Abstract
Recent rodent studies have demonstrated that parental cocaine exposure can influence offspring behavior, supporting the idea that environmental insults can impact subsequent generations. However, studies on the effects of paternal cocaine exposure are limited and multiple inconsistencies exist. In the current study, we behaviorally characterize the effects of paternal cocaine exposure in a C57BL/6J intergenerational mouse model. Male sires were administered cocaine hydrochloride (20mg/kg) or saline (0.01mL/g) once a day for 75 days, and bred with drug naïve females twenty-four hours after the final injection. Offspring, separated by sex, were tested in a battery of behaviors. We found that paternal cocaine exposure altered sensitivity to the rewarding and stimulant effects of psychostimulants and natural reward (sucrose) in female offspring; female cocaine-sired offspring showed blunted cocaine preference using cocaine conditioned place preference (CPP) at a low dose (5mg/kg), but displayed similar preference at a higher dose (10mg/kg) compared to saline-sired controls. Additionally, cocaine-sired female offspring exhibited higher psychomotor sensitivity to cocaine (10mg/kg) and amphetamine (2mg/kg) and consumed more sucrose. Cocaine-sired males exhibited increased psychomotor effects of cocaine and amphetamine. Male offspring also displayed an anxiety-like phenotype. No effect of paternal cocaine exposure was observed on depressive-like, learning and memory or social behavior in male or female offspring. Collectively, our findings show that paternal, chronic cocaine exposure induces intergenerational behavioral effects in male and female offspring with greatest impact on sensitivity to psychostimulants and sucrose in females.
Keywords: Cocaine, female, intergenerational, male, mice, reward
1. INTRODUCTION
Substance abuse and addiction, both highly comorbid with mood disorders, are prevalent problems in today’s society. Genetic and environmental factors, in part, contribute to addictive behavior and mood disorders [1]. Furthermore, multiple studies suggest an additional contribution of familial genetic and epigenetic mechanisms, an idea that is supported by the observation of higher likelihood of drug abuse amongst individuals with a family history of substance abuse [2–4]. Indeed, recent studies using animal models identify parental drug exposure as a contributing factor to behavioral, physiological, and molecular phenotypes in offspring [5, 6]. Even though males appear to abuse numerous drugs more frequently than females [7], the effects of maternal drug exposure have been investigated at greater lengths compared to paternal drug exposure.
Although there is compelling evidence for the intergenerational effects of parental cocaine exposure to subsequent generations using rat and mouse models, studies examining the behavioral consequences of paternal cocaine administration on offspring are sparse with varying results [5, 6]. For example, cocaine-sired male, but not female, rats displayed reduced reinforcing effects of cocaine using self-administration [8] whereas in cocaine-sired mice there was no difference in the stimulant effects of cocaine [9]. Similarly, investigating the cognitive impact of paternal cocaine exposure has provided conflicting results: one study indicated female offspring display learning and memory deficits [10] while another (not separated by sex), failed to show learning deficits [9]. Additionally, multiple studies have suggested that cocaine use in humans leads to the comorbidity of neuropsychiatric phenotypes such as anxiety and depression [11]. However, as with reports on drug sensitivity and cognition, there have been varying reports on the effects of parental drug exposure on depression-like phenotypes [9, 12] in animal models, suggesting further examination of neuropsychiatric phenotypes is necessary.
Thus, we sought to investigate the behavioral implications of chronic, paternal cocaine administration on the subsequent generation of male and female offspring. We aimed to comprehensively characterize the behavioral phenotype of cocaine-sired offspring with regards to responsivity to reward, affective behavior, cognition, and social behavior.
2. METHODS
2.1 Breeding Scheme
Adult male C57BL/6J mice (Jackson Labs, Bar Harbor, ME) were treated with cocaine (20 mg/kg, intraperitoneal (i.p.)) starting at postnatal day 56, once a day for 75 days. Control mice received saline injections (0.01mL/g body weight) for the same number of days. Twenty-four hours after the last cocaine or saline injection, cocaine- and saline-exposed males (designated as F0 generation) were mated with drug naïve, primiparous C57BL/6J female mice (Jackson Labs, Bar Harbor, ME). All breeding cages housed one male and one female breeder. Females were removed from breeding cage once they were plug positive to eliminate the effect of male behavior on offspring. All offspring (F1 generation) were weaned at 21 days of age (P21) and male and female offspring were separated. Female mice bred with cocaine-exposed sires relative to controls were monitored for maternal behavior. Behavioral testing was performed on adult (>P60) male and female offspring. All procedures were conducted with approval from the Weill Cornell Medicine Institutional Animal Care and Use Committee and conducted in accordance with the National Institute of Health guidelines.
2.2 Behavioral Tests
To ensure no one litter was over represented, two independent cohorts of mice were behaviorally tested, with each cohort consisting of mice from at least four different litters (2-3 mice per litter per cohort), Behaviors in offspring involving drug treatment were conducted last. All other behaviors were conducted in a semi-random fashion and data revealed that order of testing had no impact of the phenotypes reported. Behavioral tests were separated by a minimum of 48 hours.
2.2.1 Basal Locomotor activity
Basal locomotor activity was performed as previously described [13]. For each test session, animals were placed in the chamber, and distance traveled was recorded for 1h without interruption.
2.2.2 Cocaine conditioned place preference (CPP)
A three-chamber place preference protocol (Med Associates Inc., St. Albans, VT, USA) was used as previously described [14]. On Day 1, mice were allowed to freely explore all three chambers (20 min). On Day 2-4 (conditioning sessions), a biased procedure was used wherein mice were paired with cocaine (5mg/kg, i.p. or 10 mg/kg, i.p.) for 20 min in the morning on the less preferred side, and paired with saline (0.01 mL/g body weight) for 20 min in the afternoon on the opposite side. On Day 5, mice were allow to freely explore the CPP box for 20 min. Time spent in the cocaine-paired chamber minus saline-paired chamber was calculated for post-conditioning (day 5) and pre-conditioning (day 1) and is referred to as CPP score.
2.2.3 Cocaine- and amphetamine-induced locomotor activity
Animals were injected with cocaine (10mg/kg, i.p.) or amphetamine (2 mg/kg, i.p.), immediately placed in the chamber, and distance traveled was recorded for 1hr without interruption as previously described [15].
2.2.4 Sucrose Consumption test
Mice were single housed for the duration of the sucrose consumption assay. The water bottle from each cage was removed and replaced with two smaller bottles (50mL Falcon tubes, Corning, Tewksbury, MA), one containing drinking water and the other containing 1% sucrose dissolved in drinking water. A hole was drilled into each small bottle, allowing the mice to lick the solution from the drilled hole. Body weights and the mass of water and sucrose consumed were monitored once a day for four days. The first two days were considered habituation days and not used in analyses. Sucrose consumption (averaged over days 3 and 4) was calculated as (sucrose consumed (g)/water consumed (g)).
