Abstract
The etiology of thoracic aortic aneurysm and dissection (TAAD) is complex and heterogeneous. Emerging evidence has demonstrated that genetic causes may be a consideration in early-onset TAAD. Owing to overlapping clinical phenotypes and the genetic heterogeneity of TAAD, it is challenging for clinicians to make a molecular diagnosis of TAAD, particularly in those who present with non-specific syndromic features. In order to identify the causative mutation in two young patients with acute type B aortic dissection without syndromic features, whole exome sequencing (WES) was performed in the present study. A missense mutation (c.G6953A:p.C2318Y) and a nonsense mutation (c.C4786T:p.R1596X) were identified in the fibrillin 1 gene in patients T287 and T267, respectively. The present study emphasized the necessity of genetic testing for young patients with type B aortic dissection. WES is a timely, robust and inexpensive technique for molecular diagnosis, particularly for TAAD caused by numerous genes. Genetic diagnosis of Marfan syndrome could aid in periodic surveillance, prophylactic surgical measures, and genetic counseling.
Keywords: type B aortic dissection, thoracic aortic aneurysm and dissection, early-onset, fibrillin 1 mutation, whole exome sequencing
Introduction
Thoracic aortic aneurysm and dissection (TAAD) is associated with marked cardiovascular morbidity and mortality. The incidence of acute aortic dissection has been suggested to be 3–6 cases/100,000 individuals/year, with an increasing incidence in recent decades (1,2). The etiology of TAAD is complex and heterogeneous. Hypertension and male sex are the most common risk factors for TAAD in older patients, and other risk factors include a family history of aortic diseases, pre-existing aortic or aortic valve disease, a history of cardiac surgery and cigarette smoking (2,3). However, genetic disease may be a consideration in early-onset patients without the usual risk factors (4,5). Genetic predisposition to TAAD may occur in individuals with syndromic features (<5% of all cases of TAAD), including in Marfan syndrome (MFS), or in the absence of syndromic features. Of the total cases of non-syndromic TAAD, ~20% are familial (FTAAD) and the rest are sporadic TAAD (6–8).
MFS, as an autosomal dominant disease, is the most frequent heritable connective tissue disorder, associated with mutations in the fibrillin 1 (FBN1) gene (9). The incidence of MFS is estimated to be 2–3 per 10,000 individuals and patients are generally young; the disease frequently manifests by the third decade of life (10,11). MFS, a multisystem disorder, typically involves the skeletal, cardiovascular and ocular systems (10). A number of connective tissue disorders present overlapping clinical phenotypic features with MFS, including Loeys-Dietz syndrome, Ehlers-Danlos syndrome, Shprintzene-Goldberg syndrome and familial TAAD (12). With an improved understanding of MFS, the revised Ghent Nosology gives greater weight to aortic root aneurysm/dissection and FBN1 mutation in the diagnosis of MFS (13). TAAD, which frequently follows a period of progressive dilatation of the ascending aorta, remains the most life-threatening manifestation of MFS and occurs on average 20 years earlier in these patients compared with patients without MFS (14). The majority of patients suffer premature mortality from acute type A aortic dissection or rupture without surgical aortic root replacement. However, the incidence of type A aortic dissection has decreased in individuals with MFS due to prophylactic aortic root and ascending aorta replacement (15). Additionally, with the improvement in life expectancy of patients with MFS, type B aortic dissection, not involving the ascending aorta, occurs more frequently (16). FBN1 has been recognized to be the causal gene of MFS and mutations in FBN1 increase the concentration of transforming growth factor (TGF)-β1, which contributes notably to the pathology of MFS. To date, 1,847 different mutations and 1,096 protein variants have been identified (http://www.umd.be/FBN1), and affected individuals exhibit a broad phenotype although they may share the same mutation within a family (17). Therefore, the timely and accurate diagnosis of MFS remains a challenge.
