Abstract
Chloromonas nivalis (Volvocales, Chlorophyceae) is considered a cosmopolitan species of a snow-inhabiting microalga because cysts morphologically identifiable as zygotes of the species are distributed worldwide. However, recent molecular data demonstrated that field-collected cysts identified as the zygotes consist of multiple species. Recently, we demonstrated that species identification of snow-inhabiting Chloromonas species is possible based on light and electron microscopy of asexual life cycles in strains and molecular phylogenetic analyses. Vegetative cells without eyespots and of inverted-teardrop shape have been reported once in North American material of C. nivalis; however, strains with such vegetative cells in snow-inhabiting species of Chloromonas have not been examined taxonomically in detail. Here, we used light and transmission electron microscopy together with molecular analyses of multiple DNA sequences to examine several C. nivalis strains. The morphological data demonstrated that one North American strain could be identified as C. nivalis, whereas three other strains should be re-classified as C. hoshawii sp. nov. and C. remiasii sp. nov. based on vegetative cell morphology, the number of zoospores within the parental cell wall during asexual reproduction, and whether cell aggregates (resulting from repeated divisions of daughter cells retained within a parental cell wall) were observed in the culture. This taxonomic treatment was supported by multigene phylogeny and comparative molecular analyses that included a rapidly evolving DNA region. Our molecular phylogenetic analyses also demonstrated that the North American strain of C. nivalis was phylogenetically separated from the Austrian and Japanese specimens previously identified as C. nivalis based on zygote morphology.
Introduction
During the snow melt season, snowfields in polar regions and snowpacks in mountainous areas are sometimes stained green, red, or other colors. These events are typically caused by blooms of cold-adapted microalgae [1–3]. In green snow, species belonging to the genus Chloromonas Gobi (Volvocales, Chlorophyceae) are generally dominant [2,3]. The genus contains at least 16 snow-inhabiting species [4–7] in addition to approximately 130 mesophilic morphological species [8,9], all of which are unicellular, green, and biflagellate. Taxonomic studies based on consecutive light microscopy (LM) of field-collected materials from North America revealed that several previously described snow species of nonmotile chlorococcalean algae, such as Scotiella Fritsch spp., are actually identical to the zygotes of snow-inhabiting Chloromonas species [10–13].
Among the snow-inhabiting Chloromonas species, C. nivalis (Chodat) Hoham et Mullet was considered cosmopolitan because of the world-wide distribution of cysts morphologically identified as zygotes of this species [formerly classified as Scotiella nivalis (Chodat) F.E. Fritsch] based on studies of North American material [1,11]. The species was generally identified solely on the basis of zygote morphology [1,14–17] according to the species concept of previous studies [11,18]. This reliance on morphology possibly arises from the difficulty of inducing vegetative cell production from field-collected zygotes of snow-inhabiting Chloromonas. Very recently, Scotiella tatrae Kol was transferred to C. nivalis and reduced to a subspecies as C. nivalis subsp. tatrae Procházková et al., based on morphological and molecular data obtained from field-collected materials from Austria and Slovakia [19,20]. Recent molecular data also demonstrated that field-collected cysts morphologically identified as C. nivalis zygotes are composed of at least four distinct lineages or species, one of which is considered conspecific with the strains of C. miwae (Fukushima) Muramoto et al. [6]. In addition, it has been demonstrated that correct species identification of snow Chloromonas species is possible based on light and electron microscopy of asexual life cycles of cultures, as well as by molecular phylogenetic analyses [5]. Vegetative cells without eyespots and of inverted teardrop shape have been reported once in North American material of C. nivalis [11]. Subsequently, several strains from public culture collections were designated as C. nivalis [21–23], possibly based on such vegetative cell morphology. To date, three strains designated as C. nivalis (CCCryo 005–99, UTEX SNO66 and UTEX SNO74) have been examined by molecular phylogenetic analyses or LM of asexual reproductive cell morphology [19,23–26]. However, detailed descriptions of vegetative cell morphology in these strains have not been provided. In addition, molecular phylogenetic analyses suggest that these strains were not monophyletic [6,19].
Therefore, in the present study, we carried out taxonomic re-examination of strains designated as C. nivalis using detailed morphological and molecular analyses. The data demonstrate that one North American strain not previously studied could be identified as C. nivalis, whereas the other strains should be re-classified as C. hoshawii Matsuzaki et al. sp. nov. and C. remiasii Matsuzaki et al. sp. nov. We also document phylogenetic relationships between the North American strain of C. nivalis and previously examined specimens of C. nivalis zygotes.
Materials and methods
Cultures
Three strains assigned to C. nivalis in previous studies (CCCryo 005–99, UTEX SNO66 and UTEX SNO74) [19,23–25], one North American strain labeled as C. nivalis (UTEX SNO71) [21], and Chloromonas sp. strain CCCryo 047–99 (phylogenetically close to the strain CCCryo 005–99 [19]) were provided by the Culture Collection of Cryophilic Algae (CCCryo) at the Fraunhofer Institute for Cell Therapy and Immunology [22] and the Culture Collection of Algae at the University of Texas at Austin (UTEX) [21,27] (S1 Table). The cultures were maintained on AF-6 medium [28] (liquid or 1.5% agar slants) at 5°C on a 14:10-h light:dark cycle under cool-white light-emitting diodes (color temperature = 5000 K) at 35–90 μmol photons m−2·s−1.
The strain UTEX SNO74 was excluded from further analyses because light microscopic and molecular data demonstrated that it has been replaced with a species of Trebouxia (Trebouxiales, Trebouxiophyceae) (S1 Text; S2 Table; S1 Fig).
Morphological observations
Light and epifluorescence microscopy were performed using a BX51 microscope (Olympus Corp., Tokyo, Japan) equipped with Nomarski differential interference optics. Transmission electron microscopy (TEM) was performed as described previously [5] using a JEM-2010 transmission electron microscope (JEOL, Tokyo, Japan). Cells in actively growing 5- to 12-day-old cultures were investigated. In addition, we carried out LM of cultures at 1, 2 and 3 months after inoculation to detect the production of cell aggregates resulting from repeated divisions of daughter cells retained within the parental cell wall [5,29].
Molecular analysis
For molecular analysis, we used nucleotide sequences of nuclear-encoded 18S and 26S ribosomal DNA (rDNA), chloroplast-encoded ATP synthase beta subunit (atpB), P700 chlorophyll a apoprotein A2 (psaB) and the large subunit of RuBisCO (rbcL) genes, and internal transcribed spacer 2 (ITS2) region of nuclear rDNA. Sequences from five snow-inhabiting strains and of the 12 mesophilic ones (S3 Table) were determined as described previously [6,30] using newly designed specific primers (S4 Table).
For multigene phylogeny, we used four strains examined in this study (CCCryo 005–99, CCCryo 047–99, UTEX SNO66 and UTEX SNO71) as well as 28 operational taxonomic units examined in previous studies [6,9] (S3 Table). All belong to the genus Chloromonas sensu Pröschold et al. [31] or the Chloromonadinia clade [32]. The mesophilic strains (S3 Table) were treated as the outgroup according to previous results [9,24,25]. The 18S and 26S rDNA, atpB and psaB gene sequences were aligned as described previously [5,33,34]. In addition, only the first and second codon positions of the nucleotides in the atpB and psaB were used for phylogenetic analyses. This was because the third nucleotide positions of the codons had an unusual base composition and markedly higher substitution rates than the 18S and 26S rDNA and the first and second codon positions of the atpB and psaB genes [6,33,35–37]. The combined 5,497-bp data matrix of the regions was subjected to Bayesian inference (BI), maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ) analyses as described in previous studies [9,33] except that IQ-TREE v. 1.4.3 [38] was used in ML analysis instead of PAUP 4.0b10 [39]. In each analysis, identical sequences were reduced to a single operational taxonomic unit. Since rbcL gene substitutions in Chloromonas are unusual and may result in artifacts [33,40], we did not concatenate the rbcL gene sequences with the data matrix.
