Abstract
Hypoxic pulmonary hypertension (HPH) may contribute to vascular remodeling, and pulmonary artery smooth muscle cell (PASMC) proliferation has an important role in this process. However, no relevant information concerning the role and mechanism of protein kinase C (PKC)α in hypoxia-induced rat PASMC proliferation has been elucidated. The present study aimed to further investigate this by comparison of rat PASMC proliferation among normoxia for 72 h (21% O2), hypoxia for 72 h (3% O2), hypoxia + promoter 12-myristate 13-acetate control, hypoxia + safingol control, hypoxia + PD98059 control and hypoxia + U0126 control groups. The present study demonstrated that protein expression levels of PKCα in rat PASMCs were elevated. In conclusion, through activating the extracellular signal-regulated 1/2 signaling pathway, PKCα is involved in and initiates PASMC proliferation, thus bringing about pulmonary artery hypertension. These results add to the understanding of the mechanism PKCα in PH formation and lays a theoretical basis for prevention as well as treatment of HPH.
Keywords: hypoxia-induced factor, pulmonary hypertension, protein kinase Cα, extracellular signal-regulated kinase1/2, pulmonary artery smooth muscle cell
Introduction
Pulmonary hypertension (PH) is a pathophysiological syndrome caused by heterogenous diseases and diverse pathogenesis, with the dominant feature of continuous increase of pulmonary vascular resistance. Characterized by its clinical manifestations of increased load behind right ventricle, decreased activity endurance and even death out of heart failure, PH is a severe chronic pulmonary circulatory illness which could contribute to potential fatality. Provided that no effective treatment is conducted, the prognosis for majority of PH patients will be extremely poor, with ~15% mortality rate within 1 year on modern therapy (1–4).
Hypoxic pulmonary hypertension (HPH) is a clinically common type of PH, with frequent occurrence in various chronic pulmonary ailments. With interaction of long-term anoxia, chronic inflammation, a variety of active vascular substances and growth factors, the structure of pulmonary vessels may undergo transformation, resulting in vascular remodeling. On the other hand, remodeling of pulmonary arterial smooth muscle cells (PASMCs) is a key feature known to result from an imbalance between apoptosis and cell proliferation (5).
As a member of the family of serine/threonine protein kinases, protein kinase C (PKC) was first discovered by Nishizuka in 1997 (6). So far, at least 11 subtypes of PKC have been isolated and purified from different species and genuses of tissue organs (7). According to dissimilarity of molecular structure and sensitivity to activators, PKC subtypes could be grouped into three categories involving the conventional (cPKCα, βI, βII and γ), the novel (nPKCδ, ε, η and θ) as well as the atypical (aPKCλ/ι and ζ). The classical PKCs are diacylglycerol (DAG) and Ca2+-dependent enzymes; whereas, the novel PKCs require DAG, but not calcium, for activation. The atypical are not responsive to activation by DAG or calcium, but are activated by other lipid-derived second messengers (8).
PKC may exist in almost all the tissue cells, including PASMCs (9,10). In addition, PKC not only participates in the process of cell growth, differentiation, apoptosis and contraction of smooth muscle cells (SMCs), but also serves a significant part in signal transduction pathways mediated by hormones, neurotransmitters, growth factors and antigens (11,12). PKC has been reported to act as a signaling mediator in hypoxia-induced cell proliferation (13). Recently, PKCα has been demonstrated to serve a vital role in proliferation of diverse cells, including PASMCs (14–17). An study investigating ox pulmonary arterial smooth muscle cells demonstrated that PKC initiates and promotes PASMC proliferation (14). In a study of rat thoracic aortic smooth muscle cells, however, Sasaguri et al (18) revealed that PKC activation inhibits SMC proliferation. These studies suggested that PKC and its mediated cell signaling pathways may occupy an important position in SMC proliferation, but with inconsistent and conflicting findings.
To investigate hypoxia-induced PASMC proliferation, the present study aimed to establish an external model of hypoxic pulmonary hypertension and to observe the change and underlying molecular mechanism of PKCα expression in hypoxia-induced rat PASMCs, as well as its impact upon PASMC proliferation. The present study may further uncover the molecular mechanism of PH pulmonary vascular remodeling, providing a theoretical basis for its prevention and treatment.
