Abstract
Clinical and experimental reports indicate that aldosterone (ALD) contributes to the progression of renal failure independent of its hemodynamic effects. However, the mechanisms remain to be completely elucidated. The aim of the present study was to investigate the alterations of long non-coding RNA (lncRNA) in mesangial cells (MCs) treated with ALD. The present study used MCs treated with 10−6 M ALD as experimental cells. Microarray techniques performed by Agilent Technologies were used to identify the profiles of differentially expressed lncRNAs between the ALD group and the control group. Pathway and gene ontology analysis were applied to determine the roles of the differentially expressed lncRNAs. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was used to quantify the differentially expressed lncRNAs. A total of 8,459 lncRNA and 13,214 mRNAs with differential expression between MCs treated with and without ALD were identified. The expression of lncRNAs was confirmed by RT-qPCR and the results were consistent with the lncRNA array. The biological functions of lncRNAs are associated with responding to external stimuli, positive regulation of biological and apoptotic processes, cell division, mitosis and nuclear division. The pathways include cell cycle and peroxisome proliferator-activated receptor signaling pathways. The present study revealed distinct sets of lncRNA expressed in MCs treated with ALD, suggesting that this class of transcripts may be involved in the pathogenesis of chronic kidney diseases.
Keywords: long non-coding RNA, aldosterone, chronic kidney disease, mesangial cells
Introduction
Chronic kidney disease (CKD) has been increasingly recognized as a major public health problem in the world (1). Therefore, improvement in the current knowledge of molecular alterations associated with CKD is required to investigate novel strategies of diagnostics and treatment of this disease. The progression of CKD is characterized by glomerular hypertrophy, mesangial cell (MC) proliferation, extracellular matrix (ECM) accumulation, glomerulosclerosis and ultimately end-stage kidney disease (2). The ECM produced by damaged MCs is a major factor in mesangial proliferation and this is observed in light-chain-related glomerular disease which is associated with an increased synthesis of tenascin by MCs (3). MC proliferation is reported in humans and in experimental animal models with chronic nephron loss, and precedes the development of secondary focal glomerulosclerosis (4–6). In addition, progressive glomerulosclerosis is a common route for the development of end-stage renal failure of any etiology. Therefore MCs serve a critical role in the maintenance of renal function, supporting the glomerular capillaries and regulating their blood flow (7). Various growth factors and cytokines, produced by the infiltrating cells during the disease process and by the local kidney cells, have been implicated in the fibrotic process (8). Among these, aldosterone (ALD) produced by the adrenal cortex and the MCs, in addition to other extra-adrenal tissues, including cardiac myocytes and vascular smooth muscle cells, serves a significant role in the pathogenesis of mesangial matrix expansion (9–19). Part of the intracellular mechanisms involved in the proliferative and fibrotic effect of ALD in CKD has been reported. For example, ALD upregulates protein synthesis, and mRNA expression of fibronectin and transforming growth factor-β1 (TGF-β1) in cultured rat MCs partly by enhancing extracellular signal-regulated kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK) activities, and subsequent activity of transcription factor AP-1 (AP-1) (20,21). ALD stimulates intracellular adhesion molecule-1 and connective tissue growth factor transcription via activation of serine/threonine-protein kinase Sgk1 and nuclear transcription factor p65, which may be involved in the progression of ALD-induced mesangial fibrosis and inflammation (22). ALD stimulates the mitogen-activated protein kinase pathway, which promotes the proliferation of MCs (23). ALD can also increase plasminogen activator inhibitor-1 mRNA and protein expression in cultured MCs (24). Although these signaling pathways have been identified to serve important roles during the pathogenesis of CKD, there remain few effective therapeutic treatments to cure CKD by targeting these molecules. Therefore, it is necessary for novel molecular potential targets to be investigated.
Long non-coding RNA (lncRNA) has received attention in the investigation of the complex mechanisms underlying malignant processes, including tumorigenesis, drug-resistance and metastasis of different types of cancer (25). In the beginning, the majority of transcriptional outputs of the mammalian genome were confirmed to be protein noncoding genes (26) and the lncRNAs were identified as transcriptional ‘noise’ or cloning artifacts (27). During the past decade, multiple lncRNAs have been demonstrated and confirmed to be involved in the regulation of gene transcription, chromatin methylation, post-transcriptional modification and other biological progresses (28). However, the systematic analysis of aberrant expression profiles of lncRNA in MCs treated with ALD remains to be performed. In the present study, the expression pattern of lncRNAs was investigated by high-throughput microarray in MCs treated with ALD. Furthermore, to couple the observed differential expression of lncRNA with the expression of mRNAs, an mRNA transcriptome analysis by microarray was conducted. The aim of the present study was to clarify the roles of differentially expressed lncRNAs in MCs treated with ALD and provide a novel insight into CKD pathogenesis, and to identify potential biomarkers and therapeutic targets for CKD.
Materials and methods
Cell culture
Cultured rat MCs were purchased from the China Center for Type Culture Collection (Wuhan, China). MCs from passages 7–9 were used in the experiments. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal calf serum (FCS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humid atmosphere containing 5% CO2 at 37°C. MCs were grown to 75% confluence in 100 cm2 flasks (BD Biosciences, Franklin Lakes, NJ, USA) were incubated in serum-free medium for 24 h and then treated with ALD (R&D Systems, Inc., Minneapolis, MN, USA) at a concentration of 10−6 M in a 96-well plate for 24 h at 37°C.