For comparison of the previously published sequence data from field-collected cysts identified as C. nivalis zygotes, we performed single-gene phylogenetic analyses using 18S rDNA or rbcL gene sequences as described above. In addition, we set three partitions (first, second, and third codon positions) for BI and ML analysis of rbcL gene sequences according to a previous study [4]. Additional operational taxonomic units were selected from previous studies [19,20,41] and shown in S3 Table.
Substitution models for each phylogenetic analysis are described in S5 Table. The data matrices used in the present study are available from TreeBASE [42] (matrix accession number S22105).
Methods for annotation and prediction of secondary structures of nuclear rDNA ITS2 region were described in a previous study [5]. For detecting compensatory base changes (CBCs), the ITS2 sequences were aligned on the basis of sequence-structure analysis [43] using 4SALE [44,45].
Nomenclature
The electronic version of this article in Portable Document Format (PDF) in a work with an ISSN or ISBN will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) (http://iapt-taxon.org/nomen/main.php), and hence the new names contained in the electronic publication of a PLOS article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies.
Results
Morphological observation
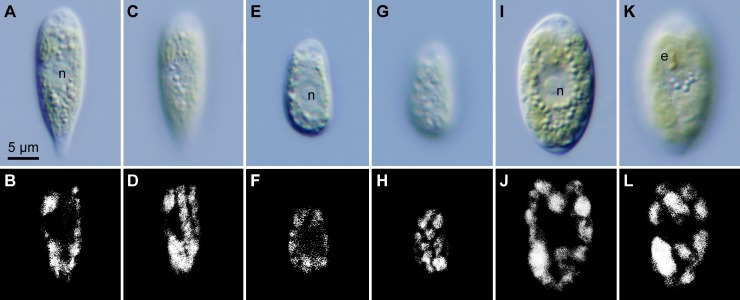
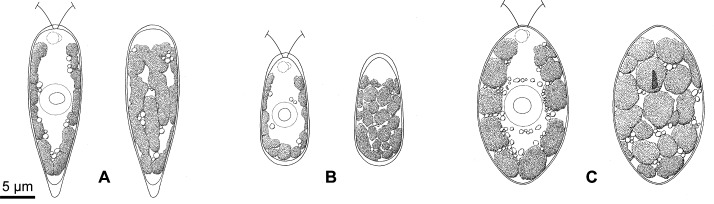
Light and epifluorescence microscopy (Figs 1 and 2) demonstrated that the strains could be subdivided into three morphological species (C. hoshawii, C. nivalis, and C. remiasii) based on differences in cell shape and size, chloroplast morphology, presence of eyespots, number of zoospores formed within the parental cell wall during asexual reproduction, and presence of cell aggregates (aggregates of 16 or more cells resulting from repeated divisions of daughter cells retained within a parental cell wall [5,29]) in culture (Table 1). In C. nivalis strain UTEX SNO71, vegetative cells had an inverted-teardrop shape with a prominent posterior tail (Figs 1A and 2A). On the other hand, vegetative cells of C. hoshawii strain UTEX SNO66 were ellipsoidal to elongate-ovoid (Figs 1E and 2B), and those of C. remiasii strains CCCryo 005–99 and CCCryo 047–99 were ellipsoidal to spindle-shaped (Figs 1I and 2C); a prominent posterior tail was not observed in the cells of the latter two species in culture. The three species lacked a prominent anterior papilla (Figs 1A, 1E, 1I and 2A–2C). In some cells of C. hoshawii, cell wall became thicker at the anterior and posterior cell end (Fig 2B). The vegetative cell length of C. hoshawii (13.8–18.6 μm) was smaller than that of C. nivalis (20.2–28.6 μm) or of C. remiasii (18.2–30.8 μm) (Table 1). Although the chloroplasts of the three species were cup-shaped (Figs 1A, 1B, 1E, 1F, 1I, 1J and 2A–2C), the surface view of the chloroplast of C. nivalis appeared as elongate-ovoid or elongate-cylindrical platelets (Figs 1C, 1D and 2A), whereas that of C. hoshawii and C. remiasii appeared as angular discs (Figs 1G, 1H, 1K, 1L, 2B and 2C). Vegetative cells of C. remiasii possessed an ellipsoidal or elongate-D-shaped eyespot positioned in the anterior third of the cell (Figs 1K and 2C). In contrast, eyespots were not observed in the cells of C. hoshawii or of C. nivalis (Figs 1C, 1G, 2A and 2B).
Fig 1. Vegetative cells of the three snow-inhabiting Chloromonas species: Light micrographs.
Identical magnification throughout. Abbreviations: e, eyespot; n, nucleus. (A-D) C. nivalis (Chodat) Hoham et Mullet strain UTEX SNO71. (A) Optical section. (B) Epifluorescence image of (A). (C) Surface view. (D) Epifluorescence image of (C). (E-H) C. hoshawii Matsuzaki et al. sp. nov. strain UTEX SNO66. (E) Optical section. (F) Epifluorescence image of (E). (G) Surface view. (H) Epifluorescence image of (G). (I-L) C. remiasii Matsuzaki et al. sp. nov. strain CCCryo 005–99. (I) Optical section. (J) Epifluorescence image of (I). (K) Surface view. (L) Epifluorescence image of (K).
Fig 2. Vegetative cells of the three snow-inhabiting Chloromonas species: Line drawings.
Identical magnification throughout. Left, optical section. Right, surface view. (A) C. nivalis (Chodat) Hoham et Mullet. (B) C. hoshawii Matsuzaki et al. sp. nov. (C) C. remiasii Matsuzaki et al. sp. nov.
Table 1. Morphological characteristics of the three snow-inhabiting Chloromonas species.
| C. nivalis | C. hoshawii sp. nov. | C. remiasii sp. nov. | |
|---|---|---|---|
| Strain(s) | UTEX SNO71 | UTEX SNO66 | CCCryo 005–99, CCCryo 047–99 |
| Cell shape | inverted teardrop, prominent posterior tail | ellipsoidal to elongate-ovoid, rounded posterior end | ellipsoidal to spindle, rounded posterior end |
| Cell width × cell length | 6.6–12.4 μm × 20.2–28.6 μm | 4.9–9.3 μm × 13.8–18.6 μm | 10.2–15.6 μm × 18.2–30.8 μm |
| Chloroplast shape | cup-shaped, seemingly composed of elongate platelets | cup-shaped, seemingly composed of angular discs | cup-shaped, seemingly composed of angular discs |
| Eyespot | absent | absent | present |
| Number of zoospores formed within the parental cell wall | up to 16 | 2 or 4 (rarely 8) | 2 or 4 (rarely 8) |
| Cell aggregates in culture | not observed | not observed | observed |
Asexual reproduction of the three species (S2 Fig) occurred through zoospore formation by successive cell divisions, as described in a report of C. nivalis from North America [11]. Immediately prior to the first cell division, the parental contractile vacuoles moved to the equator of the cell by protoplast rotation (arrows in S2A, S2C and S2E Fig). Typically, up to four zoospores were seen in C. hoshawii (S2D Fig) and C. remiasii (S2F Fig), and up to 16 in C. nivalis (S2B Fig) (Table 1). In addition, cell aggregates resulting from repeated divisions of daughter cells retained within the parental cell wall [5,29] were produced in fresh (5- to 12-day-old) cultures as well as in old (almost or more than one-month-old) cultures of C. remiasii (S3 Fig). In contrast, such cell aggregates were not observed in the other two species (Table 1). Sexual reproduction or hypnospore formation was not observed in the three species. All three species failed to grow at 20°C after two weeks of cultivation, as described in previous reports of other species of snow-inhabiting Chloromonas [5,6,46].