Whole exome sequencing (WES) is an efficient tool to identify the underlying genetic cause in TAAD (18,19). In contrast with routine Sanger sequencing, WES is more efficient, sensitive and cost-effective due to its high-throughput property. In the present study, two patients were assessed who presented with acute type B aortic dissection, and were subsequently identified to harbor FBN1 pathogenic mutations by WES analysis. According to the revised Ghent Nosology, the patients were diagnosed with MFS. The two patients would have been diagnosed with sporadic TAAD without the results of the WES analysis. It was hypothesized that WES may contribute to molecular diagnosis for patients without the typical features of MFS, thus providing genetic counseling and timely intervention for affected individuals.
Patients and methods
Patients and clinical evaluation
A total of two male patients, not related by birth, were respectively admitted in Marth and May 2015 to the department of Vascular Surgery, Nanjing Drum Tower Hospital, Nanjing, China. The two individuals, designated T287 and T267, complained of acute severe chest and back pain. They were reported to be in good health prior to this acute incidence. Regular physical examination and clinical testing was performed on the two patients, including electrocardiography, computed tomography angiography (CTA) and echocardiography. Z-score was used to estimate the degree of aortic root dilatation (http://www.marfan.org/dx/zscore). Genomic DNA was extracted from peripheral blood samples. The present study was approved by the Institutional Research Ethics Committee of Nanjing Drum Tower Hospital and informed written consent was obtained. A total of 100 ethnically-matched unrelated subjects (aged 29.5±6.5 years) were recruited between June and August 2016 as controls. Peripheral blood was drawn and DNA were subsequent extracted.
WES and data analysis
Genomic DNA was extracted from peripheral whole blood samples from the subjects using the QIAamp DNA Blood Mini kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer's protocol. WES analysis was performed by Beijing Novogene Bioinformatics Technology, Co., Ltd. (Beijing, China). Target enrichment was performed to construct the exome library using the Agilent SureSelect Human All Exon V5 kit (Agilent Technologies, Inc., Santa Clara, CA, USA), according to manufacturer's protocol, and sequenced on the Illumina HiSeq 2000 platform (Illumina, Inc., San Diego, CA). The clean reads from the Illumina Genome HiSeq 2000 were aligned to the human genome reference (University of California Santa Cruz hg19; genome.ucsc.edu) using the Burrows-Wheeler Alignment tool. The Sequence Alignment/Map tools were used to identify single nucleotide polymorphisms (SNPs) and insertions/deletions (INDELs). Picard (http://sourceforge.net/projects/picard/) was employed to mark duplicate reads. An average sequencing depth of 171.32× was achieved and >99.4% of targeted variants were covered at least by 20× for T287, while a 199.80× average sequencing depth and >99.6% of targeted variants covered at least by 20× was achieved for T267.
All annotated variants were screened with databases including the SNP database (dbSNP142, http://www.ncbi.nlm.nih.gov/projects/SNP/snp_summary.cgi), 1000 Genomes Project (version 2014 October, http://www.1000genomes.org/), and National Heart, Lung, and Blood Institute Exome Sequencing Project (ESP) 6500 (http://evs.gs.washington.edu/EVS/). Functional prediction was assessed by sorts intolerant from tolerant (SIFT; http://sift.jcvi.org/) and polymorphism phenotyping version 2 (Polyphen-2; http://genetics.bwh.harvard.edu/pph2/). Candidate SNPs or INDELs were annotated using ANNOVAR software (http://annovar.openbioinformatics.org).
Sanger sequencing confirmation
For Sanger sequencing verification of variants detected by WES, oligonucleotide primers were designed using the Primer3 program (primer3.ut.ee). The regions containing the suspected variants were amplified by standard polymerase chain reaction (PCR) analysis using GoTaq polymerase (Promega). Primers used to amplify the mutant sequence were FBN1-Ex39-F (5′-AACTTACTTCAGACGGGCAGAG-3′), FBN1-Ex39-R (5′-TAGCTCCTGGCACTCATCAATA-3′), FBN1-Ex57-F (5′-ATGTGAGAGAGGGAAGGAAGGT-3′), FBN1-Ex39-R (5′-GTCAATACGGCATCTCCAAAAT-3′). The amplification reaction mixture (50 µl) was subjected to denaturation at 98°C for 3 min followed by 35 cycles at 95°C for 30 sec, annealing temperature 60°C for 30 sec, 72°C for 30 sec and by a final extension at 72°C for 15 min. PCR products were purified and sequenced on the ABI PRISM3730 automated sequencer (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) using the BigDye terminator v3.1 cycle sequencing kit (Thermo Fisher Scientific, Inc.).
