Abstract
The thymus is critical in establishing and maintaining the appropriate microenvironment for promoting the development and selection of T cells. The function and structure of the thymus gland has been extensively studied, particularly as the thymus serves an important physiological role in the lymphatic system. Numerous studies have investigated the morphological features of thymic involution. Recently, research attention has increasingly been focused on thymic proteins as targets for drug intervention. Omics approaches have yielded novel insights into the thymus and possible drug targets. The present review addresses the signaling and transcriptional functions of the thymus, including the molecular mechanisms underlying the regulatory functions of T cells and their role in the immune system. In addition, the levels of cytokines secreted in the thymus have a significant effect on thymic functions, including thymocyte migration and development, thymic atrophy and thymic recovery. Furthermore, the regulation and molecular mechanisms of stress-mediated thymic atrophy and involution were investigated, with particular emphasis on thymic function as a potential target for drug development and discovery using proteomics.
Keywords: thymic function, T cells, molecular mechanisms, cytokines, stress signaling
1. Introduction
The thymus is a bilobed organ located in the superior mediastinum of the thorax, above the heart and behind the sternum. It can be divided into two main subcompartments: The cortex and the medulla. Each subcompartment contains numerous subtypes of thymic epithelial cells (TECs), in addition to dendritic cells, mesenchymal cells and endothelial cells (1–3). In addition, the thymus establishes and maintains thymic microenvironments, which are capable of supporting the efficient development of T cells. The maintenance of these microenvironments is dependent upon the specialized functions of thymic stromal cells, and other major components of the thymic microenvironment (4,5). During the development and maturation of thymocytes from bone marrow-derived T cell progenitors, three main events serve a critical role in each T cell bearing a unique T cell receptor (TCR): The rearrangement and expression of TCRα and β loci, which depends on their somatic assembly; positive selection [the identification of cells that are able to recognize self-major histocompatibility complex (MHC) in antigen presentation to T cells]; and negative selection (the elimination of T cells that are potentially autoreactive). T cells that survive the selection processes eventually become mature cluster of differentiation (CD)4+ or CD8+ single positive T cells (Fig. 1). These processes ensure a population of non-autoreactive peripheral T cells. T cell migration is directed by several mediators, including chemokine receptors and G protein-coupled receptors (GPCR), which are supported by guiding stromal structures and by TECs, including cortical TECs and medullary TECs (mTEC). The TECs form a three-dimensionally oriented network, rather than the more ‘typical’ two-dimensional (2D) epithelial structures (6,7). It is important to determine the molecular mechanisms underlying the thymic regulation of T cell development and of the proteins involved in T cell recognition. However, to the best of our knowledge, the mechanisms underlying these processes have not yet been fully explored.
Figure 1.
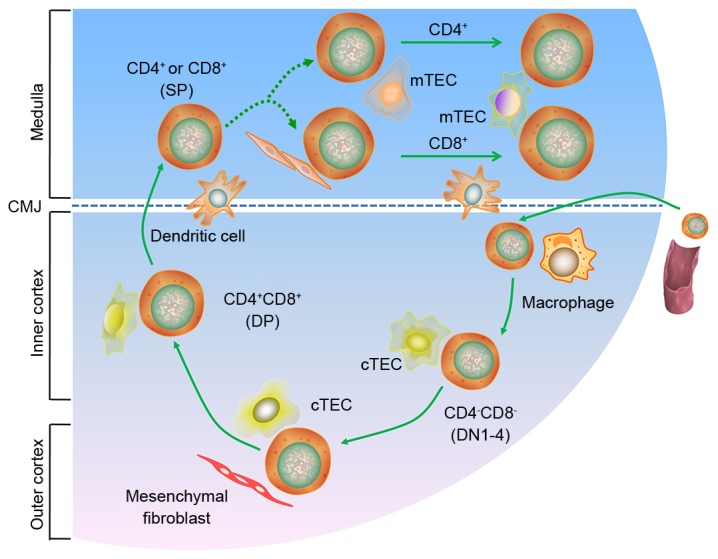
T cell development in the thymus. CD, cluster of differentiation; CMJ, corticomedullary junction; cTEC, cortical thymic epithelial cell; DN, differentiation; DP, double positive; mTEC, medullary thymic epithelial cell; SP, single positive.
2. Molecular mechanisms underlying regulatory T cell generation in the thymus
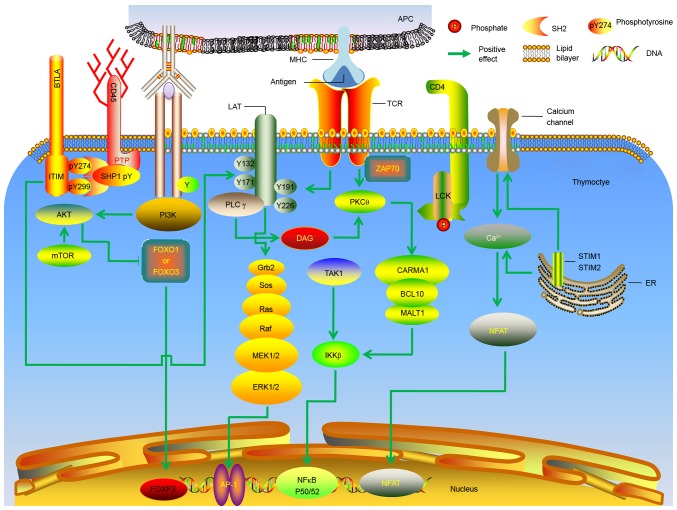
In 1969, Nishizuka and Sakakura were the first to present a mechanism for the generation of regulatory T (Treg) cells in the thymus, based on a neonatal thymectomy experiment (8). Treg cells in the thymus are vital for the negative regulation of immune-mediated inflammation, which features prominently in autoimmune and autoinflammatory disorders, acute allergies, cancer, chronic infections and commensal microbiota. They are also important for the regulation of metabolic inflammation for homeostasis and peripheral tolerance (9–11). Recent studies have demonstrated that mice lacking the forkhead box P3 (Foxp3) transcription factor experience overwhelming autoimmune pathology, which they succumb to in a matter of weeks (12,13). Although CD25 is not a specific marker expressed exclusively on Treg cells, using specific anti-CD25 antibodies for the depletion or inactivation of Treg cells, in combination with immunostimulation, is an attractive treatment modality, particularly in anti-tumour immunotherapy (14). The current understanding is that Treg cell development occurs when the TCR avidity for self-antigens lies between the TCR avidities that drive positive and negative selection (15–19). TCR engagement is also known to stimulate various downstream signaling molecules and transcription factors. This stimulation leads to an intricate web of downstream intracellular signaling events. Proteins important in thymic Treg cell function include phosphoinositide 3-kinase, protein kinase B (AKT), mammalian target of rapamycin (mTOR), nuclear factor of activated T cells, transcription factor activator protein 1 and nuclear factor-κB (NF-κB). Numerous pathways contribute to Treg cell development, including the TCR, AKT-mTOR and NF-κB pathways, among others (20–22). Various types of antigen-presenting cells (APCs) capture and present antigens to thymocytes through a complex network of signaling pathways (Fig. 2). In addition, calcium signaling appears to be involved in thymic Treg cell development (23). Furthermore, increased generation of the Foxp3 protein in developing thymic Treg cells may have a positive role in Ca2+ signaling (24,25). However, calcium is also a powerful negative regulator of Foxp3 in the AKT-mTOR pathway. Phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT signaling regulates the phosphorylation and inhibition of forkhead box O (FoxO) transcription factors. The FoxO transcription factors have recently been reported to facilitate the expression of Foxp3 and Treg cell development (26–28). Although natural Treg (nTreg) and induced Treg (iTreg) cells can enforce tolerance, iTreg cells, such as those derived from commensal bacteria in the gut, may have a particularly important role as they increase antigen receptor diversity (29,30). The mechanisms underlying the development and antigen specificities of nTreg and iTreg cells are likely to differ.
