Abstract
Viruses in avian hosts can pose threats to avian health and some have zoonotic potential. Hospitals that provide veterinary care for avian patients may serve as a site of exposure of other birds and human staff in the facility to these viruses. They can also provide a useful location to collect samples from avian patients in order to examine the viruses present in wild birds. This study aimed to investigate viruses of biosecurity and/or zoonotic significance in Australian birds by screening samples collected from 409 birds presented to the Australian Wildlife Health Centre at Zoos Victoria’s Healesville Sanctuary for veterinary care between December 2014 and December 2015. Samples were tested for avian influenza viruses, herpesviruses, paramyxoviruses and coronaviruses, using genus- or family-wide polymerase chain reaction methods coupled with sequencing and phylogenetic analyses for detection and identification of both known and novel viruses. A very low prevalence of viruses was detected. Columbid alphaherpesvirus 1 was detected from a powerful owl (Ninox strenua) with inclusion body hepatitis, and an avian paramyxovirus most similar to Avian avulavirus 5 was detected from a musk lorikeet (Glossopsitta concinna). Two distinct novel avian alphaherpesviruses were detected in samples from a sulphur-crested cockatoo (Cacatua galerita) and a tawny frogmouth (Podargus strigoides). Avian influenza viruses and avian coronaviruses were not detected. The clinical significance of the newly detected viruses remains undetermined. Further studies are needed to assess the host specificity, epidemiology, pathogenicity and host-pathogen relationships of these novel viruses. Further genome characterization is also indicated, and would be required before these viruses can be formally classified taxonomically. The detection of these viruses contributes to our knowledge on avian virodiversity. The low level of avian virus detection, and the absence of any viruses with zoonotic potential, suggests low risk to biosecurity and human health.
Introduction
Avian species serve as hosts for a diversity of viruses that pose both biosecurity and zoonotic risks. Numerous outbreaks of disease in collections of caged birds and commercial domestic poultry have been attributed to viruses introduced by direct or indirect contact with wild birds [1–7]. Some avian viruses can also cause disease in humans [8–14]. As such, understanding the viruses present in wild birds is important for managing potential risks to human and animal health, and for understanding the biology of these viruses in their natural hosts. Studying viruses in wild birds can be challenging as gaining access to these birds for the purpose of sample collection can be difficult, and their capture may be a cause of stress or injury. Collecting and testing samples from avian patients presenting to veterinary hospitals can be a convenient and useful approach to detecting and studying avian viruses. Importantly this approach does not involve additional capturing or handling of birds, and allows the risks to staff and other avian patients/collections within the facility to be more accurately assessed, since the species from which the samples are collected reflects the species being handled at the participating facility. This approach has been successful in previous studies utilizing veterinary hospitals and wildlife rescue centres in several countries including Australia, the United Kingdom, France and Belgium [15–18].
In Australia, the Australian Wildlife Health Centre (AWHC) at Zoos Victoria’s Healesville Sanctuary functions as a large primary care and referral centre for sick, injured and orphaned native wildlife, including providing veterinary care for approximately 700–900 wild birds each year, as well as Healesville Sanctuary’s collection of captive animals. Patients may be presented to the AWHC by members of the public, wildlife carers, keepers, government wildlife officers or by referral from other veterinary clinics for veterinary diagnosis and treatment. During this period in captivity there is an elevated risk of stress-induced shedding of pathogens, presenting a risk for both the veterinary staff and other animals in care, including the Sanctuary’s collection animals and captive breeding programs. Close contact between infected and susceptible animals can facilitate the transmission of pathogens between species, particularly when the hosts are taxonomically related. The large number of avian patients passing through the AWHC provides an opportunity to study the viruses present in Australian birds and better understand the potential risks these viruses present.
Of particular importance and conservation value at Healesville Sanctuary are the captive breeding and insurance programs for threatened species. This includes a captive breeding program for the critically endangered orange-bellied parrot (Neophema chrysogaster), which is facing imminent extinction in the wild [19]. The orange-bellied parrot is arguably the most threatened parrot species in the world, with the wild-bred population declining to only three females and 13 male birds in the 2016/2017 breeding season [19]. Infectious diseases, including viral diseases, are a recognized threat to this and other threatened bird species both within Australia and around the world. Beak and feather disease virus (BFDV), a circovirus, is of particular concern [20]. Our past work has shown that BFDV is prevalent in both psittacine and non-psittacine birds presenting to the AWHC, and has revealed that non-psittacine birds may represent a previously unrecognized risk for spreading BFDV infection [21]. Other viruses are also a potential threat to Psittaciformes in Australia. Psittacid alphaherpesvirus 1 (PsHV1) is the aetiologic agent of both Pacheco’s disease and mucosal papillomas in Psittaciformes [22]. PsHV1 has not been reported in wild Psittaciformes in Australia [23], but has been detected in Australia in captive green-winged macaws (Ara chloropterus) imported from the United Kingdom in the 1990s [23, 24]. It is not known how far the virus has been transmitted from these birds but it is likely to be present in at least some avicultural collections in Australia [23]. The inadvertent introduction of such a virus into the remaining population of a threatened avian species, such as through the Neophema chrysogaster recovery program, would be potentially devastating. Avian paramyxoviruses (APMVs) also pose a threat to Australian Psittaciformes. Avian avulavirus 3 (previously avian paryamyxovirus serotype 3, APMV-3) has been documented causing mortality rates of up to 70% in captive collections of Neophema species and other Australian psittacines overseas [25–28]. APMV-3 strains have been isolated from a diversity of avian species in different parts of the world, but has not yet been reported in Australia [25–31]. However we do know that the Australian parrot genus Neophema is highly susceptible to APMV-3, with infection frequently resulting in fatal neurological disease [26, 28]. Another avian paramyxovirus, Avian avulavirus 5 (avian paramyxovirus 5, APMV-5) has caused outbreaks of severe disease with up to 100% mortality in captive budgerigars (Melopsittacus undulatus), including an outbreak in a captive budgerigar flock in Queensland in 1972 [25, 32–34].
Few avian viruses represent a risk to veterinary staff and others handling birds, but avian influenza viruses (AIVs) can threaten human health [8, 9, 11–13]. Wild birds, particularly waterfowl, waders and sea birds, are the natural reservoirs of low pathogenicity avian influenza (LPAI) viruses and play important roles in the circulation of AIVs [35–38], with potential for transmission of AIVs both to and from poultry and the potential for mutation to generate highly pathogenic avian influenza (HPAI) viruses [6, 7, 39]. Some AIVs can be transmitted from poultry to humans, and some have caused severe disease or even death in humans [8, 9, 11–13, 40, 41]. Transmission of AIVs from wild birds to commercial poultry has occurred recently in Australia in Victoria, New South Wales and Queensland [10, 42–45]. The Australian National Avian Influenza Wild Bird Surveillance Program has been in place since 2006 but primarily targets Anseriformes (ducks, swans, geese) and Charadriiformes (gulls, terns and shorebirds) [46, 47]. The prevalence of AIV infection in birds presenting to veterinary care facilities, such as the AWHC, has not been as comprehensively studied [15]. In Australia, avian coronaviruses (AvCoVs) are also of particular interest in wild birds because of the presence of variant forms of the poultry pathogen infectious bronchitis virus (IBV) in Australian commercial poultry [48–50]. It is suspected that the variant IBV strains contain genetic material derived from AvCoVs hosted by wild birds, but this requires further investigation [48].
This study aimed to collect samples from birds presenting to the AWHC and use them for the molecular detection and characterization of viruses with potential significance to biosecurity, human health or animal health. Four virus families were targeted in this study: avian influenza viruses, herpesviruses, paramyxoviruses and coronaviruses. Samples were screened using a combination of genus- or family-wide polymerase chain reaction methods coupled with sequencing and phylogenetic analyses for detection and identification of both known and novel viruses. Two novel viruses were detected in this study, hence contributing to our knowledge of avian virodiversity, while the overall low level of virus detection adds to our understanding about the presence or absence of viruses of birds in Australia and their potential risk to avian and human health.
