Abstract
Lucilia sericata maggots have beneficial properties; however, their protective effects on burn wounds have yet to be fully elucidated. In the present study, a deep second-degree burn rat model was used to investigate the burn wound healing properties of aqueous extract of maggots (MAE). The anti-inflammatory, antioxidative and antibacterial activities were examined. In addition, the protein expression levels of Akt, vascular endothelial growth factor A (VEGFA) and nuclear factor-κB (NF-κB) were detected by western blotting. The findings of the present study revealed that MAE treatment increased burn wound healing and hydroxyproline content in the burn-treated rats. A total of seven compounds (MAE-P1-P7) were separated from MAE and a comparative study was performed to identify the major active component. The results demonstrated that MAE-P6 exerted greater antibacterial activity compared with the other compounds. MAE-P6 treatment reduced tissue levels of malondialdehyde, tumor necrosis factor-α and interleukin-6, and increased superoxide dismutase activity. Furthermore, MAE-P6 increased the expression levels of VEGFA and reduced NF-κB expression through Akt, which was verified by treatment with the Akt-specific inhibitor, LY294002. In conclusion, the present study demonstrated that the beneficial effects of MAE on burn wound healing were due to its antibacterial, antioxidative and anti-inflammatory activities. MAE-P6 reduced the release of inflammatory cytokines via the Akt/NF-κB signaling pathway, and regulated angiogenesis and vasopermeability via the Akt/VEGFA pathway.
Keywords: aqueous extract of maggots, anti-inflammatory, antioxidative activity, antibacterial activity, burn
Introduction
The number of patients suffering from burn injuries has increased over time (1); this type of chronic and non-healing wound, and its associated treatment, is considered a major medical and economic issue (2,3). Natural and synthetic drugs have been used to treat burn injuries, in order to identify an efficient method to control the damage. Natural remedies are considered safe and cheap therapeutic alternatives; therefore, the demand for them is increasing in developing countries, as well as in certain developed countries. Various plants or animals with antibacterial and healing properties have been identified as effective treatments for burn injuries (4–6), including Lucilia sericata maggots.
L. sericata maggots have been clinically used to treat decubital necrosis; gastrointestinal problems, such as indigestion and malnutrition; ecthyma and burn injuries since the Ming/Qing dynasty (1368 AD) in China. This therapeutic method became popular worldwide for the treatment of chronic and infected wounds in the 1930s (7). Maggot debridement therapy (MDT) is a therapeutic method that has proven effective for the cleansing of non-healing wounds. Previous comparative clinical trials have investigated the efficacy of MDT (8,9), and demonstrated that MDT is more effective compared with conventional therapies in terms of debridement (8). Paul et al (10) used MDT to clinically treat diabetic foot ulcers; the findings revealed that MDT was effective when used as a debridement technique. In addition, the ward stay time and amputation rate were reduced in MDT-treated patients (10). Maggots have been reported to exert several beneficial effects on wounds, including cleansing, disinfection, debridement and healing (11,12).
Maggot therapy has been adopted as an effective debridement technique for the management of chronic wounds (13,14); however, it remains cosmetically unappealing to the majority of patients and nursing staff, and the maggots themselves have a limited shelf life (15). For more fields to benefit from the use of maggots, a switch to contemporary biosurgery, involving the application of maggot-derived substances instead of live insects, should be investigated for future treatments. A previous study isolated and characterized the beneficial molecules in maggot secretions, with the aim of producing them synthetically or as recombinant proteins that may be applied as topical formulations (15). However, a limited number of studies have investigated the beneficial molecules in maggot secretions and the underlying molecular mechanisms (16,17).
In the present study, antimicrobial proteins were extracted and purified from the body of maggots and their biological activity was investigated in vivo. In addition, the underlying molecular mechanisms of action on second-degree burn injury were evaluated.
Materials and methods
Sterile larvae and crude protein extract
A laboratory colony of L. sericata flies was obtained from Xi'an Furulin Biological Technology Co., Ltd. (Xi'an, China) and sterile maggots were reared in our laboratory, according to a previously described method by Mumcuoglu et al (18). The maggots were 4–5 days old prior to extraction of the proteins.
