Abstract
Long non-coding RNAs (lncRNAs) serve a critical role in various biological processes including cell growth, transcriptional regulation and differentiation. Previous studies have demonstrated that human amnion-derived mesenchymal stem cells (HAMSCs) possess the potential to promote proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells (HBMSCs). However, little is known about the roles of lncRNAs in these mechanisms. The present study investigated the expression of lncRNAs in HBMSCs co-cultured with HAMSCs to study their involvement in the mechanism of osteogenic differentiation. RNA sequencing was used to compare the lncRNA expression profiles of HBMSCs co-cultured with or without HAMSCs during osteogenic differentiation. A total of 339 differentially expressed lncRNAs were identified [log2 (fold change)>2.0 or <-2.0; P<0.05], consisting of 131 downregulated and 208 upregulated lncRNAs. Among these lncRNAs, it was identified that the lncRNA-differentiation antagonizing non-protein coding RNA (DANCR) expression level in HBMSCs was significantly decreased by co-culturing with HAMSCs, and DANCR overexpression inhibited the effect of HAMSCs on the promotion of runt-related transcription factor 2 expression. These data suggested that HAMSCs are likely to regulate differentiation processes in HBMSCs by influencing the DANCR, thus offering a novel insight into the complicated regulation mechanisms of HAMSC-derived osteogenic differentiation.
Keywords: human amnion-derived mesenchymal stem cells, human bone marrow mesenchymal stem cells, long non-coding RNA, differentiation antagonizing non-protein coding RNA, osteogenic differentiation
Introduction
Mesenchymal stem cells (MSCs) possess the potential for self-renewal and may differentiate into multiple connective tissue cell types, including chondrogenic, adipogenic and osteogenic lineages (1–3). Tissue engineering using suitable MSCs has been considered to be a novel treatment for bone deficiency. Human amnion-derived mesenchymal stem cells (HAMSCs), obtained from abundant human amniotic membrane, are associated with fewer ethical issues and low anti-inflammatory properties (4). Previous studies (5,6) have suggested that HAMSCs are capable of promoting proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells (HBMSCs). However, the complex molecular mechanisms of HAMSC-derived osteogenic differentiation remain largely unknown.
During the past decade, the ENCODE project and large-scale sequencing efforts have revealed that long non-coding RNAs (lncRNAs) belong to a novel heterogeneous class of ncRNAs that includes thousands of different species (7,8). lncRNAs have been proposed to serve crucial roles in a wide range of biological pathways and cellular processes, including the reprogramming of stem cell pluripotency (9), inactivation of chromosomes (10), modulation of invasion and apoptosis (11) and regulation at transcriptional and post transcriptional levels (12). A previous study (13) demonstrated that the lncRNA-differentiation antagonizing non-protein coding RNA (DANCR), an important regulator of osteogenic differentiation, is associated with the inhibition of runt-related transcription factor 2 (RUNX2) expression and subsequent osteogenic differentiation in human osteoblastic cell. Therefore, the potential role of lncRNAs in HAMSC-derived osteogenic differentiation was investigated.
In the present study, the differential expression profiles of mRNAs and lncRNAs of HBMSCs co-cultured with or without HAMSCs during osteogenic differentiation were obtained. RNA sequencing (RNA-seq) data was validated using the reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Based on these results, the present study investigated whether HAMSCs downregulated the DANCR expression level in HBMSCs, and whether the effect of HAMSCs on the promotion of RUNX2 expression in HBMSCs was inhibited by DANCR overexpression. The observations demonstrated that lncRNAs may possibly provide novel insights into the mechanisms by which HAMSCs regulate osteogenic differentiation.
