Abstract
The Ras-Raf-mitogen-activated protein kinase kinase (MEK)1/2-extracellular signal-regulated kinase (ERK)1/2 signaling pathway contributes to the release of chondral matrix-degrading enzymes and accelerates the degradation of articular cartilage. Electroacupuncture (EA) treatment has been widely used for the treatment of osteoarthritis (OA); however, the mechanism underlying the effects of EA on OA remains unclear. Therefore, the present study evaluated the anti-inflammatory effects and potential underlying mechanisms of EA serum (EAS) on tumor necrosis factor (TNF)-α-mediated chondrocyte inflammation. A total of 30 Sprague Dawley rats were randomly divided into three groups: The blank group; experimental group I, which received 15 min of EA treatment; and experimental group II, which received 30 min of EA treatment. Subsequently, serum samples were obtained. Chondrocytes were isolated from the knee cartilage of Sprague Dawley rats, and were identified using collagen type II immunohistochemistry. TNF-α-treated chondrocytes were used as a cell model, and subsequently the cells were treated with EAS from each group for various durations. The results demonstrated that EAS treatment significantly promoted the viability and inhibited the apoptosis of TNF-α-treated chondrocytes. In addition, interleukin (IL)-1β concentration was significantly increased in the model group compared with in the control group, whereas EAS significantly reduced IL-1β concentration in TNF-α-treated chondrocytes. Furthermore, the protein expression levels of Ras, Raf and MEK1/2 were reduced in the EAS groups compared with in the model group. EAS also significantly inhibited the phosphorylation of ERK1/2, and the expression of downstream regulators matrix metalloproteinase (MMP)-3 and MMP-13. In conclusion, these results indicated that EAS may inhibit TNF-α-mediated chondrocyte inflammation via the Ras-Raf-MEK1/2-ERK1/2 signaling pathway in vitro, thus suggesting that EAS may be considered a potential therapeutic strategy for the treatment of OA.
Keywords: electroacupuncture serum, signaling pathway, osteoarthritis, chondrocyte, inflammation
Introduction
Osteoarthritis (OA) is characterized by simultaneous bone destruction and osteophyte formation; the main clinical symptoms of OA include joint pain and dysfunction (1). Cartilage degradation induced by chondrocyte inflammation has been hypothesized to be a crucial event in the development of OA. Chondrocytes, which are the only resident cell type present in articular cartilage, serve an important role in the proliferation and degradation of cartilage (2,3). It has been demonstrated that the apoptosis of chondrocytes is significantly correlated with the severity of OA, as it can cause matrix degradation and destruction, finally resulting in OA (4,5).
It has been hypothesized that inflammation is involved in the development and progression of OA, even in the early stages of the disease. Tumor necrosis factor (TNF)-α is one of the pivotal inflammatory factors in the pathogenesis of OA. During the onset and progression of OA, the concentration of TNF-α in the synovial fluid has been reported to increase, resulting in the induction of inflammation, promotion of the release of bone matrix-degrading enzymes, and the enhancement of fibroblastic hyperplasia and histological damage (6). The Ras-Raf-mitogen-activated protein kinase kinase (MEK)1/2-extracellular signal-regulated kinase (ERK)1/2 signaling pathway regulates the TNF-α-induced inflammatory response, and finally leads to cartilage degradation (7).
The Ras-Raf-MEK1/2-ERK1/2 signaling pathway is involved in cell inflammation and is closely associated with the progression of various diseases. TNF-α interacts with its receptor on the cell surface and then initiates the Ras-Raf-MEK1/2-ERK1/2 signaling pathway inducing gene transcription. In addition, the pathway regulates the activity of apoptotic molecules that are normally sequestered in the mitochondria. Previous studies have demonstrated that the pathway is inappropriately activated in numerous types of cancer, leading to the activation of ERK via its phosphorylation. Phosphorylated (p)-ERK interacts with various substrates in the nucleus and cytosol, thus controlling the transcription and expression of numerous genes involved in inflammatory responses and cellular apoptosis (8,9).
Acupuncture has been widely used as an alternative therapy for the treatment of several diseases (10–12). Electroacupuncture (EA) is a modified acupuncture technique that utilizes electrical stimulation. EA is an effective and simple approach, which has been widely used for the treatment of OA, not only to alleviate pain and disability, but also to decrease inflammation and the progression of pathogenesis (13,14). Our previous study demonstrated that EA treatment promotes chondrocyte proliferation via promotion of G1/S checkpoint transition in the cell cycle (15). However, the precise mechanism underlying the effects of EA serum (EAS) on inflammation-induced cartilage degradation remains to be elucidated. Therefore, the present study investigated the effects of EAS on the TNF-α-mediated inflammation of chondrocytes, in order to explore the underlying molecular mechanisms.
Materials and methods
Animals
All animal procedures conducted in the present study were approved by the Institutional Animal Care and Use Committee of the Fujian University of Traditional Chinese Medicine (Fuzhou, China). A total of 30 Sprague Dawley rats (age, 2 months; weight, 210±10 g) were purchased from the Shanghai Slack Laboratory Animal Co., Ltd. (Shanghai, China; certification no. SCXK: 2012-0002). All rats were housed in cages at a relative humidity of 55±5% and at room temperature (24±2°C) under a 12-h light/dark cycle. The rats were given ad libitum access to water and food.