Preparation of model
Performed as previously described by Zhang et al (29), with 10−6 M ALD-treated MCs being used as the experimental cells. An equal amount of solvent (DMEM+10% FCS) was added to the solvent control group. Cells were cultured in 6-well plates for 24 h. MCs were washed with PBS three times, then 200 µl EDTA (Gibco; Thermo Fisher Scientific, Inc.) was added to each well followed by 750 µl TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The cells were stored at −80°C and sent to the LncRNA Expression Microarray (Arraystar, Rockville, MD, USA).
lncRNA microarray analysis
The lncRNAs were constructed using public transcriptome databases (Refseq, www.ncbi.nlm.nih.gov/refseq; University of California Santa Cruz knowngenes, genome.ucsc.edu; and Gencode, www.gencodegenes.org), in addition to a publication (30). The lncRNA expression microarray used in the present study classifies its probes as the following subtypes: i) Enhancer lncRNAs: Contains profiling data of all lncRNAs with enhancer-like function; ii) Rinn lncRNAs: Contains profiling data of all lncRNAs based on studies by Khalil et al (31) and Guttman et al (32); iii) homeobox protein (HOX) cluster: Contains profiling data of all probes in the four HOX loci, targeting 407 discrete transcribed regions, lncRNAs and coding transcripts; iv) lncRNAs located near coding genes: These contain differentially expressed lncRNAs and nearby coding gene pairs (distance, 300 kb); and v) enhancer lncRNAs located near coding genes: These contain differentially expressed enhancer-like lncRNAs and their nearby coding genes (distance, 300 kb). Following hybridization and washing, processed slides were scanned with an Agilent DNA Microarray Scanner (part no. G2505B; Agilent Technologies, Inc., Santa Clara, CA, USA). Agilent Feature Extraction software version 10.7.3.1 (Agilent Technologies, Inc.) was used to analyze all acquired array images. Quantile normalization and subsequent data processing were performed using the GeneSpring GX version 11.5.1 software package (Agilent Technologies, Inc.) (33). The profile of microarray data of the 8,459 lncRNAs was detected by third-generation lncRNA microarray. The general characteristics of the differentially-expressed lncRNAs were summarized, including chromosomal, source, relationship and fold-change distribution using the most widely-used public transcriptome databases (Ensembl, www.ensembl.org/index.html; UCSC, genome.ucsc.edu/index.html; NONCODE, www.noncode.org; NCBI, www.ncbi.nlm.nih.gov). The source of the lncRNA was collected from RefSeq_NR (RefSeq validated non-coding RNA), RefSeq_XR (RefSeq un-validated non-coding RNA), mouse_ortholog (rat lncRNAs which are obtained by sequence comparison with mouse lncRNAs), ‘ultra-conserved regions’ among human, mouse and rat (users.soe.ucsc.edu/~jill/ultra.html), and misc_lncRNA (other sources).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was used to verify the differential expression of seven lncRNAs and six associated genes that were detected by the lncRNA and mRNA expression microarray. The cDNA was synthesized using reverse transcriptase (Takara Bio, Inc., Otsu, Japan) and oligo (dTs) primers with 1 µl RNA from the same samples as those used in the microarray. The reaction consisted of 2 µl 5X PrimeScript buffer, 0.5 µl PrimeScript RT Enzyme Mix I, 0.5 µl Oligo dT Primer, 0.5 µl Random 6 mers, 500 ng total RNA and RNase free dH2O up to 10 µl, and was performed for 15 min at 37°C. Primers for each lncRNA and mRNA are reported in Table I. qPCR was performed on an Applied Biosystems ViiA™ 7 Dx (Thermo Fisher Scientific, Inc.) using the SYBR-Green method, according to the manufacturer's protocol. Each RT-qPCR reaction (in 10 µl) contained 5 µl SYBR−Green real-time PCR Master mix (Thermo Fisher Scientific, Inc.), 1.0 mM primer and 1 µl template cDNA. The cycling conditions consisted of an initial single cycle of 2 min at 50°C; 2 min at 95°C; followed by 40 cycles of 15 sec at 95°C, 15 sec at 56°C and 60 sec at 72°C. PCR amplifications were performed in triplicate for each sample. Gene expression levels were quantified relative to the expression of GAPDH (primers: Sense, 5′-CAAGTTCAACGGCACAGTCAA-3′; antisense, 5′-TGGTGAAGACGCCAGTAGACTC-3′) using an optimized comparative 2−ΔΔCq method (34). RT-qPCR was performed in triplicate on the diluted cDNA and the experiments were performed twice in the control and ALD-treated MC groups.
Table I.
Primers for quantitative polymerase chain reaction of long non-coding RNA and mRNAs.
| Primers (5′-3′) | ||
|---|---|---|
| Genes | Forward | Reverse |
| BC168211 | CACCTGGCCACTGTTTCCTAA | TGATACTCGGCTAGGGAAGCA |
| BC088254 | CCCAGAAAGCTCTCAGGTCCTA | TGCTGGGTGCTTTATTTACACAA |
| AF336872 | TGGCCAGGAGTGCCATTC | CCCCCCAATGCCATGA |
| AY325162 | CCCATGTCCCTCATTCATTACC | GGTGACACGAAGCATCCAAGT |
| BC168687 | CATTGCTCCTGTCTTAGGTCGTT | GGTGGCGATAGGGTTAATTTCC |
| AF230638 | TCCTTTTGCAAGAATCCATACTCA | CGGTGCTAACGGTGAATCAGA |
| BC167085 | TGGAGGCCGCCAAGTGT | GAATCCCACCGGGTCACA |
| NM_001108598 | CACCTGGCCACTGTTTCCTAA | TGATACTCGGCTAGGGAAGCA |
| NM_001109190 | CCAGGCTATGAACGGTTTCC | AGTAGGGTCTGTTTGCATCCTTAGG |
| NM_001101018 | TCACCAAGACCCAGTTCAGTTAGA | GAAGGCCGTGCCAATGAG |
| NM_019347 | ACACACCTGTTGGCACTTGTCT | CGGTGGCACACCAACCA |
| NM_177962 | CCTTGCACCTGTTTCAAATCAA | GGGCAGAGGGAACGAATCA |
| NM_001108823 | AACCCTCAGGAGCCATGCT | TGGGCACTGCAGGTGAGA |
Gene ontology (GO) analysis and pathway analysis
Previous studies have demonstrated that lncRNAs are preferentially located next to genes with developmental functions (26). For each lncRNA locus the nearest protein-coding neighbor within 100 kb was identified. For antisense overlapping and intronic overlapping lncRNAs, the overlapping gene was identified. GO (http://www.geneontology.org) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses (http://www.genome.jp/kegg) were applied to determine the roles of these closest coding genes in GO terms or biological pathways.