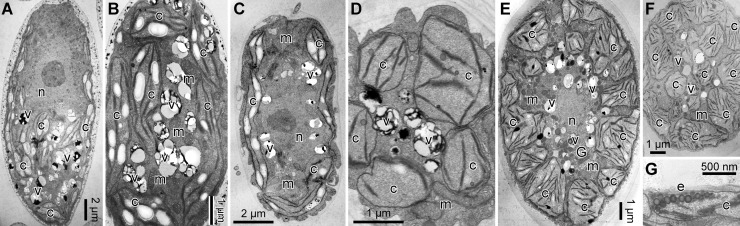
TEM (Fig 3) showed that each cell of the three species possessed a nucleus, and a cup-shaped chloroplast without pyrenoid matrices (Fig 3A, 3C and 3E). As in other snow-inhabiting Chloromonas species [5], mitochondria and Golgi bodies were present mainly between the nucleus and chloroplast. Several small vacuoles with crystalline content were observed in the cytoplasm of the three species (Fig 3A–3F). Tangential sections of C. nivalis showed the chloroplast profiles to be almost elongate in shape (Fig 3B). In contrast, chloroplasts of C. hoshawii and C. remiasii were generally angular in shape (Fig 3D and 3F). LM surface views of the chloroplasts correlated with TEM images; the chloroplasts appeared to be composed of elongate platelets or angular discs (Figs 1C, 1D, 1G, 1H, 1K, 1L and 2A–2C). The eyespot of C. remiasii was comprised of a single layer of electron-dense globules (Fig 3G). Such structures were not seen in C. hoshawii or in C. nivalis, even under TEM.
Fig 3. Vegetative cells of the three snow-inhabiting Chloromonas species: Transmission electron micrographs.
Abbreviations: c, chloroplast; e, eyespot; G, Golgi body; m, mitochondrion; n, nucleus; v, vacuole with crystalline content. (A, B) C. nivalis (Chodat) Hoham et Mullet strain UTEX SNO71. (A) Longitudinal cell section. (B) Tangential cell section. (C, D) C. hoshawii Matsuzaki et al. sp. nov. strain UTEX SNO66. (C) Longitudinal cell section. (D) Tangential cell section. (E-G) C. remiasii Matsuzaki et al. sp. nov. strain CCCryo 005–99. (E) Longitudinal cell section. (F) Tangential cell section. (G) Eyespot composed of a single layer of electron-dense globules.
Molecular phylogenetic analyses
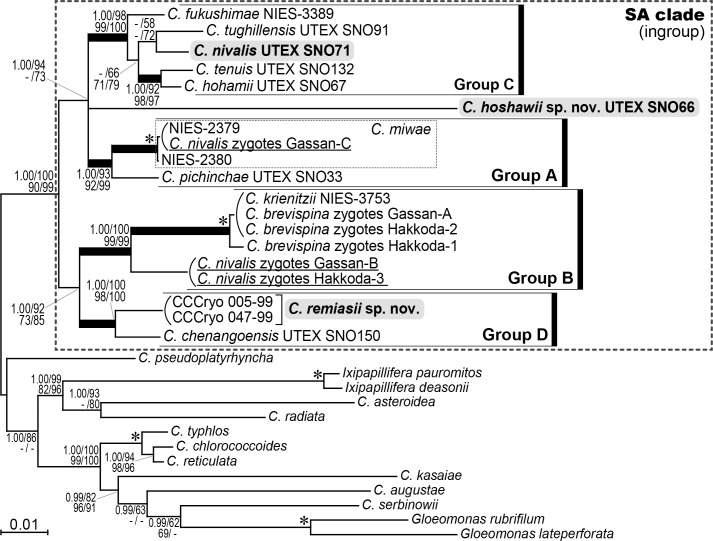
Phylogenetic analyses (based on the sequences of 18S and 26S rDNA, and the first and the second codon positions of atpB and psaB), revealed four robust monophyletic groups of snow-inhabiting Chloromonas species (A–D) resolved with 1.00 posterior probabilities (PP) in BI and 92–100% bootstrap values (BV) in ML, MP and NJ analyses (Fig 4). Chloromonas nivalis strain UTEX SNO71 and both C. remiasii strains (CCCryo 005–99 and CCCryo 047–99) were included within groups C and D, respectively, whereas C. hoshawii strain UTEX SNO66 was positioned outside of the four groups and therefore represents an independent lineage. Group C contained C. fukushimae Matsuzaki et Nozaki, C. hohamii H.U. Ling et Seppelt, C. nivalis, C. tenuis Matsuzaki et Nozaki, and C. tughillensis Hoham et al. Within the group, C. nivalis was sister to C. tughillensis with 58% and 72% BV in ML and NJ analyses, respectively. Chloromonas hohamii and C. tenuis formed another clade supported by 1.00 PP in BI and 92–98% BV in ML, MP and NJ analyses. The two subclades were sister to each other (66–79% BV in ML, MP and NJ analyses), and C. fukushimae was the most basally located strain. Group D was composed of C. chenangoensis Hoham et al. and C. remiasii. Importantly, Japanese specimens identified as C. nivalis zygotes (Gassan-B, Gassan-C and Hakkoda-3 [6]) were positioned within groups A and B, and were well separated from the North American C. nivalis strain UTEX SNO71. Group A comprised C. pichinchae Wille strain UTEX SNO33 together with a small robust clade containing the two strains of C. miwae and a specimen of C. nivalis zygotes, Gassan-C; this subclade was considered a single species in a recent molecular analysis [6]. Group B contained two C. nivalis zygote specimens (Gassan-B and Hakkoda-3), three C. brevispina (F.E. Fritsch) Hoham et al. zygote specimens (Gassan-A, Hakkoda-1 and Hakkoda-2 [6]) and C. krienitzii Matsuzaki et Nozaki strain NIES-3753. In the present multigene phylogenetic tree, the four robust monophyletic groups and one independent lineage of C. hoshawii were subdivided into two large clades: one composed of groups A and C together with C. hoshawii (1.00 PP in BI and 94% and 73% BV in ML and NJ analyses, respectively), and the other constructed of groups B and D (1.00 PP in BI and 73–92% BV in ML, MP and NJ analyses). Within the former clade, phylogenetic relationships among groups A and C and C. hoshawii were not resolved.
Fig 4. Bayesian phylogenetic tree of snow-inhabiting Chloromonas spp. based on 5,497 base pairs from 18S and 26S rDNA, and the first and the second codon positions of atpB and psaB.
C. nivalis zygote specimens (field-collected samples) are underlined. Corresponding posterior probabilities (PP; 0.95 or more) are shown at top left. Numbers shown in top right, bottom left and bottom right indicate bootstrap values (BV; 50% or more) in maximum likelihood (ML), maximum parsimony (MP) and neighbor-joining (NJ) analyses, respectively. Branches within the SA clade (recovered at 1.00 PP and 90% or more BV in ML, MP and NJ analyses) are shown by thick lines. Asterisk indicates 1.00 PP in BI and 100% BV in ML, MP and NJ analyses.
Further comparison of phylogenetic relationships between C. nivalis strain UTEX SNO71 and field-collected C. nivalis zygote specimens examined in previous studies [19,20,41] was performed by single-gene phylogenetic analyses using 18S rDNA and rbcL sequences (S4 and S5 Figs). Both trees reconstructed the monophyletic groups B–D which were robustly resolved in the multigene phylogenetic tree (Fig 4), but statistical support values for monophyly were lower. Group A in Fig 4 was recovered only in the rbcL-based tree (S5 Fig). In the 18S rDNA- and rbcL-based trees (S4 and S5 Figs), the Austrian C. nivalis zygote specimen (P24/DR4 [19,20]) and the Slovak C. nivalis subsp. tatrae zygote specimen (LP01 [20]) were positioned within group B and formed a small robust clade (1.00 PP in BI and >89% BV in ML, MP and NJ analyses). This subclade was sister to the Japanese C. nivalis zygote specimens (Gassan-B and Hakkoda-3 [6]) with 1.00 PP in BI and 74–83% and 83–94% BV in ML, MP and NJ analyses in 18S rDNA- and rbcL-based tree, respectively. In addition, the two Japanese C. nivalis zygote specimens (Gassan-NIV1 and Gassan-NIV2 [41]) were positioned outside of groups A–D in the phylogenetic tree of rbcL sequences (S5 Fig). However, C. nivalis strain UTEX SNO71 was included within group C in 18S rDNA- and rbcL-based trees, and was phylogenetically separated from the Austrian, Japanese and Slovak C. nivalis zygote specimens.