Figure 2.
Molecular mechanisms underlying the generation of thymic regulatory T cells. Molecular signals downstream of the TCR are presented. AP, activator protein; APC, antigen-presenting cell; BCL, B cell lymphoma; BTLA, B and T lymphocyte attenuator; Ca, calcium; CARMA, CARD-containing MAGUK protein; CD, cluster of differentiation; DAG, diacylglycerol; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; IKKβ, inhibitor of nuclear factor κB; ITIM, immunoreceptor tyrosine-based inhibition motif; MEK, mitogen-activated extracellular signal-regulated kinase; MHC, major histocompatibility complex; FoxO, forkhead box protein O; FOXP3, forkhead box protein 3; NFAT, nuclear factor of activated T; Grb, growth factor receptor-bound protein; LAT, linker for activation of T cells; LCK, lymphocyte-specific protein tyrosine kinase p56; MALT, mucosa-associated lymphoid tissue lymphoma translocation protein; mTOR, mechanistic target of rapamycin; NF, nuclear factor; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; PK, protein kinase; PL, phospholipase; PTP, protein-tyrosine phosphatase; Ras, rat sarcoma also known as p21; Raf, rapidly accelerated fibrosarcoma; SHP, SH2-containing protein tyrosine phosphatase; SOS, Son of Sevenless; STIM, stromal interaction molecule; TAK, transforming growth factor beta-activated kinase; ZAP70, ζ-associated protein of 70 kD.
Over the past few years, substantial progress has been made in understanding the developmental process of thymic Treg cells and the molecular mechanism underlying their regulation in the thymus. However, there remain numerous unanswered questions. For example, the molecular differences between immature CD4+ single positive (SP) thymocytes in the thymus and naive peripheral T cells remain unknown. In addition, it remains to be elucidated why Foxp3 expression occurs predominantly in CD4+, and not CD8+, SP thymocytes, The present review aimed to understand these molecular mechanisms and how these molecular components are ‘wired’ into regulatory signaling and transcriptional networks. Achieving this may aid in the improvement of therapeutic strategies used to treat autoimmune and inflammatory disorders.
3. Effects of cytokines on thymic function
Cytokines serve as molecular messengers between immune cells, and have been reported to be of major importance to thymic function. The effects of cytokine cascades on thymic function are generally well understood. Almost all types of thymic cells can produce cytokines, either spontaneously or following stimulation with stimulating agents, including lipopolysaccharides, phytohemagglutinin and ionomycin. The most important of the thymic cell subsets are TECs, which are the principal source of cytokines and chemokines required in early T cell development (31). The differential expression of major cytokines produced by TECs can be divided into four branches: Hemopoietins, proinflammatory cytokines, suppressor cytokines and interleukin (IL)-6 and IL-7 cytokines (32,33). Notably, cytokines and other growth factors serve important roles in thymic function, regulating various cellular processes. However, the functions of numerous cytokines in the thymus are not well understood. Understanding the effects of intrathymic cytokines may reveal some unknown aspects of thymic physiology.
The thymus produces hormones and cytokines that regulate immune function. A previous study identified at least six types of thymic cells (34). The histological features of the thymus are broadly divided into the central medulla and a peripheral cortex. Previous research has demonstrated that cytokine secretion by T lymphocytes has a vital role in mounting adaptive immune responses (35). In addition, the large number of cytokines produced by the thymus maintains a fine balance between thymocyte proliferation, maturation, activation, differentiation and survival inhibition. Thymic cells also secrete the peptides IL-1, IL-3, IL-4 and IL-6, and three major thymic hormones, thymosins, thymopoietin and thymulin (36–39). Thymic hormones serve a major role in preserving the functions of the immune system, and cytokines have essential roles in the control of immune responses.
Cytokines are small polypeptides that regulate cell function and are predominantly secreted by immune cells. Numerous cytokines responsible for the modulation of T cell differentiation are produced by thymocytes and TECs. The ability of thymocytes to produce cytokines is important in the regulation of thymic cytokine production and the responses to their action (Fig. 3). Of these regulators, IL-7 serves a particular role in thymocyte differentiation; IL-7 has been reported to promote the rearrangement of TCR genes by enhancing the production and activity of recombinases (40,41). The thymic production of Treg cells requires IL-2, which is also required during T cell development in the thymus and for the maturation of Treg cells. Recent studies have reported that IL-2 receptor is functionally active within the thymus; it increases the number of CD4+Foxp3+ thymocytes and the expression of Foxp3 and CD25 to normal levels (42–44). IL-4 is another cytokine produced by T cells whose receptor contains a γ(c)-chain. It has previously been demonstrated that IL-4 is synergistic with IL-2 in the induction of thymocyte proliferation in fetal thymic organ culture. In addition, IL-4 supports thymocytes through successive phases of proliferation, acting alongside stimulatory agents (45,46). Recently, research has been directed at the cytokine IL-10, which is produced by Treg cells, and other chronically stimulated T helper cells, B cells and APCs. IL-10 is important for maintaining immune homeostasis at mucosal surfaces and also contributes to immune suppression (47–49).
Figure 3.
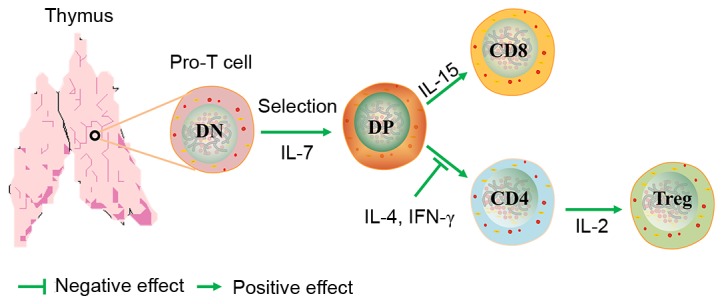
Role of cytokines in T cell development. CD, cluster of differentiation; DN, double negative; DP, double positive; IL, interleukin; Treg, regulatory T cell.
Interferon (IFN)-γ has numerous effects on TECs; it activates TECs and increases surface expression of MHC classes I and II, and other membrane proteins (50). Furthermore, IFN-γ stimulates the secretion of IL-6 by TECs (51). IFN-γ also supports thymocyte differentiation, through its action on TEC functions. Tumor necrosis factor (TNF)-α has been reported to have an important role in the regulation of thymocyte production, inducing apoptosis and the proliferation of immature CD3−CD4−CD8− T cells in the presence of IL-7 (52). Furthermore, TNF-α and IL-1 participate as cofactors in the induction of CD4−CD8− thymocyte commitment and differentiation (53). TNF-α also stimulates the production of IL-6 and enhances the apoptosis of CD4+CD8+ cells induced by glucocorticoids (54,55).