To extract the active ingredients from the maggots, the maggots were frozen in liquid nitrogen and homogenized using a mortar, subsequently the homogenate was lyophilized and the pulverized material was dissolved in 0.1 µg/ml aprotinin in 0.1% trifluoroacetic acid (1:10 v/v); the suspension was maintained on ice in the dark for 1 h and centrifuged 3 times at 20,000 × g at 4°C for 30 min to remove residues. The supernatant was collected and stored at −80°C until further use.
Purification of the crude protein extract
To purify the crude extract, the Sephadex G-50 fine column (26×1,000 mm; GE Healthcare Life Sciences, Chalfont, UK) was used and the extract was eluted at 2 ml/min. Eluted components were collected corresponding to the absorbance at 280 nm and lyophilized. To analyze the proteins, SDS-PAGE was conducted; MAE samples were separated on a 5.0% stacking gel and 12.5% separation gel, followed by Coomassie staining (Nanjing Jiancheng Bioengineering Institute). To determine the antibacterial activity of the extract, and for the performance of other bioassays, the fractions were dissolved in water to 50 µg/ml.
Antibacterial tests
Antibacterial activity was tested using a method described by Huberman (19). Briefly, Escherichia coli (ATCC 25922) was purchased from the China Center of Type Culture Collection (Wuhan, China). E. coli was cultured in 20 ml tryptic soy broth (TSB; BD Biosciences, Franklin Lakes, NJ, USA) at 30°C for 17 h. Subsequently, 100 µl culture was mixed with 10 ml TSB and incubated for a further 4 h (density ~2×108/ml). Nutrient agar (Oxoid, Ltd., Basingstoke, UK) was inoculated with 10 µl bacterial culture, at a final density of 4×104/ml. After gentle mixing, the agar was immediately distributed into Petri dishes (85-mm). After cooling and solidification, holes (4-mm-diameter) were bored into the agar, and 10 µl samples [aqueous extract of maggots (MAE) P1-P7] were loaded into the holes. Sterile ultrapure water was included as a control. Plates were incubated at 30°C overnight and the diameters of bacterial growth inhibition were measured.
Burn injury model and drug administration
Male Wistar rats (n=100; age, 20±1 weeks; weight, 200–250 g) were provided by the Experimental Animal Center of the Fourth Military Medical University (Xi'an, China). Animals were housed in a room at a controlled temperature (25°C) under a 12 h light/dark cycle with free access to food and water. The present study was approved by the Ethics Committee of the Fourth Military Medical University.
Rats were attached to a homemade wooden support and anesthetized by 100 mg/kg ketamine and 8 mg/kg xylazine through intraperitoneal injection. Back hair was removed and the exposed skin was immersed in 98°C water for 15 sec to induce a second-degree burn. A group of animals (n=8) was not burned and served as controls. Jingwanhong ointment (JWH; Tianjin Darentang Jingwanhong Pharmaceutical Co., Ltd., Tianjin, China) was used as a positive control. The animals were divided into the following four groups (n=8/group): i) Control group; ii) burn group (model); iii) burn + MAE (0.2 g per wound) group; and iv) burn + JWH (0.2 g per wound) group. All the tested medicines were applied on the surface of the wound slowly. The wounds were treated every 24 h for 14 days. To evaluate effects of MAE-P1-P7 on burn wound, MAE-P1-P7 (0.2 g per wound; n=8 rats/treatment) was administered to rats in the burn + MAE group. In some experiments, LY294002 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was intraperitoneally administered together with MAE-P6, at a dose of 0.3 mg/kg. The burn wound area was observed after all treatments on the 14th day and the rats were anesthetized with ketamine (75 mg/kg) and sacrificed by decapitation. Blood was subsequently collected, and wound tissues were collected and frozen at −80°C until use.
Determination of hydroxyproline levels
A hydroxyproline assay was used to quantify collagen deposition in the wound area. Tissue samples (7 mg) were obtained, subcutaneous fat was removed, and they were incubated at 60°C in open microtubes for 20 h to obtain the dry weight. Subsequently, the samples were hydrolyzed with 6 mol/l HCl by stirring for 15 h at 120°C. The lysate was diluted with deionized water and neutralized with NaOH. Subsequently, the lysate was centrifuged at 1,000 × g at 25°C for 15 min, and the supernatant was collected for hydroxyproline assay. The hydroxyproline levels were quantified using the hydroxyproline assay kit (catalog no. A030-1; Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's protocol. Hydroxyproline levels were expressed as mg/g of tissue.