2.2.5 Forced swim test
The test was performed in a 2L beaker containing 1800mL of 26°C water, for 10 minutes. Each mouse was video-recorded using a camera directed to the front of the beaker, as well as a camera above the beaker. Time spent immobile was scored by an experimenter blind to the conditions using the computer assisted software ButtonBox v5.1 (Behavioral Research Solutions, Madison, WI).
2.2.6 Elevated Plus Maze
Elevated plus maze test was performed as previously described [13]. Mice were placed in the center of a cross-shaped maze elevated 38 cm above the floor and consisting of two open and two closed arms (arm: 50.0 cm × 6.4 cm, height of closed arm: 15.2 cm). All trials were recorded with a video camera mounted above the maze. Time spent in the open and closed arms was obtained using the ANY-maze software (Stoelting Co., Wood Dale, IL).
2.2.7 Three-Chambered Social interaction
Social interaction was conducted as previously published [16] in a rectangular plastic apparatus (67.3 cm × 41.9 cm × 22.9 cm) containing three separate chambers that could be divided by plastic walls (40.6 cm × 15.2 cm). Following habituation, the experimental mouse was given access to all three chambers for five minutes. The experimental mouse was then contained in the center chamber for 1 minute while a pencil cup containing a novel object and a pencil cup containing an aged-matched stranger mouse (C57BL/6J) were placed in opposite chambers of the apparatus. The experimental mouse was then allowed to explore the full chamber for five minutes, and the total time spent with each cup was recorded. A virtual three-inch contact zone was created around each pencil cup. Sociability was assessed by comparing the amount of time spent within the stranger contact zone versus the novel object contact zone. All trials were recorded using a video camera mounted above the plastic apparatus and mouse position and interaction time was recorded using ANY-maze software (Stoelting Co., Wood Dale, IL).
2.2.8 Morris Water Maze
A modified version of the Morris water maze was utilized [17]. A black plastic pool with a radius of 48.3 cm and height of 36.8 cm was used. The maze was filled with water (25-26°C) and clouded with non-toxic, white tempura paint to conceal the position of an underwater escape platform submerged 1-1.5 cm beneath the surface. Four different starting points labeled North, East, South, and West were designated, and four distal cues (27.9 cm × 21.6 cm) were scattered on the walls surrounding the maze at a height of 49.5 cm. The maze was virtually split into triparts, with one tripart containing the platform (goal), and the other two containing nothing (lures). Mice were first habituated to the pool and allowed to swim freely for 60 seconds. Following habituation, animals underwent training, wherein 12 training trials were administered over the span of three days (four trials/day). During training, mice were released from different starting points on the maze and allowed to find the escape platform, which remained stationary throughout the experiment. The latency (secs) to locate the hidden platform was recorded. Mice were given a maximum of 60 seconds to find the platform, before being placed on the platform by the experimenter and removed from the maze. Following the last training trial, mice were given 48h to consolidate memory, and on day six, animals underwent seven more training trials as described previously. On day seven, a 24h probe to test long-term memory was conducted in the absence of the platform. The amount of time spent in the “goal” tripart was compared to time in the other two “lure” triparts. All trials were recorded using a video camera mounted above the maze and mouse position was tracked using ANY-maze software (Stoelting Co., Wood Dale, IL).
2.2.9 Water-based Y-maze
The apparatus consisted of three equal sized plastic arms (33.0 cm × 7.6 cm × 38.1 cm) 120° apart. The maze was filled with water (at 25-26°C, height: 22.9 cm) and clouded with non-toxic, white tempura paint. On day one, mice were habituated to the maze for three separate one-minute trials. On day two, ten training trials were administered. Mice were allowed to swim freely for a maximum of 60 seconds to discover an underwater escape platform in an adjacent arm of the Y-maze. The escape platform always remained in the same arm. If the platform was not found, the mouse was picked-up, placed on the platform for an additional 15 seconds, and then removed from the maze. A long-term memory test consisting of five trials was run 24 h after the last training trial. For each trial, mice were placed in the same start arm as training and allowed to swim freely. The latency (secs) to locate the platform during each trial was recorded.
2.3 Statistical Analyses
For all experiments, data were analyzed by a two-way or repeated measure ANOVA, or an independent samples t-test. For ANOVA analyses, if a significant interaction was achieved, post hoc comparisons were performed using the Bonferroni-Dunn post-hoc test. A value of p ≤ 0.05 was considered to be statistically significant. GraphPad Prism (La Jolla, CA) was used for all statistical analyses.
3. RESULTS
3.1 Generation of a mouse model to investigate intergenerational paternal cocaine exposure
To study the behavioral phenotypic effect of paternal cocaine exposure in male and female offspring, we generated mice as shown in Figure 1 and described in methods. There was no effect of paternal cocaine exposure on female pregnancy or male to female ratio of offspring. No qualitative differences in maternal behaviors, such as nest building or care of pups were observed between dams bred with cocaine-exposed sires relative to controls. Examination of basal locomotor activity, as cumulative distance measured at five minute time points over a total of 60 minutes, revealed a main effect of paternal cocaine exposure and time in male offspring, but no significant interaction (Fig. 2a, time, F11, 204 = 90.48, p < 0.0001; paternal exposure, F1, 204 = 6.799, p = 0.0098; time × paternal exposure, F11, 204 = 0.03250, p = 1.000). A main effect of time on distance traveled was observed in female offspring with no main effect of paternal exposure and no significant interaction (Fig 2b, time, F11, 228 = 91.71, p = < 0.0001; paternal exposure, F1, 228 = 0.1893, p = 0.6639; time × paternal exposure, F11, 228 = 0.2257, p = 1.000). Examination of body weights across three different ages (postnatal day (P) 85, P125, P245) revealed significant differences in both male (Fig. 2c, age × paternal exposure, F2, 47 = 9.173, p = 0.0004) and female cocaine-sired offspring (Fig. 2d, age × paternal exposure, F2, 54 = 27.32, p = 0.0129) relative to their saline-sired counterparts. Male cocaine-sired offspring weighed significantly less than saline-sired offspring at P85 (Bonferroni, p < 0.05), significantly more at P125 (p < 0.05) but had normalized by P245 (p > 0.05). For female offspring, cocaine-sired and saline-sired counterparts had similar weights at P85 and P125 (p > 0.05) but at P245, cocaine-sired females weighed significantly more (p < 0.01), demonstrating an effect of paternal cocaine exposure later in life for female offspring.
Figure 1.