Some molecules are multifunctional and serve different functions in the cytokine system within the thymus than they do in peripheral compartments of the immune system. For example, some cytokines are pleiotropic in their biological activities and exhibit different roles in these different systems. The principal roles of thymic cytokines are in constitutive processes, including thymocyte migration and development, and the mediation of cell populations, but not inducible ones, such as immune response/tolerance or inflammation, as in the periphery. The synthesis of cytokines and the expression of their receptors in the thymus is usually spontaneous, or is induced by cell-cell interactions, unlike in the periphery. Information regarding the production of cytokines in the thymus and the biological activity of these cytokines is summarized in Table I.
Table I.
Biological activity of cytokines affects T cell-associated thymic function.
| Author (year) | Name | Molecular weight (kDa) | Cell producers | Biological activity | (Refs.) |
|---|---|---|---|---|---|
| Coto et al (1992); Dalloul et al (1991) | IFN-γ | 17.1 | Activated T cells; Natural killer cells | Affects T-cell, B-cell, and macrophage differentiation and maturation | (36,37) |
| Nitta and Suzuki (2016); Galy et al (1990); Savino et al (2016) | IL-1 | 17.3; 17.5 | Thymic epithelial cells; Macrophages; Monocytes | Can act as a growth factor for thymocytes and promote thymocyte T cell activation, proliferation and differentiation; Members of the IL-1 family of receptors contain activators and suppressors of inflammation | (31,38,39) |
| Savino and Dardenne (2000); Muegge et al (1993) | IL-2 | 13–17 | Activated T cells | Promotes the development of Treg cells within the inner thymus; Promotes the activation of T cell proliferation, differentiation and cytokine productio | (40,41) |
| Bayer et al (2007); Varas et al (1997); Weist et al (2015); Meilin et al (1997) | IL-4 | 18–19 | Activated T cells | T cell growth factor | (42–45) |
| Zlotnik et al (1987); Shevach (2009); Barnes and Powrie (2009) | IL-6 | 19–28 | Monocytes; Macrophages; Fibroblasts; T cells; Endothelial cells; B cells | Promotes the development and maturation of thymocytes | (46–48) |
| Mittal and Roche (2009); Patel et al (1995) | IL-7 | 20–28 | Stromal cells; Keratinocytes Hepatocytes; Dendritic cells | Promotes differentiation of CD8+ T cells in the thymus; Maintains T cell proliferation | (49,50) |
| Meilin et al (1995); Baseta and Stutman (2000) | IL-9 | 14–25 | Activated T cells | T cell growth factor | (51,52) |
| Zúñiga-Pflücker et al (1995); Arzt et al (2000) | IL-12 | 75 | T cells | Maintains thymic integrity and function | (53,54) |
| Cohen-Kaminsky et al (1991); Wang et al (2016) | IL-17 | 34–52 | T lymphocytes | Activates CD4+ memory T lymphocytes; Produces Treg 17 cells | (55,56) |
| Shanley et al (2009); Dooley and Liston (2012); Kappler et al (1987) | IL-21 | 15–18 | Activated CD4 T cells | Treg 17 cells Promotes CD4 T cell differentiation; Reduces the Th17 pathway; Costimulates activated natural killer and CD8 lymphocytes; Desensitizes responding cells to the inhibitory effects of; Treg cells act as a switch for immunoglobulin G production in B cells | (57–59) |
| Xing and Hogquist (2012); Roberts et al (1990) | IL-22 | 17–22 | T helper 17 cells | Promotes thymic epithelial cells proliferation and survival; Affects T cell development | (60,61) |
| Kisielow et al (1988); Ramsdell and Fowlkes (1990); Howard et al (1999) | TGF-β | 12.5 | Activated T cells; Activated B cells | Inhibits the IL-1, IL-2 and IL-7-dependent proliferation of thymocytes | (62,63,67) |
| Wang et al (1994); Müller-Hermelink et al (1987); Gruver and Sempowski (2008) | TNF-α | 17–26 | Monocytes; Macrophages | Promotes T cells and B cell proliferation | (68–70) |
| Boyd (1932); Gruver et al (2007) | TSLP | 18.1 | Epithelial and dendritic cells | Promotes T helper 2 differentiation of naïve CD4 T cells; Activates natural killer T cells, basophils and other innate immune cells | (71,72) |
CD, cluster of differentiation; IFN, interferon; IL, interleukin; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; Treg, regulatory T; TSLP, Thymic stromal lymphopoietin.
4. Regulation of molecular mechanisms in stress-mediated thymic atrophy and involution
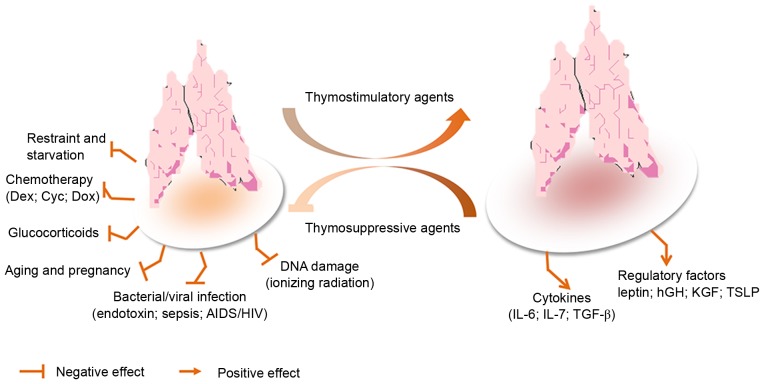
Stress is able to disrupt homeostasis of the immune system, and various stressful conditions cause acute thymic involution, including emotional distress, malnutrition and pregnancy (56,57). Furthermore, numerous processes can trigger thymic involution during pathological conditions, such as bacterial and viral infections, inflammation, disease, clinical cancer treatment and preparative regimens for bone marrow transplants (58), as presented in Fig. 4. Therefore, mechanisms must exist to regulate these processes in various contexts. It is well known that the thymus serves an important role in the body's immune response. It provides the microenvironment essential for the development of T cells from hematopoietic stem cells. The central functions of the thymus are critical to immune tolerance in several rodent and large animal models under normal or pathological conditions. These functions act through various mechanisms, such as clonal deletion or clonal anergy of self-reactive T cells, elimination or control of self-reactive T cells, and anergy of self-reactive T cells (59–63). Recent mechanistic studies regarding central and peripheral T cell tolerance have assisted in the design of novel, immunomodulating therapeutic strategies for the treatment of autoimmune diseases, and improve the prevention, detection and treatment of cancer and associated diseases, as well as exert immunoregulatory effects in transplantation outcomes using pharmacological or biological interventions (64–66). Immunosenescence and immune atrophy, which are associated with reduced immunity, are complex processes that have yet to be fully understood. Numerous factors exert a negative effect on thymopoiesis, acute stress-induced thymic atrophy and on chronic thymic involution associated with aging. These factors include starvation, environmental stressors, bacterial infection, and irradiation or immunosuppressive therapies (67–70).
Figure 4.