EA treatment
Following 1 week of acclimation, the rats were randomly divided into three groups: The blank group and two experimental groups (n=10 rats/group). Rats in the two experimental groups were treated with EA using the bilateral Neixiyan (EX-LE4) and Waixiyan (ST 35) acupuncture points, which are located on the inside and outside of the depression of the patellar ligament, respectively. Disposable needles (diameter, 0.25 mm; length, 15 mm; Huatuo; Suzhou Medical Appliance Factory, Suzhou, China) were inserted to a depth of 4 mm at the acupuncture points. The acupuncture points of rats in experimental groups I and II were stimulated using the SDZ-II nerve and muscle stimulator (Huatuo; Suzhou Medical Appliance Factory) with an intensity of 2 mA and a frequency of 2 Hz for 15 and 30 min, respectively. The positively and negatively charged clips were connected to the right and left needles of the bilateral knee joints for 15 or 30 min each day for 3 days.
Preparation of serum
Arterial blood was collected from the abdominal aorta. Processing and storage of blood was conducted in accordance with a previously described method (16). The collected blood was placed at room temperature for 4 h and centrifuged at 1,500 × g for 15 min at room temperature. The serum fraction was isolated and was then filtered through a 0.22 µm filter. The resulting EAS was then aliquoted and stored at −20°C.
Chondrocyte culture and identification
Chondrocytes were isolated (stepwise 0.2% collagenase type II digestion) from the knee cartilage of 4-week-old male Sprague Dawley rats (weight, 80±10 g), which were purchased from the Shanghai Slack Laboratory Animal Co., Ltd. (certification no. SCXK: 2012-0001). The chondrocytes were cultured and identified by collagen type II immunohistochemistry (17–19). Briefly, chondrocytes from the knee articular cartilage of 4-week-old rats were isolated using 0.2% type II collagenase (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in magnesium- and calcium-free PBS (pH 7.4; HyClone; GE Healthcare, Logan, UT, USA) for 1 h at 37°C. The cells were then resuspended in Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 µg/ml streptomycin and 100 U/ml penicillin (HyClone; GE Healthcare), and were seeded in a monolayer at a density of 5×105 cells/cm2. Third generation chondrocytes were seeded on cover slips and cultured for 72 h, the cover slips were then rapidly washed in ethanol, dried and mounted for microscopic examination. Subsequently, the chondrocytes were incubated with rat monoclonal antibodies against type II collagen (BS1071; 1:100; Bioworld Technology, Inc., St. Louis Park, MN, USA) at 4°C overnight. The expression of type II collagen was observed using a phase-contrast microscope (BH2; Olympus Corporation, Tokyo, Japan). The primary chondrocytes were termed passage (P)1, and the P3 chondrocytes were used in subsequent experiments.
EAS treatment of chondrocytes
P3 chondrocytes were seeded into 6-well plates at a density of 1.3×105/ml, and were incubated in DMEM (HyClone; GE Healthcare) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) for 72 h at 37°C in a 5% CO2 incubator. The cells were divided into four groups: Control group, which was treated with 10% serum (100 µl/ml) obtained from the blank group; model group, which was cotreated with 10 µg/l TNF-α and 10% serum (100 µl/ml) obtained from the blank group; EAS I group, which was cotreated with 10 µg/l TNF-α and 10% serum (100 µl/ml) obtained from experimental group I; and EAS II group, which was cotreated with 10 µg/l TNF-α and 10% serum (100 µl/ml) obtained from experimental group II. All groups were treated for 24, 48, and 72 h at 37°C in a 5% CO2 incubator.
Assessment of cell viability
Following treatment with EAS, the chondrocytes were seeded into 6-well plates at a density of 1.3×105/ml for 24 h. Following treatment with or without TNF-α and EAS for 24, 48 and 72 h, the medium was then discarded and 100 µl 0.5% MTT solution was added to replace the medium. Following a 4 h incubation at 37°C, the wells were emptied, and 150 µl dimethyl sulfoxide was added to the plate and agitated for 10 min. The absorbance was measured at 490 nm using an ELISA reader (BioTek Instruments, Inc., Winooski, VT, USA), and the means were calculated.
Observation of morphologic alterations
The cells were cultured in 6-well plates at a density of 1.3×105/ml for 24 h. Following treatment with or without TNF-α and EAS for 48 h, cell morphology was observed using a phase-contrast microscope (BH2; Olympus Corporation).
ELISA
The concentration of interleukin (IL)-1β in conditioned medium was measured using an ELISA kit (RLB00; R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer's protocol. Briefly, following treatment with or without EAS for 48 h at 37°C in a 5% CO2 incubator, the cell culture medium was collected and centrifuged at 1,500 × g for 15 min at room temperature to remove cell fragments, and IL-1β concentration was determined. Absorbance of the samples was measured using a microplate spectrophotometer (Omega Bio-Tek, Inc., Norcross, GA, USA). A standard curve was generated, from which IL-1β concentration was determined.
Assessment of chondrocyte apoptosis by DAPI staining
Treated chondrocytes were collected and fixed in 4% paraformaldehyde for 15 min. Subsequently, the cells were stained with 5 µg/ml DAPI for 5 min and washed three times with PBS. The cells were then observed under a Fluo-View confocal fluorescent microscope (Fluo-View FV10i; Olympus Corporation). A total of 10 visual fields in each group were randomly selected and the number of apoptotic chondrocytes in each of these fields was counted using ZEN 2009 Light Edition (Carl Zeiss AG, Oberkochen, Germany).