Results
Clinical findings
The two patients complained of acute episodes of chest and back pain. T267 is a 35-year-old man, 182 cm in height and 60 kg in weight. He was diagnosed with descending aortic dissection by CTA. Echocardiography of T267 demonstrated that the diameter of the aortic sinus was 4.3 cm, and identified hypertrophy of the left ventricle. T287 is a 32-year-old male, 178 cm in height and 95 kg in weight. A CTA scan revealed descending aortic dissection and ascending aortic dilatation. Echocardiography of T287 demonstrated that the diameter of the aortic sinus was 4.5 cm, in addition to identifying mild aortic valve regurgitation, and mild bicuspid and tricuspid valve regurgitation. Additionally, the grandfather of T287 died suddenly of an unknown cause at age of 40. Clinical manifestations of other systems, including the skeletal and ocular systems, were absent. The clinical data of the two patients are presented in Table I.
Table I.
Clinical characteristics of the two individuals affected with acute type B aortic dissection.
| Cardiovascular | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age, years | Sex | Height, cm | Weight, kg | Diameter of aortic sinus, cma | Z-Scoreb | Aorta | Other | Skeletal | Ocular |
| T267 | 35 | M | 182 | 60 | 4.3 | 3.86 | TAD | Hypertension; left ventricular hypertrophy | None | None |
| T287 | 32 | M | 178 | 95 | 4.5 | 4.12 | TAD | Hypertension; dilatation of ascending aorta; mild valve regurgitation | None | None |
Diameter of the aortic sinus was measured by echocardiography.
Z-score was calculated via a calculator at www.marfan.org. TAD, thoracic aortic dissection.
WES
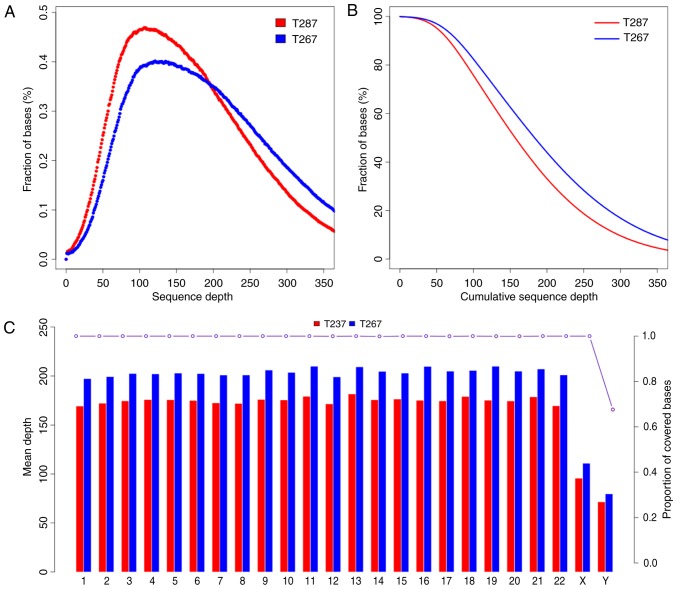
The present study generated a total of 58,189,201 and 50,222,226 raw reads of 180–280 bp paired-end read sequences from the patients T267 and T287, respectively. The raw depth of T267 and T287 was 346.49× and 299.06×, respectively; and the Phred-like Q20 (that is, a 99% accuracy of the base call) was 95.02 and 94.26%. The average depth of targeted sequences was 199.80× and 171.32× for T267 and T287, respectively. The sequence depth and proportion covered bases of every sequence and chromosome of T267 and T287 are presented in Fig. 1. Variant genes were filtered with dbSNP142, the 1000 Genomes Project, and ESP6500. Subsequently, variant genes were identified in either the exonic regions or the splice sites, and synonymous SNPs or INDELs were excluded. SIFT and PolyPhen-2 were used to excluded synonymous mutations and to predict the functions of the mutations.