Model of stress-induced thymic atrophy, and thymosuppressive and thymostimulatory mediators. AIDS, acquired immunodeficiency syndrome; Cyc, cyclophosphamide; Dex, dexamethasone; Dox, doxorubicin; HIV, human immunodeficiency virus; hGH, human growth hormone; IL, interleukin; KGF, keratinocyte growth factor; TGF-β, transforming growth factor-β; TSLP, thymic stromal lymphopoietin.
The shrinkage of the thymus was reported >80 years ago by Boyd (71); however, the underlying mechanisms are not well understood. Immunosenescence is defined as deterioration in the immune system, which is associated with aging (72–74), and has attracted increasing interest in the scientific and health-care sectors alike. Thymic atrophy has often been observed due to the direct or indirect influences of drugs or the environment on the thymus. However, one other major consideration in thymic atrophy is a systemic rise in glucocorticoids and inflammatory cytokines. Unfortunately, the thymus is acutely sensitive to various stresses and injuries; therefore, it is often considered as a ‘barometer of stress’ for the body. Prolonged thymic atrophy in stress situations can contribute to peripheral T cell deficiency or can inhibit immune reconstitution, thus resulting in a decrease in thymopoiesis (75,76). Therefore, mechanistic studies have increasingly focused on thymic atrophy. A commonly used mouse model of endotoxemia-induced acute thymic atrophy has been used to reveal the effects of acute stress on thymopoiesis. For example, in a lipopolysaccharide (LPS)-induced acute thymic atrophy model, microarray analysis revealed >11,000 probe sets with significant alterations (>1.4-fold), 1 day after an LPS challenge. This finding has important implications regarding how the direct intrathymic response to an endotoxin challenge contributes to thymic involution during endotoxemia (77). In endotoxin-stressed mice, it has previously been reported that leptin administration augments thymopoiesis in LPS-treated leptin-deficient (ob/ob) mice, but not in normal mice (78). Furthermore, a recent study indicated that the number of thymocytes and TECs was significantly decreased in LPS-treated neonatal thymic involution (79).
Age-associated thymic involution must also be considered. Aging is accompanied by a decline in the function and development of the immune system. Understanding the aging process, and how that process can be delayed or reversed, may allow us to take action to adopt healthier lifestyles and live longer. Age-associated thymic involution is characterized by progressive diminution of novel T cell production (80). However, many previous findings are contradictory. Some studies have reported the effects of aging on the function of neutrophils, macrophages and natural killer cells, whereas other studies have reported no association (81,82). In addition, some studies have demonstrated that the systemic administration of keratinocyte growth factor (KGF) enhances T cell lymphopoiesis by stimulating TECs to secrete various cytokines that then act on developing thymocytes in young and old mice (83,84). Furthermore, a previous study was conducted on C57BL/6×DBA/2 recombinant inbred strains of mice to identify the genetic loci influencing age-associared thymic involution, and demonstrated that the strongest quantitative trait loci influencing the rate of thymic involution in the recombinant-inbred mice were mapped to chromosome (Chr) 9 (D9Mit20 at 62 cM) and Chr 10 (D10Mit61 at 32 cM) (85).
It is well known that stress on the immune system leads to the suppression of immune cell functions, such as in T cells, macrophages, dendritic cells and B cells, and the atrophy of immune organs, predominantly the thymus and spleen. The thymus is one of the central organs of the immune system, and is essential for the development of the adaptive immune system. Insult, infection, dysregulation of positive and negative selection, suppression of cell adhesion, chemotaxis, cytotoxicity, increased apoptosis or antigen presentation in the thymus, may all lead to autoimmunity or immunosuppression (86,87). Previous studies have suggested that exposure to immunosuppressive agents, such as diethylstilbestrol, dexamethasone (DEX), azathioprine, cyclophosphamide (Cyc), 2,3,7,8-tetrachlorodibenzo-p-dioxin or cyclosporin A may induce immunotoxic effects resulting in hypocellularity, apoptosis and atrophy in the thymus (88–92). This provides evidence regarding the molecular mechanisms and cellular targets involved in thymic atrophy-induced immunosuppression. DEX is a synthetic glucocorticoid compound with potent anti-inflammatory activity, which is associated with clinically significant side effects that severely limit its therapeutic use. In a previous study, DEX (20 mg/kg) was administered to C57Bl/6 mice via intraperitoneal injection; the thymuses were then harvested 5 days after treatment. Analysis of the thymic tissues detected a depletion of CD4+CD8+ double positive thymocytes, and upregulation of IL-22 and IL-23 in wild-type mice (93). In another study, the immunosuppressant cyclosporin A was reported to induce extensive reductions in the autoimmune regulator tolerance-inducing MHC class IIhigh mTECs (mTEChigh). The most distinctive effects of Cyc and DEX exposure were extensive reductions in thymocytes and stromal cells, and, as with cyclosporin A, severely depleted tolerance-inducing mTEChigh (91).
5. Prediction of potential drug targets on the thymus using proteomics
The thymus remains still largely uncharted territory that invites further investigation. Understanding the role of the thymus in T cell generation and homeostasis, and understanding exactly how such systems work and what proteins are involved has resulted in greater interest in thymus organogenesis. The application of systems biology, combined with more traditional methods, is essential to uncover and optimize the molecular mechanisms underlying effects (drug-induced or otherwise) on the thymus. These methods will allow the study of novel aspects of thymic function and aid understanding regarding thymic function, morphogenesis and development. This knowledge may then be used to identify potential drug targets. In addition, these methods will prove useful not only for studying gene and protein function in thymus organogenesis, but also for clarifying the origin and lineage relationship between cortical and medullary epithelial cell types. Recently, modern approaches to chemical genomics, metabolomics, genomics, transcriptomics, pharmacogenomics, microbiomics and proteomics have proved to be useful in the identification and characterization of molecular mechanisms underlying all aspects of pharmacological sciences and physiological processes, and in other areas (94,95). Therefore, evidence suggests that proteomics may be effectively used in the in-depth study of the thymus in different models and pathological conditions.
Proteomics is the large-scale study of proteins, and facilitates the systematic analysis of protein molecules in complicated biological systems. Turiák et al (96) focused on the proteomic characterization of thymocyte-derived microvesicles (MVs) and apoptotic bodies in BALB/c mice; 195 and 142 proteins were identified in MVs and apoptotic bodies, respectively. This previous study also identified numerous molecules known to serve important roles in the immune system, such as MHCI, MHCII, CD5 and CD97 in MVs, and CD45 in both types of vesicles. Similarly, Billing et al (97) used proteomic profiling analysis to measure the non-genomic and concomitant genomic effects of acute restraint stress on rat thymocytes. In recent years, several methods have been developed for relative and absolute quantitative proteomics. The most widely used quantitative techniques include gel-based [2D gel electrophoresis, difference gel electrophoresis (DIGE)] and liquid chromatography-mass spectrometry (MS)-based methods (isotope-coded affinity tag, stable isotope labeling with amino acids in cell culture, isobaric tags for relative and absolute quantitation). MS-based proteomics methods are typically divided into two categories: Label-free or label-based approaches (98). Proteomics research is applied to a wide range of biological systems for the study of differentially expressed proteins, particularly candidates for biomarker discovery and validation, understanding disease processes and clinical proteomics (99). Notably, in a previous study quantitative 2D-DIGE with matrix-assisted laser desorption/ionization-time of flight (TOF)/TOF MS was used to identify 108 proteins with differential subcellular localizations in rat thymocytes; this may be the first study to determine the rapid effects of stress-induced hypothalamus-pituitary-adrenal activation at the proteome level in vivo (97). According to our current understanding, doxorubicin (DOX) treatment leads to degeneration of the thymus. Proteomics analysis is consistent with the notion that DOX treatment in vivo leads to thymic senescence (100). Cyc has also been reported to induce immunosuppression and thymic atrophy. Proteomic analysis indicated that possible target-related processing was instigated following Cyc-treatment in mice (101). Apoptosis serves an essential role in the development and maturation of T lymphocytes during mammalian thymus maturation. Experiments have indicated that several proteins were differentially regulated in the cytosol of T cell precursors by a signal from TCR, as identified using proteomic techniques (102). Proteomics has been widely used to study the experimentally induced acute phase reaction, and to study numerous disease models associated with cancer and inflammatory diseases (103,104). A previous study revealed the cellular and molecular mechanisms using proteomic approaches combined with bioinformatics analysis (105). Despite the increased use of proteomics, knowledge of protein interactions and pathway networks remains largely incomplete; however, data generated by quantitative proteomics can still provide valuable insights (106).