Comparative molecular analyses
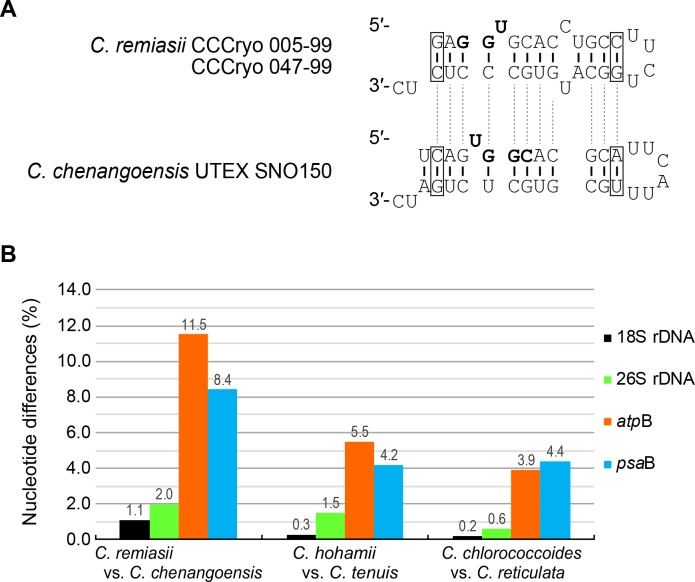
To verify separation of C. remiasii and C. chenangoensis, which were sister to each other (Fig 4), we compared the secondary structures of the nuclear rDNA ITS2 region. The predicted secondary structures (S6 and S7 Figs) possessed four helices, a U-U mismatch in helix II (S6 and S7 Figs, arrowheads), and the YGGY motif on the 5′ side near the apex of helix III (S6 Fig and S7 Fig, boldface). All these features are common structural hallmarks of eukaryote nuclear rDNA ITS2 secondary structures [47–50]. In C. remiasii and C. chenangoensis, at least two CBCs were detected near the apex of helix III encompassing the YGGY motif (the most conserved region of nuclear rDNA ITS2 secondary structures [48,49]) (Fig 5A). In addition, we estimated the uncorrected p-distances in nuclear-encoded 18S and 26S rDNA, and in chloroplast-encoded atpB and psaB genes, for C. remiasii and C. chenangoensis. The nucleotide differences between the two species were much larger than those between snow-inhabiting C. hohamii and C. tenuis, and also between mesophilic C. chlorococcoides (H. Ettl et K. Schwarz) Matsuzaki et al. and C. reticulata (Goroschankin) Gobi, each pair being sister species previously delineated by morphological and molecular data [5,51] (Fig 5B).
Fig 5. Genetic differences between Chloromonas remiasii Matsuzaki et al. sp. nov. and C. chenangoensis Hoham et al.
(A) Comparison of the most conserved region (near the apex of helix III encompassing the YGGY motif) of nuclear rDNA ITS2 secondary structures. Open box indicates compensatory base change. Boldface marks the YGGY motif. For the complete nuclear rDNA ITS2 secondary structures, see S6 and S7 Figs. (B) Nucleotide differences (%) from pairwise comparisons in four genes. Black: nuclear-encoded 1,748 bases of 18S ribosomal DNA (rDNA). Green: nuclear-encoded 2,020 bases of 26S rDNA. Red: chloroplast-encoded 1,128 bases of ATP synthase beta subunit gene (atpB). Blue: chloroplast-encoded 1,392 bases of P700 chlorophyll a apoprotein A2 gene (psaB). Note that the sequences from Chloromonas remiasii strains CCCryo 005–99 and CCCryo 047–99 were identical. The nucleotide differences between snow-inhabiting and mesophilic sister species [C. hohamii H.U. Ling et Seppelt vs. C. tenuis Matsuzaki et Nozaki; and C. chlorococcoides (H. Ettl et K. Schwarz) Matsuzaki et al. vs. C. reticulata (Goroschankin) Gobi] are according to the previous study [5].
Discussion
Zygotes or cysts morphologically identified as C. nivalis have been reported from various localities of the world [1,11,18]. However, motile vegetative cells directly obtained from such dormant cells have never been reported [11,19]; the partial life cycle (from vegetative cells to zygotes) of C. nivalis was observed only in North American field-collected material [11]. The type locality of Pteromonas nivalis Chodat (the basionym of C. nivalis) is in the French Alps [52]; however, the original species description lacks information on motile vegetative cells, and neither a strain nor sequences are available. Recent robust molecular data indicated that Japanese field-collected cysts morphologically identical to the North American C. nivalis zygotes (= P. nivalis and S. nivalis [11]) contain multiple species [6]. Thus, C. nivalis should be circumscribed by the vegetative morphology reported from the North American material [11].
The light microscopic features of the North American strain UTEX SNO71, which has not been examined in previous studies, were consistent with those of North American C. nivalis [11] with respect to cell shape, chloroplast morphology, and the number of zoospores formed within the parental cell wall (Table 1; S2 Text). Thus, we consider the strain UTEX SNO71 as C. nivalis, although zygotes were not observed in our study. Contrary, the vegetative morphology of strains previously designated as C. nivalis (CCCryo 005–99 and UTEX SNO66) [19,23,24] differed from that of strain UTEX SNO71 (Table 1). In addition, these three strains were phylogenetically well separated from each other (Fig 4). Therefore, based on morphology and phylogeny of vegetative cells, we re-classified strains CCCryo 005–99 and UTEX SNO66 as C. remiasii and C. hoshawii, respectively.
LM and TEM showed that chloroplasts of C. hoshawii and C. remiasii lack pyrenoids (Figs 1E, 1I and 3C–3F), and the species are robustly positioned within Chloromonadinia clade (Fig 4). These characteristics correspond to both traditional [8,53] and phylogenetically revised [31] concepts of the genus Chloromonas. Among the snow-inhabiting species of the genus, C. hoshawii resembles C. chenangoensis and C. pichinchae in having an ellipsoidal or elongate-ovoid vegetative cell with rounded anterior and posterior ends, and a chloroplast which appeared to be composed of angular discs and had no eyespot (Figs 1E and 2B; S2 Text; S6 Table) [5,8,10,25]. However, C. hoshawii differs from C. pichinchae in that it does not produce cell aggregates in old cultures (S2 Text; S6 Table) [5]. Maximum cell width is less than 10 μm in C. hoshawii, whereas vegetative cell width of C. chenangoensis is up to 17.5 μm (S2 Text; S6 Table) [5,25]. Furthermore, the phylogenetic position of C. hoshawii strain UTEX SNO66 is separated from those of C. chenangoensis strain UTEX SNO150 and C. pichinchae strain UTEX SNO33 (Fig 4). On the other hand, C. remiasii is very similar to C. alpina Wille in possessing an ellipsoidal vegetative cell with rounded anterior and posterior ends, and a chloroplast seemingly composed of angular discs and having an eyespot (Figs 1I, 1K and 2C; S2 Text; S6 Table) [8,53,54]. However, C. remiasii differs from C. alpina (of which no sequences are available) in cell size (10.2–15.6 μm wide × 18.2–30.8 μm long vs. 4–7 μm wide × 9–12 μm long, respectively; S6 Table) [8,53,54]. Although the present phylogenetic results demonstrate that C. remiasii is sister to C. chenangoensis (Fig 4), C. remiasii can be distinguished from C. chenangoensis in having an eyespot on the chloroplast and in producing cell aggregates in culture (Figs 1K and 2C; S3 Fig; S2 Text; S6 Table) [5,25]. In addition, the two species had at least two CBCs in the most conserved region of nuclear rDNA ITS2 secondary structures (Fig 5A). The CBCs correlate with the separation of biological species, according to [49]. Furthermore, genetic differences in the four genes between these two species were much larger than those between snow-inhabiting C. hohamii and C. tenuis, or between mesophilic C. chlorococcoides and C. reticulata, each pair being sister species delineated by morphological and molecular data [5,51] (Fig 5B). Therefore, the separation of C. remiasii and C. chenangoensis was supported by morphological and molecular data, and apparently they have different patterns of geographic distribution (Arctic Svalbard vs. Arizona, USA [21,22,23,25]).