Materials and methods
Sample collection
Samples were collected from 409 birds presented to the AWHC at Healesville Sanctuary (37°68'35"S, 145°53'46"E) for veterinary diagnosis and treatment between December 2014 and December 2015 (see S1 Table). This study was approved by the Animal Ethics Committee of the Faculty of Veterinary and Agricultural Sciences of the University of Melbourne (Ethics ID #1413397). Of these 409 birds, 299 were wild avian patients presented to the AWHC for veterinary care, while 110 were captive (part of the Healesville Sanctuary collection). Information regarding the location, date, species and any other available and relevant details about the bird’s history, including reason for admittance, were collected at the time of admission by AWHC staff. A clinical examination was performed by a veterinarian after admission, with any observed clinical signs and diagnostic findings being recorded, including body condition as judged by palpation of pectoral muscle mass. Samples were collected from live avian patients or at necropsy (from birds that had been euthanased or died for reasons unrelated to this study). Most samples collected from live birds were opportunistically taken whilst birds were anesthetised using inhalational isoflurane (Delvet Isoflurane) and oxygen delivered via mask to facilitate clinical examination and diagnostic investigations, and all efforts were made to minimise animal stress and discomfort. Sterile rayon-tipped dry swabs (Copan Italia) were first moistened by dipping in sterile phosphate buffered saline, then used to separately swab the choana and cloaca of 199 live birds (107 wild and 92 captive avian patients). The tips of these swabs were cut into 500 μL of RNAlater® (Ambion) and stored at -20°C. For the 210 birds sampled at post-mortem (comprising 192 deceased wild birds and 18 birds originating from the Healesville Sanctuary collection), sterile dry swabs were used to separately swab the trachea and intestine/caecum, cut into 500 μL of RNAlater® and stored at -20°C. A piece of liver was also aseptically collected and stored at either -20°C or -80°C. The collection of these liver samples, and their subsequent testing for BFDV, has been previously reported in a separate study [21]. Instruments used for post-mortem sample collection were cleaned and autoclaved between each necropsy. Samples of liver, spleen, kidney, lung, heart, gonad, pancreas, proventriculus, gizzard, small intestine, large intestine (including caecae if present, depending on bird species), pectoral muscle, skin, brain and any lesions were also collected and fixed by submersion in 10% phosphate-buffered formalin at necropsy. For a few select birds of interest, formalin-fixed, paraffin-embedded tissue sections were stained with haematoxylin and eosin for histological examination.
Nucleic acid extraction
Swabs in RNAlater® were vortexed for 10 seconds and then centrifuged briefly before 100 μL aliquots of each choanal/tracheal and cloacal/intestinal swab in RNAlater® from the same bird were pooled to produce 200 μL samples for genomic nucleic acid extraction. Nucleic acid extraction was performed manually using a QIAamp Viral RNA Mini Kit (Qiagen), using the spin protocol according to the manufacturer’s instructions, eluting into two lots of 40 μL of Buffer AVE, and robotically using VX Universal Liquid Sample DNA Extraction Kits (Qiagen) and a Corbett X-tractor Gene Robot (Corbett Robotics), according to the manufacturer’s instructions, with products being eluted in 70 μL of elution buffer. Samples of liver tissue collected at post-mortem were pulverised with a swab and the swab placed into 500 μL RNAlater®. Liver swabs in RNAlater® were vortexed for 10 seconds then centrifuged briefly before taking 200 μL aliquots from which nucleic acid was extracted as above. Whenever pooled clinical samples tested positive by PCR, nucleic acid was extracted from 140 μL aliquots of the corresponding original choanal/tracheal and cloacal/intestinal swab samples separately using the QIAamp Viral RNA Mini Kit spin protocol, and eluted in 60 μL of Buffer AVE. Laboratory stocks of Influenza A/Memphis/1/1971 (H3N2), Chlamydia felis, Macropodid alphaherpesvirus 1 3076/08 and Newcastle disease virus vaccine strain (NDV.V4) were pooled to give 200 μL positive extraction control samples. Negative extraction control samples utilised 200 μL aliquots of RNAlater®.
PCR detection of avian herpesviruses
Samples were tested for the presence of herpesvirus DNA using a universal herpesvirus PCR assay utilising degenerate, deoxyinosine-substituted oligonucleotide primers specifically targeting a highly conserved region of the herpesvirus DNA polymerase gene, as previously described [51]. Macropodid alphaherpesvirus 1 or Equid alphaherpesvirus 4 DNA were used as template for positive control reactions, and sterile H2O for negative control reactions. When herpesvirus DNA was detected, additional viral DNA sequence information was obtained by first repeating the first round of the nested universal herpesvirus PCR assay, using DNA extracted from separate choanal/tracheal and cloacal/intestinal swab samples as template. Further PCRs using forward primer TGV paired with reverse primer KG1, and forward primer DFA paired with reverse primer IYG, were then performed using 5 μL aliquots of the first round reaction as template.
PCR detection of RNA viruses
cDNA synthesis
SuperScript™ III Reverse Transcriptase (Invitrogen) was used to synthesise cDNA from aliquots of extracted nucleic acid in preparation for screening of samples for the presence of paramyxoviruses and coronaviruses by PCR. The reverse transcriptase (RT) reaction was performed according to the manufacturer’s instructions using 100 ng random primer oligonucleotides (Invitrogen), and 10 μL of nucleic acid extract as template. All assays included a no template control with sterile water, and the appropriate positive control as described for each assay.
Avian paramyxoviruses
Samples were tested for the presence of paramyxovirus RNA using a family-wide RT-PCR assay utilising consensus degenerate and inosine-containing oligonucleotide primers annealing to conserved motifs in domain III of the RNA-dependent RNA polymerase gene, as described previously [52]. cDNA synthesised from RNA extracted from Newcastle disease virus vaccine strain (NDV.V4) was used as the template for positive control reactions. Products amplified by PCR were separated on agarose gels using SYBR Safe (Invitrogen) and visualised by UV transillumination using Bio-Rad Image Lab Software. When Paramyxoviridae RNA was detected by RT-PCR, forward and reverse primers PAR-F1 and PAR-R [53] were used in combination with the degenerate oligonucleotide primer pair PMX1 and PMX2 [52] to obtain additional sequence information. Both sets of degenerate primers target the same conserved region of the L-protein-coding sequences of the RNA-dependent RNA polymerase gene–the most conserved viral gene in the family Paramyxoviridae [53]. PAR-F1 was coupled with PMX2 to produce amplicons of approximately 120 bp in size, while PMX1 coupled with PAR-R produced amplicons of approximately 650 bp in size. The PCR mixtures contained 0.5 μM of each forward and reverse primer (GeneWorks), 2.5 μL of cDNA template, 200 μM (each) deoxynucleoside triphosphate, Green GoTaq® Flexi buffer, 3 mM MgCl2 and 1 U GoTaq® Flexi DNA Polymerase in a 25 μL reaction volume. PCR mixtures were incubated at 94°C for 2 minutes prior to 40 cycles of 94°C for 15 seconds, 41°C for 30 seconds and 72°C for 30 seconds, followed by a final extension at 72°C for 7 minutes.
Avian coronaviruses
A real-time RT-PCR assay using primers targeting conserved sequences within the 5’-untranslated region (UTR) gene and a TaqMan® dual-labelled probe [54] was used to screen samples for the presence of avian gammacoronaviruses of the IBV lineage. The 25 μL reactions used TaqMan® GTXpress™ Master Mix (Life Technologies) according to the manufacturer’s instructions, with forward and reverse primers to a final concentration of 0.5 μM each, 5-carboxyfluorescein (FAM)-labelled probe with 3’-BHQ1-quencher (GeneWorks) to a final concentration of 0.1 μM, and 5 μL of cDNA template. cDNA synthesised from RNA extracted from laboratory stocks of infectious bronchitis virus (strain VIC-S) was used as the template for positive controls reactions. Reactions were conducted in a Stratagene Mx3000P™ quantitative PCR thermocycler, with initial enzyme activation at 95°C for 20 seconds, then 40 cycles of a denature step at 94°C for 1 second followed by 60°C for 60 seconds for annealing and extension. Results were analysed using Stratagene MxPro software, with CT values above 40 considered negative.
Influenza A viruses
Samples were tested for the presence of influenza A virus RNA using a TaqMan Real-Time matrix gene-specific RT-PCT assay [55] within a commercial kit (AgPath-ID™ One-Step RT-PCR Kit, Ambion). RNA extracted from Influenza A/Memphis/1/1971 was used as template for positive control reactions, while sterile water was used for the negative control reactions. Reactions were performed in a Stratagene Mx3000P™ thermocycler. Cycle threshold values above 40 were considered negative.
Throughout this study 95% confidence intervals (95% C. I.) for sample proportions and prevalence estimates were calculated using the Jeffreys method [56], while statistical analysis was carried out using the Fisher exact chi-squared test [57].
DNA sequencing and phylogenetic analyses
PCR products were purified from PCR reaction mixtures and sequenced as described previously [21]. Geneious® 9.1.8 bioinformatics software (Biomatters Ltd., Auckland, New Zealand) [58] was used to trim and align all obtained sequences. Nucleotide sequences were compared with publicly available sequences in the GenBank® database (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/genbank/) using the NCBI Nucleotide Basic Local Alignment Search Tool (BLASTN®) online algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and ClustalW2 alignments with their closest matches were generated within Geneious R9 to determine nucleotide identities for the region of available sequence. PhyML 2.2.0 maximum likelihood phylogenetic trees for the herpesviruses were generated from ClustalW2 [59] nucleotide and amino acid sequence alignments using the general-time-reversible nucleotide substitution and Jones-Taylor-Thornton amino acid substitution models respectively, each with four substitution rate categories [60]. For the phylogenetic analysis of the family Paramyxoviridae, a ClustalW2 alignment [59] was used to draw a PhyML 2.2.0 maximum likelihood phylogenetic tree using the HKY85 nucleotide substitution model with four substitution rate categories [60]. For each of these trees the reliability of each branch was tested using 100 replicates in a bootstrap resampling analysis.