Determination of malondialdehyde (MDA) levels
MDA is an index of lipid peroxidation. The MDA level was quantified using a commercial kit (catalog no. A003; Nanjing Jiancheng Bioengineering Institute), which results in the formation of thiobarbituric acid-reactive substances during an acid-heating reaction. To precipitate proteins, tissue samples were mixed with 600 µl 10% (w/v) trichloroacetic acid and then centrifuged at 1,000 × g for 10 min at 25°C. Supernatants were collected and treated with an equal volume of 0.67% thiobarbituric acid in a boiling water bath for 15 min. After cooling for 5 min the reaction products were measured at 535 nm. MDA (nmol/mg)=(ODtest-ODblank)/(ODstandard-ODblank)x10/protein concentration, where OD refers to optical density. The level of MDA was expressed as nmol/mg of protein.
Determination of superoxide dismutase (SOD) levels
The enzyme activity of SOD was quantified using a spectrophotometric assay kit (catalog no. A001-3; Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's protocol. Briefly, the proteins in the tissue samples were extracted using a protein extraction kit (catalog no. P0033; Beyotime Institute of Biotechnology, Nanjing, China). The protein samples were incubated with the provided radical detector reagent and xanthine oxidase was subsequently added to initiate the reaction. Following incubation at room temperature for 20 min with agitation, the reaction system was measured at 450 nm. SOD (U/mg prot)=(ODstandard-ODtest)/ODstandard/0.5/protein concentration, where OD refers to optical density. The levels of SOD were expressed as U/mg of protein.
Determination of serum levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)
Blood samples were collected 14 days after burns. To separate serum, blood samples were maintained at room temperature for 1 h, and were centrifuged at 1,000 × g for 10 min at 25°C. Serum levels of TNF-α and IL-6 were quantified using rat TNF-α ELISA kit (catalog no. PT516) and rat IL-6 ELISA kit (catalog no. PI328; Beyotime Institute of Biotechnology), according to the manufacturer's protocols. The level was expressed as ng/l.
Western blot analysis
Proteins in excised tissues were homogenized using a protein extraction kit (catalog no. P0033; Beyotime Institute of Biotechnology) and were centrifuged at 12,000 × g for 15 min at 4°C. Protein concentration was determined using a bicinchoninic acid kit (catalog no. A045-3; Nanjing Jiancheng Bioengineering Institute). The supernatant was then collected, mixed with 2X SDS loading buffer and denatured at 100°C for 5 min. Equal amounts of protein (40 µg) were separated by 10% SDS-PAGE. Subsequently the proteins were transferred to nitrocellulose membranes (Pall Corporation, New York, NY, USA), which were blocked with 5% non-fat dry milk in 1X Tris-buffered saline. The membranes were then incubated with Akt (catalog no. ab79360), phosphorylated (p)-Akt (catalog no. ab81283), nuclear factor (NF)-κB (catalog no. ab28856) and vascular endothelial growth factor A (VEGFA; catalog no. ab51745) rabbit anti-rat monoclonal antibodies (1:1,000; Abcam, Cambridge, UK) at 4°C for 8 h and were then incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (catalog no. sc-2357; 1:5,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or horseradish peroxidase-conjugated anti-mouse secondary antibody (catalog no. ab97255; 1:5,000; Abcam). β-actin (1:1,000; catalog no. ab8226; Abcam) was used as a control. All bands were detected by enhanced chemiluminescence (ECL) using the ECL Western Blot detection kit (GE Healthcare Life Sciences). Western blots were semi-quantified by densitometric analysis using Quantity One 1-D software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Analysis of microvessel density (MVD) and VEGFA expression