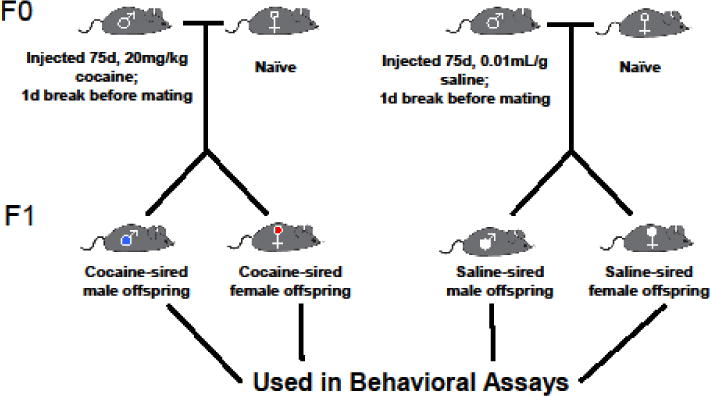
Breeding Scheme and Experimental Design. Sires received either cocaine (20mg/kg, i.p.) or saline (0.01mL/g, i.p.) for 75 days consecutively. On day 76, sires were given a rest day before being breeding with naïve females on day 77. Adult (> P60) male and female offspring were used in behavioral assays.
Figure 2.
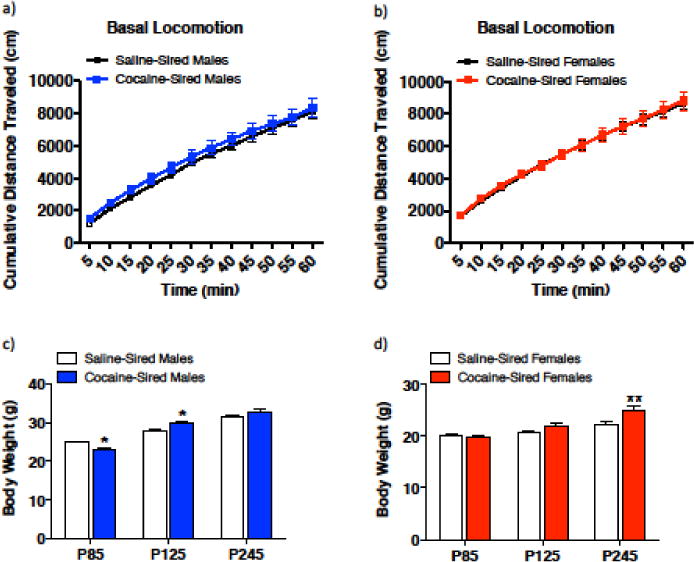
Paternal cocaine exposure influences basal locomotor activity in males and influences body weight in both male and female offspring. (a) There was a main effect of time as well as paternal exposure on cumulative distance traveled at five-minute time points between saline-sired males (n = 9) and cocaine-sired males (n = 8), but no interaction. (b) A main effect of time was observed with no main effect of paternal exposure or interaction on distance traveled between saline-sired females (n = 9) and cocaine-sired females (n = 9). (c) There was a significant difference between the weights of cocaine-sired males (n = 8) and saline-sired males (n = 9) at P85 and P125. At P85, cocaine-sired males weighed significantly less than saline-sired males. However, at P125, cocaine-sired males weigh significantly more than saline-sired males. (d) There was also a significant difference between the weights of cocaine-sired females (n = 9) compared to saline-sired females (n = 9). At P245, cocaine-sired females weighed significantly more than saline-sired females. *p < 0.05, **p < 0.01. Error bars represent mean ± SEM.
3.2 Altered cocaine conditioned place preference in cocaine-sired female mice
The rewarding effect of cocaine was first evaluated using the cocaine conditioned place preference (CPP) test with two different cocaine doses (5 and 10 mg/kg, i.p.). In males, using a two-way ANOVA, a significant main effect of conditioning was obtained with 5mg/kg (Fig. 3a, F1, 26 = 12.20, p = 0.0017) with no effect of paternal cocaine exposure or significant interaction (paternal exposure, F1, 26 = 0.2445, p = 0.6251; conditioning x paternal exposure, F1, 26 = 0.4032). At 10mg/kg, there was a main effect of conditioning but no effect of paternal cocaine exposure or significant interaction (Fig. 3b, conditioning, F1, 40 = 60.08, p < 0.0001; paternal exposure, F1, 40 = 0.01184, p = 0.9139; conditioning × paternal exposure, F1, 40 = 0.03241, p = 0.8580).
Figure 3.
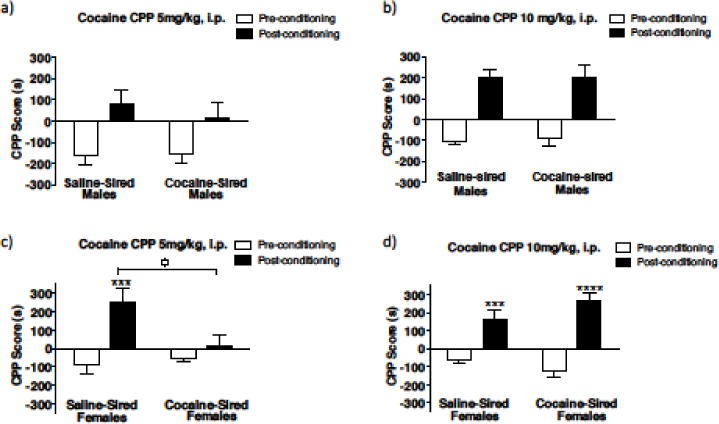
Female cocaine-sired mice exhibit altered cocaine conditioned place preference (CPP). (a) Cocaine CPP with 5mg/kg, i.p. cocaine in saline-sired (n = 8) and cocaine-sired (n = 7) male offspring. (b) Cocaine CPP with 10m/kg, i.p. cocaine in saline-sired (n=14) and cocaine-sired (n=10) male offspring. (c) With 5mg/kg, i.p. cocaine, saline-sired (n = 8) but not cocaine-sired (n = 9) female offspring exhibit a significantly higher CPP score post-conditioning compared to pre-conditioning. During post-conditioning, cocaine-sired females also display significantly lower CPP score compared to saline-sired females. (d) With 10mg/kg, i.p. cocaine, saline-sired (n = 8) and cocaine-sired (n = 9) male offspring both displayed significantly higher CPP scores on post-conditioning compared to pre-conditioning. ***p < 0.001,, post- vs. pre-conditioning. °p < 0.05, saline-sired female versus cocaine-sired female post-conditioning CPP score. Error bars represent mean ± SEM.