Materials and methods
Animals and agents
A total of 20 adult rats (age, 8 weeks; weight, ~200 g) purchased from the Experimental Animal Center of Shanxi Medical University (Taiyuan, China) were maintained in a temperature-(22°C) and humidity (between 60 and 65%)-controlled room on a 12-h light/dark cycle with free access to food and water for 1 week prior to use. All procedures were approved by the Animal Management Guidelines of the Ministry of Health of the People's Republic of China, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Dulbecco's modified Eagle's medium (DMEM) and 20% fetal bovine serum (FBS) were obtained from Hyclone; GE Healthcare Life Sciences (Logan, UT, USA). Monoclonal antibodies against ERK (cat. no. 9102) and phosphorylated (p)-ERK (cat. no. 9101) were from Cell Signaling Technology, Inc. (Beverly, MA, USA). Polyclonal antibodies against smoothlin (cat. no. sc-20481), PKCα (cat. no. sc-208) were from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Polyclonal antibody against β-actin (cat. no. AP0060) was from Bioworld Technology, Co., Ltd. (Nanjing, China). Polyclonal antibodies against smooth muscle myosin heavy chain (cat. no. ab53219) were obtained from Abcam (Cambridge, MA, USA).
Isolation and culture of PASMCs
Rat PASMCs were isolated and cultured in accordance with previously described methods (19). Rats were anaesthetized by intraperitoneal injection of pentobarbital sodium (Sinopharm Chemical Reagent Co., Ltd., Beijing, China; 50 mg/kg body weight), then the main trunk of pulmonary arteries and the right and left branches were isolated under a dissecting light microscope (Olympus Corporation, Tokyo, Japan). After connective tissues of arteries were cleaned and vessels cut open longitudinally, luminal endothelia were removed by gentle scraping with cotton swabs. The isolated pulmonary arteries were dissected into small pieces of 1×1 mm, maintained in DMEM supplemented with 20% FBS and incubated in a humidified atmosphere with 5% CO2 at 37°C. Culture medium was changed twice per week and cells were harvested with trypsin (0.25%; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing EDTA. Passages ranging from 4 to 6 were used for all experiments, which were divided into three groups: Normoxia, hypoxia and control. In the normoxia group, PASMCs were placed at 37°C in a humidified atmosphere containing 5% CO2. In the hypoxia group, PASMCs were placed into three-gas chambers containing 3% O2, 5% CO2 and 92% N2 for 24, 48 and 72 h, respectively. In the control group, cells were pre-treated with drugs (12-myristate 13-acetate, safingol, PD98059 and U0126) and placed into three-gas chambers containing 3% O2, 5% CO2 and 92% N2 for 72 h. Prior to exposure to hypoxia or treatment, cells were incubated in DMEM with free FBS for 24 h and then exposed to hypoxia or treated in DMEM supplemented with 2% FBS.
Immunofluorescence staining of PASMCs
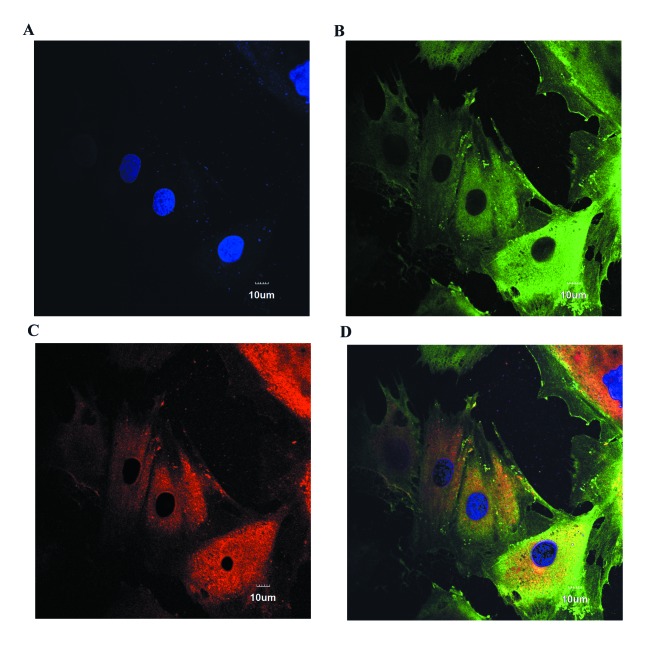
PASMCs were plated in glass chambers, fixed with 4% paraformaldehyde for 10 min, and added to 0.2% permeable Triton X-100 for 15 min. Following three washes in PBS, cells were blocked with goat serum (Solarbio, Beijing, China) for 1 h, followed by incubation with anti-smoothlin (1:100) and anti-smooth muscle heavy chain (1:80) primary antibodies overnight at 4°C. Cells were then washed with PBS three times, followed by incubation with fluorescein isothiocyanate-conjugated secondary antibody (cat. no. A16000; dilution, 1:500; Thermo Fisher Scientific, Inc.) and tetramethylrhodamine-conjugated secondary antibody (cat. no. A16040; dilution, 1:500; Thermo Fisher Scientific, Inc.) for 1 h in the dark. Following three washes with PBS, nuclei were stained with DAPI for 10 min and observed using confocal laser scanning microscope. All the aforementioned procedures were performed at room temperature.