Western blot analysis
Total proteins were isolated from cells using radioimmunoprecipitation assay lysis buffer (P0013B; Beyotime Institute of Biotechnology, Shanghai, China), after which they were stored on ice for 30 min and the protein concentration was determined using the bicinchoninic acid assay (P0009; Beyotime Institute of Biotechnology). Protein (20 µg) was separated by 10 or 12% SDS-PAGE. Following electrophoresis, proteins were transferred to polyvinylidene fluoride membranes using a semidry blotting system, and the membranes were blocked with 5% w/v nonfat dry milk for 1 h at room temperature (20). The membranes were then incubated with rat monoclonal antibodies against Ras (ab52939; 1:5,000), Raf (ab33899; 1:2,000), MEK1/2 (ab178876; 1:5,000) and β-actin (ab6276; 1:1,000) (Abcam, Cambridge, UK), and ERK1/2 (4695s; 1:1,000) and p-ERK1/2 (4094s; 1:1,000) (Cell Signaling Technology, Inc., Beverly, MA, USA) at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (ZB-2301; 1:5,000; OriGene Technologies, Inc., Beijing, China; or bs-0296G; 1:5,000; BIOSS, Beijing, China) for 1 h at room temperature. Blots were visualized using Pierce™ Enhanced Chemiluminescence Western Blotting Substrate (32106; Thermo Fisher Scientific, Inc.). The intensity of each band was semi-quantified using the Image Lab gel analyzing system (Image Lab 3.0™ Software; Bio-Rad Laboratories, Inc., Hercules, CA, USA) and was normalized to the band intensity of β-actin.
Laser confocal scanning microscopy
Following treatment, the cells were fixed in ice-cold methanol and permeabilized with 1% Triton X-100 for 10 min, after which they were blocked with 5% bovine serum albumin (A8010; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 1 h at room temperature. The fixed cells were washed and incubated with rat monoclonal antibodies against matrix metalloproteinase (MMP)-3 (sc-30070; 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and MMP-13 (PA5-16566; 1:100; Thermo Fisher Scientific, Inc.) at 4°C overnight, followed by incubation with Alexa Fluor 488 goat anti-rabbit immunoglobulin G (H+L) secondary antibodies (A-11008; 1:300; Thermo Fisher Scientific, Inc.) and DAPI (D1306; 1:100; Thermo Fisher Scientific, Inc.) for 5 min at room temperature in the dark. The signal was visualized and images were acquired using a laser confocal scanning microscope (Olympus Corporation).
Statistical analysis
All data were collected from at least three independent experiments. Statistical analysis was performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). All data are presented as the mean ± standard deviation. The differences among the four groups were compared using one-way analysis of variance, and multiple comparisons were performed using the Student-Newman-Keuls-q test. P<0.05 was considered to indicate a statistically significant difference.
Results
EAS enhances chondrocyte viability
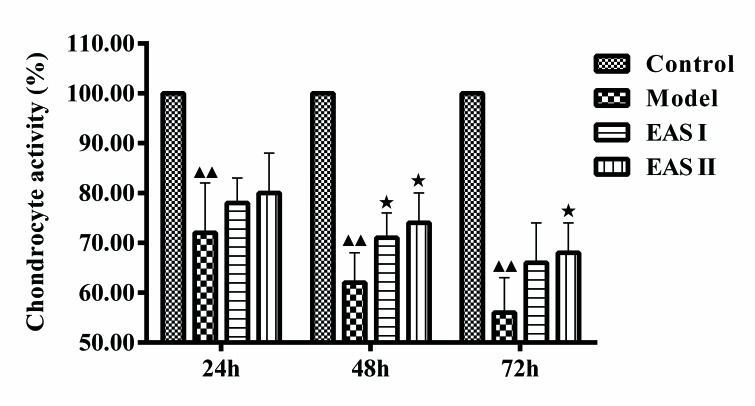
The present study examined whether EAS could promote the viability of TNF-α-treated chondrocytes using an MTT assay. The groups were treated with or without EAS for various durations. The results demonstrated that the viability of the model group was significantly lower than that of the control group (P<0.01), and the viability of the EAS I and II groups was significantly higher than that of the model group after 48 h (P<0.05; Fig. 1). Therefore, EAS treatment for 48 h was used in the subsequent experiments.
Figure 1.
EAS enhances the viability of TNF-α-treated chondrocytes. Chondrocytes were treated with or without TNF-α, and with or without EAS, for 24, 48 and 72 h, and an MTT assay was performed to detect cell viability. Data are presented as the mean ± standard deviation. ▲▲P<0.01 compared with the control group; ★P<0.05 compared with the model group. EAS, electroacupuncture serum; TNF-α, tumor necrosis factor-α.
Effects of EAS on the morphology of TNF-α-treated chondrocytes
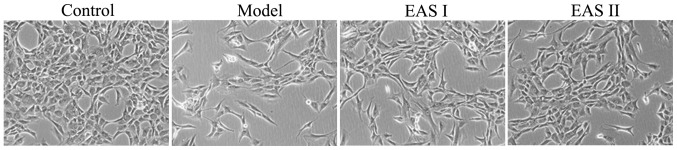
To determine the effects of EAS treatment on the morphology of cells, the morphological alterations of the cells in the various groups were observed by phase-contrast microscopy (Fig. 2). The morphology of the untreated control cells exhibited a healthy status, whereas TNF-α-treated chondrocytes presented more apoptotic cells that detached from each other and became bright, elongated and shrunken, or floated in the medium, as compared with cells in the EAS I and II groups. However, the TNF-α-induced alterations in cell morphology were not observed, or were less evident, in the TNF-α-stimulated chondrocytes treated with EAS.
Figure 2.
Effects of EAS on the morphology of TNF-α-treated chondrocytes. Chondrocytes were treated with or without TNF-α, and with or without EAS, for 48 h, and a phase-contrast microscope was employed to detect the morphological alterations (magnification, ×200). EAS, electroacupuncture serum; TNF-α, tumor necrosis factor-α.