GO analysis was used to assess the main function of the closest coding genes according to the GO database, which provides the key functional classifications for the National Center for Biotechnology Information (35). The ontology covers two domains: Biological processes and molecular function. Fisher's exact test is used to identify if there is more overlap between the differentially expressed list and the GO annotation list than would be expected by chance. The P-value denotes the significance of GO term enrichment in the differentially expressed genes. Pathway analysis is a functional analysis mapping genes to the KEGG pathways. The P-value (expression analysis systematic explorer-score, Fisher-P-value or Hypergeometric-P-value) denotes the significance of the pathway correlated with the conditions. P<0.05 was considered to indicate a statistically significant difference.
Statistical analysis
Each qPCR experiment was performed at least three times. Numerical data were presented as the mean ± standard error of the mean. Relative expression levels of lncRNAs between the two groups were analyzed using the Student's t-test. All statistical analyses were performed using SPSS software (version 18; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Profile of lncRNA microarray data
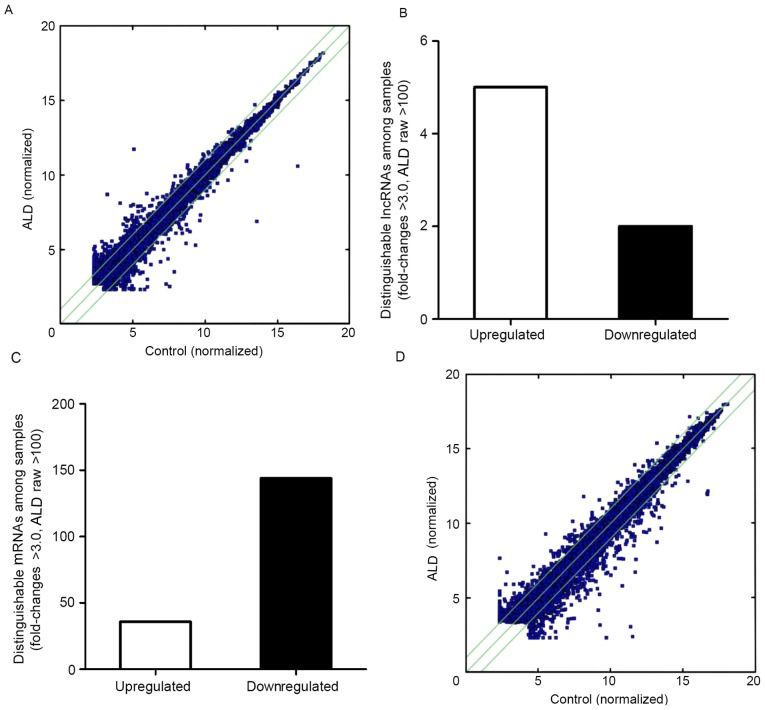
A gene chip study was performed in the normal and ALD-treated MC group to investigate the possible lncRNA alteration in expression using the Arraystar probe dataset, which included 8,459 lncRNAs. The lncRNAs were constructed using public transcriptome databases (Refseq, University of California Santa Cruz knowngenes and Gencode), in addition to a publication (30). The scatterplot is useful for assessing the variation in the expression of lncRNAs and coding transcripts between the two MCs (Fig. 1A); the dot above the green line represents a difference of >2 times. The number of points above the top and below the bottom green lines indicated lncRNAs that exhibit >2.0 fold-change when comparing the control and ALD-treated MC groups. By setting a filter of fold-change >3.0, raw >100 of the expression level between the ALD-treated group and control group, 5 upregulated and 2 downregulated lncRNAs were identified (Fig. 1B).
Figure 1.
Scatter-plots illustrating the distinct expression patterns of differentially expressed RNAs between control and MCs treated with ALD. (A) Fold-change alterations for lncRNAs expressed in the control and ALD-treated MC group. The number of points above the top and below the bottom green lines indicate >2.0 fold-change when comparing the expression of lncRNAs in the control and ALD-treated MC groups. (B) The number of up- and downregulated lncRNAs from the ALD-treated MCs and the control groups. (C) The numbers of upregulated and downregulated mRNAs from the ALD-treated MCs and the control groups. (D) Fold-change alterations for mRNAs expressed in the control and ALD-treated MC group. The number of points above the top and below the bottom green lines indicate >2.0 fold-change when comparing the expression of mRNAs in the control and ALD-treated MC groups ALD, aldosterone; lncRNAs, long non-coding RNAs; MCs, mesangial cells.
Differently expressed mRNAs in the ALD-treated rat MC group
An Affymetrics gene array containing 13,214 gene transcripts was used to perform a comprehensive analysis of mRNA expression in control compared with ALD-treated rat MCs group. Comparing the rat MCs treated with ALD with the control group, 180 genes were differentially expressed (fold-change >3.0; raw >100), of which 36 genes were upregulated and 144 genes were downregulated (Fig. 1C). A scatter-plot illustrating the expression patterns of these differentially expressed mRNAs between control and ALD-treated rat MCs is exhibited in Fig. 1D.
