Abstract
The effects of microRNA-141 (miR-141) on epithelial-mesenchymal transition (EMT), and ovarian cancer cell migration and invasion were investigated. SKOV3 cells were transfected with the miR-141 mimic (mimic group), inhibitor (inhibitor group) and nonspecific sequences (NC group), and left untransfected group (blank group). The reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to detect the expression of miR-141 in SKOV3 cell lines. Then, mRNA levels and protein expression of EMT markers were determined by RT-qPCR and western blotting, respectively. Cell proliferation was assessed using an MTT assay, followed by analysis of cell invasion and migration. SPSS software was used for statistical analysis. The results demonstrated that miR-141 expression in the mimic group was increased compared with the NC or blank group. Compared with the NC or blank group, upregulation of epithelial-cadherin (E-cadherin) and integrin-β, and downregulation of zinc finger E-box-binding homeobox (ZEB) was observed in the mimic group. The rate of cell proliferation decreased in the mimic group and increased in the inhibitor group when compared with the NC group (P<0.05). The number of invasive cells significantly increased in the inhibitor group and decreased in the mimic group when compared with the NC group (P<0.01). Compared with the NC group, the migratory rate was decreased in the mimic group, and increased in the inhibitor group at 24 and 48 h (all P<0.01). In conclusion, overexpression of miR-141 caused upregulation of E-cadherin, inhibited cell proliferation and EMT, and decreased cell invasion and migration in the SKOV3 cell line.
Keywords: ovarian cancer, microRNA-141, epithelial-mesenchymal transition
Introduction
Ovarian cancer (OC) is a fatal disease in women. According to studies on the global patterns of cancer there are ~152,000 mortalities and 239,000 new OC cases annually (1). The 5-year survival rate of OC following diagnosis is only 20–30% (2). A previous study indicated that the high mortality rate and poor prognosis may be associated with OC cell invasion and metastasis (3).
Epithelial-mesenchymal transition (EMT) is a process by which an epithelial cell loses its cell-cell adhesion, gains the capacity for invasion and migration, and turns into a mesenchymal stem cell (4). EMT is recognized as one of the crucial steps in OC pathogenesis (5), and serves an important role in OC metastasis and invasion (6). Therefore, an improved understanding of the EMT process and cellular influences in OC would supply novel insights for the diagnosis and treatment of OC.
MicroRNAs (miRs) are epigenetic factors that can regulate a number of human biological processes, including cell proliferation, tissue differentiation, cancer formation and metastasis (7–10). Up- and downregulation of miR is involved in the regulation of EMT in OC progression (11,12). miR-141 has been reported to be associated with a number of types of cancer, including colonic cancer (13), gastric cancer (14) and renal cell carcinoma (15). Tamagawa et al (16) reported that miR-141 serves a key role in the regulation of migration and EMT in head and neck squamous cell carcinoma. In addition, upregulation of miR-141 is confirmed to inhibit cell proliferation and invasion by suppressing the Wnt signaling pathway in renal cell carcinoma (17). However, the effects of miR-141 on EMT and human OC migration and invasion remain to be demonstrated.
In the present study, miR-141 expression in SKOV3 cells was measured, in addition to the mRNA and protein levels of EMT markers: Vimentin; epithelial-cadherin (E-cadherin); integrin-β; β-catenin and zinc finger E-box-binding homeobox (ZEB), using the reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting, respectively. Then cell proliferation, invasion and migration assays were performed. The aims of the present study were to determine the effects of miR-141 on EMT, and on OC cell migration and invasion.
Materials and methods
Cell culture
The human OC cell line SKOV3 was obtained from the Shanghai Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai China) was cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin. The cell line was cultured at 37°C with 5% CO2. The experiment was conducted following the protocol approved by Tongji University (Shanghai China).
Cell transfection
SKOV3 cells were seeded in 6-well plates at a density of 4×105 cells/ml, 24 h prior to transfection. When the cells reached 60% confluence (~24 h), the cells were divided into four groups and transfected with 50 nM miR-141 mimic (mimic group, 5′-UAACACUGUCUGGUAAAGAUGG-3′), miR-141 inhibitor (inhibitor group, 5′-CCATCTTTACCAGACAGTGTTA-3′) or miR-141 nonspecific sequences (NC group, 5′-UUCUCCGAACGUGUCACGUTT-3′), or left untransfected (blank group) using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The mimic, inhibitor and NC of miR-141 were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
RNA extraction and RT-qPCR
Cells were harvested 48 h following transfection. RNA was extracted from the cells with TRIzol® reagent and chloroform, according to the manufacturer's protocol (Guangzhou RiboBio Co., Ltd.). The RNA was used as the template for the synthesis of DNA using an RT-PCR kit (Guangzhou RiboBio Co., Ltd.).