EAS inhibits IL-1β and apoptosis of TNF-α-treated chondrocytes
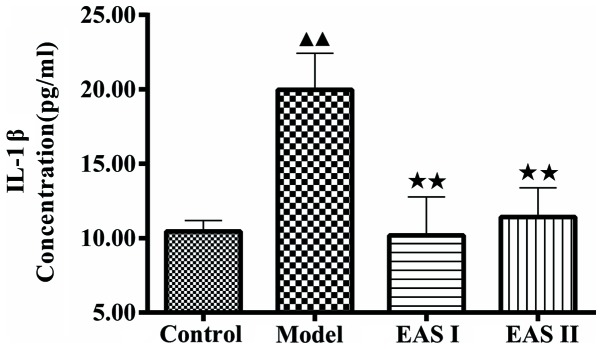
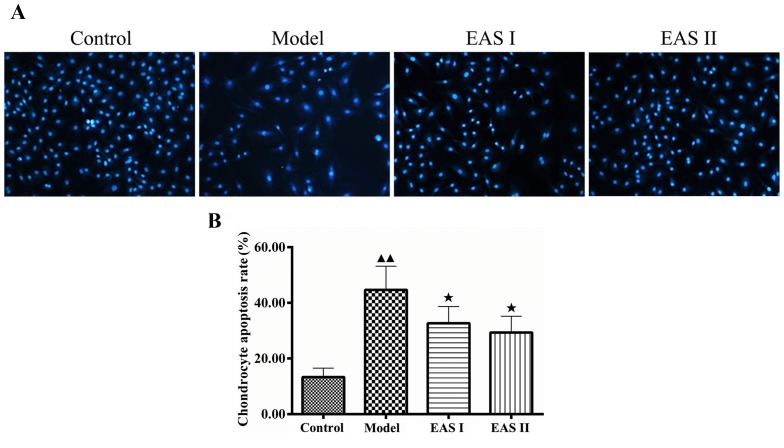
To examine the effects of EAS on inflammation in TNF-α-treated chondrocytes, an ELISA was used to measure IL-1β concentration. As presented in Fig. 3, IL-1β concentration in the model group was significantly higher compared with in the control group (P<0.01); however, treatment with EAS significantly reduced IL-1β concentration compared with the model group (P<0.01). To further determine whether EAS inhibited inflammation via apoptotic processes, DAPI staining was used to assess chondrocyte apoptosis. Apoptotic cells exhibited typical alterations, including reduced cellular volume, bright blue staining, and condensed or fragmented nuclei. This phenomenon was more obvious in the model group compared with in the EAS I and II groups (Fig. 4). The percentage of apoptotic cells in the EAS groups was significantly lower than in the model group (P<0.05), thus suggesting that EAS may inhibit the apoptosis of TNF-α-treated chondrocytes via the regulation of IL-1β.
Figure 3.
EAS inhibits IL-1β concentration in TNF-α-treated chondrocytes. Chondrocytes were treated with or without TNF-α, and with or without EAS, for 48 h, and an ELISA was performed to determine the concentration of IL-1β in the supernatant. Data are presented as the mean ± standard deviation. ▲▲P<0.01 compared with the control group; ★★P<0.01 compared with the model group. EAS, electroacupuncture serum; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α.
Figure 4.
EAS inhibits the apoptosis of tumor necrosis factor-α-treated chondrocytes. Third-passage chondrocytes were cultured for 2 days. After 48 h of treatment, chondrocyte apoptosis was detected using DAPI staining, (A) DAPI staining was employed to detect the apoptotic alterations of the cells (magnification, ×200). (B) Apoptotic chondrocytes were quantified as the percentage of total cells. Data are presented as the mean ± standard deviation. ▲▲P<0.01 compared with the control group; ★P<0.05 compared with the model group. EAS, electroacupuncture serum.
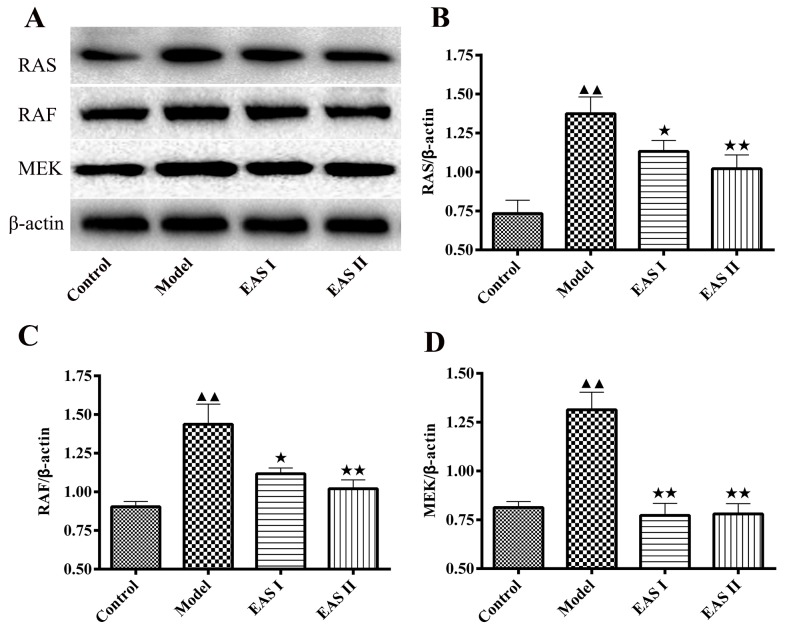
EAS inhibits the protein expression levels of Ras, Raf, MEK1/2 and p-ERK1/2 in TNF-α-treated chondrocytes
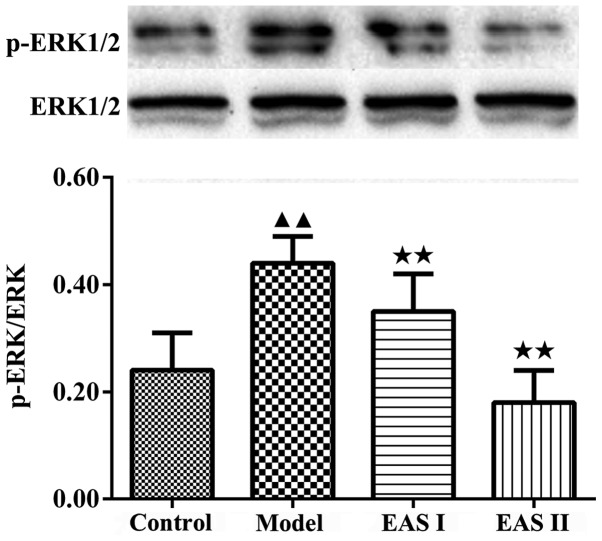
To gain insight into the mechanisms underlying the effects of EAS on the inflammation of TNF-α-treated chondrocytes, the protein expression levels of Ras, Raf and MEK1/2 were detected. The results demonstrated that the protein expression levels of Ras, Raf and MEK1/2 were lower in the EAS I and II groups compared with in the model group (P<0.05; Fig. 5). Furthermore, activation of the ERK1/2 signaling pathway has been reported to participate in chondrocyte inflammation induced by various stimuli. p-ERK1/2 is an intracellular signaling molecule that transduces extracellular responses, serves a well-known role in regulating chondrocyte inflammation and contributes to loss of the chondral matrix (21,22). Therefore, to understand the molecular mechanism by which EAS inhibits TNF-α-induced inflammation, the expression of p-ERK1/2 was detected. The results demonstrated that the protein expression levels of p-ERK1/2 were significantly reduced in the EAS groups compared with in the model group (P<0.01; Fig. 6). Taken together, these results indicated that EAS may inhibit TNF-α-mediated chondrocyte inflammation via the Ras-Raf-MEK1/2-ERK1/2 signaling pathway.