Materials and methods
Cell culture
The HBMSC cell line PTA-1058 was obtained from the American Type Culture Collection (Manassas, VA, USA). The HAMSCs were collected from abundant amniotic membrane samples using the pancreatin/collagenase digestion method as previously described (14). The two cell lines were expanded in Dulbecco's modified Eagle's medium (HyClone, Logan, UT, USA) supplemented with 10%fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/l penicillin and 100 mg/l streptomycin (both Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere of 5% CO2 at 37°C. The confluent cells were transferred to next passage using 0.25% trypsin for up to five passages and culture medium was changed every 3 days. The experiments were approved by the Ethics Committee of Nanjing Medical University (Nanjing, China). Prior informed consent was obtained from all participants enrolled in the present study.
Preparation of the co-culture system
The HBMSCs were seeded at an initial cell density of 5×104 cells/cm2 in 6-well culture plates (EMD Millipore, Billerica, MA, USA). Transwells (6-Well Millicell Hanging Cell Culture Inserts, 0.4 µm; EMD Millipore) were placed in other 6-well culture plates and HAMSCs were seeded at 1.5×105 cells/cm2 in the transwells. Following the attachment of the cells, transwells containing HAMSCs were moved into the corresponding wells of the 6-well culture plate containing HBMSCs to create the HAMSC/HBMSC co-culture system. HBMSCs in wells without transwells were designated as the control groups, while HBMSCs with transwells were served as the experiment groups.
Proliferation assay
The proliferation assay was measured at day 3, 5 and 7 by flow cytometry. The transwells containing HAMSCs were moved into the corresponding wells containing HBMSCs and then the two cell lines were treated with serum-free medium for 24 h, following which the medium was replaced with culture medium containing 10% FBS. HBMSCs were harvested at day 3, 5 and 7 and fixed with 75% ice-cold ethanol as described previously (15). DNA content was measured by a FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and the cell cycle fractions (G0, G1, S, and G2 M phases) were processed using CellQuest Pro software (BD Biosciences). Data were analyzed using Modfit LT 3.2 (Verity Software House, Topsham, ME, USA).
In vitro osteogenic differentiation
The transwells containing HAMSCs were moved into the corresponding wells containing HBMSCs and then the regular medium in all cultures was replaced with osteogenic medium containing 10 mM β-glycerophosphate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 100 nM ascorbic acid and 100 nM dexamethasone (both Sigma-Aldrich; Merck KGaA). HBMSCs co-cultured with HAMSCs were subjected to alkaline phosphatase (ALP) activity assays and alizarin red staining. ALP activity assay was performed using an ALP assay kit (Jiancheng Corp, Nanjing, China) according to the manufacturer's protocols. Alizarin red staining was performed using 40 mM alizarin red S (pH 4.4) for 10 min at room temperature. Following rinsing with PBS, mineralized nodules were visualized using an inverted microscope (Carl Zeiss AG, Oberkochen, Germany) and 10 images were captured for each group.
Plasmid construction and cell transfection
DANCR overexpression was achieved through pcDNA3.1-DANCR (Guangzhou RiboBio Co., Ltd., Guangzhou, China) transfection using lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), with an empty pCDNA3.1 vector used as a control. Plasmid vectors (pcDNA3.1-DANCR and pcDNA3.1) for transfection were extracted using Midiprep kits (Qiagen GmbH, Hilden, Germany), and respectively transfected into HBMSCs. Fusion and transfection of HBMSCs were performed when the cells were cultivated on six-well plates by lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols.
RNA isolation and RT-qPCR
Total cellular RNA was isolated from HBMSCs in the control and treatment groups using TRIzol, according to the protocols recommended by the manufacturer. The RNA was reverse-transcribed into cDNA by using a PrimeScript RT Master Mix kit. Real-time reverse transcription-PCR was performed with a SYBR Green PCR kit (Toyobo, Osaka, Japan) and ABI 7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). Amplification was performed as follows: Incubation at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec and subsequent annealing and extension at 60°C for 34 sec. For each sample, GAPDH expression was analyzed to normalize target gene expression. Relative gene expression was calculated using the 2−ΔΔCq method (16). Each sample was analyzed in triplicate. Primer sequences used in this study are provided in Table I.
Table I.