Analysis of the miR-141 expression level in the transfected OC cell line SKOV3 was performed using the Bulge-Loop™ miR RT-qPCR kits (miRQ0000432-1-1; Guangzhou RiboBio Co., Ltd.), according to the manufacturer's protocol, and U6 (MQP-0201; Guangzhou RiboBio Co., Ltd.) was measured as endogenous control to perform relative quantification. qPCR was carried out at 95°C for 10 min, followed by 40 cycles at 95°C for 10 sec, 60°C for 30 sec and 72°C for 1 min.
In order to evaluate the effects of miR-141 on EMT, qPCR assays were performed using SYBR® Green (Invitrogen; Thermo Fisher Scientific, Inc.) for the expression of vimentin (forward primer, 5′-AAGGAGGAAATGGCTCGTCAC-3′; reverse primer, 5′-CTCAGGTTCAGGGAGGAAAAGT-3′), E-cadherin (forward primer, 5′-GTCACTGACACCAACGATAATCCT-3′; reverse primer, 5′-TTTCAGTGTGGTGATTACGACGTTA-3′), integrin-β (forward primer, 5′-AATGTAACCAACCGTAGC-3′; reverse primer, 5′-GGTCAATGGGATAGTCTTC-3′), β-catenin (forward primer, 5′-GGGCGGCACCTTCCTACTTC-3′; reverse primer, 5′-AGCTCCCTCGCGGTTCAT-3′) and ZEB (forward primer, 5′-AAGTGGGCGGTAGATGGTA-3′; reverse primer, 5′-TTGTAGCGACTGGATTTT-3′). GAPDH was regarded as an internal control gene. The method of quantification was 2−ΔΔCq (18). The PCR primers (Table I) were designed by Primer Premier version 5 (Premier Biosoft International, Palo Alto, CA, USA). The reaction was performed at 95°C for 10 min, followed by 40 cycles at 95°C for 10 sec, 60°C for 30 sec, and 72°C for 1 min. Amplified products were checked using an Applied Biosystems 7300 Sequence Detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Table I.
Polymerase chain reaction primer sequences.
| Primer sequence (5′-3′) | |||
|---|---|---|---|
| Gene name | Forward | Reverse | Tm,°C |
| Vimentin | AAGGAGGAAATGGCTCGTCAC | CTCAGGTTCAGGGAGGAAAAGT | 60 |
| E-cadherin | GTCACTGACACCAACGATAATCCT | TTTCAGTGTGGTGATTACGACGTTA | 60 |
| Integrin-β | AATGTAACCAACCGTAGC | GGTCAATGGGATAGTCTTC | 52 |
| β-catenin | GGGCGGCACCTTCCTACTTC | AGCTCCCTCGCGGTTCAT | 61 |
| ZEB | AAGTGGGCGGTAGATGGTA | TTGTAGCGACTGGATTTT | 60 |
E-cadherin, epithelial-cadherin; ZEB, zinc finger E-box-binding homeobox; Tm, melting temperature.
Protein extraction and western blot analysis
The cellular proteins vimentin, β-catenin, integrin-β, E-cadherin and ZEB were extracted for western blotting as previously described (19). BCA protein assay kit (Thermo Scientific, USA) was used to determine the protein concentration, 100 µg proteins were separated on 10% SDS-PAGE for every cell line, and the gel was subsequently transferred onto a nitrocellulose (NC) filter. Then, the NC filter was immersed in the blocking buffer (Sigma, USA) and agitated for 1–3 h at room temperature. Specific proteins were detected with monoclonal mouse anti-vimentin (MAB2105), mouse anti-β-catenin (MAB13291), mouse anti-integrin-β (MAB1778), mouse anti-E-cadherin (MAB1838) and mouse anti-ZEB antibody (MAB6708; all from R&D Systems, Inc., Minneapolis, MN, USA) for the primary antibodies (1:100 dilution), agitated for 1–3 h at room temperature or overnight at 4°C, and then were washed with TBST 3 times. The secondary antibodies used were goat anti-mouse antibodies (sc-2005; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and anti-β actin (4967; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), shaking 1–3 h at room temperature. Then, they were washed with TBST 3 times. Protein bands on the membranes were visualized by chemiluminescence (ECL) reagent (WBKLS0500; Merck KGaA, Darmstadt, Germany) and imaged with ImageQuant LAS4000 mini analysis system (GE Healthcare Life Sciences, Little Chalfont, UK).
MTT assay
SKOV3 cells were seeded into a 96-well plate at a density of 5×104 cells/well and transfected as described above. At 48 h following transfection, 10 µl MTT solution (5 mg/ml, Sigma-Aldrich; Merck KGaA) was added to each well and incubated at 37°C for 4 h. Then, 100 µl solubilization solution [50% dimethylsulfoxide and 20% SDS (pH 4.8)] was added to each well followed by agitation for 10 min. The absorption values were measured at 570 nm.