Results
PCR detection of viruses
Pooled choanal/tracheal and cloacal/intestinal swab samples from the study population detailed in S1 Table were screened for the presence of viruses from three families of RNA viruses, with the exception that samples from a wild powerful owl (Ninox strenua) swabbed at the very end of the sampling period were not included. No influenza A viruses or avian coronaviruses were detected from any of these 408 birds (0% prevalence; 95% C. I. 0–0.6%), while a single avian paramyxovirus was detected from a choanal swab from a wild musk lorikeet (Glossopsitta concinna) using the pan-paramyxovirus RT-PCR assay (0.25% prevalence; 95% C. I. 0–1.1%) (Table 1). While no avian paramyxoviruses were detected from the 110 captive birds tested in this study, there was not a statistically significant difference in the prevalence of avian paramyxovirus detection between wild and captive birds (p > 0.5; Fisher exact test).
Table 1. Detection of viruses by host order (number of birds from which virus detected/total number of birds tested).
| Avian Order | Avian herpesviruses | Avian paramyxoviruses | Avian gamma-coronaviruses | Avian influenza viruses |
|---|---|---|---|---|
| Anseriformes | 0/24 | 0/24 | 0/24 | 0/24 |
| Columbiformes | 0/35 | 0/35 | 0/35 | 0/35 |
| Caprimulgiformes | 1/35 –a novel avian herpesvirus detected from a tawny frogmouth (Podargidae; Podargus strigoides), tentatively designated Podargid alphaherpesvirus 1 | 0/35 | 0/35 | 0/35 |
| Gruiformes | 0/7 | 0/7 | 0/7 | 0/7 |
| Charadriiformes | 0/4 | 0/4 | 0/4 | 0/4 |
| Procellariiformes | 0/1 | 0/1 | 0/1 | 0/1 |
| Pelecaniformes | 0/13 | 0/13 | 0/13 | 0/13 |
| Accipitriformes | 0/6 | 0/6 | 0/6 | 0/6 |
| Strigiformes | 1/10 –Columbid alphaherpesvirus 1 detected from a powerful owl (Strigidae; Ninox strenua) | 0/9 | 0/9 | 0/9 |
| Coraciiformes | 0/27 | 0/27 | 0/27 | 0/27 |
| Falconiformes | 0/3 | 0/3 | 0/3 | 0/3 |
| Psittaciformes | 1/193 –a novel avian herpesvirus detected from a sulphur-crested cockatoo (Cacatuidae; Cacatua galerita), tentatively designated Cacatuid alphaherpesvirus 1 | 1/193 –a putative novel genotype of Avian avulavirus 5 (APMV-5) detected from a musk lorikeet (Psittaculidae; Glossopsitta concinna), designated ‘unclassified avian avulavirus strain musk lorikeet/ Melbourne/ML22-141263/2014’ |
0/193 | 0/193 |
| Passeriformes | 0/51 | 0/51 | 0/51 | 0/51 |
| Total | 3/409 | 1/408 | 0/408 | 0/408 |
When DNA extracted from swab samples from the whole study population of 409 birds and separate liver swab samples from the 210 birds that had been sampled at necropsy (see S1 Table) was screened by the universal herpesvirus PCR assay [51], avian herpesvirus DNA was detected from three birds (0.7% prevalence; 95% C. I. 0.2–1.9%): a sulphur-crested cockatoo (Cacatua galerita), a tawny frogmouth (Podargus strigoides) and a powerful owl (Table 1). These three birds were all wildlife, with no avian herpesviruses being detected from the captive birds tested. However there was not a statistically significant difference in the prevalence of avian herpesvirus detection between wild and captive birds in this study (p > 0.5; Fisher exact test).
Phylogenetic analysis of detected viruses
Detection of a known avian herpesvirus from a powerful owl
The 181 bp nucleotide sequence obtained from the powerful owl aligned with the DNA polymerase genes of strains of Columbid alphaherpesvirus 1 (CoHV1) with 100% nucleotide identity [61–67]. Of the 210 liver swab samples screened by the universal herpesvirus PCR, only DNA extracted from the liver of the same powerful owl gave a strong positive band. A liver sample from this same individual owl also tested positive for CoHV1 in another study by Phalen, Alvarado, et al. [64]. The gross and histopathological lesions present in this owl were consistent with herpesviral inclusion body disease as described in Gailbreath and Lindsay Oaks [62]. Histopathology revealed extensive acute necrosis throughout the spleen, liver, pancreas and adrenal gland associated with eosinophilic to amphophilic intranuclear inclusion bodies in adjacent intact cells, with minimal associated inflammation. This female powerful owl had been found moribund on 1st May 2015 in St Kilda, Victoria (37°87'17"S, 144°97'95"E), and died shortly after admission to the AWHC. This same powerful owl has been the subject of previously reported diagnostic investigations which revealed the presence of BFDV DNA in a sample of liver [21].
Detection of two novel avian herpesviruses from a tawny frogmouth and a sulphur-crested cockatoo
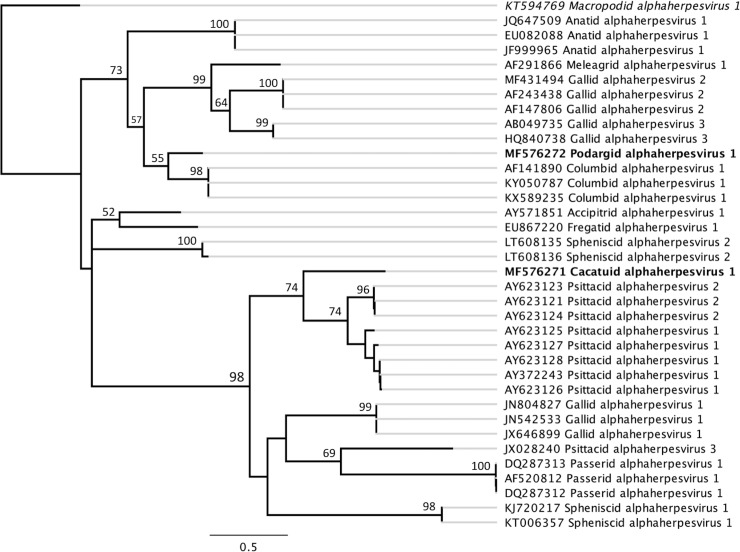
Two novel members of the family Herpesviridae were detected, one from DNA extracted from a choanal swab from a tawny frogmouth, and the other from DNA extracted from a tracheal swab collected from a sulphur-crested cockatoo. The closest matches to the 485 bp nucleotide sequence obtained from the tawny frogmouth swab were avian herpesviruses within the subfamily Alphaherpesvirinae, with highest (76%) nucleotide identity with the DNA polymerase genes of strains of CoHV1 [61, 63–65, 67]. It is unknown whether the tawny frogmouth is the natural host of this newly identified alphaherpesvirus, but lacking information to the contrary we have tentatively designated the virus Podargid alphaherpesvirus 1 (PodHV1). The nucleotide sequence of the DNA polymerase gene fragment from this novel herpesvirus has been deposited in GenBank (accession number MF576272).
The tawny frogmouth had presented at the AWHC with a minor laceration and clinical signs suggestive of head trauma (weak, depressed and ataxic with blood in the oral cavity) on 27th August 2015, but after a two week rehabilitation went on to fly off when released back where found in Badger Creek, Victoria (37°67'87"S, 145°53'99"E). The virus was not detected in DNA extracted from pooled choanal/tracheal and cloacal/intestinal swab samples from any of the other 34 tawny frogmouths tested (2.9% prevalence; 95% C. I. 0.3–12.6%), nor any of the other 82 avian species in this study. The clinical significance of this newly identified alphaherpesvirus is undetermined, but the tawny frogmouth showed no expression of clinical disease during its period of rehabilitation.
The closest match to the 489 bp nucleotide sequence from the sulphur-crested cockatoo swab was the DNA polymerase gene of PsHV1 (GenBank accession number AY372243), with 66.5% nucleotide identity [68]. This sequence also had 65% nucleotide identity with the limited DNA polymerase gene sequence available (266 bp) for Psittacid alphaherpesvirus 2 (PsHV2), which has been detected in African grey parrots (Psittacus erithacus) and a blue and gold macaw (Ara ararauna) in the USA [22, 69]. The phylogenetic trees (Figs 1 and 2) suggest that this novel herpesvirus belongs to the subfamily Alphaherpesvirinae, and we tentatively propose the name Cacatuid alphaherpesvirus 1 (CacHV1) after the host family in which it was found. The nucleotide sequence of the DNA polymerase gene fragment from this novel herpesvirus has been deposited in GenBank (accession number MF576271).
Fig 1. PhyML maximum likelihood phylogenetic tree of avian alphaherpesviruses.