Cell proliferation assay
PASMCs at a density of 5×103 cells were plated into 96-well glass chamber plates. Cell proliferation in the normoxia, hypoxia and control groups were measured. MTT (20 µl; 5 mg/ml) was added to each well and the slides were incubated in a humidified incubator with 5% CO2 at 37°C for 4 h. At the end of incubation, the supernatant was removed and dimethyl sulfoxide (DMSO; 150 µl/well) was added to the plates to solubilize for 10 min. The optical densities were determined using a multi-well scanning spectrophotometer (Spectra Max Plus 384, Molecular Devices, LLC, Sunnyvale, CA, USA) at 490 nm.
Western blot analysis
After various treatments, cells were washed three times in cold PBS, harvested and scraped with cell lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) for 2 h at 4°C. The lysates were then centrifuged at 13,000 × g for 20 min at 4°C, the supernatants collected and the total protein concentrations were determined by the Bicinchoninic Acid Protein Assay kit (Beyotime Institute of Biotechnology, Shanghai, China) following the manufacturer's protocol. Equal amounts of proteins (30 µg) were separated by 10% SDS-PAGE, and then transferred to polyvinylidene difluoride membranes. After blocking with 5% bull serum albumin (Solarbio) at room temperature for 1 h, membranes were incubated overnight at 4°C with antibodies specific for rabbit anti-p-ERK1/2 (1:1,000), rabbit anti-ERK (1:1,000), rabbit anti-PKCα (1:1,000) and rabbit anti-β-actin (1:2,000). Subsequently, the membranes were incubated with a goat anti-rabbit peroxidase-conjugated IgG secondary antibody (cat. no. ZB-2301; dilution, 1:5,000; ZSGB-BIO, Beijing, China) for 1 h at room temperature. The expressed protein amount was determined through p-ERK1/2/ERK1/2 for ERK1/2 and PKCα/β-actin for PKCα. Proteins were visualized using enhanced chemiluminescence (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Proteins were quantified using a Gel Doc XR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using Quantity One software (version 4.62; Bio-Rad Laboratories, Inc.).
Drugs
The following drugs were used for the hypoxia treated control cells. Prior to hypoxic treatment, cells were treated with the PKCα promoter 12-myristate 13-acetate (PMA; 0.4 mM; Alomone Labs, Israel), the PKCα special inhibitor safingol (0.6 mM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), the extracellular signal-regulated kinase kinase (MEK1/2) inhibitor PD98059 (20 mM, Cell Signaling Technology, Inc.) or U0126 (5 mM, Cell Signaling Technology, Inc.) and incubated in a humidified atmosphere with 5% CO2 at 37°C for 30 min one time.
Statistical analysis
Data are expressed as mean ± standard deviation. Comparisons between two groups was made with an unpaired two-tailed Student's t-test. Comparisons between multiple groups was made with a one-way analysis of variance followed by Dunnett or Tukey test. SPSS software, version 20.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Activation of hypoxia on PASMC proliferation
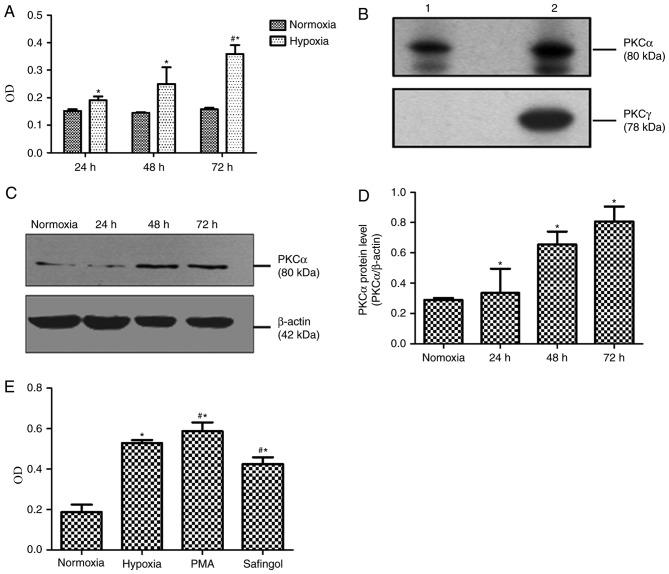
Immunofluorescence staining determined that PASMCs stain positively for smooth muscle markers (Fig. 1). MTT demonstrated that the optical densities (ODs) of cells exposed to hypoxia with 3% O2 for 24, 48 and 72 h, respectively, were significantly increased, compared with cells exposed to normoxia with 21% O2 for 24, 48 and 72 h (Fig. 2A). The mean OD values of cells exposed to normoxia for 24, 48 and 72 h were 0.152±0.006, 0.145±0.003 and 0.158±0.005, respectively; however, the OD values of cells exposed to hypoxia for 24, 48 and 72 h were 0.191±0.014, 0.250±0.060 and 0.359±0.032, respectively (Fig. 2A). Compared with cells exposed to hypoxia for 24 h, MTT absorbance of PASMCs in hypoxic exposure for 72 h was significantly increased (P<0.05; Fig. 2A).
