Abstract
Previous studies have reported that short-term vagus nerve stimulation (VNS) improves cardiac function in rats with chronic heart failure (CHF). The molecular mechanisms are unclear. The potential effect of microRNA (miR)-205 in apoptosis of short-term VNS was examined. A total of 3 weeks after inducing CHF, the rats were divided into three groups: Sham stimulation in sham operated rats, sham stimulation in CHF rats (CHF-SS), and treated with VNS in CHF rats (CHF-VNS). The right vagus nerve of the neck was stimulated for 72 h in CHF rats with rectangular pulses of 40 msec duration at 1 Hz and 5 V. miR-205 was focused on, which exhibited differential expression in the miRNA microarray analysis of CHF rats, and the effects of VNS on apoptosis were examined. It was verified that the expression level of miR-205 in the CHF-SS group was increased, and the expression was reduced in the CHF-VNS group. Furthermore, mimics or inhibitor of miR-205 was transfected into H9c2 to investigate its function on apoptosis. Baculoviral IAP repeat-containing protein 2 (Birc2) was confirmed a target of miR-205 through a dual luciferase reporter assay and western blotting. It was demonstrated that downregulated miR-205 decreased apoptosis in H9c2 cells. The apoptosis-associated proteins were further detected in H9c2 cells and rat tissue. The mRNA and protein expression levels of caspase-3 and Bcl-2-associated X protein were decreased in the CHF-VNS group, the expression of Birc2 and B-cell lymphoma 2 were increased. The results were consistent with the in vitro study in the miR-205 inhibitor group. The present study demonstrated that short-term VNS decreased apoptosis by downregulating miR-205 in rats with CHF. Therefore, the results of the present study provide basic evidence for short-term VNS in the clinical treatment of CHF.
Keywords: vagus nerve, electric stimulation, heart failure, microRNA-205, baculoviral IAP repeat-containing protein 2, apoptosis
Introduction
Chronic heart failure (CHF) is the end stage of various cardiovascular diseases and poses a serious threat to human health, as it responds poorly to treatment (1). Previous clinical investigations demonstrated that long-term vagus nerve stimulation (VNS) therapy reduces ventricular remodeling and improves cardiac function, and increases exercise tolerance and the quality of life following CHF (2–4). Our previous study has demonstrated that short-term VNS also improves left ventricular function in rats with CHF (5). However, the molecular mechanisms of short-term VNS treatment remain unclear.
MicroRNAs (miRNAs/miRs) are highly conserved, endogenous, single-stranded non-coding small RNA molecules. miRNAs regulate gene expression by promoting degradation of target mRNA and (or) inhibiting translation. miRNAs involve cellular differentiation, proliferation, apoptosis and development processes of a variety of biological tissues (6). Previous studies have identified that specific miRNAs participate in the pathology of heart disease development, particularly in the progression of heart failure (HF) (7–10). Myocardial apoptosis is also involved in the development of HF (11,12). Reducing cardiac damage is a potential therapeutic target for HF (13). miRNAs are also essential for regulation of cardiomyocyte apoptosis (14,15).
Our earlier study hypothesized that miRNAs may be cardioprotective of short-term VNS in rats with CHF. Therefore, the differential expression of miRNAs was assessed using microarray analysis (16). The present study focused on miR-205, which was upregulated after the treatment with sham stimulation (SS) and downregulated after the treatment with short-term VNS following CHF in rats. In addition, the present study selected baculoviral IAP repeat-containing protein 2 (Birc2), an inhibitor of apoptosis protein, as a predicted target gene of miR-205 through bioinformatics analysis. It was demonstrated that downregulated miR-205 increased Birc2 expression and reduced cardiac apoptosis in CHF rats.
Materials and methods
Rat model of CHF and VNS
The Animal Center of the Chinese Academy of Medical Sciences (Beijing, China) provided 58 male Wistar rats (7–8 weeks of age; weighing 250–300 g) to prepare the CHF model in the present study. The use of all rats was in strict compliance with the provisions of Animal Research Ethics Committee of Shengjing Hospital of China Medical University (Shenyang, Liaoning, China). The rats were kept in a stainless-steel net cage under standard laboratory conditions (25°C; relative humidity 60%; 12-h light/dark cycle) with free access to food and water. All animals were randomly divided into three groups: i) treated with SS in sham operated rats (SO-SS group, n=8), ii) treated with SS in CHF rats (CHF-SS group, n=8), and iii) treated with VNS in CHF rats (CHF-VNS group, n=8). As described previously, a thoracotomy was performed under 1% pelltobarbitalum natricum (40 mg/kg) anesthesia administered via an intraperitoneal injection. The left anterior descending coronary artery was ligated firmly with prolene suture (Ethicon, Inc., Cincinnati, OH, USA) to produce myocardial infarction (17,18). The left anterior descending coronary artery was tied loosely in the SO-SS group. At 3 weeks after the myocardial infarction operation, echocardiography was performed on the surviving rats. The rats with an infarction area >40% of the left ventricular wall area were enrolled for research (5). This method of research was approved by the ethics committee of Shengjing Hospital of China Medical University (Shenyang, Liaoning, China).