In female mice, with 5mg/kg, saline-sired, but not cocaine-sired, female mice had a significantly higher CPP score post-conditioning relative to pre-conditioning (Fig. 3c, conditioning x paternal exposure, F1, 34 = 6.202, p = 0.0178; saline-sired: Bonferroni, p < 0.001). Post-hoc analysis also revealed that during post-conditioning, cocaine-sired females displayed significantly lower preference for the cocaine-paired chamber relative to saline-sired females (Bonferroni, p < 0.05). In contrast, at 10mg/kg, both saline-sired and cocaine-sired female offspring had significantly higher CPP scored post-conditioning compared to pre-conditioning (Fig. 3d, conditioning × paternal exposure, F1, 32 = 4.880, p = 0.0344; saline-sired: Bonferroni, p < 0.001; cocaine-sired: Bonferroni p < 0.0001). Thus, at the lower dose of cocaine, cocaine-sired female offspring exhibited a decreased preference for cocaine compared to saline-sired females, suggesting blunted rewarding effects of cocaine. Whereas at the higher dose, cocaine-sired females showed similar preference to saline-sired female offspring suggesting paternal cocaine exposure has shifted the threshold of cocaine’s rewarding effects in female offspring.
3.3 Enhanced locomotor sensitivity to cocaine and amphetamine in male and female cocaine-sired offspring
To evaluate the acute locomotor stimulant effect of cocaine as a measure of sensitivity to cocaine, mice were injected with 10 mg/kg, i.p. cocaine. There was a significant main effect of time on distance traveled and a main effect of paternal cocaine exposure, but no significant interaction in male offspring (Fig. 4a, time, F11,204 = 32.11, p < 0.0001; paternal exposure, F1,204 = 11.56, p = 0.0008; time × paternal exposure, F1,204 = 0.06642, p = 1.000) and female offspring (Fig. 4b, time, F11,228 = 38.46, p < 0.0001; paternal exposure, F1,228 = 62.37, p < 0.0001; time × paternal exposure, F11,228 = 0.7070, p = 0.7314) when distance traveled was evaluated as cumulative distance at five minute time points over a total of 60 minutes.
Figure 4.
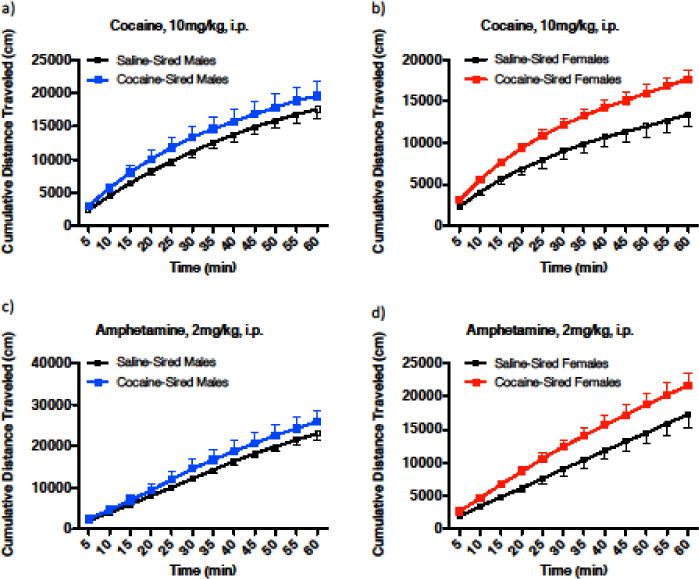
Male and female cocaine-sired mice have greater responsivity to the acute effects of cocaine and amphetamine. (a) For male offspring, there was a main effect of paternal cocaine exposure as well as a main effect of time on cocaine-induced locomotor activity, measured as cumulative distance traveled (cm) at 5-min time points (saline-sired, n = 10; cocaine-sired, n = 9). No interaction was reported. (b) For female offspring there was a main effect of paternal cocaine exposure on cocaine-induced locomotor activity and a main effect of time, when locomotor activity was measured as cumulative distance traveled (cm) at 5-min time points (saline-sired, n = 10; cocaine-sired, n = 11). No interaction was reported. (c–d) There was a main effect of paternal cocaine exposure as well as a main effect of time, but no interaction, on amphetamine-induced locomotor activity when cumulative distance was examined at 5-min time points for males (c) and females (d). Error bars represent mean ± SEM.
We additionally evaluated the locomotor effect of the psychostimulant amphetamine (2mg/kg, i.p.). Similarly, there was a main effect of paternal cocaine exposure, main effect of time but no significant interaction in male (Fig. 4c, time, F11, 204 = 45.62, p < 0.0001; paternal exposure, F1, 204 = 9.346, p = 0.0025; time × paternal exposure, F11,204 = 0.1600, p = 0.9991) and female offspring (Fig. 4d, time, F11, 228 = 41.06, p < 0.0001; paternal exposure, F1, 228 = 37.55, p < 0.0001; time × paternal exposure, F11, 228 = 0.5151, p = 0.8922). These results indicate that paternal cocaine exposure increases sensitivity to the stimulant effects of cocaine and amphetamine in both male and female offspring.
3.4 Higher sucrose consumption in cocaine-sired female offspring
As cocaine-sired offspring show altered cocaine reward response and higher sensitivity to the locomotive stimulant effects of cocaine and amphetamine, we tested mice in the sucrose consumption test, often used as a measure of natural reward [18], to evaluate if the higher sensitivity to psychostimulants was generalizable to a natural reward. Prior drug exposure has been linked to modifications in the reinforcing effects of natural rewards, such as sucrose [19]. Thus, we measured sucrose consumption in offspring prior to and following exposure to cocaine in the same cohort used in Figures 3 and 4, to evaluate baseline sensitivity to sucrose and drug-induced sensitivity. In cocaine-sired male offspring, there was no effect of timing of test on sucrose consumption, or paternal cocaine exposure, as well as no significant interaction (Fig. 5a, timing of test, F1,30 = 0.2753, p = 0.6037; paternal exposure, F1, 30 = 0.03427, p = 0.8544; timing of text × paternal exposure, F1,30 = 0.2848, p = 0.5975). However, in female offspring, there was a main effect of paternal cocaine exposure on sucrose consumption but no effect of timing of test and no interaction (Fig. 5b, timing of test, F1,34 = 0.4386, p = 0.5122; paternal exposure, F1, 34 = 8.083, p = 0.0075; timing of test × paternal exposure, F1,34 = 0.4392, p = 0.5120). Notably, paternal cocaine exposure had no effect on the total amount of liquid consumed in male (cocaine-sired, 11.04 ± 0.49g versus saline-sired, 9.80 ± 0.41g) or female (cocaine-sired, 10.66 ± 0.54g versus saline-sired, 10.37 ± 0.25g) offspring. These results indicate that cocaine-sired females have higher sensitivity for sucrose suggesting a generalized altered sensitivity to drugs of abuse and nature rewards not seen in male cocaine-sired offspring.
Figure 5.