Primers used in the reverse transcription-quantitative polymerase chain reaction.
| Gene | Sense primer (5′-3′) | Anti-sense primer (5′-3′) |
|---|---|---|
| SCN7A | TGCTGGTCAGTGTCCTGAAG | ATAGGCCATGGCAAGTATGC |
| DANCR | GCCACTATGTAGCGGGTTTC | ACCTGCGCTAAGAACTGAGG |
| TSIX | GTGGTGGCAGGCAACTTAAT | GCACATTCAGGCTCTCAACA |
| SSTR5-AS1 | AGCCCCTCATCTTGGCTAAT | TTTCCCTCTGAGCAGCAAAT |
| XIST | TTGCATATGTGGGCAAGTGT | GCTCTTGAGGCTTTTGTTGG |
| ENST00000433576.1 | ACACTGTGGGACTTCTTAGCC | TTCTGTGGTCGTGGTCTTTG |
| LINGO3 | CGCTGCAATCTCACATCACT | GCACCAGGTCTCTGAAGGAG |
| MPEG1 | CCCCAACATGCTACCTGACT | CTCCTCGTGGATCTGGGATA |
| SLPI | CTGTGGAAGGCTCTGGAAAG | AAAGGACCTGGACCACACAG |
| NPPB | TTCTTGCATCTGGCTTTCCT | TGTGGAATCAGAAGCAGGTG |
| RUNX2 | GGTTCCAGCAGGTAGCTGAG | GCCTACAAAGGTGGGTTTGA |
| MRC1 | GGCACTTGTGGAGAAGAAGC | GTGGCCTTGGTGATCTTGTT |
| GAPDH | GGGCTGCTTTTAACTCTGGT | GCAGGTTTTTCTAGACGG |
SCN7A, sodium voltage-gated channel α subunit 7; DANCR, differentiation antagonizing non-protein coding RNA; XIST, X inactive specific transcript; LINGO3, leucine rich repeat and Ig domain containing 3; MPEG1, macrophage expressed 1; NPPB, natriuretic peptide B; RUNX2, runt-related transcription factor 2; MRC1, mannose receptor C-type 1.
RNA-seq
HBMSCs co-cultured without HAMSCs at day 14 were regarded as control groups and HBMSCs co-cultured with HAMSCs at day 14 were regarded as experimental groups. Total RNA was sequenced using an Illumina HiSeq 2500 at Guangzhou RiboBio Co., Ltd. Reads were aligned to the human transcriptome. RNA-seq data was aligned to the Ensembl v73 transcript annotations using RSEM v1.1.21 and Bowtie v0.12.7, as previously described (17). The differentially expressed genes were selected if Benjamini-Hochberg corrected P-values were <0.05 and if the log2 (fold change) were >2.0 or <-2.0. Tree visualization of the data was performed with Java Treeview 3.0 software (Stanford University School of Medicine, Stanford, CA, USA).
Kyoto encyclopedia of genes and genomes (KEGG) and gene ontology (GO) analysis
Pathway analysis for the differentially expressed mRNAs was performed based on the most recent KEGG database (http://www.genome.jp/). This analysis allowed the investigation of the biological pathways for which a significant enrichment of differentially expressed mRNAs existed. GO (http://www.geneontology.org) was also derived, which provided three structured networks that described gene product attributes. The-log10 (P-value) demonstrated the significance of GO Term enrichment in the differentially expressed mRNA list. All other bioinformatic analysis was performed using glbase (18).