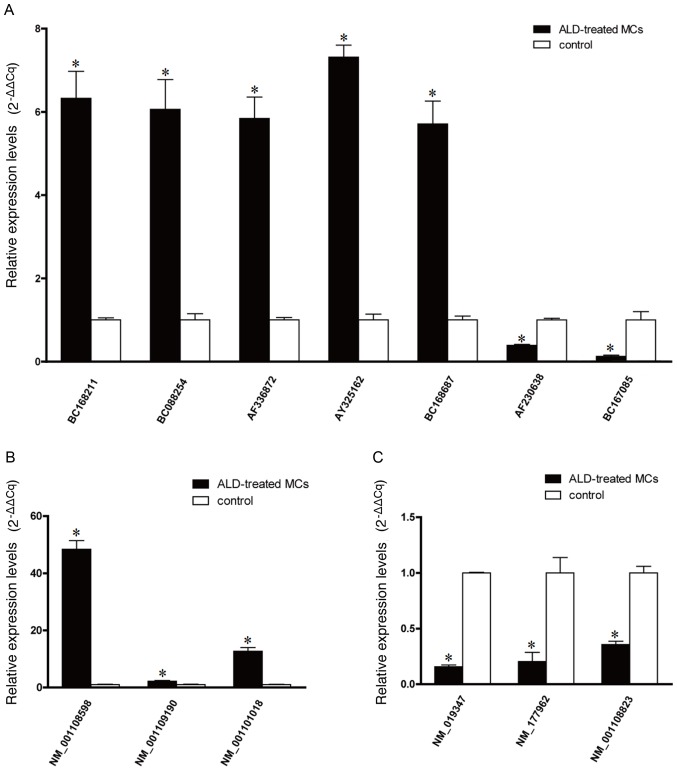
RT-qPCR analysis of microarray hybridization
To quantify the microarray hybridization results, RT-qPCR was performed on five upregulated lncRNAs (BC168211, BC088254, AF336872, AY325162 and BC168687), two downregulated lncRNAs (AF230638 and BC167085) (Fig. 2A), three upregulated mRNAs (NM_001108598, NM_001109190 and NM_001101018) (Fig. 2B) and three downregulated mRNAs (NM_019347, NM_177962 and NM_001108823) (Fig. 2C), selected on the basis of their levels of expression on the microarray and their biological significance. The RT-qPCR data was demonstrated to be consistent with the microarray results, with BC168211, BC088254, AF336872, AY325162 and BC168687 being upregulated and AF230638, BC167085 being downregulated compared with the control. The RT-qPCR data were again consistent with the microarray results for the mRNAs with NM_001108598, NM_001109190 and NM_001101018 being upregulated and NM_019347, NM_177962 and NM_001108823 being downregulated (P<0.05 vs. the control group).
Figure 2.
Reverse transcription-quantitative polymerase chain reaction quantification of microarray hybridization. (A) The relative expression level of lncRNAs BC168211, BC088254, AF336872, AY325162, BC168687, AF230638 and BC167085. (B) The relative expression levels of upregulated mRNAs, NM_001108598, NM_001109190 and NM_001101018, and (C) downregulated mRNAs, NM_019347, NM_177,962 and NM_0,011,08823. *P<0.05 vs. the control group. ALD, aldosterone; lncRNAs, long non-coding RNAs; MCs, mesangial cells.
Expression signatures of differentially expressed lncRNAs
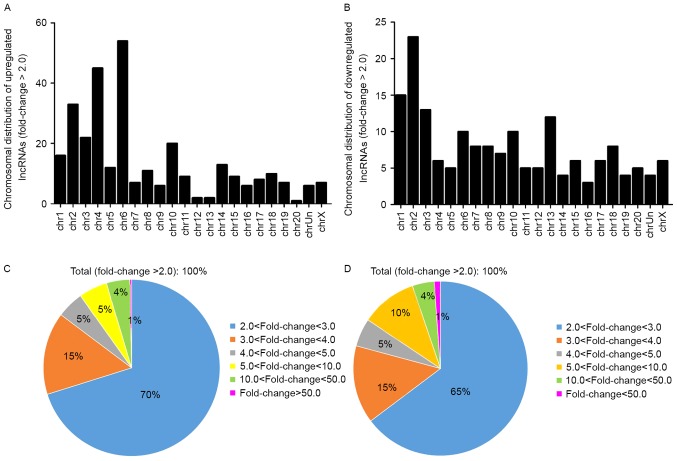
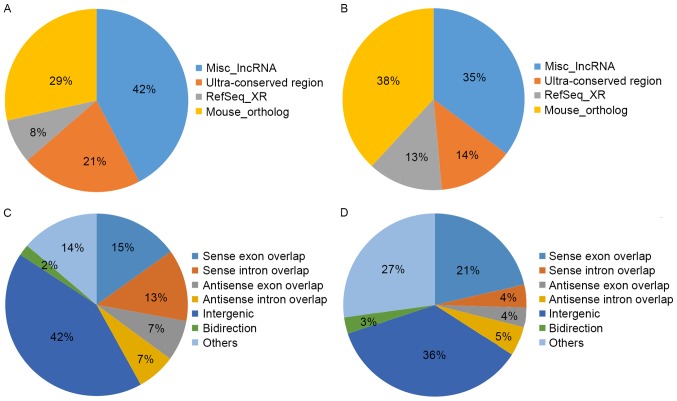
The general characteristics of the differentially expressed lncRNAs were summarized, including chromosomal, source, relationship and fold-change distribution. Chromosomal distribution of the number of up- or downregulated lncRNAs located on different chromosomes was demonstrated (Fig. 3A and B). Fold-change distribution demonstrated the differential expression of up- and downregulated lncRNAs (Fig. 3C and D), respectively. Source distribution respectively demonstrated the percentages of up- and downregulated lncRNAs collected from different sources (Fig. 4A and B), including misc_lncRNA, ultra-conserved region, Refseq-XR and mouse_ortholog. Relationship distribution demonstrated the association of up- and downregulated lncRNAs (Fig. 4C and D), respectively.