Although neither C. hoshawii nor C. remiasii could grow at 20°C, a comparison of their vegetative morphology with that of mesophilic Chloromonas species was performed: The mesophilic species C. enteromorphae (Brabez) Gerloff et H. Ettl, C. eumaculata P.C. Silva, C. gutenbrunnensis Wawrik, and C. granulata (L.Ş. Péterfi) Gerloff et H. Ettl resemble C. hoshawii and C. remiasii in that the cells are ovoid to ellipsoidal with rounded anterior and posterior ends, and they have a cup-shaped chloroplast seemingly composed of angular discs [8,53]. However, C. hoshawii differs from the mesophilic species by the lack of an eyespot on the chloroplast (Figs 1G and 2B) [8,53,55–58]. The eyespot of C. remiasii is positioned in the anterior third of the cell, whereas those of C. eumaculata and C. gutenbrunnensis are located near the equator or in the posterior third of the cell, respectively [8,53,55,58]. The cell wall of C. granulata is quite swollen; this trait was not observed in vegetative cells of C. remiasii (Figs 1I and 2C) [8,53,56]. The nucleus of C. remiasii is positioned in the middle of the protoplast (Figs 1I and 2C), whereas the nucleus is in the posterior third of the cell in C. enteromorphae [8,53,57]. Moreover, the vegetative cells of C. remiasii are smaller than those of C. enteromorphae (up to 30.8 μm long vs. up to 44 μm long, respectively) (Table 1) [57]. Thus, C. hoshawii and C. remiasii represent two new morphological species of the genus Chloromonas.
Molecular phylogenetic analyses (Fig 4; S4 and S5 Figs) demonstrated that the North American strain morphologically assignable to C. nivalis from North America is phylogenetically separated from Austrian, Japanese and Slovak field-collected zygote specimens earlier identified as C. nivalis [6,19,20,41]. Therefore, taxonomic re-examination of the latter specimens should be carried out based on their vegetative morphologies. In addition, scanning electron microscopic features of the zygotes might also help their taxonomic revision [20]. Although no one has successfully induced the production of motile vegetative cells from field-collected zygotes of snow-inhabiting Chloromonas under controlled laboratory conditions [10–13,19,23], our recent study provided a practical method for molecular identification of such zygotes by using data obtained from accurately identified cultures [6]. Thus, further taxonomic studies of cultured snow-inhabiting Chloromonas are required to reveal the correct affiliation of field-collected cysts currently identified as C. nivalis zygotes.
Taxonomic treatments
Chloromonas hoshawii Matsuzaki, Nozaki et Kawachi sp. nov.
Vegetative cells solitary, having two flagella, without a prominent anterior papilla. Cells ellipsoidal or elongate-ovoid; 4.9–9.3 μm wide and 13.8–18.6 μm long. Cells with a central nucleus and a single cup-shaped chloroplast. Chloroplast seemingly composed of angular discs, showing irregular incisions on the surface, without an eyespot and pyrenoids. Asexual reproduction by formation of generally two or four zoospores, with rotation of the protoplast before the first cell division. Cell aggregates not observed in culture.
Holotype: Specimen TNS-AL-58946 deposited at TNS (National Museum of Nature and Science, Tsukuba, Japan); material consists of resin-embedded vegetative cells from strain UTEX SNO66.
Strain examined: UTEX SNO66 (Table 1).
Etymology: The species epithet hoshawii is in honor of Dr. Robert W. Hoshaw who contributed greatly to the taxonomy of green algae (e.g. [59,60]). He participated in collection of material from which the authentic strain of this species was isolated [21].
Chloromonas remiasii Matsuzaki, Nozaki et Kawachi sp. nov.
Vegetative cells solitary, having two flagella, without a prominent anterior papilla. Cells ellipsoidal or spindle-shaped; 10.2–15.6 μm wide and 18.2–30.8 μm long. Cells with a central nucleus and a single cup-shaped chloroplast. Chloroplast seemingly composed of angular discs, showing irregular incisions on the surface, with an eyespot and without pyrenoids. Eyespot ellipsoidal to elongate D-shaped, positioned in the anterior third of the cell, composed of a single layer of globules. Asexual reproduction by formation of generally two or four zoospores, with rotation of the protoplast before the first cell division. Cell aggregates observed in culture.
Holotype: Specimen TNS-AL-58947 deposited at TNS (National Museum of Nature and Science, Tsukuba, Japan); material consists of resin-embedded vegetative cells from strain CCCryo 005–99.
Strains examined: CCCryo 005–99, CCCryo 047–99 (Table 1).
Etymology: The species epithet remiasii is in honor of Dr. Daniel Remias, who has contributed greatly to the ecology and physiology of snow-inhabiting microalgae (e.g. [19,26,61]).
Type locality: Bjørnhamna, Reuschhalvøya, Spitsbergen, Svalbard, Norway [22,23].
Remarks: A previous study [23] suggested relationship between the strain CCCryo 005–99 and field-collected cysts or zygotes, both of which were collected at the same location in Svalbard. The cysts resemble North American C. nivalis zygotes in having spindle-shaped cell with several longitudinal, slightly helical ridges on the cell wall extended partially to the poles. Since molecular data of the cysts are not available and sexual reproduction of C. remiasii has not been observed, we could not confirm this possible relationship.
Supporting information
Abbreviations: c, chloroplast; n, nucleus; p, pyrenoid. (A) Optical section focused on a pyrenoid. (B) Surface view. The strain [formerly designated as Chloromonas nivalis (Chodat) Hoham et Mullet] was not used in course of this study since the strain might be replaced with contamination by the species of the genus Trebouxia (see S1 Text; S2 Table).
(TIF)
All at identical magnification. Arrows in A, C, E indicate position of each contractile vacuole originating from the parent cell. (A, B) C. nivalis (Chodat) Hoham et Mullet strain UTEX SNO71. (A) Immediately prior to the first transverse division. (B) Sixteen daughter cells within the parental cell wall. Note that only 12 of the 16 cells are recognized. (C, D) C. hoshawii Matsuzaki et al. sp. nov. strain UTEX SNO66. (C) Immediately prior to the first transverse division. (D) Four daughter cells within the parental cell wall. (E, F) C. remiasii Matsuzaki et al. sp. nov. strain CCCryo 005–99. (E) Immediately prior to the first transverse division. (F) Four daughter cells within the parental cell wall.
(TIF)
Aggregates result from repeated divisions of daughter cells retained in parental cell walls (double arrowhead). Open arrowhead indicates a daughter cell wall surrounding offspring of a daughter cell. All at the identical magnification. (A) Strain CCCryo 005–99 after 7 days in liquid AF-6 medium. (B) Strain CCCryo 047–99 after 3 months on 1.5% agar slant of AF-6.