Figure 1.
Rat PASMCs stain positively for smooth muscle markers. PASMCs were stained with (A) DAPI, (B) anti-smoothlin and (C) anti-smooth muscle heavy chain. (D) Merged overlay of parts A-C. Magnification, ×600. PASMCs, pulmonary arterial smooth muscle cells.
Figure 2.
Expression of PKCα protein in hypoxia-induced PASMC proliferation. (A) MTT assay of PASMC proliferation. *P<0.05 vs. normoxia; #P<0.05 vs. 24 h hypoxia. (B) Identification of the PKCα isoform in rat PASMCs. Lane 1, rat PASMCs; lane 2, rat brain. (C) Representative western blot images and (D) quantification of PKCα protein expression levels following hypoxia. *P<0.05 vs. normoxia. (E) Hypoxia-induced PASMCs proliferation was stimulated by PKC promoter PMA and blocked by PKCα special inhibitor safingol. *P<0.05 vs. normoxia; #P<0.05 vs. hypoxia. Data are presented as the mean ± standard deviation of three independent experiments. PASMCs, pulmonary artery smooth muscle cells; PKCα, protein kinase Cα.
Upregulation of PKCα in hypoxia-induced PASMCs
The results of western blotting demonstrated that PKCα (80 kD) was present in normal rat PASMCs (Fig. 2B). As presented in Fig. 2C and D, PKCα protein expression levels in hypoxic group for 24, 48 and 72 h were 0.336±0.160, 0.656±0.085 and 0.808±0.098, respectively. On the other hand, PKCα protein levels in cells exposed to normoxia for 72 h were 0.290±0.013. Compared with the 72-h normoxia group, PKCα protein expression levels in PASMCs exposed to hypoxia were markedly increased in a time-dependent manner (P<0.05).
PASMC proliferation induced by PKCα
To examine the role of PKCα in hypoxia-induced proliferative responses, greatly proliferated PASMCs in hypoxia for 72 h were selected, which were treated with a PKCα promoter (PMA) and inhibitor (safingol). According to the MTT findings, the mean OD values in the 72-h normoxia, 72-h hypoxia, hypoxic PMA control and hypoxic safingol groups were demonstrated to be 0.187±0.037, 0.529±0.015, 0.587±0.044 and 0.426±0.033, respectively (Fig. 2E). In addition, the OD value in the hypoxic PMA control group was significantly increased compared with the hypoxia group (P<0.05), whereas the OD value in the hypoxic safingol group was lower than that in hypoxic PMA control (P<0.05; Fig. 2E).
PASMC proliferation activated by hypoxia through ERK1/2 phosphorylation
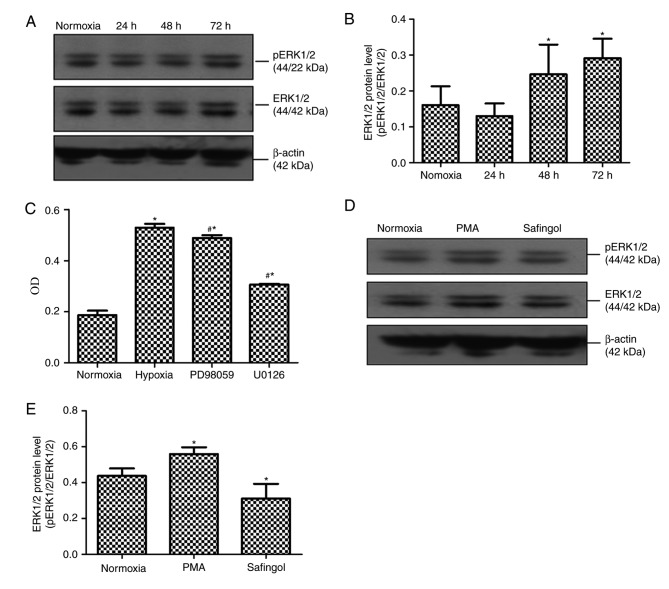
To determine the cellular pathway that may be involved in the PASMC proliferation process, the present study evaluated the possible modulation of ERK1/2 in signaling by western blotting and MTT. According to the western blotting results, p-ERK1/2 expression levels in cells exposed to hypoxia for 24, 48 and 72 h were 0.130±0.035, 0.246±0.083 and 0.291±0.054, respectively; compared with cells exposed to normoxia for 72 h, which was 0.160±0.053 (Fig. 3A and B). Compared with the 72-h normoxia group, p-ERK1/2 expression in PASMCs after exposure to hypoxia for 48 and 72 h was markedly upregulated (P<0.05, Fig. 3A and B). When PASMCs exposed to hypoxia for 72 h were pretreated with the MEK1/2 inhibitor PD98059 or U0126, the mean OD values in the normoxia 72 h, hypoxia 72 h, hypoxic PD98059 control and hypoxic U0126 control groups were 0.187±0.017, 0.529±0.015, 0.489±0.011 and 0.306±0.004, respectively. Compared with the hypoxia for 72 h group, the mean OD values in the hypoxic PD98059 control and hypoxic U0126 control groups were significantly attenuated (P<0.05; Fig. 3C).