Figure 3.
Chromosomal distribution demonstrating the number of (A) up- and (B) downregulated lncRNAs. Fold-change distribution demonstrating the differential expression of (C) up- and (D) downregulated lncRNAs. lncRNAs, long non-coding RNAs.
Figure 4.
Source distribution demonstrating the number of (A) up- and (B) downregulated lncRNAs as a percentage. Relationship distribution demonstrated the association of (C) up- and (D) downregulated lncRNAs. lncRNAs, long non-coding RNAs.
GO and pathway analysis
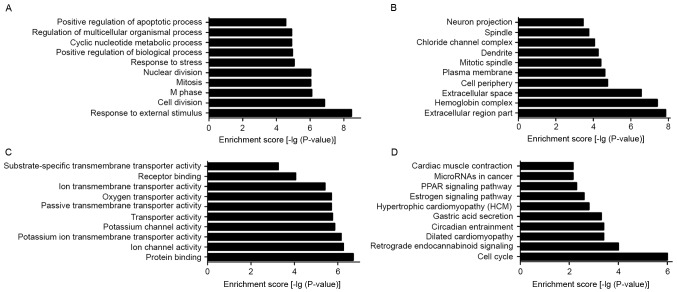
To elucidate the biological processes and functional classification of differentially expressed lncRNAs, GO and pathway analyses were performed. The functions of coding genes adjacent to dysregulated lncRNAs included (in size order, most prevalent first, the top ten): i) Response to external stimuli; ii) response to stress; iii) positive regulation of biological processes; iv) cyclic nucleotide metabolic process; v) regulation of multicellular organism processes; vi) positive regulation of the apoptotic process; vii) cell division; viii) M phase; ix) mitosis; and x) nuclear division (Fig. 5A). The cellular component containing dysregulated lncRNAs included the following (in size order, most prevalent first, the top ten): i) Cell periphery; ii) dendrite; iii) chloride channel complex; iv) neuron projection; v) extracellular region part; vi) hemoglobin complex; vii) extracellular space; viii) plasma membrane; ix) mitotic spindle; and x) spindle (Fig. 5B). The molecular function of dysregulated lncRNAs mainly consisted of the following (in size order, most prevalent first, the top ten): i) Ion channel activity; ii) potassium ion transmembrane transporter activity; iii) potassium channel activity; iv) transporter activity; v) passive transmembrane transporter activity; vi) ion transmembrane transporter activity; vii) protein binding; viii) oxygen transporter activity; ix) receptor binding; and x) substrate-specific transmembrane transporter activity (Fig. 5C). Pathway analysis is a functional analysis process that maps genes to KEGG pathways. In the present study, the top 10 pathways that were associated with coding genes of dysregulated lncRNAs involved: i) Estrogen signaling pathway; ii) peroxisome proliferator-activated receptor (PPAR) signaling pathway; iii) cell cycle; iv) retrograde endocannabinoid signaling; v) circadian entrainment; vi) hypertrophic cardiomyopathy; vii) gastric acid secretion; viii) microRNAs in cancer; ix) cardiac muscle contraction; and x) dilated cardiomyopathy-all Rattus norvegicus (Fig. 5D).
Figure 5.
Biological process classification of GO analysis of (A) downregulated and up-regulated lncRNAs (counter-clockwise from the upper right to the upper left in accordance with size order from smallest to biggest), respectively. (B) Cellular component classification of GO analysis of down- and upregulated lncRNAs, respectively (the top 10 were selected, proportionately). (C) Molecular function classification of GO analysis of down- and upregulated lncRNAs, respectively (the top 10 were selected, proportionately). (D) The pathway of down- and up-regulated lncRNAs between stimulated and normal control groups, respectively (the top 10 were selected, proportionately). GO, gene ontology; lncRNAs, long non-coding RNAs.
Bioinformatic analysis
As the transcription of non-coding genes can affect the expression of their flanking coding genes, relatively lowly and highly expressed lncRNAs were selected with a < and >3-fold-change, respectively, as well as an raw >100 in the MCs treated with ALD and control group, and an associated coding gene with a function in a developmental processes, including cell cycle and PPAR signaling. It was demonstrated that dual specificity phosphatase 15 (Dusp15) and acyl-coenzyme A thioesterase Them4 (Them4) were associated with the lncRNAs BC168211 and BC168687, respectively (data not shown).
Discussion
Currently available therapies are not efficacious in the treatment of CKD, suggesting that further understanding of the molecular mechanisms underlying the pathogenesis of CKD is required for the identification of more effective diagnostic markers and therapeutic targets. MCs have been demonstrated to be a target of local ALD action, which may serve an important role in glomerular damage in CKD (36). In addition, ALD has been proved to serve a significant role in modulating MC function (11,23,37).
The present study, to the best of the authors' knowledge, is the first to report the differential lncRNA expression in MCs treated with ALD compared with normal MCs. A threshold of >3.0 fold-change and raw >100 was set and it was demonstrated that 5 lncRNAs were upregulated and 2 were downregulated in MCs treated with ALD compared with the non-ALD treated MCs. The RT-qPCR results revealed that BC168211, BC088254, BC168687, AF336872 and AY325162 were significantly upregulated in MCs treated with ALD, and AF230638 and BC167085 were downregulated. Furthermore, it was demonstrated that lncRNAs may act through distinct transcription factors to modulate their target genes' transcription, thereby being involved in the potential mechanism of CKD. Then, mRNA microarray technology was used to evaluate differences in the mRNA expression profiles of control and MCs treated with ALD. GO and pathway analyses revealed that these lncRNAs were associated with changes in key pathogenic processes of CKD.