After the rats were anesthetized with ketamine, 4% paraformaldehyde was routinely perfused through left ventricle for 1 h, and wound tissue was collected and fixed in 4% paraformaldehyde for 24 h. Sections (5.0 µm) were obtained from paraffin-embedded tissues, and were then deparaffinized and rehydrated. To block endogenous peroxidase activity, sections were treated with 3% 2O2 methanol solution for 10 min at room temperature. For antigen retrieval, sections were boiled in 0.01 mol/l citric acid for 20 min and blocked with 3% skim milk powder solution for 30 min at 37°C. The sections were then washed with PBS and incubated with cluster of differentiation (CD)31 monoclonal antibody (catalog no. sc-376764) or rabbit anti-rat VEGFA (catalog no. sc-152) monoclonal antibody (1:10; Santa Cruz Biotechnology, Inc.) at 37°C for 1 h. Sections were washed with PBS, incubated with biotinylated goat anti-mouse (1:100; catalog no. BA1001) and biotinylated goat anti-rabbit secondary antibody (1:100; catalog no. BA1003; Boster Biological Technology Co., Ltd., Zhongshan, China) for 30 min at 37°C and were subsequently incubated with horseradish peroxidase-labeled streptavidin (catalog no. BA1088; Boster Biological Technology Co., Ltd.) for 30 min at 37°C. Sections were stained with 3,3′-diaminobenzidine/H2O2 and hematoxylin, dehydrated, cleared and mounted for viewing. The quantity of CD31-positive small vessels was observed under a microscope (×400 magnification; DM2500; Leica Microsystems GmbH, Wetzlar, Germany) to determine MVD. The number of counted microvessels per mm2 was considered the MVD. MVD of a sample was calculated as the average MVD of five selected areas. An additional five areas were randomly selected for VEGFA analysis. To calculate VEGFA expression, integrated optical density and density mean of positive expression were used; the images were analyzed with Image Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Data were recorded as fold-change.
Statistical analysis
Each experiment was repeated at least three times. Data are presented as the mean ± standard deviation. SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Statistically significant differences between treatment groups were determined by one-way analysis of variance, followed by Tukey test for multiple comparisons between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
MAE reduces the size of the wound area
To evaluate the effects of MAE treatment on burn wound closure, wound contraction was measured. As presented in Fig. 1A, an increase in wound-healing activity was observed in the rats treated with MAE and JWH, as compared with the burn model rats that did not receive treatment. In addition, it was demonstrated that the wound-healing activity of MAE was greater compared with JWH.
Figure 1.
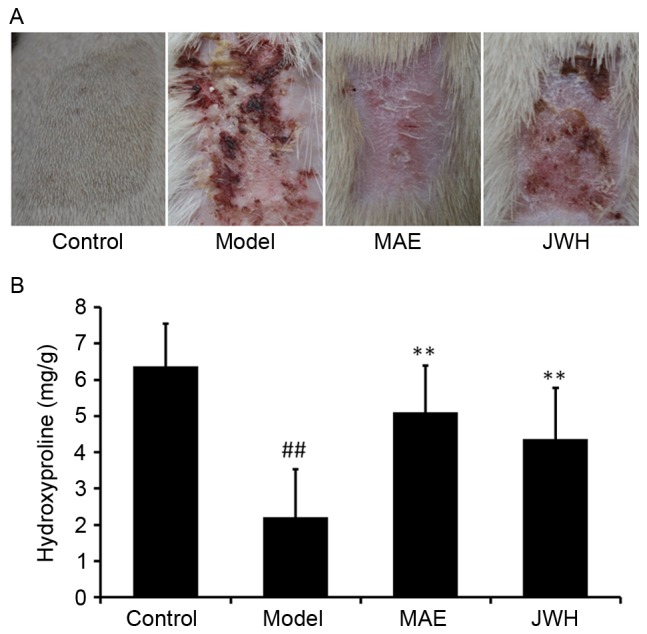
MAE exerted a burn wound healing effect. (A) Wound appearance following 14 days of treatment with MAE. MAE increased wound healing compared with the model group. (B) Effects of MAE on hydroxyproline levels in the burn wound model. Data are expressed as the mean ± standard deviation. ##P<0.05 vs. the control group, **P<0.05 vs. the model group. MAE, aqueous extract of maggots; JWH, Jingwanhong ointment.
Hydroxyproline, which is integral to collagen fibers, may promote wound healing in tissue injury (20). In various wound models, an increase in hydroxyproline content may indicate increased collagen synthesis. In the burn wound model, the level of hydroxyproline in granulation tissue was significantly reduced compared with in the control group (Fig. 1B; P<0.05). However, MAE and JWH treatment significantly increased the hydroxyproline level compared with the model group (P<0.05). These findings suggested that MAE may be an effective treatment option for second-degree burns.