Figure 3.
Expression of ERK1/2 protein in hypoxia-induced PASMC proliferation. (A) Representative western blot images and (B) quantification of ERK1/2 phosphorylation in rat PASMCs. (C) MTT assay of hypoxia-induced PAMSC proliferation following treatment with the extracellular signal-regulated kinase kinase 1/2 inhibitors PD98059 and U0126. (D) Representative western blot images and (E) quantification of ERK1/2 phosphorylation following treatment with PMA and safingol after hypoxia exposure for 72 h. Data are presented as the mean ± standard deviation*P<0.05 vs. normoxia; #P<0.05 vs. hypoxia. PASMCs, pulmonary artery smooth muscle cells; p, phosphorylated; ERK1/2, extracellular signal-regulated kinase 1/2; PMA, promoter 12-myristate 13-acetate.
ERK1/2 phosphorylation stimulated by PKCα
To investigate a potential association between PKCα and ERK1/2, the present study tested the modulation of ERK1/2 expression through hypoxic PASMCs pretreated with a PKCα promoter and inhibitor. Western blotting results indicated that p-ERK1/2 protein expression levels in the normoxia for 72 h, hypoxic PMA control and hypoxic safingol control groups were demonstrated to be 0.437±0.042, 0.558±0.039 and 0.311±0.082, respectively. p-ERK1/2 protein expression levels in the presence of the PKCα promoter was significantly increased compared with the other two hypoxic groups (P<0.05). Compared with the other two groups, ERK1/2 phosphorylation in the presence of PKCα inhibitor was greatly reduced (P<0.05, Fig. 3D and E).
Discussion
The present study primarily demonstrated that PKCα was upregulated in hypoxia-induced rat PASMC proliferation, and through activation of ERK1/2 phosphorylation, upregulated PKCα induced the proliferation of PASMCs, thus contributing to the occurrence of pulmonary artery hypertension.
Chronic hypoxic pulmonary hypertension is a common clinical problem, which has an association with increased vascular tone, and imbalance between proliferation and apoptosis of PASMCs (20,21). By MTT assay to determine PASMC proliferation, the present study demonstrated that hypoxia may induce PASMC proliferation, which is in consistent with findings by Preston et al and Li et al (22,23).
As a type of protein kinase, PKC is expressed in many tissue cells. To date, at least 11 subtypes of PKC have been identified, among which each type has a different function. As a classical subtype of PKC, PKCα is widely distributed and present in all the tissues, and has a connection with cell apoptosis, proliferation and migration (24). Although PKCα has been reported to serve an important role in cell proliferation, including in PASMCs, there is still a lack of research on the association between PKCα subtypes and signal transduction pathways in hypoxia-induced PASMC proliferation.
Using western blotting in the present study, PKCα was demonstrated to be present in rat PASMCs. In comparison, the presentation of various PKC subtypes including α, β and γ has been validated in a study by Barman et al (25). Through further investigation, the present study demonstrated that the expression level of PKCα in hypoxia-induced PASMCs was increased. In addition, the PKCα special inhibitor safingol suppressed hypoxia-induced PASMC proliferation, and the PKCα promoter PMA significantly increased proliferation. Therefore, it may be hypothesized that the promotion of PKCα expression levels serve a vital role in hypoxia-induced PASMC proliferation. Dempsey et al (26) demonstrated that hypoxia might activate PKC in neonatal bovine PASMCs, and the activated PKC could foster SMC proliferation through stimulating growth factors such as insulin-like growth factor-1. In addition, PKC with PKCα in particular has been demonstrated to serve a critical part in hypoxia and mitogen-induced PASMC proliferation (27). Furthermore, hypoxia-activated PKC in PASMCs has also been identified to be involved in hypoxia-induced PASMC proliferation, thus participating in the formation of hypoxic PH (28). The findings resulting from the present study fit in with those reported in the aforementioned investigations.