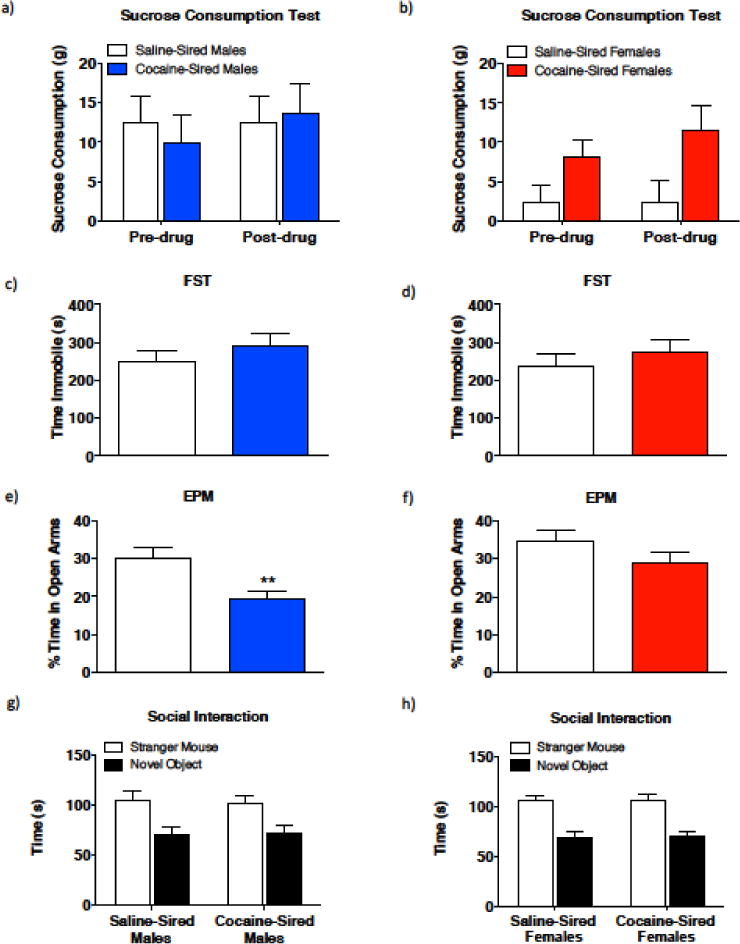
Paternal cocaine exposure increases sucrose consumption in female offspring and anxiety-like behavior in male offspring. (a) There was no significant difference between sucrose consumption of saline-sired and cocaine-sired males in both the pre- and post-testing conditions. (Saline-sired, n = 9; cocaine-sired, n = 8). (b) In the pre- and post-testing conditions, a main effect of paternal exposure was observed with no effect of timing of test and no interaction. Cocaine-sired female mice consumed higher amount of sucrose than saline-sired mice. (Saline-sired, n = 9; cocaine-sired, n = 10). (c–d) There was no significant difference in immobility time in the FST between saline-sired and cocaine-sired males (c, saline-sired, n = 12; cocaine-sired, n = 10) or females (d, saline-sired, n = 10; cocaine-sired, n = 13). (e) Compared to saline-sired males, cocaine-sired males spent significantly less time in the open arms of the elevated-plus-maze (saline-sired, n = 12; cocaine-sired, n = 11). (f) There was no significant difference in the amount of time spent in the open arms between saline-sired females and cocaine-sired females (saline-sired, n = 11; cocaine-sired, n = 13). (g–h) During the social interaction test, both saline-sired and cocaine-sired males (g, saline-sired, n = 15; cocaine-sired, n = 14) and females (h, saline-sired, n = 15; cocaine-sired, n = 15) spent more time with the stranger mouse compared to the novel object. **p < 0.01. Error bars represent mean ± SEM.
3.5 Paternal cocaine exposure has no effect on behavioral despair as a measure of depressive-like behavior in male or female cocaine-sired offspring
Because the sucrose consumption test is also used as a measure of anhedonia (depressive-like behavior) [20], we evaluated mice in the forced swim test to measure behavioral despair, another test to evaluate depressive-like behavior [21]. No significant difference was observed in time spent immobile between cocaine-sired and saline-sired males (Fig. 6c, t20 = 0.8422, p = 0.4096). Similarly no significant difference was observed between cocaine- and saline-sired female offspring (Fig. 6d, t21 = 0.7563, p = 0.4597), indicating that paternal cocaine exposure does not alter behavioral despair, and the higher sucrose consumption observed in cocaine-sired female mice is most likely indicative of altered reward sensitivity.
Figure 6.
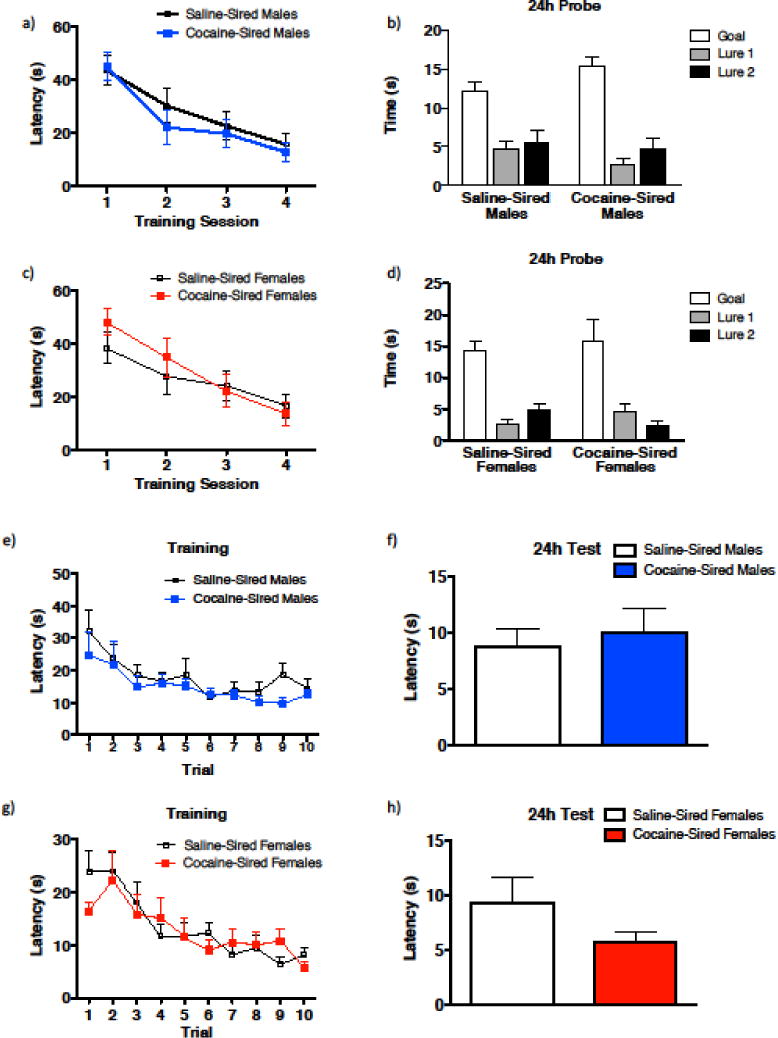
Male or female cocaine-sired offspring do not exhibit deficits in learning or memory in the Morris water maze or in the water-based Y-maze. (a, c) During the training phase of the Morris water maze, there was no significant difference in latency to find the platform between saline-sired and cocaine-sired males, and no effect of paternal exposure or interaction was observed (a. saline-sired, n = 10; cocaine-sired, n = 9) or females (c. saline-sired, n = 9; cocaine-sired, n = 9). (b, d) At the 24 hr probe test, there was a main effect of time in goal zone for both male (b) and female (d) saline- and cocaine-sired offspring but no effect of paternal exposure or interaction was observed. (e, g) During the training phase of the water-based Y-maze, there was no significant difference in latency to find the platform between saline-sired and cocaine-sired males (e, saline-sired, n = 10; cocaine-sired, n = 9) or females (g, saline-sired, n = 9; cocaine-sired, n = 9), and no effect of paternal exposure or interaction was observed. (f, h) At the 24 hr memory test, there was no significant difference in latency to find the platform between saline-and cocaine-sired males (f) or females (h). Error bars are mean ± SEM.
