meier method was used to estimate the unadjusted cancer-specific survival (CSS) of different races for the entire cohort and for each insurance subset. These survival curves were compared using the log rank test.
An initial multivariable Cox proportional hazards model was constructed using the above covariates determined a priori to describe overall racial disparities in cancer-specific mortality (CSM) after controlling for known prognostic factors. The proportional hazards assumption was confirmed by inspection of log (−log [survival]) curves.
The interaction between race and insurance was assessed in preplanned analysis by adding an interaction term for these categorical covariates to the Cox model. Stratum-specific hazard ratios (HRs) for different race within insurance subgroups and different insurance status within race subgroups were computed from the Cox model inclusive of the interaction term using white race and non-Medicaid insurance status as references, respectively. A two-sided P value of ≤ 0.05 was considered to be statistically significant. Data analysis was performed using SAS v9.4 (Cary, NC).
Results
Patient Characteristics
A total of 610,215 patients met the inclusion criteria. Of this initial group, 28,014 (5%) had unknown insurance status and 7,425 (1%) had unknown race and were excluded from further analysis. Patient and clinical characteristics of the remaining 577,716 (95%) patients are shown in Table 1. The study cohort was 67% white, 15% black, 11% Hispanic, 7% Asian, and 1% Native American. The majority of patients had non-Medicaid insurance (82%), while a minority had Medicaid (13%) or was uninsured (5%). Patients with white race were generally more likely to have non-Medicaid insurance, be married, live in a county with lower poverty levels, live in a rural residence, and present with localized disease than blacks and Hispanics.
Table 1.
Patient and clinical characteristics
| Total | White | Black | Hispanic | Asian | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
|
| |||||||||||
| Total | 577716 | 100% | 384804 | 67% | 83864 | 15% | 64729 | 11% | 40814 | 7% | |
| Age | <0.001 | ||||||||||
| Median | 56 | 57 | 56 | 54 | 55 | ||||||
| 18–29 | 6218 | 1% | 3491 | 1% | 842 | 1% | 1279 | 2% | 551 | 1% | |
| 30–39 | 25626 | 4% | 14166 | 4% | 3516 | 4% | 4989 | 8% | 2740 | 7% | |
| 40–49 | 105375 | 18% | 64850 | 17% | 15448 | 18% | 15144 | 23% | 9212 | 23% | |
| 50–59 | 260651 | 45% | 173778 | 45% | 40098 | 48% | 27713 | 43% | 17515 | 43% | |
| 60–64 | 179846 | 31% | 128519 | 33% | 23960 | 29% | 15604 | 24% | 10796 | 26% | |
| Insurance | <0.001 | ||||||||||
| Non-Medicaid | 472601 | 82% | 332689 | 86% | 60760 | 72% | 43998 | 68% | 33179 | 81% | |