As described in our previous study (5), a pair of Teflon-coated stainless steel wires (UL1330; Triumph Cable Co., Ltd., Dongguan, Guangdong, China) was looped around the right vagus nerve in the neck for electrical stimulation. The wires were connected to the output terminals of the stimulator (BL-420S Data Acquisition & Analysis System; Chengdu Tme Technology Co, Ltd., Chengdu, Sichuan, China). Rectangular pulses were used at 1 Hz, 5 V and 40 msec duration for 72 h to stimulate the vagus nerve.
Cardiac tissue preparation
The rats were sacrificed following 72 h of treatment. For each rat, the whole heart tissue was rapidly excised and washed with cold phosphate buffer. Heart tissues were frozen at −80°C for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis.
Cell culture
The H9c2 rat myoblasts and 293T human renal epithelial cells (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% (v/v) FBS (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a mixed atmosphere of 5% CO2 and 95% air.
Cell transfection
Cell transfection was carried out using Attractene Transfection reagent (Qiagen GmbH, Hilden, Germany) following the manufacturer's protocol. miR-205 mimics (miR-205m), miR-205 inhibitor (miR-205i) and matched negative controls (NCm, negative control mimics; NCi, negative control inhibitor) were synthesized by GE Healthcare Dharmacon, Inc. (Lafayette, CO, USA). The level of miRNA expression in the cells was assayed by RT-qPCR.
miRNA microarray analysis
The miRNA microarray analysis was performed by Kangchen Biotech Co., Ltd. (Shanghai, China). Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and an miRNeasy mini kit (Qiagen GmbH) according to manufacturer's protocol. The RNA quantity was measured using the NanoDrop 1000. The samples were labeled using the miRCURY™ Hy3™/Hy5™ Power labeling kit and hybridized on the miRCURY™ LNA Array (v.18.0). Following the washing steps, the slides were scanned using the Agilent Scanner G2505C. Scanned images were imported into GenePix Pro 6.0 software (Molecular Devices, LLC, Sunnyvale, CA, USA) for grid alignment and data extraction. In screening for differentially expressed miRNAs, the threshold was set at fold difference ≥1.5 or ≤0.67, and P<0.05. Hierarchical clustering was performed to demonstrate distinguishable miRNA expression profiling among samples.
RT-qPCR
All assays strictly followed the manufacturer's protocol. Total RNA isolation was achieved using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A PrimeScript RT kit (Takara Biotechnology Co., Ltd., Dalian, Liaoning, China) was used to create the cDNA library. RT-qPCR was performed using SYBR Premix Ex Taq II (Takara Biotechnology, Co., Ltd.) on a LightCycler 480 Real-Time PCR system (Roche Diagnostics, Basel, Switzerland). For qPCR, 1 µg total RNA from each sample was resuspended in 20 µl final volume of reaction buffer. Relative quantification was based on U6 and β-actin controls. All reactions were performed in triplicate and three dishes were prepared in each group in H9c2 cells. The sequences of the primer pairs used were as follows: miR-205 forward, 5′-GCGTCCTTCATTCCACCG-3′ and reverse, 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACAGACTC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse, 5′-AAAATATGGAAGGCTTCACAGATTTG-3′; Birc2 forward, 5′-CAGCTTTGTGCAGACTTTGCTTTC-3′ and reverse, 5′-CCTTGTTCCAGAGGTAGCGAGTG-3′; caspase-3 forward, 5′-AGATACCAGTGGAGGCCGAC-3′ and reverse, 5′-CACGAGTGAGGATGTGCATGA-3′; Bcl-2-associated X protein (Bax) forward, 5′-CTGACATGTTTGCAGACGGC-3′ and reverse, 5′-AAGTCCAGTGTCCAGCCCAT-3′; B-cell lymphoma 2 (Bcl-2) forward, 5′-TGGTGGACAACATCGCTCTG-3′ and reverse, 5′-GAGAAATCAAACAGAGGTCGCA-3′; β-actin forward, 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse, 5′-CACCTTCTACAATGAGCTGCGTGTG-3′. The PCR was performed as follows: 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 30 sec; 95°C for 5 sec, 60°C for 60 sec; 50°C for 30 sec. Fold changes relative to control samples were calculated using the 2−ΔΔCq method (19).
miR-205 target prediction
For predicting the potential target gene of miR-205, a bioinformatics approach was implemented, using miRanda (http://www.microrna.org/microrna/getDownloads.do), TargetScan (version 6.2; www.targetscan.org/) and miRWalk algorithm (version 2.0; zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/genepub.html). The common prediction results of three databases were used as candidate genes. Birc2, an inhibitor of apoptosis protein, was selected as a predicted target gene of miR-205.