The collected data can be used to analyze the role of lncRNA transcripts in ALD-induced CKD. The GO project provides a controlled vocabulary that can be used to describe genes and gene product attributes (38). The biological processes involving dysregulated lncRNAs was demonstrated to be associated with cell proliferation stimulated by ALD included the following: Cell division; positive regulation of biological processes; response to external stimuli; regulation of multicellular organism processes; and cyclic nucleotide metabolic processes. The biological processes associated with lncRNAs included cell division, immune system processes, immune responses and cell-cell signaling. Pathway analysis provides a method for gaining insight into the underlying biology of differentially expressed genes and proteins (16). Pathway analysis demonstrated that the associated genes of dysregulated lncRNAs between the control and MCs treated with ALD included a variety of pathways for example cell cycle-associated, PPAR signaling and estrogen signaling pathways. It has been reported that ALD serves a major part in the glomerular ECM accumulation and proliferation of MCs in several glomerular diseases, and produces renal fibrosis in rats (18–21,23,24). The lncRNAs examined in the present study were demonstrated to be involved in the progression of CKD, which is induced by cell division, cell proliferation and immune deposits, in which ALD was involved.
Due to the complexity of the transcriptome, lncRNAs are frequently overlapping or are interspersed between multiple coding and non-coding transcripts (39,40). In the present study, two genes were identified to be associated with lncRNAs through GO and pathway analysis. These results were used to investigate the association between lncRNAs and genes, further. Dusp15 and Them4 were demonstrated to be associated with the lncRNAs BC168211 and BC168687, respectively.
Dusp15, a member of the protein tyrosine phosphatase family, previously only reported to be expressed in the testes, was suggested to be a pharmacological target for promoting remyelination in multiple sclerosis (41). Dusp15 is expressed in the kidneys of spontaneously hypertensive rats, which indicates that it is associated with renal injury. Dusp15 was identified to serve a role in the regulation of cell proliferation, positive regulation of the JNK cascade and TGF-β receptor-signaling pathway. TGF-β has been recognized to be an important factor in the development of CKD (42). In rat MCs, ALD upregulates mRNA expression of TGF-β partly by enhancing the ERK1/2, JNK and AP-1 intracellular signaling pathways, and stimulating the progression of renal disease (20,21,43–45). TGF-β has also been demonstrated to induce mesangial expansion, which is caused by MC hypertrophy, proliferation and eventually apoptosis (46). In the present study, an association was demonstrated between BC168211 and Dusp15. The level of Dusp15 was revealed to be increased in stimulated MCs compared with the control cells. This may indicate that Dusp15 is involved in promoting the proliferation of MCs. However, the intrinsic association between BC168211 and Dusp15 is not completely understood. Further studies on this issue are planned.
Them4, a negative regulator of RAC-α serine/threonine-protein kinase (Akt) and activated Akt, is known to protect the cell from apoptosis. The amino-terminal domain of Them4 may bind to Akt (47,48). Human Them4 has also been linked to Akt regulation and apoptosis (49). ALD stimulates MC proliferation via the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway (50). The PI3K-Akt signaling pathway regulates fundamental cellular functions including transcription, translation, proliferation, growth and survival. PI3K catalyzes the production of phosphatidylinositol-3,4,5-triphosphate, which in turn serves as a second messenger that helps to activate Akt. Once active, Akt can control key cellular processes by phosphorylating substrates involved in apoptosis, protein synthesis, metabolism and cell cycle (50) (from KEGG source record: rno04151). Thus far, it has been clear that Them4 is associated with the cell cycle (49). In the present study it was hypothesized that Them4 is involved in the PI3K/Akt signaling pathway in the progression of mesangial expansion. Them4 was demonstrated to be upregulated in ALD stimulated MCs. However, the precise mechanism of Them4 with BC168687 in ALD induced CKD warrants further investigation.
In conclusion, to the best of the author's knowledge, the present study is the first to provide a profile of lncRNAs in ALD-induced MCs in vitro. A network of differentially expressed lncRNAs was constructed, and numerous lncRNAs are involved in the development and mechanism of CKD. Further investigation of the biological progresses and molecular mechanisms of the dysregulated lncRNAs is necessary. The present study may provide novel insights into the molecular basis of CKD, and aid the identification of potential novel biomarkers and development of therapeutic interventions for this disease.
Acknowledgements
The present study was sponsored by the Scientific Research Program of Nanjing Medical University (grant no. 2015NJMUZD028) and the Natural Science Fund Project of Colleges in Jiangsu Province, China (grant no. 15KJD320005).