Generated from a ClustalW2 alignment [59] of the partial DNA polymerase gene sequences of Podargid alphaherpesvirus 1 and Cacatuid alphaherpesvirus 1 with published avian alphaherpesvirus nucleotide sequences available in GenBank, with the two novel alphaherpesviruses detected in this study highlighted in bold [60]. The GenBank accession numbers for sequences used are included in the tip labels, and Macropodid alphaherpesvirus 1 (highlighted in italics) is included as an outgroup. Branching with greater than 50% support from 100 bootstrap replicates is indicated at major node points. The distances indicated by black horizontal lines correspond to genetic distances, with the scale bar representing nucleotide substitutions/site.
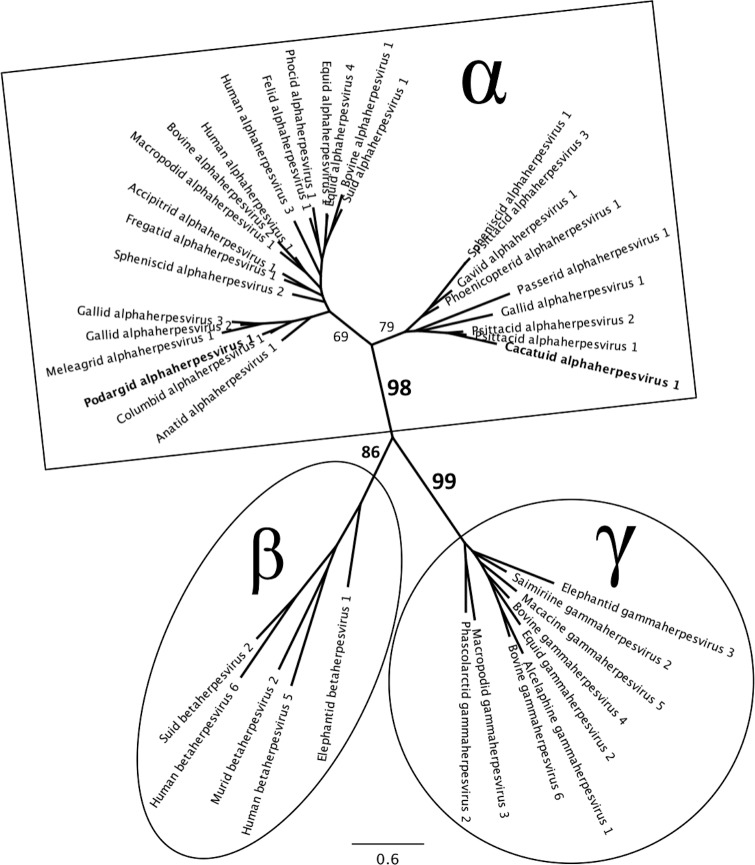
Fig 2. Unrooted maximum likelihood phylogenetic tree for the family Herpesviridae.
Generated from a ClustalW2 alignment of amino acid translations of partial DNA polymerase gene sequences from 40 representative herpesviruses retrieved from GenBank from the three subfamilies: Alphaherpesvirinae (α), Betaherpesvirinae (β) and Gammaherpesvirinae (γ) from a range of host species, and including the two novel alphaherpesviruses detected in this study (highlighted in bold) [60]. The GenBank accession numbers for sequences used are as follows: Accipitrid alphaherpesvirus 1 AY571851; Alcelaphine gammaherpesvirus 1 AF005370; Anatid alphaherpesvirus 1 EF643560; Bovine alphaherpesvirus 1 X94677; Bovine alphaherpesvirus 2 AF181249; Bovine gammaherpesvirus 4 AF031811; Bovine gammaherpesvirus 6 AF031808; Cacatuid alphaherpesvirus 1 MF576271; Columbid alphaherpesvirus 1 AF141890; Elephantid betaherpesvirus 1 AF322977; Elephantid gammaherpesvirus 3 DQ238845; Equid alphaherpesvirus 1 KF434378; Equid gammaherpesvirus 2 NC001650; Equid alphaherpesvirus 4 KT324743; Felid alphaherpesvirus 1 KR296657; Fregatid alphaherpesvirus 1 EU867220; Gallid alphaherpesvirus 1 NC006623; Gallid alphaherpesvirus 2 AF147806; Gallid alphaherpesvirus 3 HQ840738; Gaviid alphaherpesvirus 1 GU130289; Human alphaherpesvirus 1 HQ123098; Human alphaherpesvirus 3 X04370; Human betaherpesvirus 5 NC006273; Human betaherpesvirus 6 X83413; Macacine gammaherpesvirus 5 AF029302; Macropodid alphaherpesvirus 1 NC029132; Macropodid gammaherpesvirus 3 EF467663; Meleagrid alphaherpesvirus 1 AF291866; Murid betaherpesvirus 2 AY728086; Passerid alphaherpesvirus 1 AF520812; Phascolarctid gammaherpesvirus 2 JQ996387; Phocid alphaherpesvirus 1 PHU92269; Phoenicopterid alphaherpesvirus 1 KP244360; Podargid alphaherpesvirus 1 MF576272; Psittacid alphaherpesvirus 1 AY372243; Psittacid alphaherpesvirus 2 AY623124; Psittacid alphaherpesvirus 3 JX028240; Saimiriine gammaherpesvirus 2 AJ410493; Spheniscid alphaherpesvirus 1 KJ720217; Spheniscid alphaherpesvirus 2 LT608135; Suid alphaherpesvirus 1 BK001744; Suid betaherpesvirus 2 AF268042. Percentage support from 100 bootstrap replicates is indicated at major branch points. The scale bar represents amino acid substitutions/site.
The female sulphur-crested cockatoo had been found unable to fly in Lilydale, Victoria (37°75'73"S, 145°37'20"E) on 17th September 2015, presenting emaciated and weak with haemorrhagic enteritis and clinical signs suggestive of beak and feather disease virus (BFDV) infection. This cockatoo tested positive for BFDV when DNA extracted from a liver sample collected at post-mortem was used as template in a BFDV PCR assay [21, 70]. Histopathological examination of samples of liver, spleen, kidney, lung, heart, gastrointestinal tract, pancreas and ovarian tissue collected at necropsy revealed chronic enteritis with unidentified piriform protozoa in intestinal crypts and foci of mucosal and crypt necrosis with interstitial fibrosis in the submucosa of the proximal small intestine, along with a significant overgrowth of Macrorhabdus ornithogaster in the ventriculus. It is presumed that the protozoan and Macrorhabdus overgrowth were secondary to immunosuppression caused by BFDV infection, but it is unknown what role the newly identified alphaherpesvirus may have been playing in this emaciated and very unwell bird. No inclusion bodies, inflammation in the lungs nor any other sign of herpesvirus infection were seen in any of the tissues examined histologically. CacHV1 was only detected from one of the 27 sulphur-crested cockatoos tested in this study (3.7% prevalence; 95% C. I. 0.4–16.0%), and was not detected from any of the other 54 Cacatuids tested (1.8% prevalence; 95% C. I. 0.2–8.2%).
Detection of an avian paramyxovirus from a wild musk lorikeet
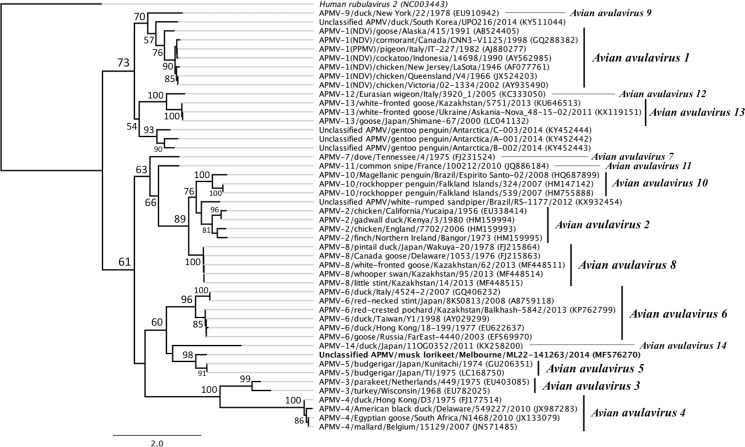
The musk lorikeet had been found unable to fly in Bundoora Park (37°42'28"S, 145°02'42"E) in December 2014, and presented to the AWHC with an old fracture of the right coracoid, on the day of sample collection. After a successful four-week rehabilitation, during which the lorikeet ate well, gained bodyweight and showed no signs of ill health, the bird was released back where it was found. On BLAST analysis, the 603 bp sequence fragment of the paramyxovirus RNA-dependent RNA polymerase (L protein) gene obtained from this musk lorikeet did not match any currently known paramyxovirus, but had highest (77.4–77.6%) nucleotide sequence identity with strains of Avian avulavirus 5 (GenBank accession numbers GU206351 and LC168750) [34, 71]. The partial RNA-dependent RNA polymerase gene sequence of the avian avulavirus detected in this study (strain musk lorikeet/Melbourne/ML22-141263/2014) has been deposited in GenBank (accession number MF576270). Phylogenetic analysis suggests that this avulavirus strain belongs to the serotype Avian avulavirus 5, and perhaps represents a novel genotype or genetic subgroup within this serotype (Fig 3). Paramyxovirus RNA was not detected from any of the other three wild musk lorikeets tested in this study, giving a prevalence of 25% (95% C. I. 2.9–71.6%) for the species.