Dual luciferase reporter gene assay
The mutant 3′UTR and wild-type 3′UTR of Birc2 were inserted downstream of firefly luciferase in pmiR-Glo to form a luciferase reporter construct. 293T human renal epithelial cells were co-transfected with miR-205 and mutant pmiR-Glo-Birc2-3′UTR or wild-type pmiR-Glo-Birc2-3′UTR (Shanghai GenePharma Co., Ltd., Shanghai, China). Using Lipofectamine 2000, plasmid DNA and miR-205m/NCm were co-transfected into 293T cells. After 24 h, luciferase activity was measured using the Dual-Luciferase Assay system (E1960, Promega Corporation, Madison, WI, USA) and a Multilabel Plate Reader (PerkinElmer, Inc., Waltham, MA, USA). Normalized luciferase activity was calculated as the ratio of Renilla and firefly luciferase activities.
Western blotting
SDS lysis buffer was used to extract total protein of rat tissues, and radioimmunoprecipitation assay lysis buffer was used for lysis of H9c2 cells (Beyotime Institute of Biotechnology, Shanghai, China). Proteins (40 µg) were separated by 12% SDS-PAGE and transferred to polyvinylidene fluoride membranes (Roche Diagnostics), blocked using 15% skimmed milk (Yili Group, Inner Mongolia, China). Membranes were incubated overnight at 4°C with primary antibodies against Bcl-2 (mouse monoclonal; 1:2,000 dilution; cat. no. sc-7382; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), caspase-3 (rabbit polyclonal; 1:500 dilution; cat. no. sc-7145; Santa Cruz Biotechnology, Inc.), Bax (mouse monoclonal; 1:500 dilution; cat. no. sc-7480; Santa Cruz Biotechnology, Inc.), Birc2 (rabbit polyclonal; 1:500 dilution; cat. no. bs-4262R; BIOSS, Beijing, China) and β-actin (mouse monoclonal; 1:1,000 dilution; cat. no. bsm-33036m; BIOSS). The blots were then incubated at 37°C for 45 min with a horseradish peroxidase-conjugated goat anti rabbit immunoglobulin G secondary antibody (1:5,000; cat. no. A0208; Beyotime Institute of Biotechnology). A UVP Bioimaging system (BioSpectrum 410; UVP Inc., Upland, CA, USA) and Gel-Pro-Analyzer software version 5.0 (Media Cybernetics, Inc., Bethesda, MD, USA) were used for detection and analysis. For H9c2 cells, three dishes were prepared in each group, and experiments were repeated three times.
TdT-mediated dUTP nick end labeling (TUNEL) assay
According to manufacturer's protocol, a TUNEL kit was used to detect cell apoptosis (cat. no. 11684817910; Roche Diagnostics). H9c2 cells were seeded into 12-well plates; 1 ml of cell suspension was inoculated with 2×105 cells/well. Cells were fixed with 4% paraformaldehyde at room temperature for 30–60 min, and then permeabilized with a 0.1% Triton X-100 solution (50 µl; cat. no. ST795; Beyotime Institute of Biotechnology) in an ice bath for 2–5 min. Cells were then quenched of endogenous peroxidase activity with 3% H2O2 (cat. no. 10011218; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) for 10 min at room temperature away from light, and incubated with the TUNEL reaction mixture (Enzyme solution vs. Label Solution, 1:9) for 1 h at 37°C. Subsequently, cells were stained with 0.8% hematoxylin for 3 min at room temperature. The apoptotic index was evaluated in 5 random fields per section under a fluorescence microscope (magnification, ×200) and counted for each group.
Statistical analysis
All statistical analyses were performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). All results are expressed as the mean ± standard deviation. Comparisons among various groups were determined using one-way analysis of variance followed by the post hoc least significant difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Inhibition of miR-205 in the myocardial tissue of CHF rats following short-term VNS treatment
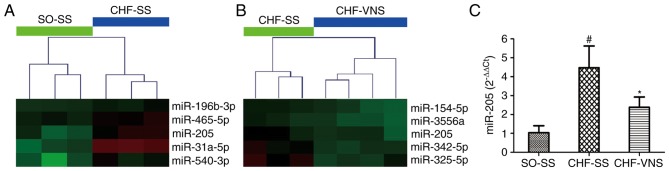
As presented in Fig. 1A and B, miR-205 was screened by microarray analysis in our previous study (16), which revealed that the expression level of miR-205 was significantly increased in the CHF-SS group (n=3; P=0.022 vs. SO-SS group) and markedly decreased in the CHF-VNS group (n=4; P=0.026 vs. CHF-SS group) (16). As presented in Fig. 1C, these results were verified in cardiac tissue by RT-qPCR. The expression level of miR-205 in rats of the CHF-SS group was significantly activated compared with the SO-SS group (4.48±1.15 vs. 1.04±0.37, P=0.002). Following short-term VNS, the expression level of miR-205 was gradually inhibited in CHF-VNS group compared with the CHF-SS group (2.38±0.55 vs. 4.48±1.15, P=0.015).