| Medicaid | 75612 | 13% | 36098 | 9% | 16637 | 20% | 15663 | 24% | 5781 | 14% | |
| Uninsured | 29503 | 5% | 16017 | 4% | 6467 | 8% | 5068 | 8% | 1854 | 5% | |
| Sex | <0.001 | ||||||||||
| Female | 278958 | 48% | 182761 | 47% | 36588 | 44% | 33982 | 52% | 23765 | 58% | |
| Male | 298758 | 52% | 202043 | 53% | 47276 | 56% | 30747 | 48% | 17049 | 42% | |
| Marital status | <0.001 | ||||||||||
| Unmarried | 198763 | 34% | 120261 | 31% | 43635 | 52% | 23213 | 36% | 10204 | 25% | |
| Married | 349868 | 61% | 245843 | 64% | 35239 | 42% | 38143 | 59% | 28983 | 71% | |
| Percent of county below poverty | <0.001 | ||||||||||
| < 11% | 153555 | 27% | 113889 | 30% | 12010 | 14% | 10912 | 17% | 15465 | 38% | |
| 11–14% | 140126 | 24% | 101033 | 26% | 13931 | 17% | 13538 | 21% | 10963 | 27% | |
| 14–17.25% | 140459 | 24% | 80498 | 21% | 22941 | 27% | 25130 | 39% | 11473 | 28% | |
| >17% | 143485 | 25% | 89321 | 23% | 34979 | 42% | 15125 | 23% | 2912 | 7% | |
| Residence type | <0.001 | ||||||||||
| Rural | 64139 | 11% | 53230 | 14% | 6760 | 8% | 2408 | 4% | 1067 | 3% | |
| Urban | 512767 | 89% | 331511 | 86% | 77101 | 92% | 62297 | 96% | 39746 | 97% | |
| Site | <0.001 | ||||||||||
| Breast | 174488 | 30% | 113767 | 30% | 21360 | 25% | 22111 | 34% | 16192 | 40% | |
| Prostate | 127657 | 22% | 86557 | 22% | 24117 | 29% | 11609 | 18% | 4945 | 12% | |
| Lung | 77943 | 13% | 56001 | 15% | 12248 | 15% | 4663 | 7% | 4616 | 11% | |
| Colorectal | 78162 | 14% | 48480 | 13% | 11694 | 14% | 10232 | 16% | 7120 | 17% | |
| Liver | 20363 | 4% | 9989 | 3% | 3265 | 4% | 4003 | 6% | 2850 | 7% | |
| Pancreas | 18318 | 3% | 11843 | 3% | 2895 | 3% | 2227 | 3% | 1224 | 3% | |
| Bladder | 22791 | 4% | 18468 | 5% | 1650 | 2% | 1674 | 3% | 902 | 2% | |
| Central nervous system | 15582 | 3% | 11330 | 3% | 1124 | 1% | 2140 | 3% | 897 | 2% | |
| Esophagus | 8019 | 1% | 5853 | 2% | 1098 | 1% | 705 | 1% | 314 | 1% | |
| Renal | 34393 | 6% | 22516 | 6% | 4413 | 5% | 5365 | 8% | 1754 | 4% | |
| Extent of disease | <0.001 | ||||||||||
| Local | 361954 | 63% | 244968 | 64% | 50859 | 61% | 39567 | 61% | 24541 | 60% | |
| Regional | 112477 | 19% | 72895 | 19% | 15792 | 19% | 14052 | 22% | 8994 | 22% | |
| Distant | 93620 | 16% | 61254 | 16% | 15550 | 19% | 9658 | 15% | 6510 | 16% | |
Unadjusted Cancer Specific Survival
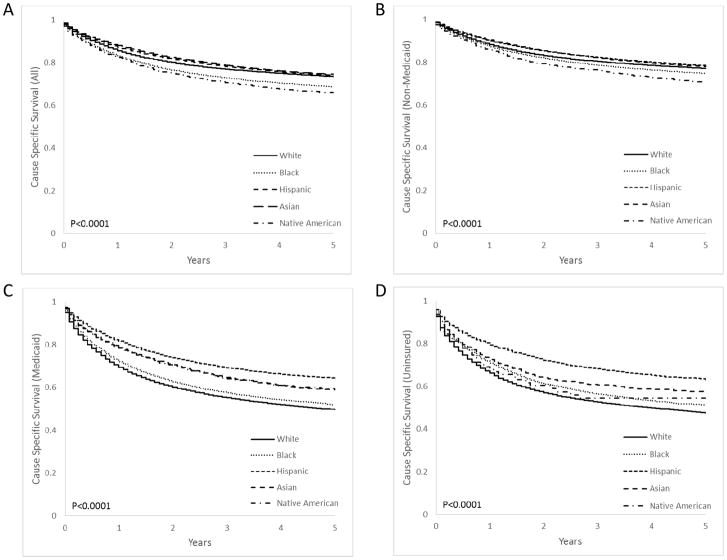
At a median time to follow up of 2.8 years, the 1- and 5-year CSS for all patients was 86% and 73%, respectively. The 1-year CSS for Asians, Hispanics, whites, blacks, and Native Americans was 88%, 87%, 86%, 83%, and 82%, respectively (P<0.001) (Figure 1A). Among patients who had Medicaid or were uninsured, those with white race had the lowest 1-year CSS at 69% and 66%, respectively, as compared to other races (P<0.001) (Figure 1C–D).