The mitogen-activated protein kinase (MAPK) family is comprised of ERK1/2, c-Jun N-terminal kinase (JNK) and p38 MAPK. MAPK has been reported to exhibit a distinct increase of activation in chronic hypoxic pulmonary arteries (29–31). In recent years, ERK1/2 has been identified to be activated by a variety of extracellular stimuli, and is involved in hypoxia-induced PASMC proliferation (20,22,23,32–34). PD98059 and U0126 are universally used as MEK1/2 inhibitors, the role of which is to inhibit mitochondrial metabolism, thus using up p-ERK. With ERK1/2 being the only known MEK1/2 downstream substrate (21), PD98059 and U0126 were used to block ERK1/2. In the present study, PASMC proliferation was markedly increased with exposure to hypoxia for 48 and 72 h, and p-ERK1/2 expression was also significantly increased. However, PD98059 and U0126 markedly weakened this proliferative response, which suggested that hypoxia may activate ERK1/2, thus promoting PASMC proliferation. Therefore, ERK1/2 may serve a vital part in hypoxia-induced PASMC proliferation.
It has been reported that activated PKCα induces the proliferation of capillary endothelial cells through the ERK1/2 signaling pathway (35). Another investigation demonstrated that activated PKCα in human saphenous vein SMCs strengthened the activity of MAPKs, which could contribute to cell proliferation (36). However, relevant information concerning the role and mechanism of PKCα in PH-induced PASMCs proliferation is not yet available. The present study demonstrated that PMA-induced PKCα activation upregulated the expression of p-ERK1/2, thus increasing PASMC proliferation. On the other hand, the PKCα inhibitor safingol may inhibit ERK1/2 phosphorylation, suppressing PASMC proliferation. Therefore, increased expression of PKCα may activate ERK1/2, leading to PASMC proliferation and PH formation.
In conclusion, protein expression levels of PKCα in rats were upregulated in hypoxia-induced rat PASMCs. Through activating ERK1/2, PKCα served part in PASMC proliferation, which adds to the understanding of its mechanism in PH formation and lays a theoretical basis for prevention as well as treatment of HPH. Whether PKCα could be considered as a target for PH treatment in the future still requires further studies on the role of other subtypes in PH formation. Limitations of the present study lie in that early pathological variations of PH are primarily involved in peripheral pulmonary artery. The present study would be of much more significance if distal PASMCs could be obtained.
Acknowledgements
The present study work was supported by the Natural Science Foundation of Shanxi Province of China (grant no. 201601D011110).
References
- 1.Hoette S, Jardim C, Souza Rd. Diagnosis and treatment of pulmonary hypertension: An update. J Bras Pneumol. 2010;36:795–811. doi: 10.1590/S1806-37132010000600018. (In English, Portuguese) [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Wang C, Han S, Pang B, Zhang N, Wang J, Li J. Determination of PKC isoform-specific protein expression in pulmonary arteries of rats with chronic hypoxia-induced pulmonary hypertension. Med Sci Monit. 2012;18:BR69–BR75. doi: 10.12659/MSM.882458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nie X, Shi Y, Yu W, Xu J, Hu X, Du Y. Phosphorylation of PTEN increase in pathological right ventricular hypertrophy in rats with chronic hypoxia induced pulmonary hypertension. Chin Med J (Engl) 2014;127:338–342. [PubMed] [Google Scholar]