6. Final remarks
More than 50 years ago, Miller (107) conducted seminal studies on the immunological function of the thymus using neonatally thymectomized mice. The importance of this primary lymphoid organ was quickly established, as the thymus provides a unique microenvironment in which T cells or T lymphocytes undergo development, differentiation and clonal expansion during the physiological development of the immune system. In recent years, there has been a marked interest in the association between the immune system and the thymus, generating results that confirmed that the thymus was endowed with an immune function. The immune system has evolved to mount an effective defense against pathogens and to minimize deleterious immune-mediated inflammation caused by commensal microorganisms, immune responses against self and environmental antigens, and metabolic inflammatory disorders. It appears that Treg cell-mediated suppression serves as a vital mechanism in the negative regulation of immune-mediated inflammation, and features prominently in autoimmune and autoinflammatory disorders, and pathologies induced by fungi, parasites, allergies, acute and chronic infections, cancer and metabolic inflammation. Treg cells are considered important to researchers in their efforts to increase the efficacy of vaccines for cancer, acquired immune deficiency syndrome and autoimmune diseases. The discovery that Foxp3 is the transcription factor that specifies the Treg cell lineage has facilitated recent progress in understanding the biology of Treg cells. These findings may provide novel targets for subsequent drug development.
There is an increasingly in-depth understanding of cytokines and their activities in biological pathways. Therefore, an improved understanding regarding the cytokine network is essential to determine the role of numerous key cytokines, and to modulate thymic function. Cytokines, such as ILs, may be useful in improving the functionality of the thymus and may be used to treat immunodeficiency or autoimmune diseases. It has been reported that cytokines, including IL-6, IL-7 receptor, IL-10 and IL-22, serve a key regulatory role in T cell growth and differentiation processes in the thymus. These cytokines may be mediated through various regulatory mechanisms and signaling pathways to establish a protective effect on the thymus. Understanding these pathways will increase the understanding of the regulatory mechanism of the thymus and the biology of Treg cells and secreted cytokine function. Previous studies have analyzed the effects of cytokine therapy as a complementary schedule to conventional therapy with γ-globulin (108–110). The results suggested that the treatment has a long-term positive effect on the immune response, relative to other therapeutic interventions. A combination of IFN-α2b, thymic factors, γ-globulin and granulocyte-macrophage colony-stimulating factor may be a promising to treat common variable immunodeficiency. In addition, a previous study reported that cytokines not only serve an essential role during early T cell development, but are also responsible for the development of other thymic cells, such as thymic dendritic cells, generated from precursors produced in bone marrow (32). At present, information on this topic is limited. An essential difference between cytokine production inside the thymus and in peripheral organs is the different levels of dependence on cell activation, and possibly cross talk, depending on the cytokine environment and situation.
Studying protective mechanisms may provide novel directions in research and the development of drugs for the treatment of various stresses to the thymus, including immunosenescence, immune atrophy and immunosuppression. There have been reports of several small molecules having a protective effect on the thymus, including leptin, KGF and IL-22. Studies have also explored the molecular mechanisms involved, predominantly using mice (70,111). In various chemical stress and thymic atrophy models, these active molecules can enhance the remodeling of the thymus, protecting the thymus from some stressors, such as those involved in aging, as well as hunger, radiation, hormones and immunosuppressants. Notably, researchers have made great progress in examining the numerous mechanisms that contribute to immune suppression and have provided a future direction for research and a novel manner of developing immune-modulating drugs (112). It must be noted that there are differences between immunosenescence, immune atrophy and immunosuppression; therefore, these situations should be treated differently when developing specific molecular signaling pathways and in targeted drug development. The development of novel drugs, and signal transduction research concerning these mechanisms, may benefit patients that are immunocompromised, in a pathological state, or a combination, to reduce the side effects of other drugs on the thymus.
During the last decade, the development of proteomics technology and protein targets for drug generation and drug screening mechanisms has provided novel tools for biomedical research (113,114). There have been several reports regarding thymic molecular mechanisms using proteomics technology; therefore, a more comprehensive analysis of protein alterations in the thymus under various circumstances has been established. This, combined with the related molecule-function databases, including UniProt, the Kyoto Encyclopedia for Genes and Genomes and the Gene Ontology database, has enabled protein network data analysis to screen for known or predicted drug-protein or protein-protein interactions in the thymus. A greater understanding of the mutual regulation of protein molecules may allow the prediction of possible molecular drug targets and drug development pathways. Existing proteomics studies have provided some pathways for protein regulation of signal transduction. These pathways are intricate webs of downstream intracellular signaling events that ultimately result in specific thymic immune response stresses. This understanding may provide novel ways of treating immunological diseases by targeting the stress protein molecules in the thymus, and may be useful in improving the functionality of the thymus. Collectively, these studies suggest that the markedly complex action mechanisms underlying immunomodulatory effects in the thymus are a promising therapeutic target for treatment of the immune system.
Acknowledgements
This review was supported by the Young Scientists Fund of the National Natural Science Foundation of China (grant no. 81403395), the Traditional Chinese Medicine Bureau of Guangdong Province [grant no. (2014) 539] and the Specific Research Fund for TCM Science and Technology of Guangdong Provincial Hospital of Chinese Medicine (grant nos. YN2015QN09, YN2016QJ11 and YN2015QN12).