Figure 1.
Fragment of miR-205 in the miR expression profile of the cluster diagram and the verification in myocardial tissue. (A) The CHF-SS vs. SO-SS group, and (B) the CHF-VNS vs. CHF-SS group. Green represents downregulation and red represents upregulation. (C) The expression level of miR-205 in myocardial tissue. Data are expressed as the mean ± standard deviation. #P<0.05 vs. SO-SS; *P<0.05 vs. CHF-SS. miR, microRNA; SO, sham-operated; CHF, chronic heart failure; SS, sham stimulation; VNS, vagus nerve stimulation.
Downregulation of miR-205 targeted to increase the expression of Birc2 and further reduce cell apoptosis
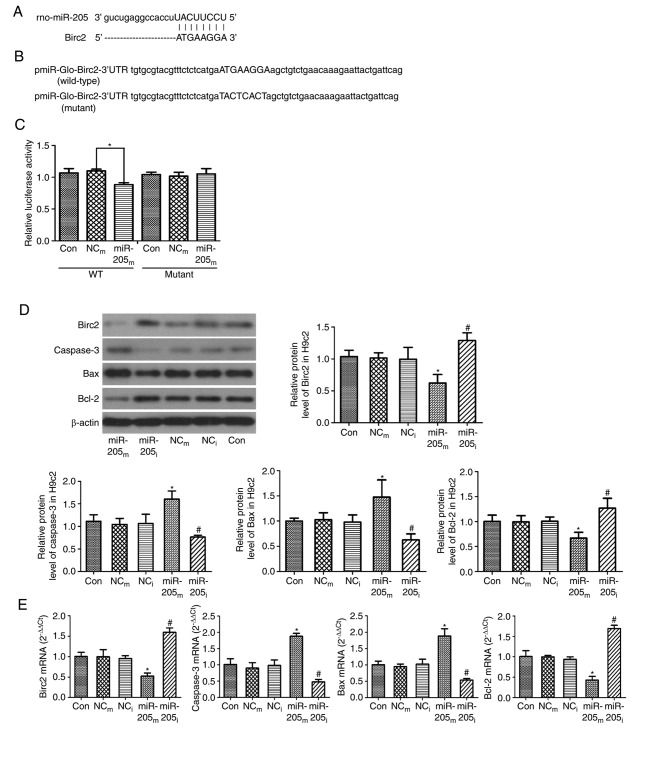
For predicting the potential target gene of miR-205, the present study implemented a bioinformatic analysis using miRanda, TargetScan and miRWalk algorithm. It was demonstrated that Birc2 theoretically included an miR-205 binding site in the 3′UTR (Fig. 2A). Birc2 is one of the inhibitor of apoptosis proteins, and its activation results in cell anti-apoptosis and cytoprotection. Studies have demonstrated that miR-205 accelerates cell apoptosis (20–22); the present study supported this by indicating that miR-205 may regulate Birc2. To verify this hypothesis, the present study cloned the 3′UTR of Birc2 into a pmiR-Glo-Birc2 vector (Fig. 2B) and co-transfected miR-205 mimics/inhibitor into the vector in 293T cells. Co-transfection of miR-205 inhibited the luciferase activity of the reporter, including wild-type pmiR-Glo-Birc2-3′UTR sequence (0.88±0.03 vs. 1.09±0.01, P<0.001, vs. negative control group; Fig. 2C), but failed to suppress that of mutant pmiR-Glo-Birc2 by dual luciferase reporter gene assay (P>0.05 vs. negative control group; Fig. 2C). These data suggested that miR-205 could directly target the 3′UTR sequences of pmiR-Glo-Birc2. Additionally, the protein expression level of Birc2 was significantly suppressed by miR-205 mimic transfection in H9c2 cells (0.62±0.13 vs. 1.02±0.08, P=0.003 vs. NCm group), while the expression was markedly increased by the miR-205 inhibitor (1.28±0.11 vs. 0.99±0.19, P=0.02 vs. NCi group; Fig. 2D). All these findings demonstrated that miR-205 targets and regulates Birc2 protein expression. They also suggested that inhibition of miR-205 expression could serve an anti-apoptotic function by increasing Birc2 expression.
Figure 2.