Figure 1.
Sequence depth and proportion covered bases. (A) Proportion of different sequence depths. (B) Cumulative proportion of different sequence depths. (C) Mean sequence depth and proportion covered bases of every chromosome.
In order to identify pathogenic mutations in the two patients, candidate genes were filtered with reported disease-causing genes for TAAD (data for the two patients are presented in Table II). Applying the above strategy, two heterozygous variants in the FBN1 gene were observed. One was a nonsense mutation, c.C4786T (p.R1596X), and was deleterious due to nonsense-mediated RNA decay. The other was a missense mutation, c.G6953A (p.C2318Y), which was reported to disrupt disulfide bond formation, and thus may be disease-causing (20).
Table II.
Number of variants present in the patients at different stages of the filtering process.
| Number | ||
|---|---|---|
| Filtering criteria | T267 | T287 |
| Total number of variants | 221,478 | 198,478 |
| Variants not in dbSNP, MAF<1% | 22,723 | 19,655 |
| Filtering using 1000 Genomes and ESP | 22,529 | 17,494 |
| Variants in exonic or splicing | 1,082 | 1,021 |
| Non-synonymous and frameshift variants | 950 | 917 |
| Functional analysis by SIFT and Polyphen-2 | 776 | 753 |
| Genes associated with TAAD | 1 | 1 |
MAF, minor allele frequency; ESP, exome sequencing project; SIFT, sorts intolerant from tolerant; Polyphen-2, polymorphism phenotyping version 2; TAAD, thoracic aortic aneurysm and dissection; SNP, single nucleotide polymorphism.
Mutation conformation
The two variants were confirmed by Sanger sequencing (Fig. 2). The variants were absent in 100 ethnically-matched unrelated subjects.
Figure 2.
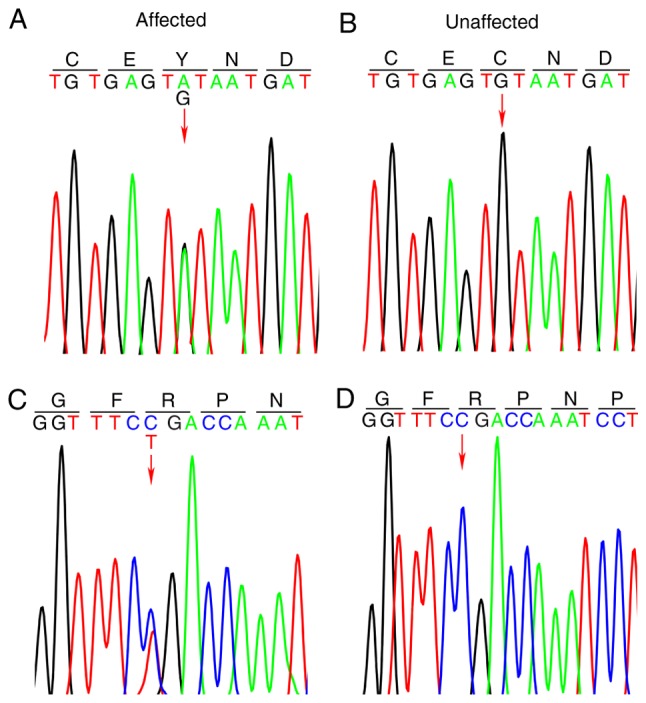
Sequencing analysis of FBN1 gene. (A) Sequence of heterozygous FBN1 c.G6953A (p.C2318Y) mutation of T287 and (B) the corresponding sequence of unaffected individuals. (C) Sequence of heterozygous FBN1 c.C4768T (p.R1596X) mutation of T267 and (D) the corresponding sequence of unaffected individuals. FBN1, fibrillin 1.