References
- 1.Zhang L, Zhang P, Wang F, Zuo L, Zhou Y, Shi Y, Li G, Jiao S, Liu Z, Liang W, Wang H. Prevalence and factors associated with CKD: A population study from Beijing. Am J Kidney Dis. 2008;51:373–384. doi: 10.1053/j.ajkd.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF, III, Werb Z, Kalluri R. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3:e100. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo YI, Cheng H, Wang S, Moeckel GW, Harris RC. Puromycin induces reversible proteinuric injury in transgenic mice expressing cyclooxygenase-2 in podocytes. Nep Exp Nephrol. 2007;107:e87–e94. doi: 10.1159/000108653. [DOI] [PubMed] [Google Scholar]
- 4.Pesce CM, Striker LJ, Peten E, Elliot SJ, Striker GE. Glomerulosclerosis at both early and late stages is associated with increased cell turnover in mice transgenic for growth hormone. Lab Invest. 1991;65:601–605. [PubMed] [Google Scholar]
- 5.Kreisberg JI, Karnovsky MJ. Glomerular cells in culture. Kidney Int. 1983;23:439–447. doi: 10.1038/ki.1983.40. [DOI] [PubMed] [Google Scholar]
- 6.Floege J, Burns MW, Alpers CE, Yoshimura A, Pritzl P, Gordon K, Seifert RA, Bowen-Pope DF, Couser WG, Johnson RJ. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992;41:297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- 7.Schlöndorff D, Banas B. The mesangial cell revisited: No cell is an island. J Am Soc Nephrol. 2009;20:1179–1187. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 8.Eddy AA, Neilson EG. Chronic kidney disease progression. J Am Soc Nephrol. 2006;17:2964–2966. doi: 10.1681/ASN.2006070704. [DOI] [PubMed] [Google Scholar]
- 9.Siragy HM, Xue C. Local renal aldosterone production induces inflammation and matrix formation in kidneys of diabetic rats. Exp Physiol. 2008;93:817–824. doi: 10.1113/expphysiol.2008.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu P, Liang X, Dai Y, Liu H, Zang Y, Guo Z, Zhang R, Lai W, Zhang Y, Liu Y. Aldosterone biosynthesis in extraadrenal tissues. Chin Med J (Engl) 1999;112:414–418. [PubMed] [Google Scholar]
- 11.Nishikawa T, Suematsu S, Saito J, Soyama A, Ito H, Kino T, Chrousos G. Human renal mesangial cells produce aldosterone in response to low-density lipoprotein (LDL) J Steroid Biochem Mol Biol. 2005;96:309–316. doi: 10.1016/j.jsbmb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Martinez D, Oestreicher E, Roubsanthisuk W, et al. proceedings of the Journal of Hypertension. Lippincott Williams & Wilkins; Philadelphia, PA: 2002. Angiotensin II, aldosterone and the caveolae. IV. Aldosterone content of the kidney after adrenalectomy; pp. 19106–3621. [Google Scholar]
- 13.Silvestre JS, Heymes C, Oubénaïssa A, Robert V, Aupetit-Faisant B, Carayon A, Swynghedauw B, Delcayre C. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation. 1999;99:2694–2701. doi: 10.1161/01.CIR.99.20.2694. [DOI] [PubMed] [Google Scholar]
- 14.Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem. 1998;273:4883–4891. doi: 10.1074/jbc.273.9.4883. [DOI] [PubMed] [Google Scholar]
- 15.Kayes-Wandover KM, White PC. Steroidogenic enzyme gene expression in the human heart. J Clin Endocrinol Metab. 2000;85:2519–2525. doi: 10.1210/jcem.85.7.6663. [DOI] [PubMed] [Google Scholar]
- 16.Takeda Y, Yoneda T, Demura M, Miyamori I, Mabuchi H. Sodium-induced cardiac aldosterone synthesis causes cardiac hypertrophy. Endocrinology. 2000;141:1901–1904. doi: 10.1210/endo.141.5.7529. [DOI] [PubMed] [Google Scholar]
- 17.Kornel L. Colocalization of 11beta-hydroxysteroid dehydrogenase and mineralocorticoid receptors in cultured vascular smooth muscle cells. Am J Hypertens. 1994;7:100–103. doi: 10.1093/ajh/7.1.100. [DOI] [PubMed] [Google Scholar]
- 18.Lai LY, Gu Y, Chen J, Yu SQ, Ma J, Yang HC, Lin SY. Production of aldosterone by rat mesangial cell and the accumulation of extracellular matrix induced by aldosterone. Zhonghua Yi Xue Za Zhi. 2003;83:1900–1905. (In Chinese) [PubMed] [Google Scholar]
- 19.Terada Y, Kuwana H, Kobayashi T, Okado T, Suzuki N, Yoshimoto T, Hirata Y, Sasaki S. Aldosterone-stimulated SGK1 activity mediates profibrotic signaling in the mesangium. J Am Soc Nephrol. 2008;19:298–309. doi: 10.1681/ASN.2007050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han JS, Choi BS, Yang CW, Kim YS. Aldosterone-induced TGF-beta1 expression is regulated by mitogen-activated protein kinases and activator protein-1 in mesangial cells. J Korean Med Sci. 2009;24(Suppl 1):S195–S203. doi: 10.3346/jkms.2009.24.S1.S195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XD, Chen XW, Tang DS, Liang D, Liu HF. Effects of aldosterone on synthesis of fibronectin and expression of transforming growth factor-beta1 mRNA in cultured rat mesangial cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2007;23:140–1422. (In Chinese) [PubMed] [Google Scholar]
- 22.Terada Y, Ueda S, Hamada K, Shimamura Y, Ogata K, Inoue K, Taniguchi Y, Kagawa T, Horino T, Takao T. Aldosterone stimulates nuclear factor-kappa B activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum-and glucocorticoid-inducible protein kinase-1. Clin Exp Nephrol. 2012;16:81–88. doi: 10.1007/s10157-011-0498-x. [DOI] [PubMed] [Google Scholar]
- 23.Terada Y, Kobayashi T, Kuwana H, Tanaka H, Inoshita S, Kuwahara M, Sasaki S. Aldosterone stimulates proliferation of mesangial cells by activating mitogen-activated protein kinase 1/2, cyclin D1, and cyclin A. J Am Soc Nephrol. 2005;16:2296–2305. doi: 10.1681/ASN.2005020129. [DOI] [PubMed] [Google Scholar]