Invasion assay
Invasion assays were performed in triplicate using polycarbonate membranes (8-µm pore size) in 6-well tissue culture plates, which were coated with Matrigel® (BD Biosciences, Franklin Lakes, NJ, USA) and were cultured for 48 h. SKOV3 cells (3×104/chamber) of the different groups and DMEM containing 10% fetal calf serum (Hangzhou Sijiqing Biological Engineering Materials Co., Ltd., Zhejiang, China) was added to the upper and to the lower chambers, respectively. Following incubation for 24 h, cells on the top filter were scrapped off, rinsed into the lower chamber tissue culture, fixed in 4% paraformaldehyde for 15 min at room temperature, washed with PBS twice and stained with 0.1% crystal violet for 20 min at room temperature. A total of five random fields was calculated.
Scratch migration assay
SKOV3 cells were transfected for 24 h then a scratch (repeated six times) in the cell monolayer was made with a cell scratch spatula. Cells were washed three times with PBS and cultured under standard conditions for 72 h. Following discarding of the supernatant, the cells were fixed in 4% paraformaldehyde for 30 min, and images were captured at 0, 24, 48, and 72 h, under an optical microscope. The potential of migration was calculated by counting the number of cells that migrated from the wound edge.
Statistical analysis
Data were presented as the mean ± standard deviation. The statistical significance of differences between groups was determined using one-way analysis of variance followed by a Fisher's least significant difference post hoc test. Statistical analysis was performed with SPSS software (version 20.0; IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
miR-141 overexpression in SKOV3 cells
Following the detection of miR-141 expression in SKOV3 cell by RT-qPCR, it was demonstrated that miR-141 levels in the mimic group (3.72±0.16) were increased compared with the blank (1.08±0.06) and NC groups (1.07±0.05; Fig. 1). In addition, the miR-141 level in the inhibitor group (0.59±0.02) was significantly decreased compared with the blank or NC groups.
Figure 1.
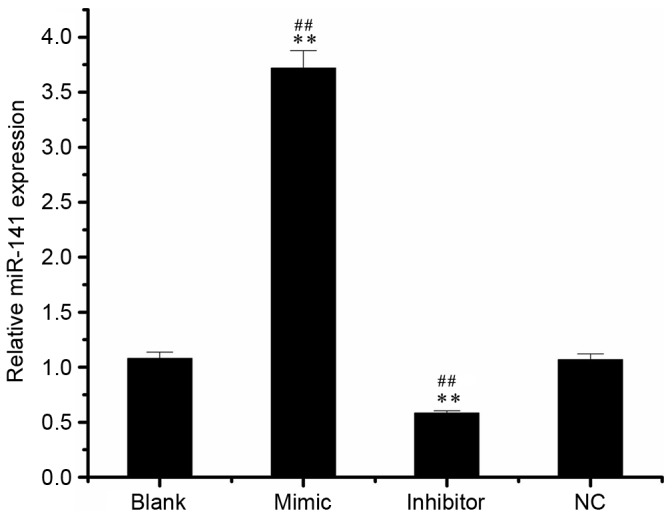
miR-141 expression in different groups of transfected-SKOV3 cells. SKOV3 cells were transfected with 50 nM miR-141 mimic, inhibitor or NC, or left untransfected. **P<0.01 vs. NC group, ##P<0.01 vs. blank group. NC, nonspecific sequences; miR, microRNA.
Expression of EMT markers in different groups
To investigate the effect of miR-141 on EMT, mRNA and protein levels of EMT markers were detected in the mimic, inhibitor, NC and blank groups. As exhibited in Fig. 2A, the mRNA expression levels of E-cadherin and integrin-β were increased in the mimic group and decreased in the inhibitor group compared with the NC or blank groups. Compared with the NC or blank groups, the mRNA level of ZEB was decreased in the mimic group and increased in the inhibitor group. In addition, the mRNA level of vimentin was increased in the mimic and inhibitor groups compared with the NC or blank groups. However, no significant differences were identified in the expression of β-catenin between mimic and blank or NC groups.
Figure 2.