Discussion
MFS is a common multisystem connective tissue disorder, inherited in an autosomal dominant manner, caused by mutations in the FBN1 gene on chromosome 15q21.1 (9). FBN1, the major component of elastin-associated microfibrils, is a large gene (>200 kb), highly fragmented into 65 exons, and ~1,847 different mutations have been described to date (http://www.umd.be/FBN1). FBN1 mutations may affect elastic fiber deposition and cytokine-regulatory functions via regulation of the TGF-β signaling pathway (21). The principal clinical features of MFS involve the skeletal system (arachnodactyly, bone overgrowth and joint laxity), cardiovascular system (particularly valve regurgitation and TAAD), and the ocular system (ectopia lentis) (13). Due to phenotypic overlap and the genetic heterogeneity of multiple Marfan-like disorders, it is challenging for clinicians to make an accurate diagnosis of MFS (17). The revised Ghent Nosology highlights the role of the Z-score of the aortic sinus, ectopia lentis and identification of a bona fide FBN1 mutation to establish the diagnosis of MFS (13).
Considering the phenotypic overlap and genetic heterogeneity of diseases featuring aortopathy, molecular genetic testing is often required for the timely and accurate diagnosis of affected individuals. Traditional Sanger sequencing is costly and laborious due to the size of the FBN1 gene. The present study identified one missense mutation (c.G6953A:p.C2318Y in exon 57) and one nonsense mutation (c.C4786T:p.R1596X in exon 39) in the FBN1 gene by WES in two early-onset affected individuals with type B aortic dissection. The two patients lacked a family history of TAAD or MFS. For T287, the diameter of the aortic sinus was measured and the Z-score was calculated to be 4.12, using the Z-score calculator at www.marfan.org. According to the revised Ghent Nosology (2010), T287 was diagnosed with MFS with manifestation of type B aortic dissection. Similarly, the Z-score of T267 was 3.86 and this individual was diagnosed with MFS subsequently. Without identification of the FBN1 mutation, the two patients may have been diagnosed with sporadic TAAD. Therefore, using the technique of WES, patients with MFS may benefit from a timely and accurate diagnosis of MFS, which aids in periodic surveillance, prophylactic surgical measures and genetic counseling for family members.
Clinical manifestations vary between TAAD caused by MFS and non-syndromic TAAD. In previous decades, without the use of surgical aortic root replacement, the incidence of type A aortic dissection was increased compared with type B aortic dissection in patients with MFS (10). Den Hartog et al (15) observed that the rate of type B aortic dissection was 9% in 600 patients with MFS without previous TAAD, during a median follow-up period of 6 years, and that dissection generally occurred in the mildly dilated proximal descending aorta. Hypertension is considered to be the most important risk factor in non-syndromic TAAD. However, TAAD caused by MFS, as the most life-threatening clinical manifestation of the cardiovascular system, is necessarily associated with the mutations in the FBN1 gene (22). Patients with MFS frequently suffer acute TAAD at a younger age compared with patients with non-syndromic TAAD. At present, patients with MFS who have undergone prophylactic surgical aortic root replacement are associated with an increased risk of acute type B aortic dissection, which frequently occurs without prior significant aortic dilatation (15,16).
In the present study, the two patients were admitted to the Department of Vascular Surgery with acute type B aortic dissection in their thirties. It was inferred that the symptoms were likely to be caused by a genetic defect. Subsequent molecular analysis confirmed this hypothesis. It is widely accepted that endovascular stent grafting may not be suitable for the treatment of type B dissection, particularly in young patients with MFS, due to weakening of the aortic walls and the lack of long-term durability of stent grafting (23–25); however, the majority of these previous studies used first-generation stent grafts. Considering the hemodynamic instability, increased surgical risk and absence of definitive diagnostic criteria for MFS, thoracic endovascular aortic repair was performed on the two patients recruited for the present study. Angiotensin-II type-1 receptor blockers have been demonstrated to reverse vascular complications in a fibrillin-deficient mouse model of MFS and losartan was recommended for used in medical therapy (26,27). With advances in thoracic endovascular stent grafting and improved recognition of MFS, improved treatment options may be identified for young patients with MFS.