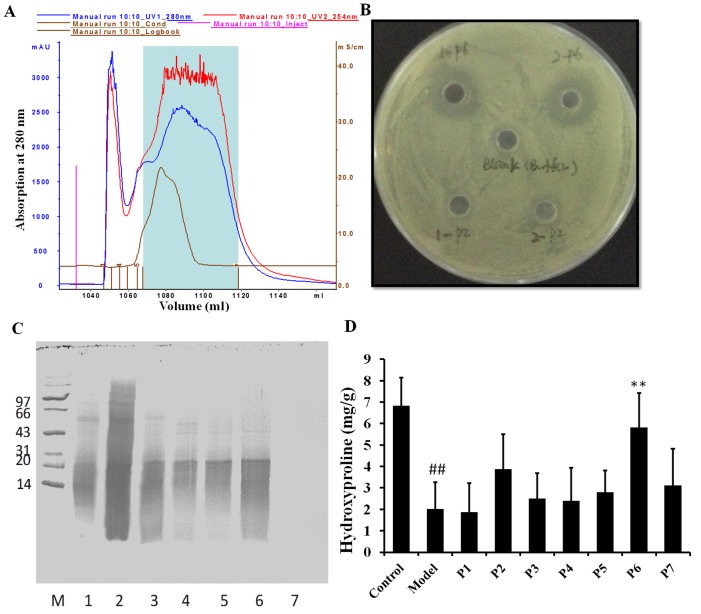
Partial purification and characterization of the antibacterial components of MAE
In order to identify the major active ingredients in MAE, Sephadex G-50 fine column was previously used (21). Different components were identified according to their UV absorption characteristics (Fig. 2A). Finally, a total of 7 compounds were collected. Antibacterial tests demonstrated that compound 6 (MAE-P6) had a high antibacterial activity, whereas the remaining compounds had little or no antibacterial activity (Fig. 2B). Examination of these fractions by SDS-PAGE revealed that the majority of proteins in the MAE-P6 were <20 kDa molecular weight (Fig. 2C). In order to determine if the various compounds exert anti-burn activity, MAE-P1-P7 were used to treat rats in the burn wound model group. The results demonstrated that MAE-P6 treatment significantly increased hydroxyproline levels compared with the burn model (P<0.05); however, no significant difference was identified between the remaining compounds and the burn model (Fig. 2D). Therefore, MAE-P6 may be responsible for the antibacterial activity and healing properties of MAE. MAE-P6 was used for the subsequent experiments.
Figure 2.
Partial purification and characterization of antibacterial components of MAE. (A) UV absorption characteristics of the various components. (B) Antibacterial tests. Various components (P1-P7) were tested on Escherichia coli (dose, 50 µg/ml). The antibacterial activity of P2 and P6 is presented. (C) SDS-PAGE analysis of the different components. Lane 1, P1; lane 2, P2; lane 3, P3; lane 4, P4; lane 5, P5; lane 6, P6; lane 7, P7. (D) Effects of MAE P1-P7 on hydroxyproline levels in the burn wound model. Data are expressed as the mean ± standard deviation. ##P<0.05 vs. the control group, **P<0.05 vs. the model group. MAE, aqueous extract of maggots.