Figure 1.
Kaplan-Meier curve estimating cancer-specific survival for different races, separated by insurance type, for (A) all patients, (B) non-Medicaid patients, (C) Medicaid patients, and (D) uninsured patients diagnosed with one of the 10 most deadly solid cancers.
Multivariable Proportional Hazards Model
On multivariable analysis, older age, male gender, more advanced disease presentation, higher county poverty level, rural residence, and unmarried status were associated with a higher risk of CSM (Table 2). Certain disease sites (liver, central nervous system, pancreas, esophagus, and lung) also had higher risk of death compared to others (breast and prostate). After adjusting for all factors, black race had a higher CSM compared to whites (HR=1.10, 95% confidence interval [CI]: 1.08–1.12, P<0.001), while Hispanic (HR=0.94, 95% CI: 0.92–0.96, P<0.001) and Asian (HR=0.85, 95% CI: 0.83–0.87, P<0.001) races were associated with lower risks of death. There was no statistical difference in CSM for Native Americans as compared to whites (HR=1.07, 95% CI: 1.00–1.16, P=0.057). Compared to patients with non-Medicaid insurance, those with Medicaid (HR=1.42, 95% CI: 1.40–1.44, P<0.001) and no insurance (HR=1.45, 95% CI: 1.42–1.48, P<0.001) had similarly elevated risk of death.
Table 2.
Multivariate Cox model for cancer-specific mortality
| Reference | HR | 95% CI | P | |
|---|---|---|---|---|
|
| ||||
| Age at diagnosis | y, Continuous | 1.02 | 1.02–1.02 | <0.001 |
| Sex | Female | |||
| Male | 1.21 | 1.19–1.22 | <0.001 | |
| Extent of disease | Local | |||
| Regional | 2.99 | 2.94–3.04 | <0.001 | |
| Distant | 9.97 | 9.81–10.13 | <0.001 | |
| County poverty level | Quartile | 1.06 | 1.05–1.06 | <0.001 |
| Urban residence | Rural | 0.93 | 0.92–0.95 | <0.001 |
| Disease Site | Breast | |||
| Prostate | 0.40 | 0.38–0.41 | <0.001 | |
| Lung | 6.32 | 6.18–6.46 | <0.001 | |
| Colorectal | 2.13 | 2.08–2.19 | <0.001 | |
| Liver | 14.18 | 13.78–14.58 | <0.001 | |
| Pancreas | 9.70 | 9.45–9.97 | <0.001 | |
| Bladder | 2.45 | 2.34–2.56 | <0.001 | |
| Central nervous system | 15.12 | 14.66–15.58 | <0.001 | |
| Esophagus | 6.68 | 6.45–6.92 | <0.001 | |
| Renal | 2.45 | 2.36–2.53 | <0.001 | |
| Married | Unmarried | 0.83 | 0.82–0.84 | <0.001 |
| Insurance | Non-Medicaid | |||
| Medicaid | 1.42 | 1.4–1.44 | <0.001 | |
| Uninsured | 1.45 | 1.42–1.48 | <0.001 | |
| Race | White | |||
| Black | 1.10 | 1.08–1.12 | <0.001 | |
| Hispanic | 0.94 | 0.92–0.96 | <0.001 | |
| Asian | 0.85 | 0.83–0.87 | <0.001 | |
| Native American | 1.07 | 1–1.16 | .057 | |
Race and Insurance Interaction Analysis
An interaction term between the categorical variables of race and insurance status was added to the Cox model, which was statistically significant (Pinteraction <0.001). All other covariates remained statistically significant and similar to the initial model (Supplementary Table S1).