- 24.Yuan J, Jia R, Bao Y. Aldosterone up-regulates production of plasminogen activator inhibitor-1 by renal mesangial cells. J Biochem Mol Biol. 2007;40:180–188. doi: 10.5483/bmbrep.2007.40.2.180. [DOI] [PubMed] [Google Scholar]
- 25.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Cai B, Song XQ, Cai JP, Zhang S. HOTAIR: A cancer-related long non-coding RNA. Neoplasma. 2014;61:379–391. doi: 10.4149/neo_2014_075. [DOI] [PubMed] [Google Scholar]
- 28.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 29.Zhang A, Han Y, Wang B, Li S, Gan W. Beyond gap junction channel function: The expression of Cx43 contributes to aldosterone-induced mesangial cell proliferation via the ERK1/2 and PKC pathways. Cell Physiol Biochem. 2015;36:1210–1222. doi: 10.1159/000430291. [DOI] [PubMed] [Google Scholar]
- 30.Xu G, Chen J, Pan Q, Huang K, Pan J, Zhang W, Chen J, Yu F, Zhou T, Wang Y. Long noncoding RNA expression profiles of lung adenocarcinoma ascertained by microarray analysis. PLoS One. 2014;9:e104044. doi: 10.1371/journal.pone.0104044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Morales D Rivea, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression; Proc Natl Acad Sci USA; 2009; pp. 11667–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Li H, Hou S, Hu B, Liu J, Wang J. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS One. 2013;8:e65309. doi: 10.1371/journal.pone.0065309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai L, Chen J, Hao CM, Lin S, Gu Y. Aldosterone promotes fibronectin production through a Smad2-dependent TGF-beta1 pathway in mesangial cells. Biochem Biophys Res Commun. 2006;348:70–75. doi: 10.1016/j.bbrc.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 37.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–448. doi: 10.1161/01.HYP.0000118519.66430.22. [DOI] [PubMed] [Google Scholar]
- 38.Balakrishnan R, Harris MA, Huntley R, Van Auken K, Cherry JM. A guide to best practices for Gene Ontology (GO) manual annotation. Database (Oxford) 2013;2013:bat054. doi: 10.1093/database/bat054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1163. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 40.Kapranov P, Drenkow J, Cheng J, Long J, Helt G, Dike S, Gingeras TR. Examples of the complex architecture of the human transcriptome revealed by RACE and high-density tiling arrays. Genome Res. 2005;15:987–997. doi: 10.1101/gr.3455305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt F, van den Eijnden M, Gobert R Pescini, Saborio GP, Carboni S, Alliod C, Pouly S, Staugaitis SM, Dutta R, Trapp B, et al. Identification of VHY/Dusp15 as a regulator of oligodendrocyte differentiation through a systematic genomics approach. PLoS One. 2012;7:e40457. doi: 10.1371/journal.pone.0040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnaper HW, Jandeska S, Runyan CE, Hubchak SC, Basu RK, Curley JF, Smith RD, Hayashida T. TGF-beta signal transduction in chronic kidney disease. Front Biosci (Landmark Ed) 2009;14:2448–2465. doi: 10.2741/3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chin BY, Mohsenin A, Li SX, Choi AM, Choi ME. Stimulation of pro-alpha(1)(I) collagen by TGF-beta(1) in mesangial cells: role of the p38 MAPK pathway. Am J Physiol Renal Physiol. 2001;280:F495–F504. doi: 10.1152/ajprenal.2001.280.3.F495. [DOI] [PubMed] [Google Scholar]
- 44.Huwiler A, Pfeilschifter J. Transforming growth factor beta 2 stimulates acute and chronic activation of the mitogen-activated protein kinase cascade in rat renal mesangial cells. FEBS Lett. 1994;354:255–258. doi: 10.1016/0014-5793(94)01132-X. [DOI] [PubMed] [Google Scholar]
- 45.Martínez-Salgado C, Rodríguez-Peña AB, López-Novoa JM. Involvement of small Ras GTPases and their effectors in chronic renal disease. Cell Mol Life Sci. 2008;65:477–492. doi: 10.1007/s00018-007-7260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.López-Hernández FJ, López-Novoa JM. Role of TGF-β in chronic kidney disease: An integration of tubular, glomerular and vascular effects. Cell Tissue Res. 2012;347:141–154. doi: 10.1007/s00441-011-1275-6. [DOI] [PubMed] [Google Scholar]
- 47.Cao J, Xu H, Zhao H, Gong W, Dunaway-Mariano D. The mechanisms of human hotdog-fold thioesterase 2 (hTHEM2) substrate recognition and catalysis illuminated by a structure and function based analysis. Biochemistry. 2009;48:1293–1304. doi: 10.1021/bi801879z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao H, Martin BM, Bisoffi M, Dunaway-Mariano D. The Akt C-terminal modulator protein is an acyl-CoA thioesterase of the Hotdog-Fold family. Biochemistry. 2009;48:5507–5509. doi: 10.1021/bi900710w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H, Lim K, Choudry A, Latham JA, Pathak MC, Dominguez D, Luo L, Herzberg O, Dunaway-Mariano D. Correlation of structure and function in the human hotdog-fold enzyme hTHEM4. Biochemistry. 2012;51:6490–6492. doi: 10.1021/bi300968n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishiyama A, Yao L, Fan Y, Kyaw M, Kataoka N, Hashimoto K, Nagai Y, Nakamura E, Yoshizumi M, Shokoji T, et al. Involvement of aldosterone and mineralocorticoid receptors in rat mesangial cell proliferation and deformability. Hypertension. 2005;45:710–716. doi: 10.1161/01.HYP.0000154681.38944.9a. [DOI] [PubMed] [Google Scholar]