(TIF)
C. nivalis zygote specimens (Field-collected samples) are underlined, and the Austrian C. nivalis zygote specimen (P24/DR4 [19]) and the Slovak C. nivalis subsp. tatrae zygote specimen (LP01 [20]) are shadowed in black. Groups A–D are as indicated in Fig 4. The corresponding posterior probabilities (PP, 0.95 or more) are shown at the top left. Numbers shown in top right, bottom left and bottom right indicate bootstrap values (BV, 50% or more) from maximum likelihood (ML), maximum parsimony (MP) and neighbor-joining (NJ) analyses. Asterisk indicates 1.00 PP in BI and 100% BV in ML, MP, and NJ analyses.
(TIF)
C. nivalis zygote specimens (Field-collected samples) are underlined, and the Austrian and Japanese C. nivalis zygote specimens examined in the previous studies (P24/DR4 [19,20], and Gassan-NIV1 and Gassan-NIV2 [41], respectively) and the Slovak C. nivalis subsp. tatrae zygote specimen (LP01 [20]) are shadowed in black. Groups A–D are as in Fig 4. The corresponding posterior probabilities (PP, 0.95 or more) are shown at the top left. Numbers shown in top right, bottom left and bottom right indicate bootstrap values (BV, 50% or more) from maximum likelihood (ML), maximum parsimony (MP) and neighbor-joining (NJ) analyses. Asterisk indicates 1.00 PP in BI and 100% BV in ML, MP and NJ analyses.
(TIF)
The 3′ end of the 5.8S ribosomal RNA (rRNA) and the 5′ end of the 26S rRNA are shown (DDBJ/ENA/GenBank accession number: HQ404862). The sequence from C. remiasii strains CCCryo 005–99 is identical to that from CCCryo 047–99 (LC360496). Note U-U mismatch in helix II (arrowheads) and the YGGY motif on the 5′ side near the apex of helix III (boldface), common structural hallmarks of eukaryotic nuclear rDNA ITS2 secondary structures [47,50].
(TIF)
The 3′ end of the 5.8S ribosomal RNA (rRNA) and the 5′ end of the 26S rRNA are shown (DDBJ/ENA/GenBank accession number: LC360497). Note U-U mismatch in helix II (arrowheads) and the YGGY motif on the 5′ side near the apex of helix III (boldface), common structural hallmarks of eukaryotic nuclear rDNA ITS2 secondary structures [47,50].
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors are grateful to Ms. Yasuko Yoshikawa and Ms. Shizuko Kinoshita (National Institute for Environmental Studies, Japan) for their kind support in transmission electron microscopy.
Data Availability
New sequence data, alignments used for our phylogenetic analyses, and the holotype specimens are available under the DDBJ/ENA/GenBank accession numbers (LC360463–LC360497, and LC361432), TreeBASE ID (S22105), and the herbarium specimen numbers for the holotypes (TNS-AL-58946 and TNS-AL-58947), respectively. All other relevant data are within the manuscript and its Supporting Information files.
Funding Statement
RM was supported by Grants-in-Aid for Research Activity Start-up (No. 15H06148) and JSPS Research Fellow (No. 16J09828) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI (https://www.jsps.go.jp/english/e-grants/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kol E. Kryobiologie. Biologie und Limnologie des Schnees und Eises. I. Kryovegetation In: Elster H-J, Ohle W, editors. Die Binnengewässer 24. Stuttgart: E. Schweizerbart'sche Verlagsbuchhandlung (Nägele u. Obermiller); 1968. p. 1–216 with 16 pls. German. [Google Scholar]
- 2.Hoham RW. Unicellular chlorophytes—snow algae In: Cox ER, editor. Phytoflagellates. New York: Elsevier-North Holland; 1980. p. 61–84. [Google Scholar]
- 3.Hoham RW, Duval B. Microbial ecology of snow and freshwater ice with emphasis on snow algae In: Jones HG, Pomeroy JW, Walker DA, Hoham RW, editors. Snow ecology. An interdisciplinary examination of snow-covered ecosystems. Cambridge: Cambridge University Press; 2001. p. 168–228. [Google Scholar]
- 4.Muramoto K, Nakada T, Shitara T, Hara Y, Nozaki H. Re-examination of the snow algal species Chloromonas miwae (Fukushima) Muramoto et al., comb. nov. (Volvocales, Chlorophyceae) from Japan, based on molecular phylogeny and cultured material. Eur J Phycol. 2010;45: 27–37. doi: 10.1080/09670260903272607 [Google Scholar]
- 5.Matsuzaki R, Hara Y, Nozaki H. A taxonomic study of snow Chloromonas species (Volvocales, Chlorophyceae) based on light and electron microscopy and molecular analysis of cultured material. Phycologia. 2014;53: 293–304. doi: 10.2216/14-3.1 [Google Scholar]
- 6.Matsuzaki R, Kawai-Toyooka H, Hara Y, Nozaki H. Revisiting the taxonomic significance of aplanozygote morphologies of two cosmopolitan snow species of the genus Chloromonas (Volvocales, Chlorophyceae). Phycologia. 2015;54: 491–502. doi: 10.2216/15-33.1 [Google Scholar]
- 7.Kirjakov IK, Velichkova KN. New species of green snow algae Chloromonas (Volvocales, Chlorophyta) from Bulgaria. International Journal of Fisheries and Aquatic Studies. 2016;4: 94–95. [Google Scholar]
- 8.Ettl H. Chlorophyta. I. Phytomonadina In: Ettl H, Gerloff J, Heynig H, Molenhauer D, editors. Süßwasserflora von Mitteleuropa 9. Stuttgart: G. Fischer Verlag; 1983. p. 1–807. German. [Google Scholar]
- 9.Matsuzaki R, Nakada T, Hara Y, Nozaki H. Description of Chloromonas kasaiae sp. nov. (Volvocales, Chlorophyceae), based on comparative electron microscopy and molecular data. Phycologia. 2013;52: 239–245. doi: 10.2216/12-083.1 [Google Scholar]
- 10.Hoham RW. The life history and ecology of the snow alga Chloromonas pichinchae (Chlorophyta, Volvocales). Phycologia. 1975;14: 213–226. doi: 10.2216/i0031-8884-14-4-213.1 [Google Scholar]
- 11.Hoham RW, Mullet JE. The life history and ecology of the snow alga Chloromonas cryophila sp. nov. (Chlorophyta, Volvocales). Phycologia. 1977;16: 53–68. doi: 10.2216/i0031-8884-16-1-53.1 [Google Scholar]
- 12.Hoham RW, Roemer SC, Mullet JE. The life history and ecology of the snow alga Chloromonas brevispina comb. nov. (Chlorophyta, Volvocales). Phycologia. 1979;18: 55–70. doi: 10.2216/i0031-8884-18-1-55.1 [Google Scholar]
- 13.Hoham RW, Mullet JE, Roemer SC. The life history and ecology of the snow alga Chloromonas polyptera comb. nov. (Chlorophyta, Volvocales). Can J Bot. 1983;61: 2416–2429. doi: 10.1139/b83-266 [Google Scholar]
- 14.Marchant HJ. Snow algae from the Australian snowy mountains. Phycologia. 1982;21: 178–184. doi: 10.2216/i0031-8884-21-2-178.1 [Google Scholar]
- 15.Ling HU. Snow algae of the Windmill Islands region, Antarctica. Hydrobiologia. 1996;336: 99–106. doi: 10.1007/BF00010823. [Google Scholar]
- 16.Müller T, Bleiß W, Martin C-D, Rogaschewski S, Fuhr G. Snow algae from northwest Svalbard: their identification, distribution, pigment and nutrient content. Polar Biol. 1998; 20: 14–32. doi: 10.1007/s003000050272 [Google Scholar]
- 17.Lukavský J, Cepák V. Cryoseston in Stara Planina (Balkan) mountains, Bulgaria. Acta Bot Croat. 2010;69: 163–171. [Google Scholar]
- 18.Hoham RW, Mullet JE. Chloromonas nivalis (Chod.) Hoh. & Mull. comb. nov., and additional comments on the snow alga, Scotiella. Phycologia. 1978;17: 106–107. doi: 10.2216/i0031-8884-17-1-106.1 [Google Scholar]
- 19.Remias D, Karsten U, Lütz C, Leya T. Physiological and morphological processes in the Alpine snow alga Chloromonas nivalis (Chlorophyceae) during cyst formation. Protoplasma. 2010;243: 73–86. doi: 10.1007/s00709-010-0123-y . [DOI] [PubMed] [Google Scholar]
- 20.Procházková L, Remias D, Řezanka T, Nedbalová L. Chloromonas nivalis subsp. tatrae, subsp. nov. (Chlamydomonadales, Chlorophyta): re-examination of a snow alga from the High Tatra Mountains (Slovakia). Fottea. 2018;18: 1–18. doi: 10.5507/fot.2017.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UTEX Culture Collection of Algae [Internet]. Texas: The University of Texas at Austin; c2017 [cited 2017 Jan 26]. Available from: https://utex.org/.