Birc2 is a target of miR-205 and downregulation of miR-205 reduces cell apoptosis in H9c2 cells. (A) Sequence alignment of miR-205 and 3′UTR of Birc2. (B) 3′UTR of Birc2 or a mutated sequence was cloned into pmiR-Glo vector. (C) Luciferase reporter assay with co-transfection of wild-type or mutant 3′UTR and miR-205m or NCm in 293T cells. (D) Protein and (E) mRNA expression levels of Birc2, caspase-3, Bax and Bcl-2 in H9c2 cells transfected with miR-205 as an internal control. β-actin served as a loading control. Data are expressed as the mean ± standard deviation. *P<0.05 vs. NCm; #P<0.05 vs. NCi. miR-205m, miR-205 mimics; miR-205i, miR-205 inhibitor; NCm, negative control mimics; NCi, negative control inhibitor; Birc2, baculoviral IAP repeat-containing protein 2; miR, microRNA; 3′UTR, 3-untranslated region; Con, control; WT, wild-type; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2.
Furthermore, for clarifying the mechanisms of apoptosis, RT-qPCR and western blot analyses were performed to measure the expression levels of Birc2, caspase-3, Bax and Bcl-2. As presented in Fig. 2D, the protein expression levels of caspase-3 and Bax were higher in the miR-205m group compared with the NCm group (1.61±0.18 vs. 1.05±0.13 and 1.48±0.34 vs. 1.03±0.14, P=0.001 and P<0.014, respectively), and were markedly decreased in the miR-205i group compared with the NCi group (0.76±0.04 vs. 1.06±0.21 and 0.63±0.12 vs. 0.98±0.14, P=0.036 and P=0.042, respectively). The protein expression level of Bcl-2 was lower in the miR-205m group (0.67±0.12 vs. 0.99±0.12, P=0.015 vs. NCm group), and was markedly higher in the miR-205i group (1.27±0.2 vs. 1.01±0.09, P=0.037 vs. NCi group).
As presented in Fig. 2E, consistent with the western blot results, the mRNA expression levels of caspase-3 and Bax were significantly increased in the miR-205m group compared with the NCm group (1.88±0.09 vs. 0.90±0.17 and 1.88±0.22 vs. 0.95±0.08, P<0.001, respectively); however, the levels of caspase-3 and Bax were lower in the miR-205i group compared with the NCi group (0.48±0.07 vs. 0.98±0.17 and 0.54±0.05 vs. 1.03±0.15, P=0.001, respectively). The mRNA expression levels of Birc2 and Bcl-2 were markedly lower in the miR-205m group compared with the NCm group (0.52±0.08 vs. 0.99±0.18 and 0.43±0.09 vs. 0.99±0.04, P<0.001 respectively). However, the levels of Birc2 and Bcl-2 were increased in the miR-205i group compared with the NCi group (1.60±0.11 vs. 0.95±0.07 and 1.69±0.08 vs. 0.94±0.06, P=0.001 respectively). These data demonstrated that downregulation of miR-205 reduced cell apoptosis.
TUNEL assay was also performed to evaluate apoptosis of H9c2 cells transfected with the miR-205 vector. As presented in Fig. 3, the apoptosis of H9c2 cells was markedly increased in the miR-205m group (13.61±1.44 vs. 8.21±1.56, P<0.001 vs. NCm group), while the apoptosis was markedly reduced in the miR-205i group (4.96±0.70 vs. 8.27±0.94, P<0.001 vs. NCi group).
Figure 3.
TUNEL assay in H9c2 cells transfected with miR-205. Representative microscope images. Magnification, ×200. The red arrows indicate the apoptotic cells. Data are expressed as the mean ± standard deviation. *P<0.05 vs. NCm; #P<0.05 vs. NCi. miR-205m, miR-205 mimics; miR-205i, miR-205 inhibitor; NCm, negative control mimics; NCi, negative control inhibitor; Con, control; TUNEL, TdT-mediated dUTP nick end labeling assay.
Anti-apoptosis effect in the myocardial tissue of rats with CHF following short-term VNS treatment
The above studies demonstrated that miR-205 mediates Birc2 to reduce myocardial apoptosis. To further determine the anti-apoptosis effect of short-term VNS treatment in the myocardial tissue of CHF rats, the present measured the expression levels of Birc2, caspase-3, Bax and Bcl-2 using RT-qPCR and western blot analyses in the three experimental groups of rats. As presented in Fig. 4A, the mRNA expression levels of caspase-3 and Bax were significantly increased in the CHF-SS group compared with the SO-SS group (2.07±0.13 vs. 1.05±0.4 and 2.63±0.48 vs. 1.01±0.19, P=0.004 and P=0.001, respectively) and were reduced in the CHF-VNS group compared with the CHF-SS group (1.39±0.23 vs. 2.07±0.13 and 1.65±0.15 vs. 2.63±0.48, P=0.023 and P=0.008, respectively). The mRNA expression levels of Birc2 and Bcl-2 were markedly lower in the CHF-SS group compared with the SO-SS group (0.37±0.14 vs. 1.01±0.21 and 0.44±0.05 vs. 1.02±0.22, P=0.002 and P=0.004, respectively) and were increased in the CHF-VNS group compared with the CHF-SS group (0.78±0.1 vs. 0.37±0.14 and 0.87±0.14 vs. 0.44±0.05, P=0.018 and P=0.016, respectively).