3.6 Male but not female cocaine-sired offspring exhibit enhanced anxiety-like behavior in the elevated plus maze
Since paternal cocaine exposure has been shown to increase anxiety-like behavior in males but not female offspring [12], we tested mice in the elevated plus maze (EPM) test. Cocaine-sired male offspring exhibited anxiety-like behavior as indicated by significantly less time spent in the open arms compared to saline-sired offspring (Fig. 5e, t21 = 2.842, p = 0.0098). No difference in anxiety-like behavior was observed in female mice (Fig. 5f, t22 = 1.337, p = 0.1948).
3.7 No effect of paternal cocaine exposure on social behavior in male or female cocaine-sired offspring
As anxiety [22] and the reward pathway have been shown to influence social behavior [23], we tested mice in the three-chamber social interaction task. A two-way ANOVA revealed a main effect of contact zone in both male and female offspring (Fig. 5g, male, contact zone, F1, 54 = 16.76, p = 0.0001; Fig. 5h, contact zone, female, F1, 56 = 42.23, p <0.0001), but no effect of paternal cocaine exposure and no interaction (male: paternal exposure, F1, 54 = 0.008104, p = 0.9286; contact zone × paternal exposure, F1,54 = 0.06510, p = 0.7996; female: paternal exposure, F1, 56 = 0.03039, p = 0.8625; contact zone × paternal exposure, F1,56 = 0.007112, p = 0.9331). Thus, the anxiety-like behavior observed in cocaine-sired male offspring and the altered reward responses observed in cocaine-sired female mice did not impact social behavior, as there was no effect of paternal exposure.
3.8 No effect of paternal cocaine exposure on learning and memory in male or female offspring
Paternal cocaine exposure has been associated with cognitive deficits; therefore, we tested learning and memory using a modified Morris water maze test (MWM) [24] and the water-based Y-maze test [25]. During MWM training, saline- and cocaine-sired males learned the task equally well (Fig. 6a, latency, F3, 68 = 11.23, p < 0.0001) with no main effect of paternal exposure or interaction (paternal exposure, F1, 68 = 0.6950, p =0.4074; latency × paternal exposure, F3, 68 = 0.2592, p = 0.8545). During the 24hour probe test (memory test; Fig. 6b), a main effect of time in target area (goal or lures) was found in males (time in target area, F2, 51 = 33.24, p < 0.0001) with no main effect of paternal exposure or interaction (paternal exposure, F1, 51 = 0.006116, p = 0.9380; time in target area × paternal exposure, F2, 51 = 2.164, p = 0.1253). Similarly, cocaine-sired female mice learned the task as well as saline-sired female mice and there was no main effect of paternal exposure or interaction (Fig. 6c, latency, F3, 64 = 8.689, p < 0.0001; paternal exposure, F1, 64 = 0.5674, p = 0.4540; latency × paternal exposure, F3, 64 = 0.6129, p = 0.6091). During the 24hour probe test (memory test; Fig. 6d), a main effect of time in target area (goal or lures) was found in females (time in target area, F2, 48 = 28.57, p < 0.0001) with no main effect of paternal exposure or interaction (paternal exposure, F1, 48 = 0.08396, p = 0.7732; time in target area × paternal exposure, F2, 48 = 0.9743, p = 0.3848).
During Y-maze training, male and female mice from all groups learned to find the platform with similar efficiency, and there was no main effect of paternal exposure and no interaction (Fig. 6e, male: latency, F9, 170= 3.952, p = 0.0001; paternal exposure, F1, 170 = 3.603, p = 0.0594; latency × paternal exposure, F9,170 = 0.3051, p = 0.9724; Fig. 6g, female: latency, F9, 160 = 6.502, p < 0.0001; paternal exposure: F1, 160 = 0.2914, p = 0.5901; latency × paternal exposure, F9,160 = 0.7089, p = 0.7001). Twenty four hours later, memory recall for the hidden platform was similar for both male and female groups (Fig. 6f, males, t17 = 0.4845, p = 0.6342; Fig. 6h, females, t16 = 1.437, p = 0.1699). Combined, the above results indicate that paternal cocaine exposure has no effect on learning and memory.
4. DISCUSSION
Here we provide a comprehensive behavioral characterization of the effects of paternal cocaine exposure on male and female offspring in an intergenerational mouse model. We find that paternal cocaine exposure alters sensitivity to the rewarding and stimulant effects of cocaine and amphetamine in female offspring. Additionally, female offspring exhibit higher sensitivity to sucrose, suggesting that paternal cocaine exposure results in alteration in generalized reward processing in female offspring. Male offspring exhibit heightened sensitivity to the stimulant effects of cocaine and amphetamine and higher anxiety-like behavior. There was no indication of behavioral despair, deficits in social behavior or learning and memory in cocaine-sired male or female offspring. Thus, this study supports and expands upon previous literature, showing paternal cocaine usage can impact behavior of offspring.