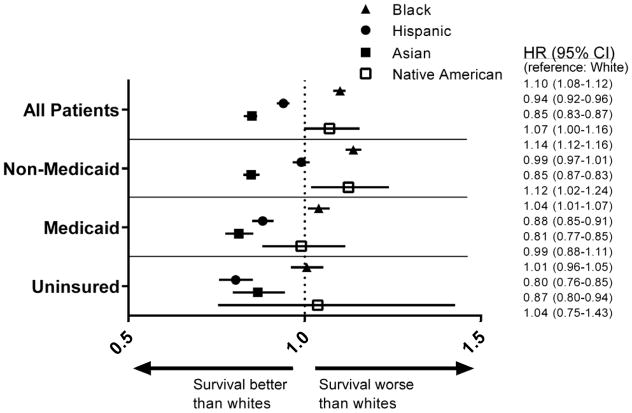
The different HR for each race within insurance subgroups using white as the reference is depicted in Figure 2. Blacks had similar CSM as whites among the uninsured (HR=1.01, 95% CI: 0.96–1.05, P=0.80), but higher CSM among the Medicaid (HR=1.04, 95% CI: 1.01–1.07, P=0.011) and non-Medicaid (HR=1.14, 95% CI: 1.12–1.16, P<0.001) strata. Hispanics had lower CSM than whites among uninsured (HR=0.80, 95% CI: 0.76–0.85, P<0.001) and Medicaid (HR=0.88, 95% CI: 0.85–0.91, P<0.001) patients, but there was no difference among non-Medicaid (HR=0.99, 95% CI: 0.97–1.01, P=0.30) patients. Asians had lower CSM as compared to whites across all insurance strata (uninsured: HR=0.87, 95% CI: 0.80–0.94, P<0.001; Medicaid: HR=0.81, 95% CI: 0.78–0.85, P<0.001; non-Medicaid: HR=0.85, 95% CI: 0.83–0.87, P<0.001). CSM for Native Americans was not statistically different from whites among uninsured (HR=1.04, 95% CI: 0.75–1.43, P=0.83) and Medicaid (HR=0.99, 95% CI: 0.88–1.12, P=0.90) patients, but was higher among non-Medicaid (HR=1.12, 95% CI: 1.02–1.24, P=0.018) patients.
Figure 2.
Forest plot of hazard ratios and 95% CIs for cancer-specific mortality for patients of different race as compared with white race, separated by insurance type. Outcomes are controlled for age, sex, marital status, residence, county poverty level, disease site, and extent of disease at presentation.
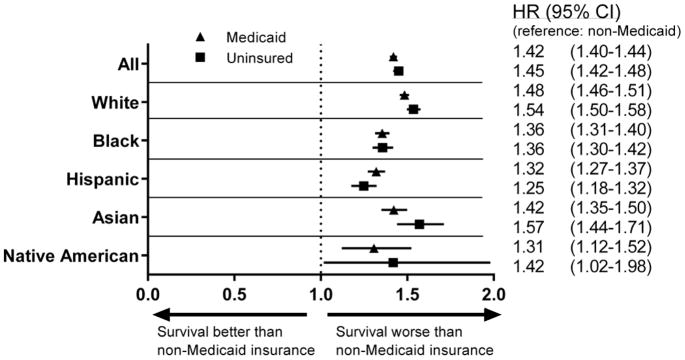
The different HR by insurance type within racial subgroups using non-Medicaid insurance as the reference is shown in Figure 3. For all races, Medicaid and no insurance was associated with a higher risk of death as compared to non-Medicaid insurance. The absence of non-Medicaid insurance conferred a greater risk of CSM for whites (HR=1.48, 95% CI: 1.46–1.51, P<0.001 for Medicaid; HR=1.54, 95% CI: 1.50–1.58, P<0.001 for uninsured) than for blacks (HR=1.36, 95% CI: 1.31–1.40, P<0.001 for Medicaid; HR=1.36, 95% CI: 1.30–1.42, P<0.001 for uninsured) and Hispanics (HR=1.32, 95% CI: 1.27–1.37, P<0.001 for Medicaid; HR=1.25, 95% CI: 1.18–1.32, P<0.001 for uninsured).
Figure 3.