Figure 5.
EAS inhibits the protein expression levels of Ras, Raf and MEK1/2 in TNF-α-treated chondrocytes. (A) Chondrocytes were treated with or without TNF-α, and with or without EAS, for 48 h, and the protein expression was measured by western blot analysis. Protein expression levels of (B) Ras, (C) Raf and (D) MEK. β-actin was used as an internal control for normalization. Data are presented as the mean ± standard deviation. ▲▲P<0.01 compared with the control group; ★★P<0.01 and ★P<0.05 compared with the model group. EAS, electroacupuncture serum; MEK, mitogen-activated protein kinase kinase; TNF-α, tumor necrosis factor-α.
Figure 6.
EAS inhibits the phosphorylation of ERK in TNF-α-treated chondrocytes. After chondrocytes were treated for 48 h, the protein expression levels of ERK1/2 and p-ERK1/2 were measured by western blot analysis. EAS significantly inhibited the TNF-α-mediated phosphorylation of ERK. The results are representative of three independent experiments. Data are presented as the mean ± standard deviation. ▲▲P<0.01 compared with the control group; ★★P<0.01 compared with the model group. EAS, electroacupuncture serum; ERK, extracellular signal-regulated kinase; p-, phosphorylated; TNF-α, tumor necrosis factor-α.
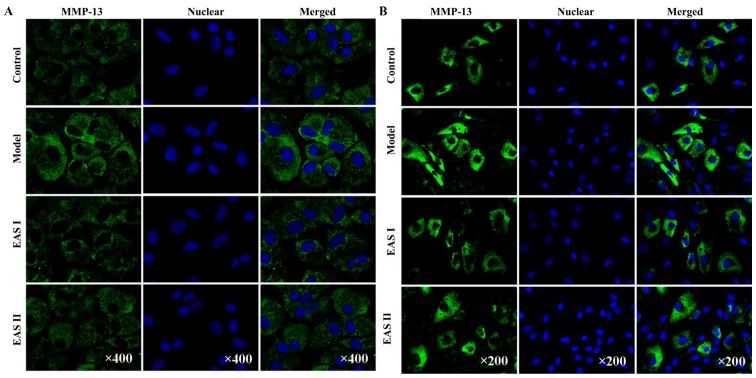
EAS decreases the protein expression levels of MMP-3 and MMP-13 in TNF-α-treated chondrocytes
It is well known that MMPs serve a critical role in OA. Furthermore, a previous study reported that EA inhibited the expression of MMP-3 in chondrocytes (23). Therefore, the present study investigated the effects of EAS on the protein expression of MMP-3 and MMP-13 using immunofluorescent staining. Chondrocytes stimulated with TNF-α exhibited an increased release of MMP-3 and MMP-13 compared with the untreated controls. However, treatment of chondrocytes with EAS markedly inhibited the TNF-α-mediated release of MMP-3 and MMP-13 (Fig. 7), which indicated that EAS may inhibit the Ras-Raf-MEK1/2-ERK1/2 pathway, resulting in reduced MMP-3 and MMP-13 expression.
Figure 7.
EAS decreases the expression of MMP-3 and MMP-13 in TNF-α-treated chondrocytes. Chondrocytes were treated with or without TNF-α, and with or without EAS, for 48 h, and the expression of (A) MMP-3 and (B) MMP-13 was determined by immunofluorescent staining (green). The nuclei of chondrocytes were stained with DAPI (blue). The results represent four independent experiments. EAS, electroacupuncture serum; MMP, matrix metalloproteinase; TNF-α, tumor necrosis factor-α.
Discussion
EA is a modern modification of the traditional acupuncture method, which stimulates acupuncture points with electrical current instead of manual manipulations and has been used to treat OA. Previous studies have demonstrated that EA treatment is able to induce a series of changes in serum (24–26). The present study used EAS to explore the anti-inflammatory effects and underlying mechanisms of EA on OA. EA treatment leads to the production of a series of factors involved in the regulation of cartilage inflammation. These factors are translocated to tissues and cells through the blood circulation; therefore, cells treated with EAS in vitro are in a similar condition to in vivo cells (27,28). The treatment of cells in vitro with EAS is easily regulated and effects are easily detected; therefore, EAS treatment is convenient in cell and molecular biology, as it can reveal the mechanisms underlying acupuncture treatment (29,30). In the present study, chondrocytes were treated with EAS with the aim to further investigate the potential mechanisms underlying the effects of EAS treatment on the regulation of chondrocyte function.