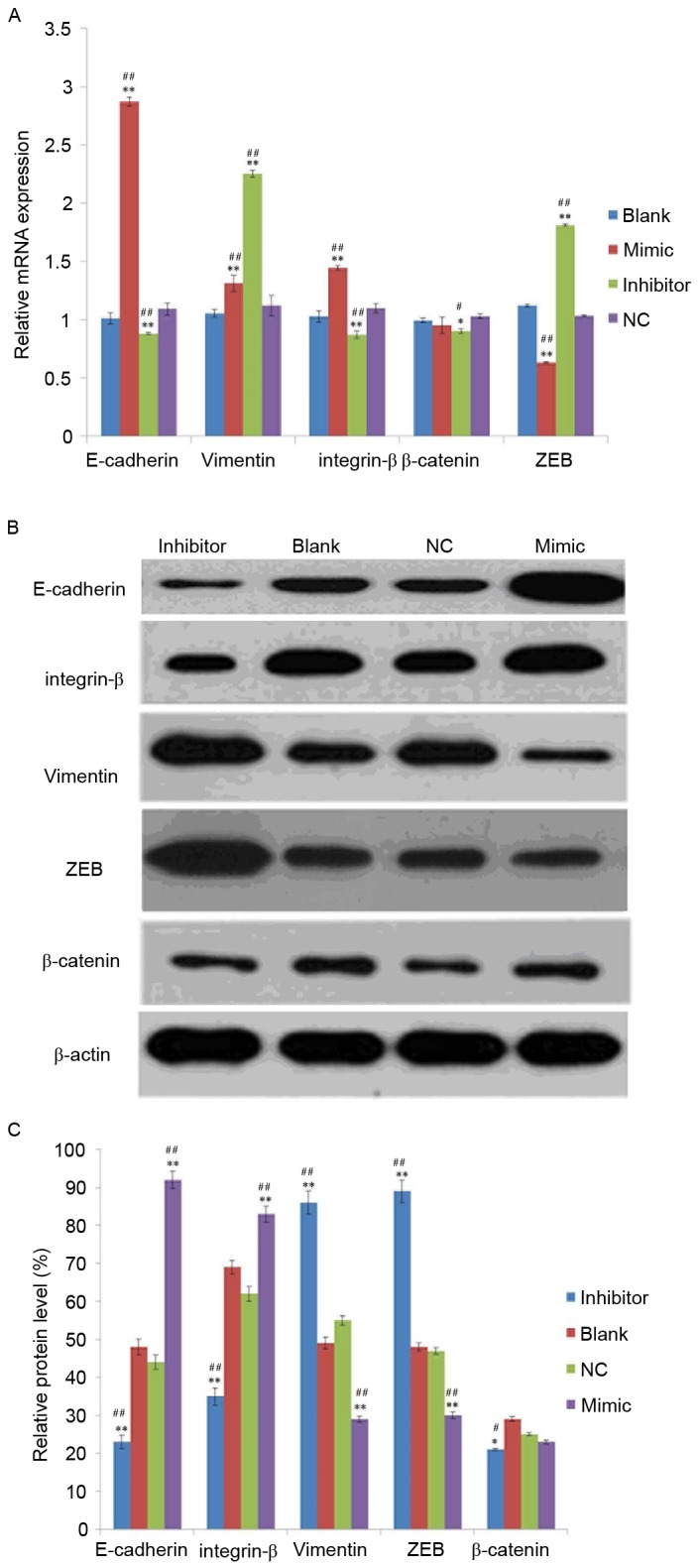
Analysis of epithelial mesenchymal transition-marker expression in SKOV3 cells. (A) mRNA and (B) protein expression of vimentin, E-cadherin, integrin-β, β-catenin and ZEB in transfected-SKOV3 cells. (C) The relative protein level was normalized using β-actin. SKOV3 cells were transfected with 50 nM microRNA-141 mimic group, inhibitor group or NC, or left untransfected. *P<0.05, **P<0.01 vs. NC group, #P<0.05, ##P<0.01 vs. blank group. E-cadherin, epithelial-cadherin; ZEB, zinc finger E-box-binding homeobox; NC, nonspecific sequences.
Following analysis of the protein expression of vimentin, β-catenin, integrin-β, E-cadherin and ZEB using western blotting, it was demonstrated that all were expressed in the SKOV3 transfected cells (Fig. 2B) and their relative protein expression exhibited a similar tend to the mRNA expression (Fig. 2C).
MTT assay
Cell proliferation was assessed using the MTT assay and the value of optical density at 570 nm (OD570) in the four groups is exhibited in Fig. 3. The rate of cell proliferation was increased in the inhibitor group (1.10±0.13) and decreased in the mimic group (0.80±0.07) compared with the NC group (0.92±0.05; both P<0.05).
Figure 3.
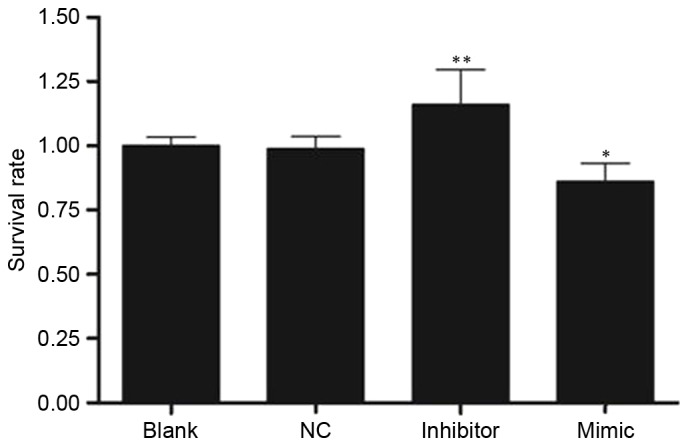
Value of optical density at 570 nm in four groups determined using an MTT assay 24 h following transfection. SKOV3 cells were transfected with 50 nM microRNA-141 mimic, inhibitor or, NC, or left untransfected. *P<0.05, **P<0.01 vs. NC group. NC, nonspecific sequences; blank, without transfection.
miR-141 inhibits the invasion and migration of SKOV3 cells in vitro
Following analysis of the effect of miR-141 on SKOV3 cell invasion, the invasive cells were visualized in the mimic, inhibitor, NC and blank groups (Fig. 4A). Compared with the NC group (137.67±4.04), the number of invaded cells was significantly increased in the inhibitor group (243.00±6.24) and decreased in the mimic group (76.33±2.08; both P<0.01; Fig. 4B).