References
- 1.Gordon J, Manley NR. Mechanisms of thymus organogenesis and morphogenesis. Development. 2011;138:3865–3878. doi: 10.1242/dev.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4:278–289. doi: 10.1038/nri1331. [DOI] [PubMed] [Google Scholar]
- 3.Skogberg G, Lundberg V, Berglund M, Gudmundsdottir J, Telemo E, Lindgren S, Ekwall O. Human thymic epithelial primary cells produce exosomes carrying tissue-restricted antigens. Immunol Cell Biol. 2015;93:727–734. doi: 10.1038/icb.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- 5.Su M, Hu R, Jin J, Yan Y, Song Y, Sullivan R, Lai L. Efficient in vitro generation of functional thymic epithelial progenitors from human embryonic stem cells. Sci Rep. 2015;5:9882. doi: 10.1038/srep09882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Y, Tajima A, Goh SK, Geng X, Gualtierotti G, Grupillo M, Coppola A, Bertera S, Rudert WA, Banerjee I, et al. Bioengineering thymus organoids to restore thymic function and induce donor-specific immune tolerance to allografts. Mol Ther. 2015;23:1262–1277. doi: 10.1038/mt.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Ewijk W, Wang B, Hollander G, Kawamoto H, Spanopoulou E, Itoi M, Amagai T, Jiang YF, Germeraad WT, Chen WF, Katsura Y. Thymic microenvironments, 3-D versus 2-D? Semin Immunol. 1999;11:57–64. doi: 10.1006/smim.1998.0158. [DOI] [PubMed] [Google Scholar]
- 8.Nishizuka Y, Sakakura T. Thymus and reproduction: Sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 9.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 11.Wang YM, Ghali J, Zhang GY, Hu M, Wang Y, Sawyer A, Zhou JJ, Hapudeniya DA, Wang Y, Cao Q, et al. Development and function of Foxp3(+) regulatory T cells. Nephrology (Carlton) 2016;21:81–85. doi: 10.1111/nep.12652. [DOI] [PubMed] [Google Scholar]
- 12.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 14.Rosalia RA, Štěpánek I, Polláková V, Šímová J, Bieblová J, Indrová M, Moravcová S, Přibylová H, Bontkes HJ, Bubeník J, et al. Administration of anti-CD25 mAb leads to impaired α-galactosylceramide-mediated induction of IFN-γ production in a murine model. Immunobiology. 2013;218:851–859. doi: 10.1016/j.imbio.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 16.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 19.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: What thymocytes see (and don't see) Nat Rev Immunol. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman NM, Chi H. mTOR links environmental signals to T cell fate decisions. Front Immunol. 2015;5:686. doi: 10.3389/fimmu.2014.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akimzhanov AM, Boehning D. IP3R function in cells of the immune system. WIREs Membr Transp Signal. 2012;1:329–339. doi: 10.1002/wmts.27. [DOI] [Google Scholar]
- 22.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR; Proc Natl Acad Sci USA; 2008; pp. 7797–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz A, Schumacher M, Pfaff D, Schumacher K, Jarius S, Balint B, Wiendl H, Haas J, Wildemann B. Fine-tuning of regulatory T cell function: The role of calcium signals and naive regulatory T cells for regulatory T cell deficiency in multiple sclerosis. J Immunol. 2013;190:4965–4970. doi: 10.4049/jimmunol.1203224. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Yang L, Silva HM, Trzeciak A, Choi Y, Schwab SR, Dustin ML, Lafaille JJ. Increased generation of Foxp3(+) regulatory T cells by manipulating antigen presentation in the thymus. Nat Commun. 2016;7:10562. doi: 10.1038/ncomms10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engel M, Sidwell T, Vasanthakumar A, Grigoriadis G, Banerjee A. Thymic regulatory T cell development: Role of signalling pathways and transcription factors. Clin Dev Immunol. 2013;2013:617595. doi: 10.1155/2013/617595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang W, Beckett O, Ma Q, Paik Jh, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 27.Kerdiles YM, Stone EL, Beisner DL, McGargill MA, Ch'en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omenetti S, Pizarro TT. The Treg/Th17 axis: A dynamic balance regulated by the gut microbiome. Front Immunol. 2015;6:639. doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitta T, Suzuki H. Thymic stromal cell subsets for T cell development. Cell Mol Life Sci. 2016;73:1021–1037. doi: 10.1007/s00018-015-2107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarilin AA, Belyakov IM. Cytokines in the thymus: Production and biological effects. Curr Med Chem. 2004;11:447–464. doi: 10.2174/0929867043455972. [DOI] [PubMed] [Google Scholar]
- 33.Shitara S, Hara T, Liang B, Wagatsuma K, Zuklys S, Holländer GA, Nakase H, Chiba T, Tani-ichi S, Ikuta K. IL-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and TCRγδ+ intraepithelial lymphocytes. J Immunol. 2013;190:6173–6179. doi: 10.4049/jimmunol.1202573. [DOI] [PubMed] [Google Scholar]
- 34.Tian T, Zhang J, Gao L, Qian XP, Chen WF. Heterogeneity within medullary-type TCRalphabeta(+)CD3(+)CD4(−)CD8(+) thymocytes in normal mouse thymus. Int Immunol. 2001;13:313–320. doi: 10.1093/intimm/13.3.313. [DOI] [PubMed] [Google Scholar]
- 35.Chemin K, Bohineust A, Dogniaux S, Tourret M, Guégan S, Miro F, Hivroz C. Cytokine secretion by CD4+ T cells at the immunological synapse requires Cdc42-dependent local actin remodeling but not microtubule organizing center polarity. J Immunol. 2012;189:2159–2168. doi: 10.4049/jimmunol.1200156. [DOI] [PubMed] [Google Scholar]
- 36.Coto JA, Hadden EM, Sauro M, Zorn N, Hadden JW. Interleukin 1 regulates secretion of zinc-thymulin by human thymic epithelial cells and its action on T-lymphocyte proliferation and nuclear protein kinase C; Proc Natl Acad Sci USA; 1992; pp. 7752–7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalloul A, Arock M, Fourcade C, Hatzfeld A, Bertho JM, Debré P, Mossalayi MD. Human thymic epithelial cells produce interleukin-3. Blood. 1991;77:69–74. [PubMed] [Google Scholar]
- 38.Galy AH, Dinarello CA, Kupper TS, Kameda A, Hadden JW. Effects of cytokines on human thymic epithelial cells in culture. II. Recombinant IL 1 stimulates thymic epithelial cells to produce IL6 and GM-CSF. Cell Immunol. 1990;129:161–175. doi: 10.1016/0008-8749(90)90195-W. [DOI] [PubMed] [Google Scholar]
- 39.Savino W, Mendes-da-Cruz DA, Lepletier A, Dardenne M. Hormonal control of T-cell development in health and disease. Nat Rev Endocrinol. 2016;12:77–89. doi: 10.1038/nrendo.2015.168. [DOI] [PubMed] [Google Scholar]
- 40.Savino W, Dardenne M. Neuroendocrine control of thymus physiology. Endocr Rev. 2000;21:412–443. doi: 10.1210/edrv.21.4.0402. [DOI] [PubMed] [Google Scholar]
- 41.Muegge K, Vila MP, Durum SK. Interleukin-7: A cofactor for V(D)J rearrangement of the T cell receptor beta gene. Science. 1993;261:93–95. doi: 10.1126/science.7686307. [DOI] [PubMed] [Google Scholar]
- 42.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 43.Varas A, Vicente A, Romo T, Zapata AG. Role of IL-2 in rat fetal thymocyte development. Int Immunol. 1997;9:1589–1599. doi: 10.1093/intimm/9.10.1589. [DOI] [PubMed] [Google Scholar]