- 4.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the pulmonary hypertension association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Wang Y, Jiang J, Wang R, Li L, Qiu Z, Wu H, Zhu D. 15-HETE protects rat pulmonary arterial smooth muscle cells from apoptosis via the PI3K/Akt pathway. Prostaglandins Other Lipid Mediat. 2010;91:51–60. doi: 10.1016/j.prostaglandins.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Hurley JH, Newton AC, Parker PJ, Blumberg PM, Nishizuka Y. Taxonomy and function of C1 protein kinase C homology domains. Protein Sci. 1997;6:477–480. doi: 10.1002/pro.5560060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitti M, Pronzato MA, Marinari UM, Domenicotti C. PKC signaling in oxidative hepatic damage. Mol Aspects Med. 2008;29:36–42. doi: 10.1016/j.mam.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Fan HC, Fernandez-Hernando C, Lai JH. Protein kinase C isoforms in atherosclerosis: Pro- or anti-inflammatory? Biochem Pharmacol. 2014;88:139–149. doi: 10.1016/j.bcp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Haller H, Baur E, Quass P, Behrend M, Lindschau C, Distler A, Luft FC. High glucose concentrations and protein kinase C isoforms in vascular smooth muscle cells. Kidney Int. 1995;47:1057–1067. doi: 10.1038/ki.1995.152. [DOI] [PubMed] [Google Scholar]
- 10.Webb BL, Lindsay MA, Seybold J, Brand NJ, Yacoub MH, Haddad EB, Barnes PJ, Adcock IM, Giembycz MA. Identification of the protein kinase C isoenzymes in human lung and airways smooth muscle at the protein and mRNA level. Biochem Pharmacol. 1997;54:199–205. doi: 10.1016/S0006-2952(97)00165-2. [DOI] [PubMed] [Google Scholar]
- 11.Newton AC. Protein kinase C: Structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 12.Throckmorton DC, Packer CS, Brophy CM. Protein kinase C activation during Ca2+-independent vascular smooth muscle contraction. J Surg Res. 1998;78:48–53. doi: 10.1006/jsre.1998.5368. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Okamoto M, Panzhinskiy E, Zawada WM, Das M. PKCδ/midkine pathway drives hypoxia-induced proliferation and differentiation of human lung epithelial cells. Am J Physiol Cell Physiol. 2014;306:C648–C658. doi: 10.1152/ajpcell.00351.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agbani EO, Coats P, Mills A, Wadsworth RM. Peroxynitrite stimulates pulmonary artery endothelial and smooth muscle cell proliferation: Involvement of ERK and PKC. Pulm Pharmacol Ther. 2011;24:100–109. doi: 10.1016/j.pupt.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: Role in the development of pulmonary hypertension. Microvasc Res. 2004;68:75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Acevedo-Duncan M, Chalfant CE, Patel NA, Watson JE, Cooper DR. The roles of protein kinase C beta I and beta II in vascular smooth muscle cell proliferation. Exp Cell Res. 1998;240:349–358. doi: 10.1006/excr.1998.3999. [DOI] [PubMed] [Google Scholar]
- 17.Zhu BH, Yao ZX, Luo SJ, Jiang LM, Xiao JW, Liu SC, Liu JB, Sun JM, Pei ZY. Effects of antisense oligonucleotides of PKC-alpha on proliferation and apoptosis of HepG2 in vitro. Hepatobiliary Pancreat Dis Int. 2005;4:75–79. [PubMed] [Google Scholar]
- 18.Sasaguri T, Kosaka C, Hirata M, Masuda J, Shimokado K, Fujishima M, Ogata J. Protein kinase C-mediated inhibition of vascular smooth muscle cell proliferation: The isoforms that may mediate G1/S inhibition. Exp Cell Res. 1993;208:311–320. doi: 10.1006/excr.1993.1251. [DOI] [PubMed] [Google Scholar]
- 19.Pisarcik S, Maylor J, Lu W, Yun X, Undem C, Sylvester JT, Semenza GL, Shimoda LA. Activation of hypoxia-inducible factor-1 in pulmonary arterial smooth muscle cells by endothelin-1. Am J Physiol Lung Cell Mol Physiol. 2013;304:L549–L561. doi: 10.1152/ajplung.00081.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li XW, Hu CP, Wu WH, Zhang WF, Zou XZ, Li YJ. Inhibitory effect of calcitonin gene-related peptide on hypoxia-induced rat pulmonary artery smooth muscle cells proliferation: Role of ERK1/2 and p27. Eur J Pharmacol. 2012;679:117–126. doi: 10.1016/j.ejphar.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Ripple MO, Kim N, Springett R. Acute mitochondrial inhibition by mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) 1/2 inhibitors regulates proliferation. J Biol Chem. 2013;288:2933–2940. doi: 10.1074/jbc.M112.430082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preston IR, Hill NS, Warburton RR, Fanburg BL. Role of 12-lipoxygenase in hypoxia-induced rat pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2006;290:L367–L374. doi: 10.1152/ajplung.00114.2005. [DOI] [PubMed] [Google Scholar]