Inflammation leads to cartilage degradation; therefore, inhibiting chondrocyte inflammation may be an efficient method for the treatment of OA. Previous studies have reported that TNF-α is a potent proinflammatory cytokine that induces apoptosis of chondrocytes, which had been enzymatically dissociated from OA knee cartilage (31,32). In order to study the effects of EAS treatment on TNF-α-mediated chondrocyte inflammation, a chondrocyte culture was established in vitro via stepwise 0.2% collagenase type II digestion; subsequently, the chondrocytes were treated with 10 µg/l TNF-α for various durations. The results of the present study are similar to those of a previous study, which indicated that TNF-α suppresses viability and induces apoptotic signaling in chondrocytes ex vivo (33). Furthermore, IL-1β serves a crucial role in the progression of OA, where it induces cartilage damage through modulating the expression of MMPs, and promotes proteoglycan degradation and apoptosis (34). In the present study, IL-1β concentration was increased in TNF-α-treated chondrocytes; however, treatment with EAS significantly reduced the concentration of IL-1β in TNF-α-treated chondrocytes. Furthermore, EAS significantly reduced the percentage of TNF-α-mediated apoptotic chondrocytes, thus suggesting that EA may prevent the degradation of articular cartilage by inhibiting chondrocyte inflammation. The concentration of TNF-α (10 µg/l) used in the present study was based on the concentration range of TNF-α (5–40 µg/l) used in a previous study, which focused on TNF-α-mediated inflammation in chondrocytes in vitro (33).
The Ras-Raf-MEK1/2-ERK1/2 signaling pathway is the best characterized of three mitogen-activated protein kinase pathways that transduce extracellular signals through an intracellular signal transduction cascade. In vitro, IL-1β leads to the activation of ERK; activation of the Erk1/2 signaling pathway can induce numerous protein kinase cascade reactions, and transmits extracellular signals into the cells. Under the stimulation of extracellular signals, Ras can be activated by binding with guanosine triphosphate (35). Through a complex series of events, activated Ras then directs plasma membrane recruitment and activation of Raf. Through the formation of homo- and heterodimers, Raf propagates downstream signaling by activating MEK1/2 at one of two serine residues. Subsequently, MEK1/2 propagates the signal by phosphorylating ERK at two threonine residues (36,37). In accordance with previous reports, the present study demonstrated that TNF-α increased p-ERK1/2 expression in chondrocytes (38). It has previously been reported that EA may reduce the protein expression levels of p-ERK1/2 in cells (39). The present study demonstrated that EAS was able to decrease the protein expression levels of p-ERK1/2 in TNF-α-treated chondrocytes. p-ERK1/2 enters the nuclei and triggers the activity of transcription factors and MMPs, including MMP-3 and MMP-13, causing a series of cellular responses that can regulate cell apoptosis (40–43). Therefore, it may be hypothesized that cartilage degradation can be reduced via inhibiting the activity of MMPs. The present study demonstrated that EAS markedly decreased the protein expression of MMP-3 and MMP-13 in TNF-α-treated chondrocytes.
In conclusion, EAS treatment efficiently suppressed inflammation by inhibiting activation of the Ras-Raf-MEK1/2-ERK1/2 signaling pathway and the expression of MMP-3 and MMP-13 in TNF-α-treated chondrocytes. Further studies are required to investigate the association between EAS and other signals involved in the inflammation of chondrocytes. An enhanced understanding of the underlying molecular mechanisms of EAS will aid the improvement of diagnoses and the development of novel therapeutic targets for the treatment of OA-associated inflammation.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81373719).
References
- 1.van den Berg WB. Osteoarthritis year 2010 in review: Pathomechanisms. Osteoarthritis Cartilage. 2011;19:338–341. doi: 10.1016/j.joca.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Huang Z, Li J, Du S, Chen G, Qi Y, Huang L, Xiao L, Tong P. Effects of UCP4 on the proliferation and apoptosis of chondrocytes: Its possible involvement and regulation in osteoarthritis. PLoS One. 2016;11:e150684. doi: 10.1371/journal.pone.0150684. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Li X, Liu C, Liang W, Ye H, Chen W, Lin R, Li Z, Liu X, Wu M. Millimeter wave promotes the synthesis of extracellular matrix and the proliferation of chondrocyte by regulating the voltage-gated K+ channel. J Bone Miner Metab. 2014;32:367–377. doi: 10.1007/s00774-013-0513-2. [DOI] [PubMed] [Google Scholar]
- 4.Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15:27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Loeser RF. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17:971–979. doi: 10.1016/j.joca.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussein MR, Fathi NA, El-Din AM, Hassan HI, Abdullah F, Al-Hakeem E, Backer EA. Alterations of the CD4(+), CD8(+) T cell subsets, interleukins-1beta, IL-10, IL-17, tumor necrosis factor-alpha and soluble intercellular adhesion molecule-1 in rheumatoid arthritis and osteoarthritis: Preliminary observations. Pathol Oncol Res. 2008;14:321–328. doi: 10.1007/s12253-008-9016-1. [DOI] [PubMed] [Google Scholar]