Statistical analysis
All assays were expressed as the mean ± standard deviation from at least three separate experiments using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Gene expression levels in osteogenic differentiation were compared between control and treatment groups. The Student's t-test was used to compare data between the two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression profiles of mRNAs and lncRNAs in HBMSCs co-cultured with HAMSCs
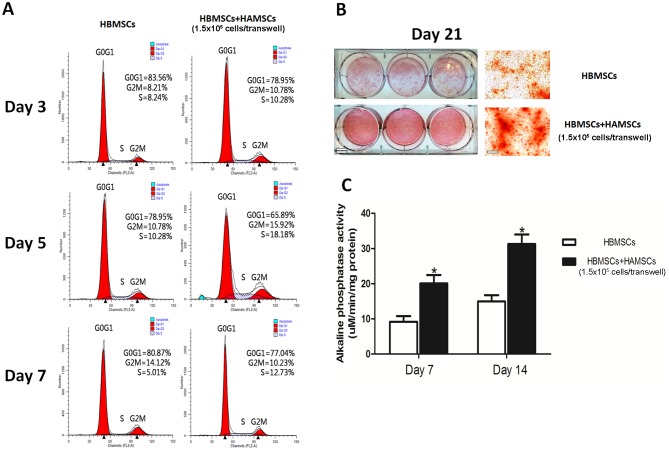
The effects of HAMSCs on HBMSCs are provided in Fig. 1. Flow cytometric analyses, ALP activity assays and alizarin red staining demonstrated the potential of HAMSCs in promoting proliferation and osteogenic differentiation of HBMSC. The expression profiles of mRNAs and lncRNAs in HBMSCs co-cultured with or without HAMSCs were identified for 14 days following osteogenic induction using RNA-seq. Expression profiles of mRNAs and lncRNAs were significantly altered between HBMSCs co-cultured with and without HAMSCs. A total of 415 differentially expressed mRNAs were identified [log2 (fold change) >2.0 or <-2.0, P<0.05]. Among these, 156 mRNAs were identified to be downregulated more than 2-fold in the control groups compared with experiment groups, while 259 mRNAs were upregulated more than 2-fold (P<0.05). Meanwhile, 339 differentially expressed lncRNAs were identified [log2 (fold change) >2.0 or <-2.0, P<0.05], consisting of 131 downregulated and 208 upregulated lncRNAs (P<0.05). When the cut-off was set at 6-fold, 17 mRNAs were downregulated and 24 were upregulated, and 6 lncRNAs were downregulated and 10 were upregulated (P<0.05; Fig. 2).
Figure 1.
Effects of HAMSCs on the promotion of proliferation and osteogenic differentiation in HBMSCs. (A) Cell cycle fractions (G0, G1, S, and G2/M phases) of HBMSCs co-cultured with or without HAMSCs were determined by flow cytometry at day 3, 5 and 7. (B) Mineralized matrix deposition was measured at day 21 by alizarin red S. Scale bar, 100 µm; double bar, 12.5 mm. (C) ALP activity was measured at day 7 and 14 using an ALP assay kit; *P<0.05. HAMSCs, human amnion-derived mesenchymal stem cells; HBMSCs, human bone marrow mesenchymal stem cells; ALP, alkaline phosphatase.
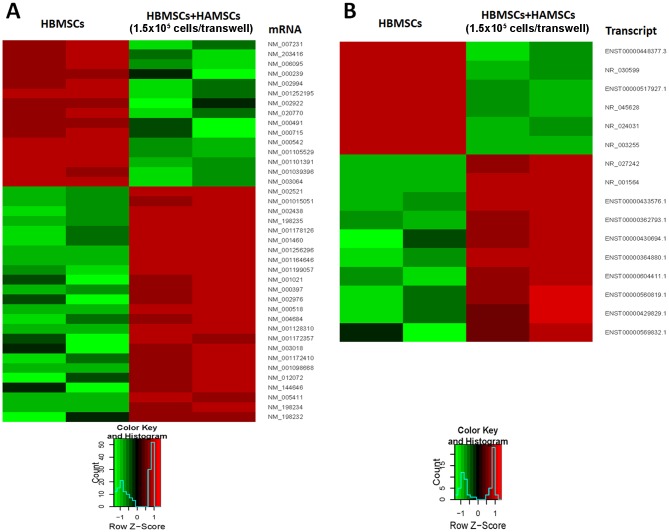
Figure 2.