Figure 4.
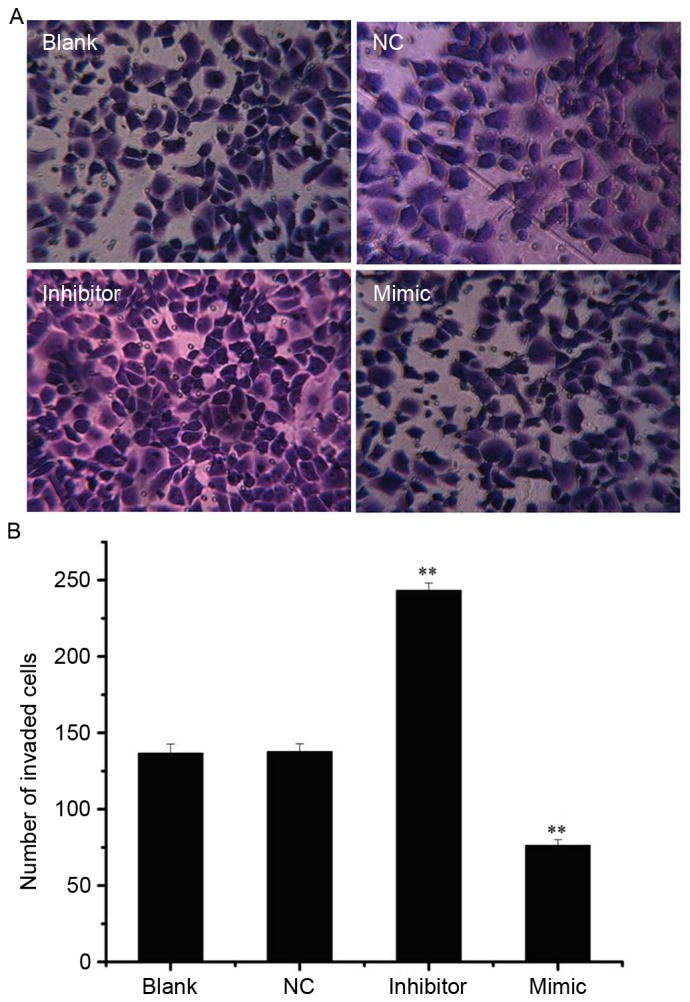
miR-141 regulates cell invasion in SKOV3 cells. (A) SKOV3 cells that invaded through Matrigel-coated invasion chambers following transfection with 50 nM miR-141 mimic, inhibitor or NC, or left untransfected (magnification, ×200). (B) The number of invasive cells. **P<0.01 vs. NC group. miR, microRNA; NC, nonspecific sequences.
In addition, the effect of miR-141 on SKOV3 cell migration was observed at 0, 24 and 48 h, respectively (Fig. 5A). The migratory rate was decreased in the mimic group and increased in the inhibitor group at 24 and 48 h compared with the NC group (all P<0.01; Fig. 5B).
Figure 5.