- 44.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol. 2015;16:635–641. doi: 10.1038/ni.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meilin A, Sharabi Y, Shoham J. Analysis of thymic stromal cell subpopulations grown in vitro on extracellular matrix in defined medium-v. Proliferation regulating activities in supernatants of human thymic epithelial cell cultures. Int J Immunopharmacol. 1997;19:39–47. doi: 10.1016/S0192-0561(96)00042-2. [DOI] [PubMed] [Google Scholar]
- 46.Zlotnik A, Ransom J, Frank G, Fischer M, Howard M. Interleukin 4 is a growth factor for activated thymocytes: Possible role in T-cell ontogeny; Proc Natl Acad Sci USA; 1987; pp. 3856–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–27. doi: 10.1016/j.coi.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel DD, Whichard LP, Radcliff G, Denning SM, Haynes BF. Characterization of human thymic epithelial cell surface antigens: phenotypic similarity of thymic epithelial cells to epidermal keratinocytes. J Clin Immunol. 1995;15:80–92. doi: 10.1007/BF01541736. [DOI] [PubMed] [Google Scholar]
- 51.Meilin A, Shoham J, Schreiber L, Sharabi Y. The role of thymocytes in regulating thymic epithelial cell growth and function. Scand J Immunol. 1995;42:185–190. doi: 10.1111/j.1365-3083.1995.tb03644.x. [DOI] [PubMed] [Google Scholar]
- 52.Baseta JG, Stutman O. TNF regulates thymocyte production by apoptosis and proliferation of the triple negative (CD3-CD4-CD8-) subset. J Immunol. 2000;165:5621–5630. doi: 10.4049/jimmunol.165.10.5621. [DOI] [PubMed] [Google Scholar]
- 53.Zúñiga-Pflücker JC, Jiang D, Lenardo MJ. Requirement for TNF-alpha and IL-1 alpha in fetal thymocyte commitment and differentiation. Science. 1995;268:1906–1909. doi: 10.1126/science.7541554. [DOI] [PubMed] [Google Scholar]
- 54.Arzt E, Kovalovsky D, Igaz LM, Costas M, Plazas P, Refojo D, Páez-Pereda M, Reul JM, Stalla G, Holsboer F. Functional cross-talk among cytokines, T-cell receptor, and glucocorticoid receptor transcriptional activity and action. Ann NY Acad Sci. 2000;917:672–677. doi: 10.1111/j.1749-6632.2000.tb05433.x. [DOI] [PubMed] [Google Scholar]
- 55.Cohen-Kaminsky S, Delattre RM, Devergne O, Rouet P, Gimond D, Berrih-Aknin S, Galanaud P. Synergistic induction of interleukin-6 production and gene expression in human thymic epithelial cells by LPS and cytokines. Cell Immunol. 1991;138:79–93. doi: 10.1016/0008-8749(91)90134-W. [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Zhuo Y, Yin L, Wang H, Jiang Y, Liu X, Zhang M, Du F, Xia S, Shao Q. Doxycycline protects thymic epithelial cells from mitomycin C-mediated apoptosis in vitro via Trx2-NF-κB-Bcl-2/Bax axis. Cell Physiol Biochem. 2016;38:449–460. doi: 10.1159/000438642. [DOI] [PubMed] [Google Scholar]
- 57.Shanley DP, Aw D, Manley NR, Palmer DB. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 2009;30:374–381. doi: 10.1016/j.it.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Dooley J, Liston A. Molecular control over thymic involution: From cytokines and microRNA to aging and adipose tissue. Eur J Immunol. 2012;42:1073–1079. doi: 10.1002/eji.201142305. [DOI] [PubMed] [Google Scholar]
- 59.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-X. [DOI] [PubMed] [Google Scholar]
- 60.Xing Y, Hogquist KA. T-Cell tolerance: Central and peripheral. Cold Spring Harb Perspect Biol. 2012;4(pii):a006957. doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts JL, Sharrow SO, Singer A. Clonal deletion and clonal anergy in the thymus induced by cellular elements with different radiation sensitivities. J Exp Med. 1990;171:935–940. doi: 10.1084/jem.171.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 63.Ramsdell F, Fowlkes B. Clonal deletion versus clonal anergy: The role of the thymus in inducing self tolerance. Science. 1990;248:1342–1348. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- 64.Nurieva R, Wang J, Sahoo A. T-cell tolerance in cancer. Immunotherapy. 2013;5:513–531. doi: 10.2217/imt.13.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xing Y, Hogquist KA. T-cell tolerance: Central and peripheral. Cold Spring Harb Perspect Biol. 2012;4(pii):a006957. doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 67.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]
- 69.Müller-Hermelink HK, Sale GE, Borisch B, Storb R. Pathology of the thymus after allogeneic bone marrow transplantation in man. A histologic immunohistochemical study of 36 patients. Am J Pathol. 1987;129:242–256. [PMC free article] [PubMed] [Google Scholar]
- 70.Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008;84:915–923. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boyd E. The weight of the thymus gland in health and disease. Am J Dis Child. 1932;43:1162–1214. [Google Scholar]
- 72.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aw D, Silva AB, Palmer DB. Immunosenescence: Emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fülöp T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol. 2013;4:271. doi: 10.3389/fimmu.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gruver AL, Ventevogel MS, Sempowski GD. Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia-induced thymus involution. J Endocrinol. 2009;203:75–85. doi: 10.1677/JOE-09-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 77.Billard MJ, Gruver AL, Sempowski GD. Acute endotoxin-induced thymic atrophy is characterized by intrathymic inflammatory and wound healing responses. PLoS One. 2011;6:e17940. doi: 10.1371/journal.pone.0017940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol. 2006;177:169–176. doi: 10.4049/jimmunol.177.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou YJ, Peng H, Chen Y, Liu YL. Alterations of thymic epithelial cells in lipopolysaccharide-induced neonatal thymus involution. Chin Med J (Engl) 2016;129:59–65. doi: 10.4103/0366-6999.172577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ann V Griffith, Venables T, Shi J, Farr A, van Remmen H, Szweda L, Fallahi M, Rabinovitch P, Petrie HT. Metabolic damage and premature thymus aging caused by stromal catalase deficiency. Cell Rep. 2015;12:1071–1079. doi: 10.1016/j.celrep.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: Is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 82.Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43:718–728. doi: 10.1016/j.exger.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Min D, Panoskaltsis-Mortari A, Kuro-o M, Holländer GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109:2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rossi SW, Jeker LT, Ueno T, Kuse S, Keller MP, Zuklys S, Gudkov AV, Takahama Y, Krenger W, Blazar BR, Holländer GA. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood. 2007;109:3803–3811. doi: 10.1182/blood-2006-10-049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu HC, Zhang HG, Li L, Yi N, Yang PA, Wu Q, Zhou J, Sun S, Xu X, Yang X, et al. Age-related thymic involution in C57BL/6J × DBA/2J recombinant-inbred mice maps to mouse chromosomes 9 and 10. Genes Immun. 2003;4:402–410. doi: 10.1038/sj.gene.6363982. [DOI] [PubMed] [Google Scholar]