- 7.Gui L, Zeng Q, Xu Z, Zhang H, Qin S, Liu C, Xu C, Qian Z, Zhang S, Huang S, Chen L. IL-2, IL-4, IFN-γ or TNF-α enhances BAFF-stimulated cell viability and survival by activating Erk1/2 and S6K1 pathways in neoplastic B-lymphoid cells. Cytokine. 2016;84:37–46. doi: 10.1016/j.cyto.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clausen BH, Degn M, Sivasaravanaparan M, Fogtmann T, Andersen MG, Trojanowsky MD, Gao H, Hvidsten S, Baun C, Deierborg T, et al. Conditional ablation of myeloid TNF increases lesion volume after experimental stroke in mice, possibly via altered ERK1/2 signaling. Sci Rep. 2016;6:29291. doi: 10.1038/srep29291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu C, Yang H, Zhao Y, Chen X, Dong Y, Li L, Dong Y, Cui J, Zhu T, Zheng P, et al. The role of inflammatory cytokines and ERK1/2 signaling in chronic prostatitis/chronic pelvic pain syndrome with related mental health disorders. Sci Rep. 2016;6:28608. doi: 10.1038/srep28608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinman RS, McCrory P, Pirotta M, Relf I, Forbes A, Crossley KM, Williamson E, Kyriakides M, Novy K, Metcalf BR, et al. Acupuncture for chronic knee pain: A randomized clinical trial. JAMA. 2014;312:1313–1322. doi: 10.1001/jama.2014.12660. [DOI] [PubMed] [Google Scholar]
- 11.White A, Cummings M. Acupuncture for knee osteoarthritis: Study by Hinman et al represents missed opportunities. Acupunct Med. 2015;33:84–86. doi: 10.1136/acupmed-2014-010719. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Spaeth RB, Retzepi K, Ott D, Kong J. Acupuncture modulates cortical thickness and functional connectivity in knee osteoarthritis patients. Sci Rep. 2014;4:6482. doi: 10.1038/srep06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu MX, Li XH, Lin MN, Jia XR, Mu R, Wan WR, Chen RH, Chen LH, Lin WQ, Huang CY, et al. Clinical study on the treatment of knee osteoarthritis of Shen-Sui insufficiency syndrome type by electroacupuncture. Chin J Integr Med. 2010;16:291–297. doi: 10.1007/s11655-010-0513-1. [DOI] [PubMed] [Google Scholar]
- 14.Plaster R, Vieira WB, Alencar FA, Nakano EY, Liebano RE. Immediate effects of electroacupuncture and manual acupuncture on pain, mobility and muscle strength in patients with knee osteoarthritis: A randomised controlled trial. Acupunct Med. 2014;32:236–241. doi: 10.1136/acupmed-2013-010489. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Wu G, Fan H, Ye J, Liu X. Electroacupuncture promotes chondrocyte proliferation via accelerated G1/S transition in the cell cycle. Int J Mol Med. 2013;31:1443–1448. doi: 10.3892/ijmm.2013.1336. [DOI] [PubMed] [Google Scholar]
- 16.Wu G, Fan H, Huang Y, Zheng C, Ye J, Liu X. Duhuo Jisheng Decoction-containing serum promotes proliferation of interleukin-1β-induced chondrocytes through the p16-cyclin D1/CDK4-Rb pathway. Mol Med Rep. 2014;10:2525–2534. doi: 10.3892/mmr.2014.2527. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Du M, Liu X, Chen W, Wu M, Lin J, Wu G. Millimeter wave treatment promotes chondrocyte proliferation by upregulating the expression of cyclin-dependent kinase 2 and cyclin A. Int J Mol Med. 2010;26:77–84. doi: 10.3892/ijmm_00000437. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Li X, Liu G, Chen J, Weng X, Liu F, Xu H, Liu X, Ye H. Bauhinia championi (Benth.) Benth. Polysaccharides upregulate Wnt/β-catenin signaling in chondrocytes. Int J Mol Med. 2013;32:1329–1336. doi: 10.3892/ijmm.2013.1527. [DOI] [PubMed] [Google Scholar]
- 19.Li XH, Wu MX, Ye HZ, Chen WL, Lin JM, Zheng LP, Liu XX. Experimental study on the suppression of sodium nitroprussiate-induced chondrocyte apoptosis by Tougu Xiaotong capsule containing serum. Chin J Integr Med. 2011;17:436–443. doi: 10.1007/s11655-011-0751-x. [DOI] [PubMed] [Google Scholar]
- 20.Liu F, Weng X, Lin P, Zheng C, Xu H, Liu X, Ye H, Li X. Duhuo Jisheng decoction inhibits endoplasmic reticulum stress in chondrocytes induced by tunicamycin through the downregulation of miR-34a. Int J Mol Med. 2015;36:1311–1318. doi: 10.3892/ijmm.2015.2331. [DOI] [PubMed] [Google Scholar]
- 21.Kim KS, Oh DH, Choi HM, Bang JS, Ryu CJ, Kim JH, Yoo MC, Yang HI. Pyrrolidine dithiocarbamate, a NF-kappaB inhibitor, upregulates MMP-1 and MMP-13 in IL-1beta-stimulated rheumatoid arthritis fibroblast-like synoviocytes. Eur J Pharmacol. 2009;613:167–175. doi: 10.1016/j.ejphar.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Li J, Cheng K, Lin Q, Wang D, Zhang H, An H, Gao M, Chen A. Effect of low-intensity pulsed ultrasound on MMP-13 and MAPKs signaling pathway in rabbit knee osteoarthritis. Cell Biochem Biophys. 2011;61:427–434. doi: 10.1007/s12013-011-9206-4. [DOI] [PubMed] [Google Scholar]