- 22.Culture Collection of Cryophilic Algae [Internet]. Brandenburg: The Fraunhofer Institute for Cell Therapy and Immunology; c2016 [cited 2017 Jan 26]. Available from: http://cccryo.fraunhofer.de/web/infos/welcome/.
- 23.Leya T. Feldstudien und genetische Untersuchungen zur Kryophilie der Schneealgen Nordwestspitzbergens [dissertation]. Berlin: Humboldt-Universität zu Berlin; 2004. German.
- 24.Hoham RW, Bonome TA, Martin CW, Leebens‐Mack JH. A combined 18S rDNA and rbcL phylogenetic analysis of Chloromonas and Chlamydomonas (Chlorophyceae, Volvocales) emphasizing snow and other cold-temperature habitats. J Phycol. 2002;38: 1051–1064. doi: 10.1046/j.1529-8817.2002.t01-1-01227.x [Google Scholar]
- 25.Hoham RW, Berman JD, Rogers HS, Felio JH, Ryba JB, Miller PR. Two new species of green snow algae from Upstate New York, Chloromonas chenangoensis sp. nov. and Chloromonas tughillensis sp. nov. (Volvocales, Chlorophyceae) and the effects of light on their life cycle development. Phycologia. 2006;45: 319–330. doi: 10.2216/04-103.1 [Google Scholar]
- 26.Remias D, Wastian H, Lütz C, Leya T. Insights into the biology and phylogeny of Chloromonas polyptera (Chlorophyta), an alga causing orange snow in maritime Antarctica. Antarct Sci. 2013;25: 648–656. doi: 10.1017/S0954102013000060 [Google Scholar]
- 27.Starr RC, Zeikus JA. UTEX-the culture collection of algae at the university of Texas at Austin. J Phycol. 1993;29 Suppl 2: 1–106. doi: 10.1111/j.0022-3646.1993.00001.x [Google Scholar]
- 28.Kawachi M, Ishimoto M, Mori F, Yumoto K, Sato M, Noël M-H. MCC-NIES. List of Strains, 9th Edition [DVD]. Tsukuba: National Institute for Environmental Studies; 2013. [Google Scholar]
- 29.Ling HU, Seppelt RD. Snow algae of the Windmill Islands, continental Antarctica. Polar Biol. 1998;20: 320–324. doi: 10.1007/s003000050309 [Google Scholar]
- 30.Nakada T, Nozaki H, Pröschold T. Molecular phylogeny, ultrastructure, and taxonomic revision of Chlorogonium (Chlorophyta): emendation of Chlorogonium and description of Gungnir gen. nov. and Rusalka gen. nov. J Phycol. 2008;44: 751–760. doi: 10.1111/j.1529-8817.2008.00525.x . [DOI] [PubMed] [Google Scholar]
- 31.Pröschold T, Marin B, Schlösser UG, Melkonian M. Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist. 2001;152: 265–300. doi: 10.1078/1434-4610-00068 . [DOI] [PubMed] [Google Scholar]
- 32.Nakada T, Misawa K, Nozaki H. Molecular systematics of Volvocales (Chlorophyceae, Chlorophyta) based on exhaustive 18S rRNA phylogenetic analyses. Mol Phylogenet Evol. 2008;48: 281–291. doi: 10.1016/j.ympev.2008.03.016 . [DOI] [PubMed] [Google Scholar]
- 33.Nozaki H, Nakada T, Watanabe S. Evolutionary origin of Gloeomonas (Volvocales, Chlorophyceae), based on ultrastructure of chloroplasts and molecular phylogeny. J Phycol. 2010;46: 195–201. doi: 10.1111/j.1529-8817.2009.00773.x [Google Scholar]
- 34.Nakada T, Tomita M, Wu J-T, Nozaki H. Taxonomic revision of Chlamydomonas subg. Amphichloris (Volvocales, Chlorophyceae), with resurrection of the genus Dangeardinia and descriptions of Ixipapillifera gen. nov. and Rhysamphichloris gen. nov. J Phycol. 2016;52: 283–304. doi: 10.1111/jpy.12397 . [DOI] [PubMed] [Google Scholar]
- 35.Nozaki H, Misumi O, Kuroiwa T. Phylogeny of the quadriflagellate Volvocales (Chlorophyceae) based on chloroplast multigene sequences. Mol Phylogenet Evol. 2003;29: 58–66. doi: 10.1016/S1055-7903(03)00089-7 . [DOI] [PubMed] [Google Scholar]
- 36.Nakada T, Nozaki H. Taxonomic study of two new genera of fusiform green flagellates, Tabris gen. nov. and Hamakko gen. nov. (Volvocales, Chlorophyceae). J Phycol. 2009;45: 482–492. doi: 10.1111/j.1529-8817.2009.00652.x . [DOI] [PubMed] [Google Scholar]
- 37.Sugasawa M, Matsuzaki R, Arakaki Y, Nozaki H. Morphology and phylogenetic position of a rare four-celled green alga, Pascherina tetras (Volvocales, Chlorophyceae), based on cultured material. Phycologia. 2015;54: 342–348. doi: 10.2216/15-27.1 [Google Scholar]
- 38.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32: 268–274. doi: 10.1093/molbev/msu300 ; PubMed Central PMCID: PMC4271533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (* and other methods) [CD-ROM]. Version 4.0b10. Sinauer: Sunderland, MA: 2002. [Google Scholar]
- 40.Nozaki H, Onishi K, Morita E. Differences in pyrenoid morphology are correlated with differences in the rbcL genes of members of the Chloromonas lineage (Volvocales, Chlorophyceae). J Mol Evol. 2002;55: 414–430. doi: 10.1007/s00239-002-2338-9 . [DOI] [PubMed] [Google Scholar]
- 41.Muramoto K, Kato S, Shitara T, Hara Y, Nozaki H. Morphological and genetic variation in the cosmopolitan snow alga Chloromonas nivalis (Volvocales, Chlorophyta) from Japanese mountainous area. Cytologia. 2008;73: 91–96. doi: 10.1508/cytologia.73.91 [Google Scholar]
- 42.TreeBASE [Internet]. Durham (NC): 2010 Mar—[cited 2017 Oct 26]. Available from: https://treebase.org/treebase-web/home.html.