Fig 3. Phylogenetic tree for the genus avulvirus of the family Paramyxoviridae based on partial RNA-dependent RNA polymerase gene sequences.
Maximum likelihood phylogenetic tree constructed using PhyML from a ClustalW2 alignment of the partial RNA-dependent RNA polymerase (large polymerase or L protein) gene sequences of the avian avulavirus detected from a musk lorikeet (Glossopsitta concinna) in this study (unclassified avian avulavirus strain musk lorikeet/Melbourne/ML22-141263/2014; highlighted in bold) and published paramyxovirus sequences retrieved from GenBank [60]. The GenBank accession numbers for sequences used are indicated in brackets in the tip labels. Human rubulavirus 2 (highlighted in italics) is included as an outgroup. Branching with greater than 50% support from 100 bootstrap replicates is indicated at major node points. The distances indicated by black horizontal lines correspond to genetic distances, with the scale bar representing nucleotide substitutions/site.
Discussion
A very low prevalence of viruses was detected in the birds examined in this study. Sampling birds presented to veterinary hospitals, such as the AWHC, is a useful approach to studying viruses in wild birds. However, this approach does involve an inherently biased population sample (for example a bias towards sick or injured birds, particular locations and common urban species) which should be considered when these results are interpreted, or compared to other studies. It is likely that the sample population in this present study, particularly the low numbers of Anseriformes and Charadriiformes (28 of the 408 birds tested) contributed to the absence or low prevalence of detection of AIVs, APMVs and AvCoVs, as previous studies have shown that these viruses are present mainly in the orders Anseriformes and Charadriiformes [3, 15, 36, 38, 46, 72–74]. The risk-based Australian National Avian Influenza Wild Bird Surveillance Program detected a low prevalence (1.7%) of AIVs in 66,987 Australian wild birds between 2005 and 2012 using a study population containing over 90% Anseriformes or Charadriiformes [46, 47]. This contrasts with the results from this study where AIVs were not detected in any of the birds sampled. Together these results suggest that, although low pathogenic AIVs are circulating at low prevalence in wild birds (particularly those belonging to the orders Anseriformes and Charadriiformes) in Australia [46, 47], there is a low risk of AIV infection in birds presenting for veterinary care at the AWHC.
Studies of avian coronaviruses in wild birds in other countries, using the same primers [54] as the present study, have detected AvCoVs at a higher prevalence than was determined in this present study, including 13% and 3.5% of wild bird samples tested in studies in Brazil and Poland, respectively [75, 76]. These primers detect gammacoronaviruses of the IBV lineage, but do not appear to detect deltacoronaviruses. Whilst the present study was interested in investigating an hypothesised wild bird AvCoV origin of genetic components of variant IBV strains infecting commercial poultry in Australia [48, 50], future use of a pan-Coronaviridae PCR assay to test Australian wild bird samples for AvCoVs would be useful for gaining a more general understanding of AvCoVs in Australia. Future studies focused on elucidating the origin of variant IBV in Australian poultry may be best focused on species of wild birds known to be in closest contact with commercial poultry, such as introduced (feral) passerine birds that commonly live in close proximity to humans and domestic animal production facilities, like European starlings (Sturnus vulgaris) and house sparrows (Passer domesticus) [77, 78].
Our results showing a very low prevalence (0.25%) of APMVs in wild birds in Victoria are consistent with the results of other similar Australian studies [15, 79, 80]. The paramyxovirus detected in a musk lorikeet had highest nucleotide sequence identity to Avian avulavirus 5, which had previously only been isolated from budgerigars [25, 32–34, 71], and for which no sequence information from Australia is available. Budgerigars and musk lorikeets both belong to the family Psittaculidae within the avian order Psittaciformes. APMV-5 has been detected in epizootic outbreaks among captive budgerigar flocks in Queensland, Australia and Japan in the 1970s and in the UK in 1993, with high mortality and clinical signs including depression, dyspnoea, diarrhoea, vomiting and torticollis [32–34]. Of the currently recognized avian avulavirus serotypes, only Newcastle disease virus and APMV-5 have been associated with 100% mortality [34]. The clinical significance of the avulavirus strain detected from the musk lorikeet in the present study is undetermined, but the bird showed no clinical disease during rehabilitation.
It is possible that the APMV strain detected in this study represents an avirulent genotype of APMV-5 that may be circulating amongst wild psittacines in Australia. The complete genome sequences of strains of other avian paramyxoviruses have provided evidence for the existence of genetic subgroups within their respective serotypes [30, 81, 82]. Analysis of the same region of the RNA polymerase gene for which we have sequence information in the present study shows the different subgroups of APMV-2, APMV-3 and APMV-6 have 72.7–78.2%, 73.1% and 75.5–76.2% nucleotide sequence identity respectively within their serotypes [30, 81, 82]. By comparison, the APMV strain detected from the musk lorikeet in this study has 77.4–77.6% nucleotide identity with the APMV-5 sequences obtained from budgerigars in Japan in the 1970s [34, 71]. This suggests that the avian avulavirus detected in this study represents a novel genetic subgroup or genotype of the serotype Avian avulavirus 5, but this putative classification can only be confirmed if the virus can be isolated in the future for antigen-based testing and more extensive genome sequencing. Formal classification of APMV strain musk lorikeet/Melbourne/ML22-141263/2014 will require more genomic sequence information than the 603 bp of the RNA polymerase gene that was obtained in the current study. The clinical significance of this possible novel APMV-5 genotype in other Psittaciformes, such as those in involved in threatened species recovery programs, is also undetermined.
Despite 193 birds belonging to the order Psittaciformes being tested in this study, which included seven orange-bellied parrots from Healesville Sanctuary’s collection, neither Avian avulavirus 3 nor Psittacid alphaherpesvirus 1 were detected. Many species of Australian parrots are susceptible to APMV-3 and/or PsHV1 infection and disease [26–28, 83], so the potential establishment of both viruses in Australia poses significant risks to Australian Psittaciformes and their conservation [23], particularly to breeding programs for threatened species, such as the orange-bellied parrot captive breeding program at Healesville Sanctuary. Both APMV-3 and PsHV1 have been reported in numerous Australian psittacine species held in captivity overseas, and have caused significant mortality events in avicultural collections in other countries [23, 26–28, 83]. APMV-3 has not yet been reported in Australia [25–31], while PsHV1 has not been reported in wild Psittaciformes in Australia [23]. However it is known that PsHV1 entered Australia in captive green-winged macaws and is likely to present at a low prevalence in some avian collections, where latently infected parrots may be potential sources of virus dissemination [22]. Ongoing surveillance of both wild and captive Psittaciformes for these viruses is vital for detecting and managing potential outbreaks of these pathogens should they be introduced into Australia’s avifauna. Our results showing an absence of PsHV1 in the sampled birds are consistent with results from a serologic survey for PsHV1 in Australian psittacines from New South Wales, Tasmania, Victoria and Western Australia, where all 411 wild and captive birds tested were negative for the presence of neutralizing antibody to PsHV1 [84].
Three avian herpesviruses were detected in the present study: Columbid alphaherpesvirus 1 in a powerful owl and two novel viruses in a sulphur-crested cockatoo and a tawny frogmouth, respectively. CoHV1 infection had previously been documented in three species of native birds of prey in Australia: powerful owls, barking owls (Ninox connivens) and an Australian hobby (Falco longipennis) [65]. The virus causes herpesviral inclusion body disease or inclusion body hepatitis in birds of prey (considered aberrant hosts of this virus) when they ingest infected natural hosts of CoHV1 (rock doves or rock pigeons (Columba livia)) [62, 64–66, 85, 86]. A recent study has suggested that CoHV1 is enzootic in feral pigeon flocks in Australia, with CoHV1 DNA detected in samples from 81% of 53 feral pigeons from five flocks in Victoria and New South Wales [64]. This recent study also tested oral swabs from 18 Australian native columbids by PCR and all were found to be negative for CoHV1 DNA [64]. The present study tested six wild native Australian pigeons and 28 captive native Australian pigeons and doves. CoHV1 DNA was not detected from any of these birds, suggesting that native columbids are not likely to be a reservoir of CoHV1 infection in Australia.