Effects of MAE on oxidative damage and inflammation
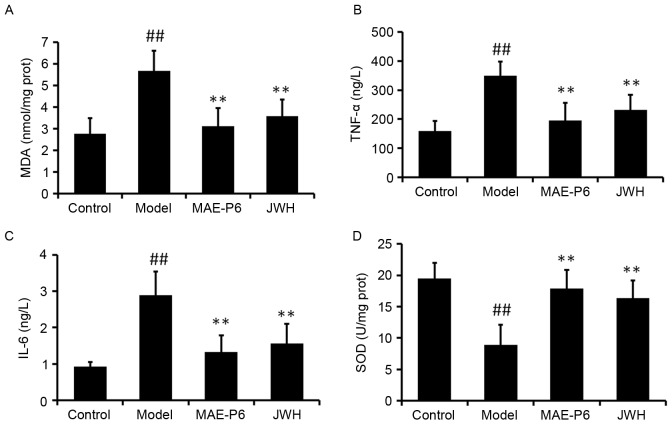
High intracellular levels of reactive oxygen species (ROS), which occur during the initial stages following a burn injury, have been identified as the inducers of oxidative damage. A previous study suggested that topical application of antioxidants may provide a photoprotective effect and may be considered an effective strategy for reducing burn-induced oxidative damage to the skin (22). The level of MDA is an indication of lipid peroxidation in tissues. The findings of the present study demonstrated that treatment with MAE-P6 significantly reduced the burn-mediated increase of epidermal MDA when compared with the model group (Fig. 3A; P<0.05). Following a burn injury, inflammatory cells are recruited to the wound site, initiating the inflammatory stage of the healing process (23). The present study examined the effects of MAE-P6 on burn-induced expression levels of cytokines TNF-α and IL-6. A significant increase in the levels of TNF-α and IL-6 was observed following the administration of the burn compared with the control group (Fig. 3B and C; P<0.05). However, topical application of MAE-P6 for 14 days post-burn significantly reduced the levels of IL-6 and TNF-α compared with in the burn model group (Fig. 3B and C; P<0.05). SOD acts as the first line of defense against superoxide anions (O2•), by dismutating these anions to H2O2. SOD activity was significantly reduced in the burn model group compared with the control group (Fig. 3D; P<0.05). However, this activity was significantly increased following 14 days of treatment with MAE-P6 when compared with the burn model group (Fig. 3D; P<0.05). These findings revealed that MAE-P6 was able to reduce levels of oxidative stress in the rats, potentially limiting the damaging effect of ROS following burn administration.
Figure 3.
Effects of MAE-P6 on oxidative damage and inflammation. The levels of (A) MDA, (B) TNF-α and (C) IL-6 were increased, whereas the levels of (D) SOD were decreased in the burn model. Application of MAE-P6 following burn treatment led to a significant increase in the activity of SOD and a reduction in the levels of MDA, TNF-α and IL-6 after 14 days of treatment. Data are expressed as the mean ± standard deviation. ##P<0.05 vs. the control group, **P<0.05 vs. the model group. MDA, malondialdehyde; MAE-P6, aqueous extract of maggots-compound 6; JWH, Jingwanhong ointment; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; SOD, superoxide dismutase.
Effects of MAE on MVD and VEGFA expression
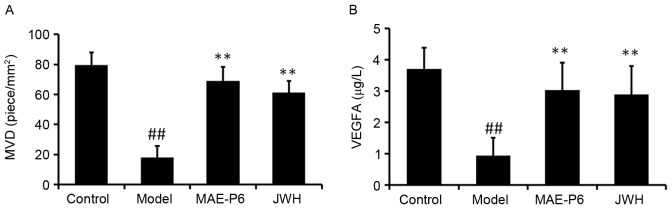
MVD and VEGFA levels in the model group were significantly reduced compared with in the control group 14 days after the burn was administered (Fig. 4; P<0.05). MVD and VEGFA levels in the MAE-P6 and JWH groups were significantly higher compared with in the model group (Fig. 4; P<0.05). In addition, the MVD and VEGFA levels in the MAE-P6 group were slightly higher when compared with the JWH group, but had no significant difference.
Figure 4.
Effects of MAE-P6 on MVD and VEGFA expression in the wound tissues. Wound tissue sections were collected 14 days after burning. (A) MVD and (B) VEGFA were detected using anti-rat cluster of differentiation 31 monoclonal antibody and mouse anti-rat VEGFA monoclonal antibody, respectively. The number of microvessels counted per mm2 was considered MVD. For VEGFA, the images were analyzed using Image Pro Plus version 6.0. Data are expressed as the mean ± standard deviation. ##P<0.05 vs. the control group, **P<0.05 vs. the model group. MVD, microvessel density; MAE-P6, aqueous extract of maggots-compound 6; JWH, Jingwanhong ointment; VEGFA, vascular endothelial growth factor A.