Expression of mRNAs and lncRNAs in HBMSCs when the cut-off was set at 6-fold. (A) mRNAs differentially expressed in HBMSCs co-cultured with or without HAMSCs. (B) lncRNAs differentially expressed in HBMSCs co-cultured with or without HAMSCs. lncRNAs, long non-coding RNAs; HBMSCs, human bone marrow mesenchymal stem cells; HAMSCs, human amnion-derived mesenchymal stem cells.
RT-qPCR analysis of lncRNA and mRNA expression
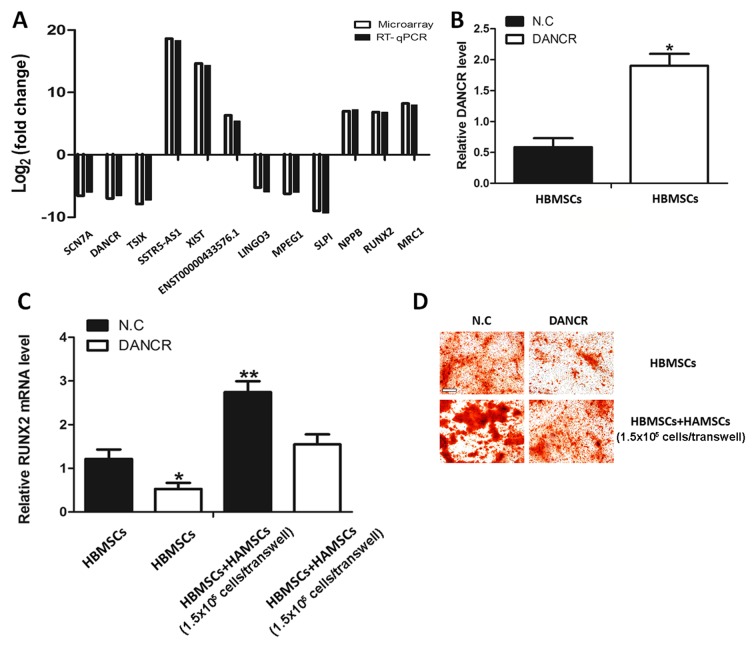
According to gene locus and fold difference, a number of candidate lncRNAs and mRNAs for validation of the RNA-seq data were investigated. Therefore, six lncRNAs (sodium voltage-gated channel α subunit 7, DANCR, TSIX, SSTR5-AS1, X inactive specific transcript and ENST00000433576.1) and six mRNAs (leucine rich repeat and Ig domain containing 3, macrophage expressed 1, SLPI, natriuretic peptide B, RUNX2 and mannose receptor C-type 1) were further examined using RT-qPCR. The results of the RT-qPCR were consistent with the RNA-seq (Fig. 3A).
Figure 3.
Results of the RNA-seq and RT-qPCR. (A) Comparison between RNA-seq data and RT-qPCR results for lncRNAs and mRNAs. The validation results suggested that the RNA-seq data correlated well with the RT-qPCR results. HBMSCs were subject to pcDNA-DANCR and then co-cultured with or without HAMSCs for 14 days under osteogenic differentiation conditions. The (B) DANCR expression and (C) RUNX2 mRNA levels were assayed by RT-qPCR. (D) Mineralized matrix deposition was measured at day 21 by alizarin red S. Scale bar: 100 µm. *P<0.05, **P<0.01 vs. N.C. RNA-seq, RNA sequencing; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; lncRNAs, long non-coding RNAs; HBMSCs, human bone marrow mesenchymal stem cells; DANCR, differentiation antagonizing non-protein coding RNA; HAMSCs, human amnion-derived mesenchymal stem cells; RUNX2, runt-related transcription factor 2; N.C., negative control.