In conclusion, the present study identified one missense mutation and one nonsense mutation via WES in two patients with early-onset type B aortic dissection without typical syndromic features, who were subsequently diagnosed with MFS. the present study emphasized the necessity of genetic testing for young patients with type B aortic dissection. WES is a timely, robust and inexpensive technique for genetic sequencing, particularly for TAAD which is caused by numerous genes. Genetic diagnosis of MFS may facilitate periodic surveillance, prophylactic surgical measures and genetic counseling.
Acknowledgements
The present study was supported by the Natural Science Foundation of Jiangsu Province, China (grant no. BK20140103) and the Natural Science Foundation of China (grant no. 81270396).
References
- 1.Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. 2012;33:26–35b. doi: 10.1093/eurheartj/ehr186. [DOI] [PubMed] [Google Scholar]
- 2.Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: Increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 3.Di Eusanio M, Trimarchi S, Patel HJ, Hutchison S, Suzuki T, Peterson MD, Di Bartolomeo R, Folesani G, Pyeritz RE, Braverman AC, et al. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: Observations from the International registry of acute aortic dissection. J Thorac Cardiovasc Surg. 2013;145:385–390.e1. doi: 10.1016/j.jtcvs.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 4.De Backer J, Campens L, De Paepe A. Genes in thoracic aortic aneurysms/dissections - do they matter? Ann Cardiothorac Surg. 2013;2:73–82. doi: 10.3978/j.issn.2225-319X.2012.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campens L, Callewaert B, Muiño Mosquera L, Renard M, Symoens S, De Paepe A, Coucke P, De Backer J. Gene panel sequencing in heritable thoracic aortic disorders and related entities results of comprehensive testing in a cohort of 264 patients. Orphanet J Rare Dis. 2015;10:9. doi: 10.1186/s13023-014-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pyeritz RE. Heritable thoracic aortic disorders. Curr Opin Cardiol. 2014;29:97–102. doi: 10.1097/HCO.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 7.Pannu H, Avidan N, Tran-Fadulu V, Milewicz DM. Genetic basis of thoracic aortic aneurysms and dissections: Potential relevance to abdominal aortic aneurysms. Ann N Y Acad Sci. 2006;1085:242–255. doi: 10.1196/annals.1383.024. [DOI] [PubMed] [Google Scholar]
- 8.Milewicz DM, Chen H, Park ES, Petty EM, Zaghi H, Shashidhar G, Willing M, Patel V. Reduced penetrance and variable expressivity of familial thoracic aortic aneurysms/dissections. Am J Cardiol. 1998;82:474–479. doi: 10.1016/S0002-9149(98)00364-6. [DOI] [PubMed] [Google Scholar]
- 9.Robinson PN, Arteaga-Solis E, Baldock C, Collod-Béroud G, Booms P, De Paepe A, Dietz HC, Guo G, Handford PA, Judge DP, et al. The molecular genetics of Marfan syndrome and related disorders. J Med Genet. 2006;43:769–787. doi: 10.1136/jmg.2005.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judge DP, Dietz HC. Marfan's syndrome. Lancet. 2005;366:1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med. 1972;286:804–808. doi: 10.1056/NEJM197204132861502. [DOI] [PubMed] [Google Scholar]
- 12.Wooderchak-Donahue W, VanSant-Webb C, Tvrdik T, Plant P, Lewis T, Stocks J, Raney JA, Meyers L, Berg A, Rope AF, et al. Clinical utility of a next generation sequencing panel assay for Marfan and Marfan-like syndromes featuring aortopathy. Am J Med Genet A. 2015;167A:1–1757. doi: 10.1002/ajmg.a.37085. [DOI] [PubMed] [Google Scholar]