Forest plot of hazard ratios and 95% CIs for cancer-specific mortality for patients with different insurance type as compared to non-Medicaid, separated by race. Outcomes are controlled for age, sex, marital status, residence, county poverty level, disease site, and extent of disease at presentation.
Discussion
This study analyzed the association of insurance status and race in CSM among a large number of patients with different cancer types. Although we found that improved insurance status benefitted patients of all races, interaction analysis demonstrated that the degree of benefit varied by race. Moreover, racial variations remained present even among patients with non-Medicaid insurance.
Our overall finding that blacks had higher risk of adjusted CSM than whites is consistent with prior studies(3, 8, 19, 20). Interestingly, we found the opposite pattern – a survival decrement for whites compared to blacks – among the uninsured and Medicaid subgroups on initial unadjusted analysis. While we hypothesized that an outcomes disparity between blacks and whites would exist across all insurance strata and narrow with improving insurance, our findings on adjusted analysis were, in fact, the opposite. There was no difference in CSM among uninsured patients, a modest survival decrement for blacks among Medicaid patients, and the most prominent disparity was among non-Medicaid patients. This suggests that whites derive a greater benefit from the improved access to healthcare despite adjustment for prognostic factors.
Our finding of Hispanics having similar or better outcomes as compared to whites is consistent with prior literature. The most recent unadjusted data from the American Cancer Society reported that Hispanics have a lower cancer incidence and death rates than whites(20) and a prior 1988–2007 SEER analysis reported that Hispanics had similar adjusted outcomes in a cohort of lung, breast, prostate, and colorectal cancer patients(3). Extensive prior literature has investigated the Hispanic health paradox(21), often attributing improved health outcomes despite less favorable SES to cultural or social factors(22, 23). Our interaction analysis revealed that this benefit was limited to uninsured and Medicaid patients, with no statistical difference among non-Medicaid patients. This finding is similar to prior research using the National Health Interview Survey that showed an overall mortality advantage for Hispanics as compared to whites that was concentrated at lower levels of SES(24).
The finding of Asians having a lower risk of adjusted CSM as compared to whites is consistent with a recent SEER study examining this question among lung, breast, prostate, and colorectal cancer patients that showed most Asian subgroups had better CSM despite adjustment for potential confounders(25). In contrast to the variation seen in the insurance stratum-specific HR for blacks and Hispanics, we found that the lower risk of death for Asians as compared to whites was consistent among all insurance subgroups. This suggests that Asians derive similar benefit from improved access to healthcare as whites.
Comparison of outcomes between Native Americans and whites showed that Native Americans had higher risk of adjusted CSM among non-Medicaid patients. There was no statistical difference between the races among Medicaid and uninsured patients, but conclusions from this study are limited by small Native American patient numbers leading to wide confidence intervals. However, prior studies focused on the Native American population have identified lagging improvements in early detection and survival as compared to whites(26, 27).
The issue of racial disparities in health outcomes is complex and the etiologies are multifactorial. Commonly studied measures in racial disparities are the extent of disease at presentation, the receipt of local treatment, and survival. Prior population-based studies have shown that blacks are more likely to fare worse across all these parameters as compared to whites in multiple disease sites(3–5, 7). More recent population-based studies have demonstrated that the lack of insurance is also associated with greater burden of disease, reduced likelihood of receiving local treatment, and worse outcomes(9, 10). To the authors knowledge, prior studies examining the relationship of race and insurance on cancer outcomes have been limited to disease-site specific studies from the National Cancer Database (NCDB)(13), which is limited to hospital settings, or studies limited by geography(14). These prior studies of breast cancer patients showed that black women had worse survival than white, even after correction for insurance status and known prognostic factors. The initial multivariable model in the current study confirms the prior findings of persistent black versus white survival disparity after adjustment for insurance status, but is more generalizable due to the inclusion of multiple disease sites and usage of the SEER dataset. While these prior studies have corrected for insurance status by inclusion into a multivariable model, the current study also includes an interaction analysis to elucidate differences in insurance stratum-specific racial variation.