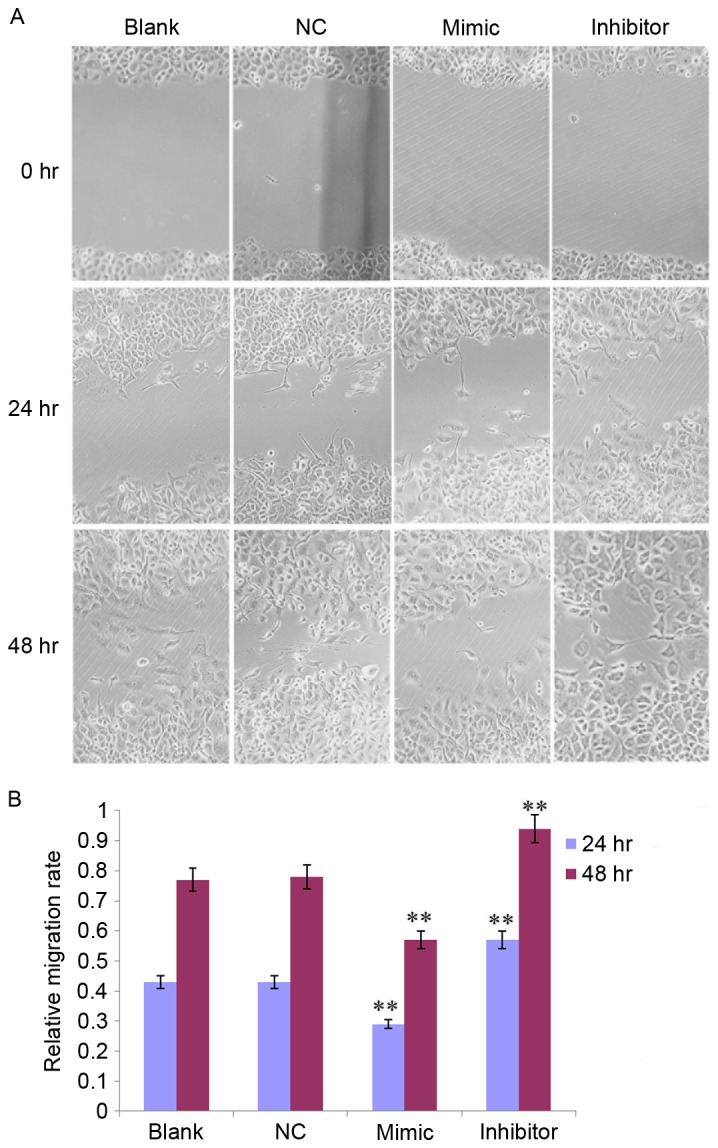
miR-141 regulates cell migration in SKOV3 cells at 0, 24 and 48 h. (A) SKOV3 cells that migrated from the wound edge following transfection with 50 nM miR-141 mimic, inhibitor or NC, or left untransfected (magnification, ×200). (B) The migratory rate of SKOV3 cells. **P<0.01 vs. NC group. NC, nonspecific sequences; miR, microRNA.
Discussion
In the present study, miR-141 expression was investigated in the OC cell line SKOV3 following transfection with an miR-141 mimic, inhibitor or NC, or no transfection. Overexpression of miR-141 caused upregulation of E-cadherin, inhibited cell proliferation and EMT in the SKOV3 cell line, and decreased cell invasion and migration.
Cancer progression has similarities with the developmental process of EMT (20). EMT results in the morphological alteration of an epithelial cell to a mesenchymal cell, and enhances the ability of cancer cells to metastasize and invade (21). The molecular characteristics of the alteration of epithelial cells to mesenchymal cells are the downregulation of epithelial cell markers, including E-cadherin, β-catenin, integrin-β, cytokeratin and mucin expression, as well as upregulation of mesenchymal markers, including vimentin and fibronectin, followed by alterations of epithelial mesenchymal transition-associated transcription factor expression (22–24). Among them, E-cadherin is an important molecule in the maintenance of the epithelial phenotype, and its decreased expression is an important marker of EMT. ZEB1 and ZEB2, two members of the ZEB family, are important regulators of EMTs, and can bind to the enhancer box motif E2 [CACCT (G)] on the E-cadherin promoter, inhibit transcription of E-cadherin, induce EMT, and enhance cell invasion and metastasis (25,26). As a subunit of integrin-β, integrin-β5 adhesion serves an important role in transforming growth factor-β-induced EMT (27). Vimentin is used as a marker of EMT. A previous study reported that vimentin is essential for alterations in cell shape, adhesion and motility during EMT (28).
In the present study, E-cadherin and integrin-β mRNA expression was increased in the mimic group and decreased in the inhibitor group compared with the NC group. In addition, ZEB was decreased in the mimic group and increased in the inhibitor group, and vimentin was increased in the mimic and inhibitor group compared with the NC or blank groups. A previous study demonstrated that the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141 and miR-29) is involved in the regulation of the EMT process, and negatively correlated with the expression of ZEB1 and ZEB2 (29). Neves et al (30) suggested that the EMT process was accompanied by DNA hypermethylation and transcriptional silencing of the miR-200c/141 promoter. Wellner et al (31) indicated that miR-200 family members, including miR-141, induce epithelial differentiation, thereby suppressing EMT by inhibiting translation of mRNA for the EMT-activators ZEB1 and ZEB2. In addition, the expression of endogenous miR-200 in normal and lung cancer cells directly acts on ZEB1 and Mothers against decapentaplegic homolog 3 interacting protein 1 mRNA, promotes E-cadherin expression and inhibition of EMT in cancer cells, and reduces the incidence of invasion in breast cancer cells (32). In addition, increased miR-141 levels increased expression of E-cadherin and reduced EMT, which agreed with a previous study reported by Tamagawa et al (16) that suggested that overexpression of miR-141 reduced the cell capacity of migration in head and neck squamous cell carcinoma through regulation of EMT. Therefore, these results suggested that miR-141 may inhibit EMT in the SKOV3 OC cell line.