The two novel herpesviruses detected in this study, tentatively designated CacHV1 and PodHV1, are interesting additions to our knowledge of herpesviruses in avian species. The genetic identification of previously undiscovered avian viruses is essential for mapping disease spread by the international trade in birds, managing biosecurity and disease outbreaks in aviaries, and managing threatened birds being bred for release for conservation of their species [87]. The 485 bp sequence obtained from the tawny frogmouth and the 489 bp sequence obtained from the sulphur-crested cockatoo showed 76% and 66.5% nucleotide identities to the most genetically similar known herpesviruses (CoHV1 and PsHV1, respectively). This level of identity in this highly conserved region of the herpesvirus DNA polymerase gene is sufficient to indicate that the viruses are new species. As a comparison, there is 82% nucleotide identity at this same region between the distinct but closely related species Equid alphaherpesvirus 1 and 4 (EHV1 and EHV4, respectively), but there is 100% nucleotide identity at this same region between different isolates of EHV1 and 100% identity between different isolates of EHV4 [88]. Serological cross neutralization has been reported between closely related alphaherpesviruses [89], thus the presence of the newly detected CacHV1 should be considered if any future serological surveys for PsHV1 return positive results. The phylogenetic analyses of sequence data in the present study is sufficient to place the newly detected viruses in the subfamily Alphaherpesvirinae (Figs 1 and 2), but insufficient to classify PodHV1 and CacHV1 beyond subfamily level. The clinical significance of these viruses remains undetermined, and more extensive genome sequencing will be required for the formal naming, description and classification of these previously unknown avian herpesviruses through the International Committee on Taxonomy of Viruses.
This study has contributed valuable information regarding viruses present (and absent) in Australian wild birds and the level of potential risk to animal and human health, and to threatened avian species in Australia. Whilst it is interesting that no avian herpesviruses nor paramyxoviruses were detected from the captive birds tested in this study, there was no significant difference in the prevalence of detection of avian viruses between the wild and captive bird study populations. The detection of two previously unknown viruses has contributed to our knowledge of avian viral communities and their diversity in Australia. Future studies incorporating deep sequencing methods could be expected to possibly uncover more avian viruses and make further contributions to our understanding of avian virodiversity.
Supporting information
Wild birds were presented to the AWHC at Healesville Sanctuary for veterinary care. Captive birds were part of the Healesville Sanctuary collection.
(DOCX)
Acknowledgments
We thank the staff at Healesville Sanctuary’s Australian Wildlife Health Centre for their assistance with sample collection–particularly Leanne Wicker, Phillipa Mason, Franciscus Scheelings, Meg Curnick, Jackie Reed and Emmajane Newton-Dinning. We are grateful to Professor Ron Slocombe and Dr Amir Noormohammadi for their histopathological examination of tissues collected from the sulphur-crested cockatoo from which Cacatuid alphaherpesvirus 1 was detected, and to Faye Docherty for preparing these histological slides. We also thank Professor Glenn Browning for his advice and guidance.
Data Availability
All relevant data are within the paper, and nucleotide sequences have been deposited in the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/, accession numbers MF576270, MF576271, MF576272).
Funding Statement
JAG was supported by The Cybec Foundation (www.cybec.com.au) and Zoos Victoria (www.zoo.org.au). JMD is supported by a grant (FT140101287) from the Australian Research Council (www.arc.gov.au). The Cybec Foundation and the Australian Research Council had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, while Zoos Victoria was involved in study design and facilitation of sample and data collection.
References
- 1.Cross TA, Arsnoe DM, Minnis RB, King DT, Swafford S, Pedersen K, et al. Prevalence of avian paramyxovirus 1 and avian influenza virus in double-crested cormorants (Phalacrocorax auritus) in eastern North America. J Wildl Dis. 2013;49(4): 965–977. doi: 10.7589/2012-06-164 [DOI] [PubMed] [Google Scholar]
- 2.Glaser LC, Barker IK, Weseloh DC, Ludwig J, Windingstad RM, Key DW, et al. The 1992 epizootic of Newcastle disease in double-crested cormorants in North America. J Wildl Dis. 1999;35(2): 319–330. doi: 10.7589/0090-3558-35.2.319 [DOI] [PubMed] [Google Scholar]
- 3.Alexander D. The epidemiology and control of avian influenza and Newcastle disease. J Comp Pathol. 1995;112(2): 105–126. [DOI] [PubMed] [Google Scholar]
- 4.Alexander D, Campbell G, Manvell R, Collins M, Parsons G, McNulty M. Characterisation of an antigenically unusual virus responsible for two outbreaks of Newcastle disease in the Republic of Ireland in 1990. Vet Rec. 1992;130(4): 65–68. [DOI] [PubMed] [Google Scholar]
- 5.Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine. 2007;25(30): 5637–5644. doi: 10.1016/j.vaccine.2006.10.051 [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Ramey AM, Bowman AS, DeLiberto TJ, Killian ML, Krauss S, et al. Low-pathogenic influenza A viruses in North American diving ducks contribute to the emergence of a novel highly pathogenic influenza A (H7N8) virus. J Virol. 2017;91(9): e02208–02216. doi: 10.1128/JVI.02208-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lycett SJ, Bodewes R, Pohlmann A, Banks J, Bányai K, Boni MF, et al. Role for migratory wild birds in the global spread of avian influenza H5N8. Science. 2016;354(6309): 213–217. doi: 10.1126/science.aaf8852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiris JM, De Jong MD, Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev. 2007;20(2): 243–267. doi: 10.1128/CMR.00037-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li K, Guan Y, Wang J, Smith G, Xu K, Duan L, et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430(6996): 209–213. doi: 10.1038/nature02746 [DOI] [PubMed] [Google Scholar]
- 10.Arzey GG, Kirkland PD, Arzey KE, Frost M, Maywood P, Conaty S, et al. Influenza virus A (H10N7) in chickens and poultry abattoir workers, Australia. Emerg Infect Dis. 2012;18(5): 814 doi: 10.3201/eid1805.111852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perdue ML, Swayne DE. Public health risk from avian influenza viruses. Avian Dis. 2005;49(3): 317–327. doi: 10.1637/7390-060305R.1 [DOI] [PubMed] [Google Scholar]
- 12.Reperant LA, Kuiken T, Osterhaus AD. Influenza viruses: from birds to humans. Hum Vaccin Immunother. 2012;8(1): 7–16. doi: 10.4161/hv.8.1.18672 [DOI] [PubMed] [Google Scholar]
- 13.Richard M, Graaf Md, Herfst S. Avian influenza A viruses: from zoonosis to pandemic. Future Virol. 2014;9(5): 513–524. doi: 10.2217/fvl.14.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capua I, Alexander DJ. Human health implications of avian influenza viruses and paramyxoviruses. Eur J Clin Microbiol Infect Dis. 2004;23(1): 1–6. doi: 10.1007/s10096-003-1059-3 [DOI] [PubMed] [Google Scholar]
- 15.Peroulis I, O'Riley K. Detection of avian paramyxoviruses and influenza viruses amongst wild bird populations in Victoria. Aust Vet J. 2004;82(1 & 2): 79–82. [DOI] [PubMed] [Google Scholar]
- 16.Sharples E, Baines SJ. Prevalence of Chlamydophila psittaci-positive cloacal PCR tests in wild avian casualties in the UK. Vet Rec. 2009;164(1): 16 [DOI] [PubMed] [Google Scholar]
- 17.Kalmar ID, Dicxk V, Dossche L, Vanrompay D. Zoonotic infection with Chlamydia psittaci at an avian refuge centre. Vet J. 2014;199(2): 300–302. doi: 10.1016/j.tvjl.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 18.Aaziz R, Gourlay P, Vorimore F, Sachse K, Siarkou VI, Laroucau K. Chlamydiaceae in North Atlantic seabirds admitted to a wildlife rescue center in western France. Appl Environ Microbiol. 2015;81(14): 4581–4590. doi: 10.1128/AEM.00778-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stojanovic D, Alves F, Cook H, Crates R, Heinsohn R, Peters A, et al. Further knowledge and urgent action required to save orange-bellied parrots from extinction. Emu. 2017: 1–9. [Google Scholar]
- 20.Raidal SR, Sarker S, Peters A. Review of psittacine beak and feather disease and its effect on Australian endangered species. Aust Vet J. 2015;93(12): 466–470. doi: 10.1111/avj.12388 [DOI] [PubMed] [Google Scholar]