Figure 4.
mRNA and protein expression levels of apoptosis-associated factors in the SO-SS, CHF-SS and CHF-VNS groups (n=8/group). (A) mRNA and (B) protein expression levels of Birc2, caspase-3, Bax and Bcl-2. β-actin served as an internal control. Data are expressed as the mean ± standard deviation. #P<0.05 vs. SO-SS; *P<0.05 vs. CHF-SS. miR, microRNA; SO, sham-operated; CHF, chronic heart failure; SS, sham stimulation; VNS, vagus nerve stimulation; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; Birc2, baculoviral IAP repeat-containing protein 2.
As presented in Fig. 4B, consistent with the RT-qPCR results, the protein expression levels of caspase-3 and Bax were significantly higher in the CHF-SS group compared with the SO-SS group (1.97±0.42 vs. 1.15±0.33 and 2.23±0.68 vs. 1.03±0.03, P=0.041 and P=0.012, respectively). Following short-term VNS, the levels of caspase-3 and Bax were markedly lower in the CHF-VNS group compared with the CHF-SS group (1.10±0.39 vs. 1.97±0.42 and 1.35±0.25 vs. 2.23±0.68, P=0.033 and P=0.042, respectively). The protein expression levels of Birc2 and Bcl-2 in the CHF-SS group were significantly reduced compared with the SO-SS group (0.75±0.04 vs. 0.94±0.06 and 0.62±0.09 vs. 1.12±0.11, P=0.002 and P=0.011, respectively). Following short-term VNS, the level of Birc2 and Bcl-2 were markedly higher in the CHF-VNS group compared with the CHF-SS group (0.97±0.03 vs. 0.75±0.04 and 0.99±0.26 vs. 0.62±0.09, P=0.001 and P=0.034, respectively). All these findings suggested that short-term VNS treatment serves an anti-apoptosis effect in the myocardial tissue of rats following CHF.
Discussion
Heart failure is the dysfunction of contraction and/or diastole, leading to insufficiency of blood circulation in the heart, and is a major cause of mortality from cardiovascular disease (1,23,24). The morbidity of HF is increased with the aging population (25). The clinical treatments of HF have made more progress; however, traditional therapies are insufficient for many patients with HF, who eventually progress to end-stage disease (1). Animal experiments and clinical investigations have reported that VNS treatment improves the cardiac function and prognosis of CHF, and reduces the fatality rate (26–28). Our previous study demonstrated that short-term VNS has a significant beneficial effect on attenuation of LV remodeling, alters the components of cardiac excitation-contraction coupling associated protein, and improves the cardiac function in rats with CHF (5).
miRNAs participate in regulating gene expression in different diseases. Previous research has demonstrated that regulating the expression of miRNAs serves a protective role in HF (29–32). The present study demonstrated that short-term VNS reduces miR-205 expression in CHF-VNS rats. miR-205 serves a different role in diverse range of tumor cell types (33–36). miR-205 induces inflammation and atherosclerosis in vascular endothelial cells and targets to regulate tissue inhibitor of metalloproteinase 3, which serves a protective role in vascular endothelial cells through interfering the expression of miR-205 (37,38). miR-205 was also demonstrated to regulate the expression of phosphatase and tensin homolog directly in endometrial cancer cells, as well as inhibit apoptosis (39). Myocardial apoptosis serves an important role in the development of HF. A variety of myocardial cell injuries, such as myocardial infarction, ischemia/reperfusion, and genetic and metabolic cardiomyopathy, can induce myocardial apoptosis and eventually develop into HF; thus, it is a potential therapeutic target to reduce myocardial damage. Apoptosis is tightly regulated by a variety of active and programmed death processes under certain physiological or pathological conditions, and it is an important homeostatic mechanisms (40). Birc2 is a member of the anti-apoptotic gene family, which mainly serves its function by inhibiting the activation of caspase-3, 7, 9 and other enzymes (41). Endoplasmic reticulum stress induces the Birc2 expression of inhibition apoptosis through the phosphoinositide 3-kinase/protein kinase B signaling pathway and serves an important role in the adaptation of cellular stress (42,43). Caspase-3 is a common downstream effector of the apoptosis signaling pathway. Bcl-2 and Bax are involved in cell survival and apoptosis via regulating the mitochondria. Bcl-2 is a direct effect of the substrate caspase-3, and the N-terminal variable loop region can be cut by caspase-3 at Asp34, and cleave fragments of similar Bax, overall promoting apoptosis (44). Birc2, caspase-3, Bcl-2 and Bax can interact and influence each other, so as to participate in the regulation of apoptosis.