- 23.Liang CX, Guo Y, Tao L, Xiao H, Liu QG, Ma HF, Guo CQ. Effects of acupotomy intervention on regional pathological changes and expression of carti-lage-mechanics related proteins in rabbits with knee osteoarthritis. Zhen Ci Yan Jiu. 2015;40(140):119–124. (In Chinese) [PubMed] [Google Scholar]
- 24.Yang EJ, Jiang JH, Lee SM, Hwang HS, Lee MS, Choi SM. Electroacupuncture reduces neuroinflammatory responses in symptomatic amyotrophic lateral sclerosis model. J Neuroimmunol. 2010;223:84–91. doi: 10.1016/j.jneuroim.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Liao HY, Sun MF, Lin JG, Chang SL, Lee YC. Electroacupuncture plus metformin lowers glucose levels and facilitates insulin sensitivity by activating MAPK in steroid-induced insulin-resistant rats. Acupunct Med. 2015;33:388–394. doi: 10.1136/acupmed-2014-010724. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, Li X, Li N, Zhou J. Electroacupuncture protects against articular cartilage erosion by inhibiting mitogen-activated protein kinases in a rat model of osteoarthritis. Acupunct Med. 2016;34:290–295. doi: 10.1136/acupmed-2015-010949. [DOI] [PubMed] [Google Scholar]
- 27.Pavão TS, Vianna P, Pillat MM, Machado AB, Bauer ME. Acupuncture is effective to attenuate stress and stimulate lymphocyte proliferation in the elderly. Neurosci Lett. 2010;484:47–50. doi: 10.1016/j.neulet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Kim SK, Bae H. Acupuncture and immune modulation. Auton Neurosci. 2010;157:38–41. doi: 10.1016/j.autneu.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Li X, Li L, Liu B, Lin J. Effect of electro acupuncture serum on MAPK signal pathway of apoptosis chondrocyte induced by TNF-α. Fujian J TCM. 2011;42:43–45. [Google Scholar]
- 30.Wu M, Li X, Wu G, Li L, Chen W, Liu X. Effects of serum on tumor necrosis factor alpha induced chondrocyte apoptosis following electro-acupuncture. J Clin Rehabilitative Tissue Engineering Res. 2011;15:8551–8555. [Google Scholar]
- 31.Markway BD, Cho H, Anderson DE, Holden P, Ravi V, Little CB, Johnstone B. Reoxygenation enhances tumour necrosis factor alpha-induced degradation of the extracellular matrix produced by chondrogenic cells. Eur Cell Mater. 2016;31:425–439. doi: 10.22203/eCM.v031a27. [DOI] [PubMed] [Google Scholar]
- 32.Malemud CJ, Sun Y, Pearlman E, Ginley NM, Awadallah A, Wisler BA, Dennis JE. Monosodium Urate and tumor necrosis factor-α increase apoptosis in human chondrocyte cultures. Rheumatology (Sunnyvale) 2012;2:113. doi: 10.4172/2161-1149.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Wu G, Wu M, Chen W, Liu X. In vitro study of inhibitory millimeter wave treatment effects on the TNF-α-induced NF-κB signal transduction pathway. Int J Mol Med. 2011;27:71–78. doi: 10.3892/ijmm.2010.549. [DOI] [PubMed] [Google Scholar]
- 34.Lu S, Xiao X, Cheng M. Matrine inhibits IL-1β-induced expression of matrix metalloproteinases by suppressing the activation of MAPK and NF-κB in human chondrocytes in vitro. Int J Clin Exp Pathol. 2015;8:4764–4772. [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Li F, Fan C, Wang C, Ruan H. Effects and relationship of ERK1 and ERK2 in interleukin-1β-induced alterations in MMP3, MMP13, type II collagen and aggrecan expression in human chondrocytes. Int J Mol Med. 2011;27:583–589. doi: 10.3892/ijmm.2011.611. [DOI] [PubMed] [Google Scholar]
- 36.Uehling DE, Harris PA. Recent progress on MAP kinase pathway inhibitors. Bioorg Med Chem Lett. 2015;25:4047–4056. doi: 10.1016/j.bmcl.2015.07.093. [DOI] [PubMed] [Google Scholar]
- 37.Xu WH, Zhang JB, Dang Z, Li X, Zhou T, Liu J, Wang DS, Song WJ, Dou KF. Long non-coding RNA URHC regulates cell proliferation and apoptosis via ZAK through the ERK/MAPK signaling pathway in hepatocellular carcinoma. Int J Biol Sci. 2014;10:664–676. doi: 10.7150/ijbs.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appleton CT, Usmani SE, Mort JS, Beier F. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab Invest. 2010;90:20–30. doi: 10.1038/labinvest.2009.111. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Peng B, Li FY, YU X, Lu HY, Wei XY. Study on the effect of electro-acupuncture ouch point in rabbits with muscle regeneration after contusion based on the ERK signal pathway inhibited by U0126. China J Traditional Chin Med Pharmacy. 2014;29:2304–2308. [Google Scholar]
- 40.Chambard JC, Lefloch R, Pouysségur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Yu SM, Kim SJ. The thymoquinone-induced production of reactive oxygen species promotes dedifferentiation through the ERK pathway and inflammation through the p38 and PI3K pathways in rabbit articular chondrocytes. Int J Mol Med. 2015;35:325–332. doi: 10.3892/ijmm.2014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, Wang Z, Xu D, Liu Y, Gao Y. Aquaporin 3 promotes prostate cancer cell motility and invasion via extracellular signal-regulated kinase 1/2-mediated matrix metalloproteinase-3 secretion. Mol Med Rep. 2015;11:2882–2888. doi: 10.3892/mmr.2014.3097. [DOI] [PubMed] [Google Scholar]
- 43.Zhong HM, Ding QH, Chen WP, Luo RB. Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF-κB nuclear translocation. Int Immunopharmacol. 2013;17:329–335. doi: 10.1016/j.intimp.2013.06.027. [DOI] [PubMed] [Google Scholar]