The upregulation of DANCR inhibits RUNX2 expression and osteogenic differentiation in HBMSCs co-cultured with HAMSCs
Previous studies have indicated that DANCR is an essential mediator of the osteogenic differentiation of mesenchymal stem cells and that overexpression of DANCR results in the inhibition of RUNX2 expression and subsequent osteogenic differentiation (13). To investigate whether HAMSCs regulates osteogenic differentiation in HBMSCs by influencing DANCR, the DANCR levels in HBMSCs co-cultured with HAMSCs were first analyzed. It was identified that the DANCR levels were decreased and the RUNX2 expression was increased (Fig. 3A). To further investigate the biological role of DANCR in the regulation of HAMSCs-derived osteogenic differentiation, HBMSCs treated with pcDNA-DANCR were analyzed. Fig. 3B demonstrates that the pcDNA-DANCR increased endogenous DANCR expression in HBMSCs. Overexpression of DANCR significantly inhibited the effects of HAMSCs in promoting RUNX2 expression and osteogenic differentiation of HBMSC (Fig. 3C and D). These observations suggested that the HAMSCs are likely to regulate differentiation process in HBMSCs by downregulating the DANCR.
GO and pathway analysis of mRNAs differentially expressed between HBMSCs co-cultured with and without HAMSCs
GO analysis demonstrated that the most significant biological processes consisted of growth factor or chemokine activity, cytokine or chemokine receptor binding, extracellular region, extracellular matrix, response to type I interferon and other functions. Pathway analysis demonstrated that the most significant pathways consisted of retinoic acid-induced protein I-like receptor signaling pathway, nucleotide oligomerization domain-like receptor signaling pathway, nuclear factor kB signaling pathway and pathways in cancer (Fig. 4). These results aid the improved identification of the mechanisms of HAMSC-derived osteogenic differentiation.
Figure 4.
Pathway analysis. (A) Gene Ontology and (B) Kyoto Encyclopedia of Genes and Genomes pathway analysis of mRNAs differentially expressed between HBMSCs co-cultured with and without HAMSCs. HBMSCs, human bone marrow mesenchymal stem cells; HAMSCs, human amnion-derived mesenchymal stem cells.
Discussion
Stem cells are present in a variety of mesenchymal tissues. They possess self-renewable capacities and multi-directional differentiation potentials (19,20). Given appropriate culture conditions, mesenchymal stem cells (MSCs) are capable of differentiating into adipocytes (21), chondrocytes (22,23), endothelial cells (24), osteocytes (25) and cardiomyocytes (26). Recently, tissue engineering, using suitable MSCs that mimic the natural healing process, has demonstrated great potential in treating bone deficiency. Human amniotic membrane, composed of a single layer of epithelial cells, an avascular collagenous stroma and underlying fibroblasts, is a readily available and highly abundant tissue (27). HAMSCs, obtained from discarded human amniotic membrane, possess low anti-inflammatory properties and fewer ethical concerns compared with other sources of MSCs (4). Previous studies have demonstrated that although HAMSC osteogenesis was lower compared with other MSCs, they are capable of driving proliferation and osteogenic differentiation of HBMSCs (5). However, the regulatory mechanisms of MSC-derived osteogenic differentiation remains to be elucidated.
lncRNAs are evolutionarily conserved ncRNAs that lack protein encoding capacity and are >200 nucleotides in length. They are abundantly encoded in mammalian genomes, numbering in the tens of thousands. The ratio of lncRNAs in total ncRNAs is >80% (28,29). Recently, numerous lncRNAs have been characterized and a number of pieces of evidence suggest that their transcriptional activity may serve a major biological role in cell differentiation and human diseases (30,31). Among them, certain lncRNAs express differently during lineage commitment and cell development (32). Previous studies suggested that lncRNAs serve a key role in the circuitry controlling cell state (33) and also modulate pluripotency in mouse embryonic stem cells (34). The lncRNA DANCR is highly expressed in the epidermis and its loss eradicates the normal blockade of differentiation in the progenitor-containing compartment; it is fundamental to maintaining the undifferentiated cell state within the epidermis. Enhancer of zeste homolog 2 (EZH2), an important epigenetic regulatory factor and highly expressed in MSCs, mediates modifications in histone methylation resulting in the regulation of the differentiation of MSCs into different cell lineages, including hepatocytes, neurons and osteoblasts (35,36). A previous study demonstrated that DANCR is an important regulator of osteogenic differentiation in human fetal osteoblastic cells. By associating with EZH2, DANCR inhibits RUNX2 expression and subsequent osteogenic differentiation (13). However, the role of lncRNAs in the complex mechanisms of MSC-derived osteogenic differentiation has not been studied.