The ACA has extended insurance coverage to the US population primarily through expanding Medicaid eligibility and creating state-based insurance exchanges. Estimates suggest that an additional 17 million residents under the age of 65 would have been uninsured in 2015 if the ACA had never been enacted(28). Based on the findings from prior studies (9, 10), one would expect there to be only marginal benefit for Medicaid insurance and a more substantial benefit for non-Medicaid insurance as compared to no insurance in terms of extent of disease at presentation, receipt of local treatment, and survival. The unadjusted survival analysis and initial multivariate model of the current study confirm these prior findings by showing that all races benefit from non-Medicaid insurance. However, the interaction analysis showed that non-Medicaid insurance provides a larger relative benefit to white patients as compared to blacks and Hispanics. Thus, while all patients should benefit in absolute terms from expanded insurance coverage, it may paradoxically widen specific pairwise racial disparities, such as between black and white.
Although the HRs for some race-insurance combinations are either not statistically significant or modestly clinically significant, it is important to note that they are adjusted for other prognostic factors (notably disease site and extent of disease) and to highlight how the HRs for race change across different insurance strata. Blacks had similar outcomes to whites among uninsured and Medicaid patients, but a HR of 1.14 among the largest insurance subgroup of non-Medicaid patients. Hispanics had a HR of 0.80 and 0.88 among uninsured and Medicaid patients, respectively, but similar outcomes among non-Medicaid patients. Asians had a HR of 0.81–0.87 compared to whites across all insurance subgroups. The magnitude of eliminating these differences may appear modest, but such a change in the outcome of CSM for a large group of cancers by a therapeutic intervention would be considered a landmark study.
The limitations of this study are largely a function of the limitations of the SEER dataset. The registry does not include individual level SES data, so patient county-level income data was used as a surrogate. Additionally, the dataset does not include other factors that may affect survival, most notably performance status and comorbid conditions, although we tried to mitigate this by using CSM as the primary outcome measure. While the SEER dataset codes the type of surgery received, it lacks details about radiotherapy treatment beyond the delivery modality and does not include information about systemic treatment. We thus chose to only include covariates present at the time of diagnosis in our multivariable model, but a more complete understanding of the effect of race and insurance status on survival requires further details regarding treatment course, subsequent surveillance, and supportive care. SEER coding of insurance status does not include details about the type of insurance beyond Medicaid versus non-Medicaid nor accounts for patients who may have switched insurance status after diagnosis. The patients with unknown insurance status were excluded from analysis, although prior sensitivity analysis showed no changes to a multivariable survival model(10). The inclusion of multiple cancer sites fulfills our study aim of presenting an overall model of racial disparities in cancer outcomes, but a more nuanced understanding would require disease-specific models that include unique prognostic factors (e.g. hormone receptor status for breast cancer) and potentially exclude less relevant prognostic factors (e.g. extent of disease in CNS tumors). Finally, the generalizability of the findings of this study are somewhat limited by exclusion of patients ages ≥65 years due to the inability of the dataset to accurately account for Medicare status. While the subset of patients <65 years account for 47% all SEER patients diagnosed with the top 10 deadly malignancies, the HR of CSM for each covariate in our model, including race, may differ between the included younger and excluded older populations.
For patients diagnosed with the 10 cancers causing the greatest mortality, black race was associated with higher risk of CSM and Hispanic and Asian races were associated with lower risk of death than whites after adjustment for prognostic factors. Interaction analysis showed that the disparity between blacks and whites was most prominent among non-Medicaid patients, the lower risk of death for Hispanics was present only among uninsured and Medicaid subgroups, and Asians had a lower risk of CSM than whites across all insurance strata. Further research is necessary to determine why there is a differential benefit of insurance between races and how these disparities can be improved.