- 21.Amery-Gale J, Marenda MS, Owens J, Eden PA, Browning GF, Devlin JM. A high prevalence of beak and feather disease virus in non-psittacine Australian birds. J Med Microbiol. 2017;66(7): 1005–1013. doi: 10.1099/jmm.0.000516 [DOI] [PubMed] [Google Scholar]
- 22.Tomaszewski EK, Wigle W, Phalen DN. Tissue distribution of psittacid herpesviruses in latently infected parrots, repeated sampling of latently infected parrots and prevalence of latency in parrots submitted for necropsy. J Vet Diagn Invest. 2006;18(6): 536–544. doi: 10.1177/104063870601800603 [DOI] [PubMed] [Google Scholar]
- 23.Phalen DN, Woods R. Psittacid herpesviruses and mucosal papillomas of birds in Australia: fact sheet. Wildlife Health Australia; 2009. [Google Scholar]
- 24.Gallagher A, Sullivan ND. Internal papilloma disease in greenwinged macaws (Ara chloroptera). Aust Vet J. 1997;75(9): 675–675. doi: 10.1111/j.1751-0813.1997.tb15372.x [DOI] [PubMed] [Google Scholar]
- 25.Alexander DJ. Avian paramyxoviruses—other than Newcastle disease virus. Worlds Poult Sci J. 1982;38(2): 97–104. [Google Scholar]
- 26.Jung A, Grund C, Muller I, Rautenschlein S. Avian paramyxovirus serotype 3 infection in Neopsephotus, Cyanoramphus, and Neophema species. J Avian Med Surg. 2009;23(3): 205–208. doi: 10.1647/2008-022.1 [DOI] [PubMed] [Google Scholar]
- 27.Shihmanter E, Weisman Y, Lublin A, Mahani S, Panshin A, Lipkind M. Isolation of avian serotype 3 paramyxoviruses from imported caged birds in Israel. Avian Dis. 1998;42(4): 829–831. [PubMed] [Google Scholar]
- 28.Smit T, Rondhuis P. Studies on a virus isolated from the brain of a parakeet (Neophema sp.). Avian Pathol. 1976;5(1): 21–30. doi: 10.1080/03079457608418166 [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Nayak B, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus type 3 reveals an unusually long trailer region. Virus Res. 2008;137(2): 189–197. doi: 10.1016/j.virusres.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Nayak B, Samuel AS, Xiao S, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus-3 strain Wisconsin: evidence for the existence of subgroups within the serotype. Virus Res. 2010;149(1): 78–85. doi: 10.1016/j.virusres.2009.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shihmanter E, Weisman Y, Lublin A, Mechani S, Gruenberg R, Horowith H, et al. Avian paramyxoviruses serotype 3 isolated from captive birds in Israel: clinical signs, pathology, and antigenic characterization. Avian Dis. 1998;42(2): 418–422. [PubMed] [Google Scholar]
- 32.Mustaffa-Babjee A, Spradbrow P, Samuel JL. A pathogenic paramyxovirus from a budgerigar (Melopsittacus undulatus). Avian Dis. 1974;18(2): 226–230. doi: 10.2307/1589130 [PubMed] [Google Scholar]
- 33.Nerome K, Nakayama M, Ishida M, Fukumi H, Morita A. Isolation of a new avian paramyxovirus from budgerigar (Melopsittacus undulatus). J Gen Virol. 1978;38(2): 293–301. doi: 10.1099/0022-1317-38-2-293 [DOI] [PubMed] [Google Scholar]
- 34.Samuel AS, Paldurai A, Kumar S, Collins PL, Samal SK. Complete genome sequence of avian paramyxovirus (APMV) serotype 5 completes the analysis of nine APMV serotypes and reveals the longest APMV genome. PLoS ONE. 2010;5(2): e9269 doi: 10.1371/journal.pone.0009269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arzey G. The role of wild aquatic birds in the epidemiology of avian influenza in Australia. Aust Vet J. 2004;82(6): 377–378. [DOI] [PubMed] [Google Scholar]
- 36.van Dijk JG, Verhagen JH, Wille M, Waldenström J. Host and virus ecology as determinants of influenza A virus transmission in wild birds. Curr Opin Virol. 2018;28: 26–36. doi: 10.1016/j.coviro.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 37.Olson SH, Parmley J, Soos C, Gilbert M, Latorre-Margalef N, Hall JS, et al. Sampling strategies and biodiversity of influenza A subtypes in wild birds. PLoS ONE. 2014;9(3): e90826 doi: 10.1371/journal.pone.0090826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansbro PM, Warner S, Tracey JP, Arzey KE, Selleck P, O’Riley K, et al. Surveillance and analysis of avian influenza viruses, Australia. Emerg Infect Dis. 2010;16(12): 1896–1904. doi: 10.3201/eid1612.100776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill NJ, Hussein IT, Davis KR, Ma EJ, Spivey TJ, Ramey AM, et al. Reassortment of influenza A viruses in wild birds in Alaska before H5 Clade 2.3.4.4 outbreaks. Emerg Infect Dis. 2017;23(4): 654–657. doi: 10.3201/eid2304.161668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poovorawan Y, Pyungporn S, Prachayangprecha S, Makkoch J. Global alert to avian influenza virus infection: from H5N1 to H7N9. Pathog Glob Health. 2013;107(5): 217–223. doi: 10.1179/2047773213Y.0000000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prosser DJ, Hungerford LL, Erwin RM, Ottinger MA, Takekawa JY, Ellis EC. Mapping avian influenza transmission risk at the interface of domestic poultry and wild birds. Front Public Health. 2013;1(28): 1–11. doi: 10.3389/fpubh.2013.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Australian Government Department of Agriculture and Water Resources. Notification of low pathogenic avian influenza virus outbreak in poultry. World Organisation for Animal Health (OIE); 2012. Available from: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?reportid=11554
- 43.Australian Government Department of Agriculture and Water Resources. Notification of highly pathogenic avian influenza virus H7N2 outbreak. World Organisation for Animal Health (OIE); 2013. Available from: http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2013/
- 44.Vijaykrishna D, Deng Y-M, Su YCF, Fourment M, Iannello P, Arzey GG, et al. The recent establishment of North American H10 lineage influenza viruses in Australian wild waterfowl and the evolution of Australian avian influenza viruses. J Virol. 2013;87(18): 10182–10189. doi: 10.1128/JVI.03437-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernandez-Jover M, Schemann K, East I, Toribio J-A. Evaluating the risk of avian influenza introduction and spread among poultry exhibition flocks in Australia. Prev Vet Med. 2015;118(1): 128–141. doi: 10.1016/j.prevetmed.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 46.Grillo VL, Arzey KE, Hansbro PM, Hurt AC, Warner S, Bergfeld J, et al. Avian influenza in Australia: a summary of 5 years of wild bird surveillance. Aust Vet J. 2015;93(11): 387–393. doi: 10.1111/avj.12379 [DOI] [PubMed] [Google Scholar]
- 47.Haynes L, Arzey E, Bell C, Buchanan N, Burgess G, Cronan V, et al. Australian surveillance for avian influenza viruses in wild birds between July 2005 and June 2007. Aust Vet J. 2009;87(7): 266–272. doi: 10.1111/j.1751-0813.2009.00446.x [DOI] [PubMed] [Google Scholar]
- 48.Quinteros JA, Lee S-W, Markham PF, Noormohammadi AH, Hartley CA, Legione AR, et al. Full genome analysis of Australian infectious bronchitis viruses suggests frequent recombination events between vaccine strains and multiple phylogenetically distant avian coronaviruses of unknown origin. Vet Microbiol. 2016;197: 27–38. doi: 10.1016/j.vetmic.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinteros JA, Markham PF, Lee S-W, Hewson KA, Hartley CA, Legione AR, et al. Analysis of the complete genomic sequences of two virus subpopulations of the Australian infectious bronchitis virus vaccine VicS. Avian Pathol. 2015;44(3): 182–191. doi: 10.1080/03079457.2015.1022857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mardani K, Noormohammadi AH, Hooper P, Ignjatovic J, Browning GF. Infectious bronchitis viruses with a novel genomic organization. J Virol. 2008;82(4): 2013–2024. doi: 10.1128/JVI.01694-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chmielewicz B, Goltz M, Lahrmann K-H, Ehlers B. Approaching virus safety in xenotransplantation: a search for unrecognized herpesviruses in pigs. Xenotransplantation. 2003;10(4): 349–356. doi: 10.1034/j.1399-3089.2003.02074.x [DOI] [PubMed] [Google Scholar]
- 52.van Boheemen S, Bestebroer TM, Verhagen JH, Osterhaus AD, Pas SD, Herfst S, et al. A family-wide RT-PCR assay for detection of paramyxoviruses and application to a large-scale surveillance study. PLoS ONE. 2012;7(4): e34961 doi: 10.1371/journal.pone.0034961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tong S, Chern SW, Li Y, Pallansch MA, Anderson LJ. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J Clin Microbiol. 2008;46(8): 2652–2658. doi: 10.1128/JCM.00192-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Callison SA, Hilt DA, Boynton TO, Sample BF, Robison R, Swayne DE, et al. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J Virol Methods. 2006;138(1–2): 60–65. doi: 10.1016/j.jviromet.2006.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heine HG, Trinidad L, Selleck P, Lowther S. Rapid detection of highly pathogenic avian influenza H5N1 virus by TaqMan reverse transcriptase–polymerase chain reaction. Avian Dis. 2007;51(s1): 370–372. doi: 10.1637/7587-040206r.1 [DOI] [PubMed] [Google Scholar]