Based on the observation that HAMSCs may promote proliferation and osteogenic differentiation in HBMSCs by providing a preferential environment, the present study first demonstrated lncRNA and mRNA expression profiles, during the osteogenic differentiation of HBMSCs co-cultured with or without HAMSCs using RNA-seq. The RNA-seq data was validated by RT-qPCR. Bioinformatic analyses were subsequently applied to further study these differentially expressed lncRNAs and mRNAs, including GO and pathway analysis.
It was identified that during osteogenic differentiation, the DANCR lncRNA level was significantly decreased and the RUNX2 mRNA level was significantly increased in HBMSCs co-cultured with HAMSCs. Based on these results, the biological role of DANCR in the mechanisms of HAMSC-derived osteogenic differentiation was further investigated. It was identified that the overexpression of DANCR in HBMSCs neutralized the positive effects of HAMSCs on the promotion of RUNX2 expression and osteogenic differentiation. These results suggested that HAMSCs are likely to exercise their function by downregulating DANCR expression and upregulating RUNX2 expression.
In conclusion, the present study revealed, for first time to the authors' knowledge, the roles of lncRNAs in the mechanisms of osteogenic differentiation derived by HAMSCs. By bioinformatics analysis, certain target genes were obtained that correlated with the biological processes and signaling pathways of the candidate lncRNAs. Furthermore, it was demonstrated that the lncRNA DANCR is an important regulator in the course of osteogenic differentiation and this may provide a guide for further investigation into the complicated regulatory mechanisms of HAMSC-derived osteogenic differentiation. Further studies in the functional analysis of more lncRNAs involved this system may help clarify the regulatory mechanisms underlying this process and provide more conclusive evidence for HAMSCs in the field of tissue engineering.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81271109) and Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant no. KYZZ15_0266).
References
- 1.Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735–749. doi: 10.1089/ten.tec.2009.0432. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Xu Z. miR-27 promotes osteoblast differentiation by modulating Wnt signaling. Biochem Biophys Res Commun. 2010;402:186–189. doi: 10.1016/j.bbrc.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 3.Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem. 2010;285:25221–25231. doi: 10.1074/jbc.M110.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leyva-Leyva M, Barrera L, López-Camarillo C, Arriaga-Pizano L, Orozco-Hoyuela G, Carrillo-Casas EM, Calderón-Pérez J, López-Díaz A, Hernandez-Aguilar F, González-Ramírez R, et al. Characterization of mesenchymal stem cell subpopulations from human amniotic membrane with dissimilar osteoblastic potential. Stem Cells Dev. 2013;22:1275–1287. doi: 10.1089/scd.2012.0359. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Yin Y, Jiang F, Chen N. Human amnion mesenchymal stem cells promote proliferation and osteogenic differentiation in human bone marrow mesenchymal stem cells. J Mol Histol. 2015;46:13–20. doi: 10.1007/s10735-014-9600-5. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Jiang F, Liang Y, Shen M, Chen N. Human amnion-derived mesenchymal stem cells promote osteogenic differentiation in human bone marrow mesenchymal stem cells by influencing the ERK1/2 signaling pathway. Stem Cells Int. 2016;2016:4851081. doi: 10.1155/2016/4851081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batista PJ, Chang HY. Long noncoding RNAs: Cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson CS, Forman D. Cancer survival in England and the influence of early diagnosis: What can we learn from recent EUROCARE results? Br J Cancer. 2009;101(Suppl 2):S102–S109. doi: 10.1038/sj.bjc.6605399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Ørom UA, Shiekhattar R. Long noncoding RNAs usher in a new era in the biology of enhancers. Cell. 2013;154:1190–1193. doi: 10.1016/j.cell.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Wu Z, Fu X, Han W. Long noncoding RNAs: Insights from biological features and functions to diseases. Med Res Rev. 2013;33:517–553. doi: 10.1002/med.21254. [DOI] [PubMed] [Google Scholar]