- 43.Schultz J, Wolf M. ITS2 sequence–structure analysis in phylogenetics: a how-to manual for molecular systematics. Mol Phylogenet Evol. 2009;52: 520–523. doi: 10.1016/j.ympev.2009.01.008 . [DOI] [PubMed] [Google Scholar]
- 44.Seibel PN, Müller T, Dandekar T, Schultz J, Wolf M. 4SALE–A tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinformatics. 2006;7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seibel PN, Müller T, Dandekar T, Wolf M. Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4SALE. BMC Res Notes. 2008;1: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoham RW, Frey FM, Mohn WW, Felio JH, Todd S, Duncan JE, Banghart JB. Optimum growth temperatures of three species of green Chloromonas snow algae from Upstate New York and the White Mountains, Arizona. Arct Antarct Alp Res. 2008;40: 355–363. doi: 10.1657/1523-0430(07–038)[HOHAM]2.0.CO;2 [Google Scholar]
- 47.Coleman AW. ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends Genet. 2003;19: 370–375. doi: 10.1016/S0168-9525(03)00118-5 . [DOI] [PubMed] [Google Scholar]
- 48.Coleman AW. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Res. 2007;35: 3322–3329. doi: 10.1093/nar/gkm233 ; PubMed Central PMCID: PMC1904279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman AW. Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Mol Phylogenet Evol. 2009;50: 197–203. doi: 10.1016/j.ympev.2008.10.008 . [DOI] [PubMed] [Google Scholar]
- 50.Schultz J, Maisel S, Gerlach D, Müller T, Wolf M. A common core of secondary structure of the internal transcribed spacer 2 (ITS2) throughout the Eukaryota. RNA. 2005;11: 361–364. doi: 10.1261/rna.7204505 ; PubMed Central PMCID: PMC1370725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuzaki R, Hara Y, Nozaki H. A taxonomic revision of Chloromonas reticulata (Volvocales, Chlorophyceae), the type species of the genus Chloromonas, based on multigene phylogeny and comparative light and electron microscopy. Phycologia. 2012;51: 74–85. doi: 10.2216/11-18.1 [Google Scholar]
- 52.Chodat R. Algues vertes de la Suisse. PleurococcoÏdes-chloolépoÏdes. Beiträge zur Kryptogamenflora der Schweiz. 1902;1: 1–373. French. [Google Scholar]
- 53.Ettl H. Die gattung Chloromonas Gobi emend. Wille (Chlamydomonas und die nächstverwandten gattungen I). Nova Hedwigia Beih. 1970;34: 1–283. German. [Google Scholar]
- 54.Wille N. Algologische notizen IX–XIV. Nyt Magazin for Naturvidenskaberne. 1903;41: 89–185, with 3 pls. German. [Google Scholar]
- 55.Playfair GI. New and rare freshwater algae. Proc Linn Soc N S W. 1918;43: 497–543. [Google Scholar]
- 56.Péterfi S. Beiträge zur Kenntnis der Algen Transsylvaniens (Rumänien). Bulletin du Jardin et du Musée botaniques de l’Université de Cluj, Roumanie. 1939;19: 87–104. German. [Google Scholar]
- 57.Brabez R. Zur Kenntnis der Algen des Franzensbader und Sooser Thermenbereiches. Beihefte zum Botanischen Centralblatt. 1941;61/A: 13–236. German. [Google Scholar]
- 58.Wawrik F. Drei neue flagellaten aus streckteichen des waldviertels. Nova Hedwigia 1974;25: 665–671. German. [Google Scholar]
- 59.Hoshaw RW, Ettl H. Chlamydomonas smithii sp. nov.—a chlamydomonad interfertile with Chlamydomonas reinhardtii. J Phycol. 1966;2: 93–96. doi: 10.1111/j.1529-8817.1966.tb04600.x . [DOI] [PubMed] [Google Scholar]
- 60.Hoshaw RW, McCourt RM. The Zygnemataceae (Chlorophyta): a twenty-year update of research. Phycologia. 1988;27: 511–548. doi: 10.2216/i0031-8884-27-4-511.1 [Google Scholar]
- 61.Remias D, Pichrtová M, Pangratz M, Lütz C, Holzinger A. Ecophysiology, secondary pigments and ultrastructure of Chlainomonas sp. (Chlorophyta) from the European Alps compared with Chlamydomonas nivalis forming red snow. FEMS Microbiol Ecol. 2016;92: fiw030 doi: 10.1093/femsec/fiw030 ; PubMed Central PMCID: PMC4815433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abbreviations: c, chloroplast; n, nucleus; p, pyrenoid. (A) Optical section focused on a pyrenoid. (B) Surface view. The strain [formerly designated as Chloromonas nivalis (Chodat) Hoham et Mullet] was not used in course of this study since the strain might be replaced with contamination by the species of the genus Trebouxia (see S1 Text; S2 Table).
(TIF)
All at identical magnification. Arrows in A, C, E indicate position of each contractile vacuole originating from the parent cell. (A, B) C. nivalis (Chodat) Hoham et Mullet strain UTEX SNO71. (A) Immediately prior to the first transverse division. (B) Sixteen daughter cells within the parental cell wall. Note that only 12 of the 16 cells are recognized. (C, D) C. hoshawii Matsuzaki et al. sp. nov. strain UTEX SNO66. (C) Immediately prior to the first transverse division. (D) Four daughter cells within the parental cell wall. (E, F) C. remiasii Matsuzaki et al. sp. nov. strain CCCryo 005–99. (E) Immediately prior to the first transverse division. (F) Four daughter cells within the parental cell wall.
(TIF)
Aggregates result from repeated divisions of daughter cells retained in parental cell walls (double arrowhead). Open arrowhead indicates a daughter cell wall surrounding offspring of a daughter cell. All at the identical magnification. (A) Strain CCCryo 005–99 after 7 days in liquid AF-6 medium. (B) Strain CCCryo 047–99 after 3 months on 1.5% agar slant of AF-6.
(TIF)
C. nivalis zygote specimens (Field-collected samples) are underlined, and the Austrian C. nivalis zygote specimen (P24/DR4 [19]) and the Slovak C. nivalis subsp. tatrae zygote specimen (LP01 [20]) are shadowed in black. Groups A–D are as indicated in Fig 4. The corresponding posterior probabilities (PP, 0.95 or more) are shown at the top left. Numbers shown in top right, bottom left and bottom right indicate bootstrap values (BV, 50% or more) from maximum likelihood (ML), maximum parsimony (MP) and neighbor-joining (NJ) analyses. Asterisk indicates 1.00 PP in BI and 100% BV in ML, MP, and NJ analyses.
(TIF)
C. nivalis zygote specimens (Field-collected samples) are underlined, and the Austrian and Japanese C. nivalis zygote specimens examined in the previous studies (P24/DR4 [19,20], and Gassan-NIV1 and Gassan-NIV2 [41], respectively) and the Slovak C. nivalis subsp. tatrae zygote specimen (LP01 [20]) are shadowed in black. Groups A–D are as in Fig 4. The corresponding posterior probabilities (PP, 0.95 or more) are shown at the top left. Numbers shown in top right, bottom left and bottom right indicate bootstrap values (BV, 50% or more) from maximum likelihood (ML), maximum parsimony (MP) and neighbor-joining (NJ) analyses. Asterisk indicates 1.00 PP in BI and 100% BV in ML, MP and NJ analyses.
(TIF)
The 3′ end of the 5.8S ribosomal RNA (rRNA) and the 5′ end of the 26S rRNA are shown (DDBJ/ENA/GenBank accession number: HQ404862). The sequence from C. remiasii strains CCCryo 005–99 is identical to that from CCCryo 047–99 (LC360496). Note U-U mismatch in helix II (arrowheads) and the YGGY motif on the 5′ side near the apex of helix III (boldface), common structural hallmarks of eukaryotic nuclear rDNA ITS2 secondary structures [47,50].
(TIF)
The 3′ end of the 5.8S ribosomal RNA (rRNA) and the 5′ end of the 26S rRNA are shown (DDBJ/ENA/GenBank accession number: LC360497). Note U-U mismatch in helix II (arrowheads) and the YGGY motif on the 5′ side near the apex of helix III (boldface), common structural hallmarks of eukaryotic nuclear rDNA ITS2 secondary structures [47,50].
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
New sequence data, alignments used for our phylogenetic analyses, and the holotype specimens are available under the DDBJ/ENA/GenBank accession numbers (LC360463–LC360497, and LC361432), TreeBASE ID (S22105), and the herbarium specimen numbers for the holotypes (TNS-AL-58946 and TNS-AL-58947), respectively. All other relevant data are within the manuscript and its Supporting Information files.