As a member of miR-200 family, miR-141 overexpression has been reported to inhibit invasion and migration in a number of types of cancer, including colorectal cancer, breast cancer and pancreatic cancer (30,33,34). When miR-141 was downregulated, cancer cell migration was induced via targeting E-cadherin transcriptional repressor genes (35), which improved tumor motility and increased carcinogenicity. Consistent with previous studies, in the present study, it was demonstrated that the number of invasive cells was increased in the inhibitor group and decreased in the mimic group compared with the NC group. In addition, the migratory rate was decreased in the mimic group and increased in the inhibitor group, at 24 and 48 h compared with the NC group. These data suggest that miR-141 can inhibit invasion and migration in SKOV3 cells in vitro.
In the present study, overexpression of miR-141 in the OC cell line SKOV3 significantly inhibited cell proliferation. As a member of miR-200, miR-141 has been demonstrated to be decreased and served as a tumor suppressor in numerous cancer types (36). miR-141 has been reported to be downregulated in childhood renal neoplasms (37). Poell et al (38) demonstrated that miR-141 could inhibit the proliferation of melanoma cells. In addition, miR-141 was decreased in gastric cancer and involved in gastric cancer cell growth (14). Van Jaarsveld et al (39) identified that miR-141 could promote cisplatin sensitivity in OC cells by targeting kelch-like ECH-associated protein 1. In the present study, the MTT assay results demonstrated that the inhibitor group exhibited an increased OD570 value compared with the blank or NC groups, suggesting that overexpression of miR-141 could decrease proliferation of SKOV3 cells.
Certain limitations remain in the present study. For example, the effects of miR-141 on OC are long and complex, and cell cycle progression and apoptosis were not included in the present study. Therefore, additional studies are required to confirm the results of the present study and the long-term impact of miR-141 on OC development should be further investigated.
In conclusion, the present study revealed that overexpression of miR-141 caused upregulation of E-cadherin, inhibited cell proliferation and EMT in the SKOV3 cell line, and decreased cell invasion and migration. The results of the present study provide novel insight into the functional mechanism of OC progression and suggest that miR-141 represents a novel molecular target for OC therapy.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Holschneider CH, Berek JS. Ovarian cancer: Epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::AID-SSU2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 6.Vergara D, Merlot B, Lucot JP, Collinet P, Vinatier D, Fournier I, Salzet M. Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett. 2010;291:59–66. doi: 10.1016/j.canlet.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Ju JA, Huang YC, Lan SH, Wang TH, Lin PC, Lee JC, Niu KC, Tian YF, Liu HS. Identification of colorectal cancer recurrence-related microRNAs. Gen Med Biomark Health Sci. 2012;4:19–20. [Google Scholar]
- 8.Blenkiron C, Miska EA. miRNAs in cancer: Approaches, aetiology, diagnostics and therapy. Hum Mol Genet. 2007;16:R106–R113. doi: 10.1093/hmg/ddm056. [DOI] [PubMed] [Google Scholar]
- 9.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 10.Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Wang L, Matyunina LV, Hill CG, McDonald JF. Overexpression of miR-429 induces mesenchymal-to-epithelial transition (MET) in metastatic ovarian cancer cells. Gynecol Oncol. 2011;121:200–205. doi: 10.1016/j.ygyno.2010.12.339. [DOI] [PubMed] [Google Scholar]
- 12.Bendoraite A, Knouf EC, Garg KS, Parkin RK, Kroh EM, O'Briant KC, Ventura AP, Godwin AK, Karlan BY, Drescher CW, et al. Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: Evidence supporting a mesothelial-to-epithelial transition. Gynecol Oncol. 2010;116:117–125. doi: 10.1016/j.ygyno.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng H, Zhang L, Cogdell DE, Zheng H, Schetter AJ, Nykter M, Harris CC, Chen K, Hamilton SR, Zhang W. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One. 2011;6:e17745. doi: 10.1371/journal.pone.0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura M, Watanabe M, Nakajima A, Kurai D, Ishii H, Takata S, Nakamoto K, Sohara E, Honda K, Nakamura M, et al. Serial quantification of procalcitonin (PCT) predicts clinical outcome and prognosis in patients with community-acquired pneumonia (CAP) J Infect Chemother. 2014;20:97–103. doi: 10.1016/j.jiac.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Nakada C, Matsuura K, Tsukamoto Y, Tanigawa M, Yoshimoto T, Narimatsu T, Nguyen LT, Hijiya N, Uchida T, Sato F, et al. Genome-wide microRNA expression profiling in renal cell carcinoma: Significant down-regulation of miR-141 and miR-200c. J Pathol. 2008;216:418–427. doi: 10.1002/path.2437. [DOI] [PubMed] [Google Scholar]