The present study demonstrated that miR-205 is involved in VNS treatment by regulating myocardial apoptosis in CHF, and downregulating miR-205 increased the expression of Birc2 in vitro. The present study also investigated the mRNA and protein expression levels of caspase-3, Bax and Bcl-2. Prior to short-term VNS treatment, the expression levels of caspase-3 and Bax were higher and that of Bcl-2 was significantly lower in the CHF-SS group compared with the SO-SS group. After short-term VNS therapy, the expression levels of Bax and caspase-3 were lower and Bcl-2 was significantly higher in the CHF-VNS group compared with the CHF-SS group. To confirm these results in vitro, the present study demonstrated that the upregulation of miR-205 increased the mRNA and protein expression levels of caspase-3 and Bax, and decreased Birc2 and Bcl-2; however, the downregulation of miR-205 served the opposite effect. TUNEL assay revealed consistent results; myocardial apoptosis was increased by upregulation of miR-205, but decreased by downregulation of miR-205. These results indicated that the downregulation of miR-205 was one significant reason for which short-term VNS treatment improved heart function in CHF.
In conclusion, the present study demonstrated that short-term VNS therapy decreases apoptosis and serves a protective role in cardiomyocytes by downregulating miR-205, and improved cardiac function in rats with CHF. Therefore, the results of the present study provide basic evidence for the application of short-term VNS in clinical treatments for CHF.
Acknowledgements
The present study was supported by Liaoning Provincial Science and Technology Department (grant no. 2011404013-7).
References
- 1.Akat KM, Moore-McGriff D, Morozov P, Brown M, Gogakos T, Da Rosa J Correa, Mihailovic A, Sauer M, Ji R, Ramarathnam A, et al. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers; Proc Natl Acad Sci USA; 2014; pp. 11151–11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz PJ, De Ferrari GM, Sanzo A, Landolina M, Rordorf R, Raineri C, Campana C, Revera M, Ajmone-Marsan N, Tavazzi L, Odero A. Long term vagal stimulation in patients with advanced heart failure: First experience in man. Eur J Heart Fail. 2008;10:884–891. doi: 10.1016/j.ejheart.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. 2011;16:171–178. doi: 10.1007/s10741-010-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein KM, et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: Results of the neural cardiac therapy for heart failure (NECTAR-HF) randomized controlled trial. Eur Heart J. 2015;36:425–433. doi: 10.1093/eurheartj/ehu345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Xuan YH, Liu SS, Dong J, Luo JY, Sun ZJ. Short-term vagal nerve stimulation improves left ventricular function following chronic heart failure in rats. Mol Med Rep. 2015;12:1709–1716. doi: 10.3892/mmr.2015.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Barringhaus KG, Zamore PD. MicroRNAs: Regulating a change of heart. Circulation. 2009;119:2217–2224. doi: 10.1161/CIRCULATIONAHA.107.715839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 10.Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6:419–429. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 11.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 12.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz MG, Fargnoli AS, Williams RD, Kendle AP, Steuerwald NM, Bridges CR. MiRNAs as potential molecular targets in heart failure. Future Cardiol. 2014;10:789–800. doi: 10.2217/fca.14.64. [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, Xiao J, Shan H, Wang Z, Yang B. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci. 2007;120:3045–3052. doi: 10.1242/jcs.010728. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50:377–387. doi: 10.1536/ihj.50.377. [DOI] [PubMed] [Google Scholar]
- 16.Liu SS, Xuan YH, Li Y, Dong J, Luo JY, Sun ZJ. Short-term vagal nerve stimulation improves chronic heart failure via miR-133a-3p upregulation in a rat model. Int J Clin Exp Pathol. 2017;10:50–60. [Google Scholar]
- 17.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000;101:1960–1969. doi: 10.1161/01.CIR.101.16.1960. [DOI] [PubMed] [Google Scholar]
- 18.Gajarsa JJ, Kloner RA. Left ventricular remodeling in the post-infarction heart: A review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail Rev. 2011;16:13–21. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Salajegheh A, Vosgha H, Md Rahman A, Amin M, Smith RA, Lam AK. Modulatory role of miR-205 in angiogenesis and progression of thyroid cancer. J Mol Endocrinol. 2015;55:183–196. doi: 10.1530/JME-15-0182. [DOI] [PubMed] [Google Scholar]