- 13.Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 14.Rylski B, Hoffmann I, Beyersdorf F, Suedkamp M, Siepe M, Nitsch B, Blettner M, Borger MA, Weigang E. Multicenter Prospective Observational Study: Acute aortic dissection type A: Age-related management and outcomes reported in the German registry for acute aortic dissection type A (GERAADA) of over patients. Ann Surg. 2014;259:598–604. doi: 10.1097/SLA.0b013e3182902cca. [DOI] [PubMed] [Google Scholar]
- 15.den Hartog AW, Franken R, Zwinderman AH, Timmermans J, Scholte AJ, van den Berg MP, de Waard V, Pals G, Mulder BJ, Groenink M. The risk for type B aortic dissection in Marfan syndrome. J Am Coll Cardiol. 2015;65:246–254. doi: 10.1016/j.jacc.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 16.Engelfriet PM, Boersma E, Tijssen JG, Bouma BJ, Mulder BJ. Beyond the root: Dilatation of the distal aorta in Marfan's syndrome. Heart. 2006;92:1238–1243. doi: 10.1136/hrt.2005.081638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeys B, Nuytinck L, Delvaux I, De Bie S, De Paepe A. Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med. 2001;161:2447–2454. doi: 10.1001/archinte.161.20.2447. [DOI] [PubMed] [Google Scholar]
- 18.Poninska JK, Bilinska ZT, Franaszczyk M, Michalak E, Rydzanicz M, Szpakowski E, Pollak A, Milanowska B, Truszkowska G, Chmielewski P, et al. Next-generation sequencing for diagnosis of thoracic aortic aneurysms and dissections: Diagnostic yield, novel mutations and genotype phenotype correlations. J Transl Med. 2016;14:115. doi: 10.1186/s12967-016-0870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziganshin BA, Bailey AE, Coons C, Dykas D, Charilaou P, Tanriverdi LH, Liu L, Tranquilli M, Bale AE, Elefteriades JA. Routine genetic testing for thoracic aortic aneurysm and dissection in a clinical setting. Ann Thorac Surg. 2015;100:1604–1611. doi: 10.1016/j.athoracsur.2015.04.106. [DOI] [PubMed] [Google Scholar]
- 20.Stheneur C, Collod-Béroud G, Faivre L, Buyck JF, Gouya L, Le Parc JM, Moura B, Muti C, Grandchamp B, Sultan G, et al. Identification of the minimal combination of clinical features in probands for efficient mutation detection in the FBN1 gene. Eur J Hum Genet. 2009;17:1121–1128. doi: 10.1038/ejhg.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 22.Brautbar A, LeMaire SA, Franco LM, Coselli JS, Milewicz DM, Belmont JW. FBN1 mutations in patients with descending thoracic aortic dissections. Am J Med Genet A. 2010;152A:1–416. doi: 10.1002/ajmg.a.32856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacini D, Parolari A, Berretta P, Di Bartolomeo R, Alamanni F, Bavaria J. Endovascular treatment for type B dissection in Marfan syndrome: Is it worthwhile? Ann Thorac Surg. 2013;95:737–749. doi: 10.1016/j.athoracsur.2012.09.059. [DOI] [PubMed] [Google Scholar]
- 24.Ptaszek LM, Kim K, Spooner AE, MacGillivray TE, Cambria RP, Lindsay ME, Isselbacher EM. Marfan syndrome is associated with recurrent dissection of the dissected aorta. Ann Thorac Surg. 2015;99:1616–1623. doi: 10.1016/j.athoracsur.2014.12.066. [DOI] [PubMed] [Google Scholar]
- 25.Demers P, Miller DC, Mitchell RS, Kee ST, Sze D, Razavi MK, Dake MD. Midterm results of endovascular repair of descending thoracic aortic aneurysms with first-generation stent grafts. J Thorac Cardiovasc Surg. 2004;127:664–673. doi: 10.1016/j.jtcvs.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 26.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]