- 16.Tamagawa S, Beder LB, Hotomi M, Gunduz M, Yata K, Grenman R, Yamanaka N. Role of miR-200c/miR-141 in the regulation of epithelial-mesenchymal transition and migration in head and neck squamous cell carcinoma. Int J Mol Med. 2014;33:879–886. doi: 10.3892/ijmm.2014.1625. [DOI] [PubMed] [Google Scholar]
- 17.Yamamura S, Sharanjot S, Shahana M, Hirata H, Ueno K, Chang I, Chiyomaru T, Tanaka Y, Dahiya R. MicroRNA-141 inhibits proliferation and invasion by suppressing the Wnt signaling pathway in renal cell carcinoma. Cancer Res. 2012;72:3149. doi: 10.1158/1538-7445.AM2012-3149. [DOI] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Gong Z, Shi Y, Zhu Z, Li X, Ye Y, Zhang J, Li A, Li G, Zhou J. JWA deficiency suppresses dimethylbenz[a]anthracene-phorbol ester induced skin papillomas via inactivation of MAPK pathway in mice. PLoS One. 2012;7:e34154. doi: 10.1371/journal.pone.0034154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savagner P. Leaving the neighborhood: Molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Pinera P, Alcantara S, Dimitrov T, Vega JA, Deuel TF. Pleiotrophin disrupts calcium-dependent homophilic cell-cell adhesion and initiates an epithelial-mesenchymal transition; Proc Natl Acad Sci USA; 2006; pp. 17795–17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 23.de Herreros AG, Peiró S, Nassour M, Savagner P. Snail family regulation and epithelial mesenchymal transitions in breast cancer progression. J Mammary Gland Biol Neoplasia. 2010;15:135–147. doi: 10.1007/s10911-010-9179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang SY, Miah A, Pabari A, Winslet M. Growth Factors and their receptors in cancer metastases. Front Biosci (Landmark Ed) 2011;16:531–538. doi: 10.2741/3703. [DOI] [PubMed] [Google Scholar]
- 25.Dave N, Guaita-Esteruelas S, Gutarra S, Frias À, Beltran M, Peiró S, de Herreros AG. Functional cooperation between Snail1 and twist in the regulation of ZEB1 expression during epithelial to mesenchymal transition. J Biol Chem. 2011;286:12024–12032. doi: 10.1074/jbc.M110.168625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, Ding Q, Sakoda M, Iino S, Ishigami S, et al. Epithelial-mesenchymal transition and mesenchymal-epithelial transition via regulation of ZEB-1 and ZEB-2 expression in pancreatic cancer. J Surg Oncol. 2012;105:655–661. doi: 10.1002/jso.23020. [DOI] [PubMed] [Google Scholar]
- 27.Bianchi A, Gervasi ME, Bakin A. Role of β5-integrin in epithelial-mesenchymal transition in response to TGF-β. Cell Cycle. 2010;9:1647–1659. doi: 10.4161/cc.9.8.11517. [DOI] [PubMed] [Google Scholar]
- 28.Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neves R, Scheel C, Weinhold S, Honisch E, Iwaniuk KM, Trompeter HI, Niederacher D, Wernet P, Santourlidis S, Uhrberg M. Role of DNA methylation in miR-200c/141 cluster silencing in invasive breast cancer cells. BMC research notes. 2010;3:219. doi: 10.1186/1756-0500-3-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, Hausen AZ, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 32.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Zhao Y, Cao C, Hao R, Li C, Yi Y, Gao S, Hui L, Liang A. Material and mechanisms induced pseudo allergic reactions of Yuxingcao injection. Zhongguo Zhong Yao Za Zhi. 2010;35:1603–1606. doi: 10.4268/cjcmm20101222. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 34.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshino H, Enokida H, Itesako T, Tatarano S, Kinoshita T, Fuse M, Kojima S, Nakagawa M, Seki N. Epithelial-mesenchymal transition-related microRNA-200s regulate molecular targets and pathways in renal cell carcinoma. J Hum Genet. 2013;58:508–516. doi: 10.1038/jhg.2013.31. [DOI] [PubMed] [Google Scholar]
- 37.Senanayake U, Das S, Vesely P, Alzoughbi W, Fröhlich LF, Chowdhury P, Leuschner I, Hoefler G, Guertl B. miR-192, miR-194, miR-215, miR-200c and miR-141 are downregulated and their common target ACVR2B is strongly expressed in renal childhood neoplasms. Carcinogenesis. 2012;33:1014–1021. doi: 10.1093/carcin/bgs126. [DOI] [PubMed] [Google Scholar]
- 38.Poell JB, van Haastert RJ, de Gunst T, Schultz IJ, Gommans WM, Verheul M, Cerisoli F, van Noort PI, Prevost GP, Schaapveld RQ, Cuppen E. A functional screen identifies specific microRNAs capable of inhibiting human melanoma cell viability. PLoS One. 2012;7:e43569. doi: 10.1371/journal.pone.0043569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Jaarsveld MT, Helleman J, Boersma AW, van Kuijk PF, van Ijcken WF, Despierre E, Vergote I, Mathijssen RH, Berns EM, Verweij J, et al. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene. 2013;32:4284–4293. doi: 10.1038/onc.2012.433. [DOI] [PubMed] [Google Scholar]