- 86.Frawley R, White K, Jr, Brown R, Musgrove D, Walker N, Germolec D. Gene expression alterations in immune system pathways in the thymus after exposure to immunosuppressive chemicals. Environ Health Perspect. 2010;119:371–376. doi: 10.1289/ehp.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boehm T, Swann JB. Thymus involution and regeneration: Two sides of the same coin? Nat Rev Immunol. 2013;13:831–838. doi: 10.1038/nri3534. [DOI] [PubMed] [Google Scholar]
- 88.Bluth MH, Kohlhoff S, Norowitz KB, Silverberg JI, Chice S, Nowakowski M, Durkin HG, Smith-Norowitz TA. Immune responses in autoimmune hepatitis: Effect of prednisone and azathioprine treatment: Case report. Int J Med Sci. 2009;6:177–183. doi: 10.7150/ijms.6.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marchetti MC, Marco BD, Santini MC, Bartoli A, Delfino DV, Riccardi C. Dexamethasone-induced thymocytes apoptosis requires glucocorticoid receptor nuclear translocation but not mitochondrial membrane potential transition. Toxicol Lett. 2003;139:175–180. doi: 10.1016/S0378-4274(02)00431-9. [DOI] [PubMed] [Google Scholar]
- 90.Gould KA, Shull JD, Gorski J. DES action in the thymus: Inhibition of cell proliferation and genetic variation. Mol Cell Endocrinol. 2000;170:31–39. doi: 10.1016/S0303-7207(00)00336-1. [DOI] [PubMed] [Google Scholar]
- 91.Fletcher AL, Lowen TE, Sakkal S, Reiseger JJ, Hammett MV, Seach N, Scott HS, Boyd RL, Chidgey AP. Ablation and regeneration of tolerance-inducing medullary thymic epithelial cells after cyclosporine, cyclophosphamide, and dexamethasone treatment. J Immunol. 2009;183:823–831. doi: 10.4049/jimmunol.0900225. [DOI] [PubMed] [Google Scholar]
- 92.Camacho IA, Singh N, Hegde VL, Nagarkatti M, Nagarkatti PS. Treatment of mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin leads to aryl hydrocarbon receptor-dependent nuclear translocation of NF-kappaB and expression of Fas ligand in thymic stromal cells and consequent apoptosis in T cells. J Immunol. 2005;175:90–103. doi: 10.4049/jimmunol.175.1.90. [DOI] [PubMed] [Google Scholar]
- 93.Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Larance M, Lamond AI. Multidimensional proteomics for cell biology. Nat Rev Mol Cell Biol. 2015;16:269–280. doi: 10.1038/nrm3970. [DOI] [PubMed] [Google Scholar]
- 95.Leung EL, Cao ZW, Jiang ZH, Zhou H, Liu L. Network-based drug discovery by integrating systems biology and computational technologies. Brief Bioinform. 2013;14:491–505. doi: 10.1093/bib/bbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Turiák L, Misják P, Szabó TG, Aradi B, Pálóczi K, Ozohanics O, Drahos L, Kittel A, Falus A, Buzás EI, Vékey K. Proteomic characterization of thymocyte-derived microvesicles and apoptotic bodies in BALB/c mice. J Proteomics. 2011;74:2025–2033. doi: 10.1016/j.jprot.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 97.Billing AM, Revets D, Hoffmann C, Turner JD, Vernocchi S, Muller CP. Proteomic profiling of rapid non-genomic and concomitant genomic effects of acute restraint stress on rat thymocytes. J Proteomics. 2012;75:2064–2079. doi: 10.1016/j.jprot.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 98.Schulze WX, Usadel B. Quantitation in mass-spectrometry-based proteomics. Annu Rev Plant Biol. 2010;61:491–516. doi: 10.1146/annurev-arplant-042809-112132. [DOI] [PubMed] [Google Scholar]
- 99.Matt P, Fu Z, Fu Q, Van Eyk JE. Biomarker discovery: Proteome fractionation and separation in biological samples. Physiol Genomics. 2008;33:12–17. doi: 10.1152/physiolgenomics.00282.2007. [DOI] [PubMed] [Google Scholar]
- 100.Sultana R, Di Domenico F, Tseng M, Cai J, Noel T, Chelvarajan RL, Pierce WD, Cini C, Bondada S, St Clair DK, Butterfield DA. Doxorubicin-induced thymus senescence. J Proteome Res. 2010;9:6232–6241. doi: 10.1021/pr100465m. [DOI] [PubMed] [Google Scholar]
- 101.Ma C, Yue QX, Guan SH, Wu WY, Yang M, Jiang BH, Liu X, Guo DA. Proteomic analysis of possible target-related proteins of cyclophosphamide in mice thymus. Food Chem Toxicol. 2009;47:1841–1847. doi: 10.1016/j.fct.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 102.Kawakami T, Nagata T, Muraguchi A, Nishimura T. Proteomic approach to apoptotic thymus maturation. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787:223–229. doi: 10.1016/S1570-0232(02)00174-5. [DOI] [PubMed] [Google Scholar]
- 103.Tyanova S, Albrechtsen R, Kronqvist P, Cox J, Mann M, Geiger T. Proteomic maps of breast cancer subtypes. Nat Commun. 2016;7:10259. doi: 10.1038/ncomms10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chan PP, Wasinger VC, Leong RW. Current application of proteomics in biomarker discovery for inflammatory bowel disease. World J Gastrointest Pathophysiol. 2016;7:27–37. doi: 10.4291/wjgp.v7.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peng F, Zhan X, Li MY, Fang F, Li G, Li C, Zhang PF, Chen Z. Proteomic and bioinformatics analyses of mouse liver microsomes. Int J Proteomics. 2012;2012:832569. doi: 10.1155/2012/832569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goh WW, Lee YH, Chung M, Wong L. How advancement in biological network analysis methods empowers proteomics. Proteomics. 2012;12:550–563. doi: 10.1002/pmic.201100321. [DOI] [PubMed] [Google Scholar]
- 107.Miller JF. Immunological function of the thymus. Lancet. 1961;2:748–749. doi: 10.1016/S0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 108.Burns JC, Franco A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert Rev Clin Immunol. 2015;11:819–825. doi: 10.1586/1744666X.2015.1044980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shankar-Hari M, Spencer J, Sewell WA, Rowan KM, Singer M. Bench-to-bedside review: Immunoglobulin therapy for sepsis - biological plausibility from a critical care perspective. Crit Care. 2012;16:206. doi: 10.1186/cc10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gupta M, Noel GJ, Schaefer M, Friedman D, Bussel J, Johann-Liang R. Cytokine modulation with immune gamma-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J Clin Immunol. 2001;21:193–199. doi: 10.1023/A:1011039216251. [DOI] [PubMed] [Google Scholar]
- 111.Chaudhry MS, Velardi E, Malard F, van den Brink MR. Immune reconstitution after allogeneic hematopoietic stem cell transplantation: Time to T Up the thymus. J Immunol. 2017;198:40–46. doi: 10.4049/jimmunol.1601100. [DOI] [PubMed] [Google Scholar]
- 112.Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2013;54:683–687. doi: 10.3109/10428194.2012.728597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ekins S, Gupta RR, Gifford E, Bunin BA, Waller CL. Chemical space: Missing pieces in cheminformatics. Pharm Res. 2010;27:2035–2039. doi: 10.1007/s11095-010-0229-0. [DOI] [PubMed] [Google Scholar]
- 114.Dobson CM. Chemical space and biology. Nature. 2004;432:824–828. doi: 10.1038/nature03192. [DOI] [PubMed] [Google Scholar]