Using the CPP model, we observed that at a lower dose of cocaine, but not higher dose, female offspring exhibited decreased preference for cocaine. Although this is the first report of the use of cocaine CPP in offspring following paternal exposure to any class of drug, the use of cocaine CPP with the same lower dose in offspring following maternal cocaine exposure in rats showed that cocaine-sired offspring (not separated by sex) display a lack of CPP as well [26]. Using a natural reward test, we found cocaine-sired females consumed significantly more sucrose than saline-sired controls. These results indicate that there may be a shift in the threshold to experience the rewarding effects of cocaine caused by paternal cocaine exposure and response to a natural reward, such as sucrose. In this scenario, it is plausible that cocaine-sired females needed to consume a larger amount of sucrose to experience the same rewarding effects as seen in saline-sired controls, suggesting a blunted rewarding effect of sucrose similar to that observed using a low dose of CPP. As the cocaine CPP procedure involves a fixed, predetermined dose of cocaine, future studies using cocaine self-administration will better address this hypothesis.
Interestingly, we find that with age, cocaine-sired females weighed significantly more than saline-sired females. As parental drug-induced epigenetic alterations in genes within the brain’s reward pathway that have been linked with obesity-related diseases in offspring [27], like maternal cigarette smoking [28–30], it is possible that paternal cocaine exposure could cause later-life obesity in female offspring. While weight effects due to maternal drug use have been documented, weight effects due to paternal drug use are very limited, and rodent studies have not previously examined weight later in life.
Previous data using self-administration in rats has shown that cocaine-sired males, but not females, find cocaine less rewarding [8]. The inconsistency with our male and female offspring findings may arise from the mode of cocaine exposure to sires (investigator administered in this study versus sires self-administering cocaine) and the behavioral test employed (CPP versus self-administration).
We found that both cocaine-sired males and females were more sensitive to the locomotor activating effects of cocaine and amphetamine. This is inconsistent with the only study that examined effect of paternal exposure on psychomotor activity in C57BL/6 mice using the same cocaine dose we used [9]. However, consistent with our data, paternal alcohol exposure (rats) results in increased sensitivity to amphetamine in both male and female offspring [31] and maternal cocaine exposure (rats) results in increased sensitivity to cocaine in male offspring [32]. This higher activity may be due to alterations in the reward pathway such as higher psychomotor-induced dopamine release and related postsynaptic changes as suggested by Sasaki et al. [32]. Future experiments will address molecular mechanisms and pathways that underlie altered sensitivity to rewarding stimuli in cocaine-sired offspring.
To assess behavioral alterations that relate to neuropsychiatric disorders, such as anxiety, sociability, and depression, we ran multiple behavioral assays. The EPM test revealed that cocaine-sired male offspring have increased anxiety-like behavior while cocaine-sired females do not, consistent with previous reports of increased anxiety in cocaine-sired male rats using the novelty-induced hypophagia and defensive burying tests [12]. However, Killinger, Robinson and Stanwood [9] reported a lack of an effect of paternal cocaine exposure on anxiety-like behavior using the open field test. This study did not separate offspring by sex, which may explain the lack of a phenotype, which appears to be sex-dependent based on our findings and those of White et al. [12]. Elevated levels of corticotrophin-releasing factor receptor 2 (CRF-R2), known to influence anxiety-related behavior [33] and cocaine responses [34] have been found in cocaine-sired offspring [12], suggesting that this pathway may also contribute to the heightened anxiety and blunted cocaine response seen in cocaine-sired male offspring in our model.
Our study is the first to test sociability in cocaine-sired offspring and found no impairments in male or female cocaine-sired offspring. Similarly, we found no effect on depressive-like behavior in male or female cocaine-sired offspring, consistent with observations of White et al. [12] in rats. Conversely, Killinger, Robinson and Stanwood [9] used the tail suspension test (TST) and observed a depressive-like phenotype in C57BL/6 cocaine-sired offspring. The differences could possibly be due to utilization of different behavioral tests and the TST not being ideal for the C57BL/6 strain as tail climbing seen in this strain usually confounds results [35].
Previous literature has also been inconsistent with regards to the effect of paternal cocaine exposure on spatial learning and memory. Here, we see no deficits using the MWM test or the water Y-maze test, which supports previous findings [9]. However, using the five-arm radial maze (RAM) test, He, Lidow and Lidow [10] found that spatial working memory was impaired in both male and female cocaine-sired mice, with larger deficits present in cocaine-sired female mice. Similarly, Abel et al. [36] reported that the larger the dose of cocaine that was administered to the sires (30mg/kg, 15mg/kg, 0mg/kg), the more trials it took female offspring to reach criterion in spontaneous alternation in a T-Maze. The differences observed may be due to the different behavioral tests utilized assessing different aspects of memory; the T-Maze [37] and RAM [38] have been associated with working memory while the MWM [24] and Y-maze [39] have been associated with spatial learning and memory. Furthermore, differences may also be due to different underlying motivational basis of these tasks. In the MWM, the main goal is to escape the pool of water. Conversely, in the RAM test, the main motivation is to collect a food reward [40]. Given our findings, particularly in female offspring that suggest altered reward processing based on response to drugs and sucrose, it is possible that we would find differences due to paternal cocaine exposure in learning and memory tasks involving a food reward.
5. CONCLUSION
In summary, using an intergenerational mouse model, we find that paternal cocaine exposure impacts behaviors that recruit the reward pathway in female offspring to a much larger extent than in male offspring, while paternal cocaine exposure increases anxiety-like behaviors in male, but not female, offspring. Our study provides new input to the field and suggests that lack of separation of sex (male versus female versus combination of sexes) in previous literature may account for numerous discrepancies. Here, we show striking behavioral differences in male and female offspring. It is possible altered levels of certain sex-related hormones, such as estrogen, are an underlying cause of these sex-dependent phenotypes. Future experiments will examine the impact of paternal cocaine exposure on estrogen signaling as an imbalance has been associated with increased drug taking behaviors and increased locomotor drug sensitization [19]. Future studies will also examine the molecular and epigenetic basis of the transmitted behavioral phenotypes, as mechanisms have been suggested [6, 41] and investigate if the observed behavioral phenotypes are transmitted to subsequent generations.
Highlights.
Cocaine-sired female offspring show altered sensitivity to the psychomotor effects of psychostimulants and rewarding effect of the natural reward, sucrose
Cocaine-sired female offspring show blunted cocaine preference using cocaine at a low dose (5mg/kg), but display similar preference at a higher dose (10mg/kg)
Cocaine-sired male offspring exhibit increased psychomotor effects of cocaine and amphetamine
Cocaine-sired male offspring display an anxiety-like phenotype
Paternal cocaine exposure does not influence depressive-like, learning and memory, or social behavior in male or female offspring
Acknowledgments
This work was supported by the NIDA/NIH Grants [5RO1DA029122-04 (AM Rajadhyaksha)], and the NIDA Diversity Supplement [3R01DA029122-04S2 (A Martínez-Rivera)]. We thank Charlotte Bavley for critical reading of the manuscript. The authors declare that they have no conflicts of interest with the contents of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTION SECTION: DF, RCR, AM-R, MED and AMR designed research and wrote the manuscript; DF, RCR and AM-R performed research.