- 13.Zhu L, Xu PC. Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochem Biophys Res Commun. 2013;432:612–617. doi: 10.1016/j.bbrc.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Jiang M, Miao D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One. 2011;6:e16789. doi: 10.1371/journal.pone.0016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Yan M, Yu Y, Wu J, Yu J, Fan Z. Estrogen deficiency inhibits the odonto/osteogenic differentiation of dental pulp stem cells via activation of the NF-kB pathway. Cell Tissue Res. 2013;352:551–559. doi: 10.1007/s00441-013-1604-z. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Pike KA, Hutchins AP, Vinette V, Théberge JF, Sabbagh L, Tremblay ML, Miranda-Saavedra D. Protein tyrosine phosphatase 1B is a regulator of the interleukin-10-induced transcriptional program in macrophages. Sci Signal. 2014;7:ra43. doi: 10.1126/scisignal.2005020. [DOI] [PubMed] [Google Scholar]
- 18.Hutchins AP, Jauch R, Dyla M, Miranda-Saavedra D. glbase: A framework for combining, analyzing and displaying heterogeneous genomic and high-throughput sequencing data. Cell Regen (Lond) 2014;3:1. doi: 10.1186/2045-9769-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 20.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47:126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 21.Karagianni M, Brinkmann I, Kinzebach S, Grassl M, Weiss C, Bugert P, Bieback K. A comparative analysis of the adipogenic potential in human mesenchymal stromal cells from cord blood and other sources. Cytotherapy. 2013;15:76–88. doi: 10.1016/j.jcyt.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 23.Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozóky B, Crowley C, Einarsson O, Grinnemo KH, Gudbjartsson T, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: A proof-of-concept study. Lancet. 2011;378:1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 24.Crisan M. Transition of mesenchymal stem/stromal cells to endothelial cells. Stem Cell Res Ther. 2013;4:95. doi: 10.1186/scrt306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heino TJ, Hentunen TA. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr Stem Cell Res Ther. 2008;3:131–145. doi: 10.2174/157488808784223032. [DOI] [PubMed] [Google Scholar]
- 26.Zhao JW, Zhang MR, Ji QY, Xing FJ, Meng LJ, Wang Y. The role of slingshot-1L (SSH1L) in the differentiation of human bone marrow mesenchymal stem cells into cardiomyocyte-like cells. Molecules. 2012;17:14975–14994. doi: 10.3390/molecules171214975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourne GL. The microscopic anatomy of the human amnion and chorion. Am J Obstet Gynecol. 1960;79:1070–1073. doi: 10.1016/0002-9378(60)90512-3. [DOI] [PubMed] [Google Scholar]
- 28.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 31.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kung JT, Colognori D, Lee JT. Long noncoding RNAs: Past, present, and future. Genetics. 2013;193:651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohamed J Sheik, Gaughwin PM, Lim B, Robson P, Lipovich L. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. RNA. 2010;16:324–337. doi: 10.1261/rna.1441510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu YL, Chou RH, Chen LT, Shyu WC, Hsieh SC, Wu CS, Zeng HJ, Yeh SP, Yang DM, Hung SC, Hung MC. EZH2 regulates neuronal differentiation of mesenchymal stem cells through PIP5K1C-dependent calcium signaling. J Biol Chem. 2011;286:9657–9667. doi: 10.1074/jbc.M110.185124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juan AH, Kumar RM, Marx JG, Young RA, Sartorelli V. Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells. Mol Cell. 2009;36:61–74. doi: 10.1016/j.molcel.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]