- 56.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16(2): 101–117. [Google Scholar]
- 57.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc. 1922;85(1): 87–94. [Google Scholar]
- 58.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12): 1647–1649. doi: 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21): 2947–2948. doi: 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 60.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52(5): 696–704. doi: 10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- 61.Ehlers B, Borchers K, Grund C, Frölich K, Ludwig H, Buhk H-J. Detection of new DNA polymerase genes of known and potentially novel herpesviruses by PCR with degenerate and deoxyinosine-substituted primers. Virus Genes. 1999;18(3): 211–220. [DOI] [PubMed] [Google Scholar]
- 62.Gailbreath KL, Lindsay Oaks J. Herpesviral inclusion body disease in owls and falcons is caused by the pigeon herpesvirus (columbid herpesvirus 1). J Wildl Dis. 2008;44(2): 427–433. doi: 10.7589/0090-3558-44.2.427 [DOI] [PubMed] [Google Scholar]
- 63.Guo Y, Li S, Sun X, He Y, Zhao H, Wang Y, et al. Complete genome sequence and evolution analysis of a columbid herpesvirus type 1 from feral pigeon in China. Arch Virol. 2017;162: 2131–2133. doi: 10.1007/s00705-017-3329-x [DOI] [PubMed] [Google Scholar]
- 64.Phalen DN, Alvarado C, Grillo V, Mason P, Dobson E, Holz P. Prevalence of columbid herpesvirus infection in feral pigeons (Columba livia) from New South Wales and Victoria, Australia, with spillover into a wild powerful owl (Ninox struena). J Wildl Dis. 2017;53(3): 543–551. doi: 10.7589/2016-07-158 [DOI] [PubMed] [Google Scholar]
- 65.Phalen DN, Holz P, Rasmussen L, Bayley C. Fatal columbid herpesvirus-1 infections in three species of Australian birds of prey. Aust Vet J. 2011;89(5): 193–196. doi: 10.1111/j.1751-0813.2011.00706.x [DOI] [PubMed] [Google Scholar]
- 66.Pinkerton ME, Wellehan JFX Jr, Johnson AJ, Childress AL, Fitzgerald SD, Kinsel MJ. Columbid herpesvirus-1 in two Cooper's hawks (Accipiter cooperii) with fatal inclusion body disease. J Wildl Dis. 2008;44(3): 622–628. doi: 10.7589/0090-3558-44.3.622 [DOI] [PubMed] [Google Scholar]
- 67.Zhang L, Li Z, Li S, Hu X, Sun H, Li M, et al. Characterization of the first columbid herpesvirus 1 isolate from a hybrid meat-type pigeon flock in China. Arch Virol. 2015;160(2): 459–464. doi: 10.1007/s00705-014-2247-4 [DOI] [PubMed] [Google Scholar]
- 68.Thureen DR, Keeler CL Jr, Psittacid herpesvirus 1 and infectious laryngotracheitis virus: comparative genome sequence analysis of two avian alphaherpesviruses. J Virol. 2006;80(16): 7863–7872. doi: 10.1128/JVI.00134-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Styles DK, Tomaszewski EK, Phalen DN. A novel psittacid herpesvirus found in African grey parrots (Psittacus erithacus erithacus). Avian Pathol. 2005;34(2): 150–154. doi: 10.1080/03079450500059032 [DOI] [PubMed] [Google Scholar]
- 70.Hulbert CL, Chamings A, Hewson KA, Steer PA, Gosbell M, Noormohammadi AH. Survey of captive parrot populations around Port Phillip Bay, Victoria, Australia, for psittacine beak and feather disease virus, avian polyomavirus and psittacine adenovirus. Aust Vet J. 2015;93(8): 287–292. doi: 10.1111/avj.12350 [DOI] [PubMed] [Google Scholar]
- 71.Hiono T, Matsuno K, Tuchiya K, Lin Z, Okamatsu M, Sakoda Y. Complete genome sequence of the avian paramyxovirus serotype 5 strain APMV-5/budgerigar/Japan/TI/75. Genome Announc. 2016;4(5): e01005–01016. doi: 10.1128/genomeA.01005-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes LA, Savage C, Naylor C, Bennett M, Chantrey J, Jones R. Genetically diverse coronaviruses in wild bird populations of northern England. Emerg Infect Dis. 2009;15(7): 1091–1094. doi: 10.3201/eid1507.090067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muradrasoli S, Balint A, Wahlgren J, Waldenström J, Belak S, Blomberg J, et al. Prevalence and phylogeny of coronaviruses in wild birds from the Bering Strait area (Beringia). PLoS ONE. 2010;5(10): e13640 doi: 10.1371/journal.pone.0013640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stallknecht DE, Shane SM. Host range of avian influenza virus in free-living birds. Vet Res Commun. 1988;12(2–3): 125–141. [DOI] [PubMed] [Google Scholar]
- 75.Durães-Carvalho R, Caserta LC, Barnabé AC, Martini MC, Simas PV, Santos MM, et al. Phylogenetic and phylogeographic mapping of the avian coronavirus spike protein-encoding gene in wild and synanthropic birds. Virus Res. 2015;201: 101–112. doi: 10.1016/j.virusres.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Domanska-Blicharz K, Jacukowicz A, Lisowska A, Wyrostek K, Minta Z. Detection and molecular characterization of infectious bronchitis-like viruses in wild bird populations. Avian Pathol. 2014;43(5): 406–413. doi: 10.1080/03079457.2014.949619 [DOI] [PubMed] [Google Scholar]
- 77.Nemeth NM, Oesterle PT, Poulson RL, Jones CA, Tompkins SM, Brown JD, et al. Experimental infection of European starlings (Sturnus vulgaris) and house sparrows (Passer domesticus) with pandemic 2009 H1N1 and swine H1N1 and H3N2 triple reassortant influenza viruses. J Wildl Dis. 2013;49(2): 437–440. doi: 10.7589/2012-09-224 [DOI] [PubMed] [Google Scholar]
- 78.Nesterowicz A, Kawaoka Y, Bean W, Webster R. Molecular analysis of the haemagglutinin genes of Australian H7N7 influenza viruses: role of passerine birds in maintenance of transmission. Virology. 1987;160: 411–418. [DOI] [PubMed] [Google Scholar]
- 79.Garnett S, Flanagan M. Survey for Newcastle disease virus in northern Queensland birds. Aust Vet J. 1989;66(5): 129–134. [DOI] [PubMed] [Google Scholar]
- 80.Hoque MA, Burgess GW, Karo-Karo D, Cheam AL, Skerratt LF. Monitoring of wild birds for Newcastle disease virus in north Queensland, Australia. Prev Vet Med. 2012;103(1): 49–62. doi: 10.1016/j.prevetmed.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 81.Subbiah M, Nayak S, Collins PL, Samal SK. Complete genome sequences of avian paramyxovirus serotype 2 (APMV-2) strains Bangor, England and Kenya: evidence for the existence of subgroups within serotype 2. Virus Res. 2010;152(1): 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao S, Subbiah M, Kumar S, De Nardi R, Terregino C, Collins PL, et al. Complete genome sequences of avian paramyxovirus serotype 6 prototype strain Hong Kong and a recent novel strain from Italy: evidence for the existence of subgroups within the serotype. Virus Res. 2010;150(1): 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomaszewski EK, Kaleta EF, Phalen DN. Molecular phylogeny of the psittacid herpesviruses causing Pacheco's disease: correlation of genotype with phenotypic expression. J Virol. 2003;77(20): 11260–11267. doi: 10.1128/JVI.77.20.11260-11267.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raidal SR, Cross GM, Tomaszewski E, Graham DL, Phalen DN. A serologic survey for avian polyomavirus and Pacheco's disease virus in Australian cockatoos. Avian Pathol. 1998;27(3): 263–268. doi: 10.1080/03079459808419334 [DOI] [PubMed] [Google Scholar]
- 85.Maré CJ, Graham DL. Falcon herpesvirus, the etiologic agent of inclusion body disease of falcons. Infect Immun. 1973;8(1): 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rose N, Warren AL, Whiteside D, Bidulka J, Robinson JH, Illanes O, et al. Columbid herpesvirus-1 mortality in great horned owls (Bubo virginianus) from Calgary, Alberta. Can Vet J. 2012;53(3): 265–268. [PMC free article] [PubMed] [Google Scholar]
- 87.Shivaprasad HL, Phalen DN. A novel herpesvirus associated with respiratory disease in Bourke's parrots (Neopsephotus bourkii). Avian Pathol. 2012;41(6): 531–539. doi: 10.1080/03079457.2012.732692 [DOI] [PubMed] [Google Scholar]
- 88.Vaz P, Horsington J, Hartley C, Browning G, Ficorilli N, Studdert M, et al. Evidence of widespread natural recombination among field isolates of equine herpesvirus 4 but not among field isolates of equine herpesvirus 1. J Gen Virol. 2016;97(3): 747–755. doi: 10.1099/jgv.0.000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel J, Heldens J. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)–epidemiology, disease and immunoprophylaxis: a brief review. Vet J. 2005;170(1): 14–23. doi: 10.1016/j.tvjl.2004.04.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild birds were presented to the AWHC at Healesville Sanctuary for veterinary care. Captive birds were part of the Healesville Sanctuary collection.
(DOCX)
Data Availability Statement
All relevant data are within the paper, and nucleotide sequences have been deposited in the GenBank database (https://www.ncbi.nlm.nih.gov/genbank/, accession numbers MF576270, MF576271, MF576272).