References
- 1.Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35(2):495–519. doi: 10.1016/j.psc.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55(11):973–9. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- 3.Keegan J, Parva M, Finnegan M, Gerson A, Belden M. Addiction in pregnancy. J Addict Dis. 2010;29(2):175–91. doi: 10.1080/10550881003684723. [DOI] [PubMed] [Google Scholar]
- 4.Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38(7):892–9. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Vassoler FM, Byrnes EM, Pierce RC. The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology. 2014;76(Pt B):269–75. doi: 10.1016/j.neuropharm.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol. 2015;118(1–2):21–33. doi: 10.1016/j.pbiomolbio.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Bruffaerts R, de Girolamo G, Gureje O, Huang Y, Karam A, Kostyuchenko S, Lepine JP, Mora ME, Neumark Y, Ormel JH, Pinto-Meza A, Posada-Villa J, Stein DJ, Takeshima T, Wells JE. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5(7):e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci. 2013;16(1):42–7. doi: 10.1038/nn.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killinger CE, Robinson S, Stanwood GD. Subtle biobehavioral effects produced by paternal cocaine exposure. Synapse. 2012;66(10):902–8. doi: 10.1002/syn.21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He F, Lidow IA, Lidow MS. Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol. 2006;28(2):198–209. doi: 10.1016/j.ntt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Herrero MJ, Domingo-Salvany A, Torrens M, Brugal MT, I. Investigators Psychiatric comorbidity in young cocaine users: induced versus independent disorders. Addiction. 2008;103(2):284–93. doi: 10.1111/j.1360-0443.2007.02076.x. [DOI] [PubMed] [Google Scholar]
- 12.White SL, Vassoler FM, Schmidt HD, Pierce RC, Wimmer ME. Enhanced anxiety in the male offspring of sires that self-administered cocaine. Addict Biol. 2016;21(4):802–10. doi: 10.1111/adb.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL, Lee A, Moosmang S, Hofmann F, Pieper AA, Rajadhyaksha AM. Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry. 2012;17(11):1054–5. doi: 10.1038/mp.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem. 2008;106(4):1780–90. doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schierberl K, Hao J, Tropea TF, Ra S, Giordano TP, Xu Q, Garraway SM, Hofmann F, Moosmang S, Striessnig J, Inturrisi CE, Rajadhyaksha AM. Cav1.2 L-type Ca(2)(+) channels mediate cocaine-induced GluA1 trafficking in the nucleus accumbens, a long-term adaptation dependent on ventral tegmental area Ca(v)1.3 channels. J Neurosci. 2011;31(38):13562–75. doi: 10.1523/JNEUROSCI.2315-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inan M, Zhao M, Manuszak M, Karakaya C, Rajadhyaksha AM, Pickel VM, Schwartz TH, Goldstein PA, Manfredi G. Energy deficit in parvalbumin neurons leads to circuit dysfunction, impaired sensory gating and social disability. Neurobiol Dis. 2016;93:35–46. doi: 10.1016/j.nbd.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 18.Levine AS, Kotz CM, Gosnell BA. Sugars: hedonic aspects, neuroregulation, and energy balance. Am J Clin Nutr. 2003;78(4):834S–842S. doi: 10.1093/ajcn/78.4.834S. [DOI] [PubMed] [Google Scholar]
- 19.Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiol Behav. 2013;112–113:96–103. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14(9):609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4(9):775–90. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 22.Beery AK, Kaufer D. Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress. 2015;1:116–127. doi: 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16(3):e92–123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. Removal of FKBP12 enhances mTOR-Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron. 2008;60(5):832–45. doi: 10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heyser CJ, Miller JS, Spear NE, Spear LP. Prenatal exposure to cocaine disrupts cocaine-induced conditioned place preference in rats. Neurotoxicol Teratol. 1992;14(1):57–64. doi: 10.1016/0892-0362(92)90029-a. [DOI] [PubMed] [Google Scholar]
- 27.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12(11):638–51. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 28.von Kries R, Toschke AM, Koletzko B, Slikker W., Jr Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol. 2002;156(10):954–61. doi: 10.1093/aje/kwf128. [DOI] [PubMed] [Google Scholar]
- 29.Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. Int J Epidemiol. 2002;31(2):413–9. [PubMed] [Google Scholar]
- 30.Wideroe M, Vik T, Jacobsen G, Bakketeig LS. Does maternal smoking during pregnancy cause childhood overweight? Paediatr Perinat Epidemiol. 2003;17(2):171–9. doi: 10.1046/j.1365-3016.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 31.Abel EL. Paternal alcohol exposure and hyperactivity in rat offspring: effects of amphetamine. Neurotoxicol Teratol. 1993;15(6):445–9. doi: 10.1016/0892-0362(93)90063-t. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki A, Constantinof A, Pan P, Kupferschmidt DA, McGowan PO, Erb S. Cocaine exposure prior to pregnancy alters the psychomotor response to cocaine and transcriptional regulation of the dopamine D1 receptor in adult male offspring. Behav Brain Res. 2014;265:163–70. doi: 10.1016/j.bbr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Krysiak R, Obuchowicz E, Herman ZS. Role of corticotropin-releasing factor (CRF) in anxiety. Polish journal of pharmacology. 1999;52(1):15–25. [PubMed] [Google Scholar]
- 34.Kasahara M, Groenink L, Bijlsma EY, Olivier B, Sarnyai Z. Lifelong, central corticotropin-releasing factor (CRF) overexpression is associated with individual differences in cocaine-induced conditioned place preference. Eur J Pharmacol. 2015;753:151–7. doi: 10.1016/j.ejphar.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 35.Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology (Berl) 2001;155(1):110–2. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- 36.Abel EL, Moore C, Waselewsky D, Zajac C, Russell LD. Effects of cocaine hydrochloride on reproductive function and sexual behavior of male rats and on the behavior of their offspring. J Androl. 1989;10(1):17–27. doi: 10.1002/j.1939-4640.1989.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 37.Chrobak JJ, Hanin I, Walsh TJ. AF64A (ethylcholine aziridinium ion), a cholinergic neurotoxin, selectively impairs working memory in a multiple component T-maze task. Brain Res. 1987;414(1):15–21. doi: 10.1016/0006-8993(87)91322-9. [DOI] [PubMed] [Google Scholar]
- 38.Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2(2):97. [Google Scholar]
- 39.Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8(2):151–4. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodges H. Maze procedures: the radial-arm and water maze compared. Brain Res Cogn Brain Res. 1996;3(3–4):167–81. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- 41.Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2014;264:198–206. doi: 10.1016/j.neuroscience.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]