Supplementary Material
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.US Department of Health and Human Services. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Vol. 2003 Washington DC: 2003. [Google Scholar]
- 2.US Department of Health and Human Services. HHS Action Plan to Reduce Racial and Ethnic Disparities: A Nation Free of Disparities in Health and Health Care. Vol. 2011 Washington DC: 2011. Apr, [Google Scholar]
- 3.Aizer AA, Wilhite TJ, Chen MH, Graham PL, Choueiri TK, Hoffman KE, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120:1532–9. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 4.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112:900–8. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith GL, Shih YC, Xu Y, Giordano SH, Smith BD, Perkins GH, et al. Racial disparities in the use of radiotherapy after breast-conserving surgery: a national Medicare study. Cancer. 2010;116:734–41. doi: 10.1002/cncr.24741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Society of Clinical Oncology. The State of Cancer Care in America, 2016: A Report by the American Society of Clinical Oncology. Journal of oncology practice / American Society of Clinical Oncology. 2016 doi: 10.1200/JOP.2015.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 8.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers: Results From the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015;1:88–96. doi: 10.1001/jamaoncol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aizer AA, Falit B, Mendu ML, Chen MH, Choueiri TK, Hoffman KE, et al. Cancer-specific outcomes among young adults without health insurance. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2025–30. doi: 10.1200/JCO.2013.54.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker GV, Grant SR, Guadagnolo BA, Hoffman KE, Smith BD, Koshy M, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:3118–25. doi: 10.1200/JCO.2014.55.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez ME, Cohen RA. Health insurance coverage: Early release of estimates from the National Health Interview Survey, January–June 2015. Vol. 2015 Hyattsville, MD: 2015. Nov, [Google Scholar]
- 12.Obama B. United States Health Care Reform: Progress to Date and Next Steps. Jama. 2016;316:525–32. doi: 10.1001/jama.2016.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117:180–9. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 14.Roetzheim RG, Gonzalez EC, Ferrante JM, Pal N, Van Durme DJ, Krischer JP. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000;89:2202–13. doi: 10.1002/1097-0142(20001201)89:11<2202::aid-cncr8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Roetzheim RG, Pal N, Tennant C, Voti L, Ayanian JZ, Schwabe A, et al. Effects of health insurance and race on early detection of cancer. Journal of the National Cancer Institute. 1999;91:1409–15. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER) Program; National Cancer Institute, editor SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (2000–2013) Apr, 2016. [Google Scholar]
- 17.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–50. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.American Cancer Society. Cancer Facts & Figures 2016. Vol. 2016 Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 19.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016 doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 21.Morales LS, Lara M, Kington RS, Valdez RO, Escarce JJ. Socioeconomic, cultural, and behavioral factors affecting Hispanic health outcomes. J Health Care Poor Underserved. 2002;13:477–503. doi: 10.1177/104920802237532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallo LC, Penedo FJ, Espinosa de los Monteros K, Arguelles W. Resiliency in the face of disadvantage: do Hispanic cultural characteristics protect health outcomes? J Pers. 2009;77:1707–46. doi: 10.1111/j.1467-6494.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- 23.Patel MI, Schupp CW, Gomez SL, Chang ET, Wakelee HA. How do social factors explain outcomes in non-small-cell lung cancer among Hispanics in California? Explaining the Hispanic paradox. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3572–8. doi: 10.1200/JCO.2012.48.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turra CM, Goldman N. Socioeconomic differences in mortality among U.S. adults: insights into the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. 2007;62:S184–92. doi: 10.1093/geronb/62.3.s184. [DOI] [PubMed] [Google Scholar]
- 25.Trinh QD, Nguyen PL, Leow JJ, Dalela D, Chao GF, Mahal BA, et al. Cancer-specific mortality of Asian Americans diagnosed with cancer: a nationwide population-based assessment. Journal of the National Cancer Institute. 2015;107:djv054. doi: 10.1093/jnci/djv054. [DOI] [PubMed] [Google Scholar]
- 26.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 27.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health. 2014;104(Suppl 3):S377–87. doi: 10.2105/AJPH.2013.301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Congressional Budget Office. Federal Subsidies for Health Insurance Coverage for People Under Age 65: 2016–2026. Vol. 2016 Washington, DC: 2016. Mar, [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.