- 21.Guan B, Li Q, Li XH, Zhou XJ. MicroRNA-205 targeted Kruppel-like factor 12 and regulated MDA-MB-468 cells apoptosis in basal-like breast carcinoma. Zhonghua Yi Xue Za Zhi. 2016;96:2070–2075. doi: 10.3760/cma.j.issn.0376-2491.2016.26.007. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 22.Tian L, Zhang J, Ge J, Xiao H, Lu J, Fu S, Liu M, Sun Y. MicroRNA-205 suppresses proliferation and promotes apoptosis in laryngeal squamous cell carcinoma. Med Oncol. 2014;31:785. doi: 10.1007/s12032-013-0785-3. [DOI] [PubMed] [Google Scholar]
- 23.Gaddam KK, Verma A, Thompson M, Amin R, Ventura H. Hypertension and cardiac failure in its various forms. Med Clin North Am. 2009;93:665–680. doi: 10.1016/j.mcna.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Schannwell CM, Hennersdorf MG, Strauer BE. Hypertension and cardiac failure. Internist (Berl) 2007;48:909–920. doi: 10.1007/s00108-007-1913-y. (In German) [DOI] [PubMed] [Google Scholar]
- 25.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 26.Hamann JJ, Ruble SB, Stolen C, Wang M, Gupta RC, Rastogi S, Sabbah HN. Vagus nerve stimulation improves left ventricular function in a canine model of chronic heart failure. Eur J Heart Fail. 2013;15:1319–1326. doi: 10.1093/eurjhf/hft118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, et al. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 28.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: Results of the ANTHEM-HF trial. J Card Fail. 2014;20:808–816. doi: 10.1016/j.cardfail.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Zhang X, Wang T, Sun C, Jin T, Yan H, Zhang J, Li X, Geng T, Chen C, et al. Regulation by bisoprolol for cardiac microRNA expression in a rat volume-overload heart failure model. J Nanosci Nanotechnol. 2013;13:5267–5275. doi: 10.1166/jnn.2013.7530. [DOI] [PubMed] [Google Scholar]
- 30.Tolonen AM, Magga J, Szabó Z, Viitala P, Gao E, Moilanen AM, Ohukainen P, Vainio L, Koch WJ, Kerkelä R, et al. Inhibition of Let-7 microRNA attenuates myocardial remodeling and improves cardiac function postinfarction in mice. Pharmacol Res Perspect. 2014;2:e00056. doi: 10.1002/prp2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong LL, Armugam A, Sepramaniam S, Karolina DS, Lim KY, Lim JY, Chong JP, Ng JY, Chen YT, Chan MM, et al. Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2015;17:393–404. doi: 10.1002/ejhf.223. [DOI] [PubMed] [Google Scholar]
- 32.Kuosmanen SM, Hartikainen J, Hippeläinen M, Kokki H, Levonen AL, Tavi P. MicroRNA profiling of pericardial fluid samples from patients with heart failure. PLoS One. 2015;10:e0119646. doi: 10.1371/journal.pone.0119646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS, Borre M, et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer A, Jung M, Mollenkopf HJ, Wagner I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G, Jung K. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126:1166–1176. doi: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- 35.Karaayvaz M, Zhang C, Liang S, Shroyer KR, Ju J. Prognostic significance of miR-205 in endometrial cancer. PLoS One. 2012;7:e35158. doi: 10.1371/journal.pone.0035158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsushima K, Isomoto H, Yamaguchi N, Inoue N, Machida H, Nakayama T, Hayashi T, Kunizaki M, Hidaka S, Nagayasu T, et al. MiRNA-205 modulates cellular invasion and migration via regulating zinc finger E-box binding homeobox 2 expression in esophageal squamous cell carcinoma cells. J Transl Med. 2011;9:30. doi: 10.1186/1479-5876-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son DJ, Kumar S, Takabe W, Kim CW, Ni CW, Alberts-Grill N, Jang IH, Kim S, Kim W, Won Kang S, et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013;4:3000. doi: 10.1038/ncomms4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim CW, Kumar S, Son DJ, Jang IH, Griendling KK, Jo H. Prevention of abdominal aortic aneurysm by anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused mice. Arterioscler Thromb Vasc Biol. 2014;34:1412–1421. doi: 10.1161/ATVBAHA.113.303134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang G, Hou X, Li Y, Zhao M. MiR-205 inhibits cell apoptosis by targeting phosphatase and tensin homolog deleted on chromosome ten in endometrial cancer Ishikawa cells. BMC Cancer. 2014;14:440. doi: 10.1186/1471-2407-14-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- 41.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 42.Warnakulasuriyarachchi D, Cerquozzi S, Cheung HH, Holcik M. Translational induction of the inhibitor of apoptosis protein HIAP2 during endoplasmic reticulum stress attenuates cell death and is mediated via an inducible internal ribosome entry site element. J Biol Chem. 2004;279:17148–17157. doi: 10.1074/jbc.M308737200. [DOI] [PubMed] [Google Scholar]
- 43.Hamanaka RB, Bobrovnikova-Marjon E, Ji X, Liebhaber SA, Diehl JA. PERK-dependent regulation of IAP translation during ER stress. Oncogene. 2009;28:910–920. doi: 10.1038/onc.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirsch DG, Doseff A, Chau BN, Lim DS, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]