- 23.Li GW, Xing WJ, Bai SZ, Hao JH, Guo J, Li HZ, Li HX, Zhang WH, Yang BF, Wu LY. The calcium-sensing receptor mediates hypoxia-induced proliferation of rat pulmonary artery smooth muscle cells through MEK1/ERK1,2 and PI3K pathways. Basic Clin Pharm Toxicol. 2011;108:185–193. doi: 10.1111/j.1742-7843.2010.00639.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, Xu J, Hou Z, Wang F, Song Y, Wang J, Zhu H, Jin H. miRNA-34a promotes proliferation of human pulmonary artery smooth muscle cells by targeting PDGFRA. Cell Prolif. 2016;49:484–493. doi: 10.1111/cpr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barman SA, Zhu S, White RE. Protein kinase C inhibits BKCa channel activity in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L149–L155. doi: 10.1152/ajplung.00259.2003. [DOI] [PubMed] [Google Scholar]
- 26.Dempsey EC, Badesch DB, Dobyns EL, Stenmark KR. Enhanced growth capacity of neonatal pulmonary artery smooth muscle cells in vitro: Dependence on cell size, time from birth, insulin-like growth factor I, and auto-activation of protein kinase C. J Cell Physiol. 1994;160:469–481. doi: 10.1002/jcp.1041600310. [DOI] [PubMed] [Google Scholar]
- 27.Dempsey EC, Frid MG, Aldashev AA, Das M, Stenmark KR. Heterogeneity in the proliferative response of bovine pulmonary artery smooth muscle cells to mitogens and hypoxia: Importance of protein kinase C. Can J Physiol Pharmacol. 1997;75:936–944. doi: 10.1139/y97-104. [DOI] [PubMed] [Google Scholar]
- 28.Zhang HP, Xu YJ, Zhang ZX, Ni W, Chen SX. Effect of protein kinase C-nuclear factor-kappa B signal transduction pathway on proliferation and expression of vascular endothelial growth factor in human pulmonary artery smooth muscle cells. Zhonghua Jie He He Hu Xi Za Zhi. 2004;27:218–223. [PubMed] [Google Scholar]
- 29.Jin N, Hatton N, Swartz DR, Xia Xl, Harrington MA, Larsen SH, Rhoades RA. Hypoxia activates jun-N-terminal kinase, extracellular signal-regulated protein kinase, and p38 kinase in pulmonary arteries. Am J Respir Cell Mol Biol. 2000;23:593–601. doi: 10.1165/ajrcmb.23.5.3921. [DOI] [PubMed] [Google Scholar]
- 30.Weerackody RP, Welsh DJ, Wadsworth RM, Peacock AJ. Inhibition of p38 MAPK reverses hypoxia-induced pulmonary artery endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1312–H1320. doi: 10.1152/ajpheart.00977.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Church AC, Martin DH, Wadsworth R, Bryson G, Fisher AJ, Welsh DJ, Peacock AJ. The reversal of pulmonary vascular remodeling through inhibition of p38 MAPK-alpha: A potential novel anti-inflammatory strategy in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2015;309:L333–L347. doi: 10.1152/ajplung.00038.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Tian Y, Prabha M, Liu D, Chen S, Zhang R, Liu X, Tang C, Tang X, Jin H, Du J. Effects of sulfur dioxide on hypoxic pulmonary vascular structural remodeling. Lab Invest. 2010;90:68–82. doi: 10.1038/labinvest.2009.102. [DOI] [PubMed] [Google Scholar]
- 33.Xia S, Tai X, Wang Y, An X, Qian G, Dong J, Wang X, Sha B, Wang D, Murthi P, et al. Involvement of Gax gene in hypoxia-induced pulmonary hypertension, proliferation, and apoptosis of arterial smooth muscle cells. Am J Respir Cell Mol Biol. 2011;44:66–73. doi: 10.1165/rcmb.2008-0442OC. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Shen M, Xu M, Liu LL, Luo Y, Xu DQ, Wang YX, Liu ML, Liu Y, Dong HY, et al. Role of macrophage migration inhibitory factor in the proliferation of smooth muscle cell in pulmonary hypertension. Mediators Inflamm. 2012;2012:840737. doi: 10.1155/2012/840737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park MJ, Park IC, Lee HC, Woo SH, Lee JY, Hong YJ, Rhee CH, Lee YS, Lee SH, Shim BS, et al. Protein kinase C-alpha activation by phorbol ester induces secretion of gelatinase B/MMP-9 through ERK 1/2 pathway in capillary endothelial cells. Int J Oncol. 2003;22:137–143. [PubMed] [Google Scholar]
- 36.Okazaki J, Mawatari K, Liu B, Kent KC. The effect of protein kinase C and its alpha subtype on human vascular smooth muscle cell proliferation, migration and fibronectin production. Surgery. 2000;128:192–197. doi: 10.1067/msy.2000.108062. [DOI] [PubMed] [Google Scholar]