Effects of MAE on the expression levels of Akt, NF-κB and VEGFA
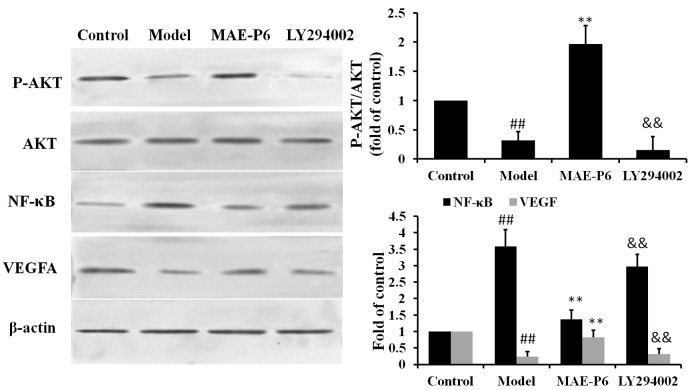
The Akt signaling pathway regulates numerous physiological processes associated with apoptosis, oxidative stress and inflammation (24). Therefore, the present study determined the effects of topical administration of MAE-P6 on the phosphorylation of Akt following burn administration using western blot analysis. The findings indicated that phosphorylation of Akt was significantly reduced post-burn, as compared with in the control group (Fig. 5; P<0.05). Conversely, MAE-P6 treatment significantly increased phosphorylation of Akt compared with the burn model group (Fig. 5; P<0.05).
Figure 5.
Effects of MAE-P6 on the expression of Akt, NF-κB and VEGFA. Rats were sacrificed 14 days after the burn treatment and tissue lysates were prepared to determine the protein expression levels of Akt, p-Akt, NF-κB and VEGFA using western blot analysis. Data are expressed as the mean ± standard deviation. ##P<0.05 vs. the control group, **P<0.05 vs. the model group, &&P<0.05 vs. the MAE-P6 group. p-Akt, phosphorylated-Akt; NF-κB, nuclear factor-κB; VEGFA, vascular endothelial growth factor A; MAE-P6, aqueous extract of maggots-compound 6.
NF-κB is a downstream target of the Akt signal transduction pathway. Western blot analysis indicated that the expression of NF-κB was significantly increased in the burn model group compared with in the control group (Fig. 5; P<0.05). Topical application of MAE-P6 significantly reduced the burn-induced increase in NF-κB expression. In addition, the effects of MAE-P6 on the possible regulation of VEGFA signaling were investigated. MAE-P6 reversed the downregulation of VEGFA protein expression, which occurred following burn exposure (Fig. 5). Furthermore, inhibition of Akt by LY294002 eliminated these effects. These findings indicated that MAE-P6 possibly inhibited NF-κB expression and increased VEGFA expression via phosphorylation of the Akt signaling pathway.
Discussion
Burns represent a devastating form of trauma, which are associated with severe pain, distress and reduced quality of life for numerous patients worldwide. Thermal injury may severely compromise the skin barrier, affecting important functions, such as protection against infection, thermoregulation and body fluid homeostasis (25,26). Effective treatments associated with wound infection control and wound healing are important for a successful recovery (27). Burn wound healing is categorized into three overlapping stages: i) Inflammation; ii) granulation tissue formation; and iii) remodeling (19). Therefore, acceleration of the rate of tissue regeneration, inhibition of inflammation or oxidative stress, and prevention of wound infection are crucial procedures for burn treatment (28). Natural products, such as insects or plants, may be considered promising for the treatment of burn injuries, and may be used worldwide. A particularly interesting therapeutic strategy in traditional Chinese medicine is the use of maggots, which are the larvae of insects and terrestrial arthropods; however, only a small number of studies regarding this treatment strategy are available (13,14). The present study aimed to determine the burn wound healing and antibacterial properties of MAE in rats, and observed that MAE treatment accelerated wound contraction 14 days after burn administration, indicating the beneficial effects of this therapeutic agent.
Fibroblasts, which are responsible for the deposition, synthesis and remodeling of the extracellular matrix, are the primary cell type involved in the wound healing process (29). At the initiation of wound repairing, fibroblasts undergo in situ proliferation and differentiation, then migrate from the edges of the wound to the wound site, proliferate again and begin to produce collagen (30). In the healing phase, collagen content in the granulation tissue may be quantified by monitoring the concentration of hydroxyproline, which is a marker of collagen biosynthesis (31). Therefore, the levels of hydroxyproline represent the rate of collagen synthesis. In the present study, 14 days after treatment, an increase in hydroxyproline content was observed in the MAE group (5.11±1.28 mg/g dry weight of tissue), which was significantly higher compared with in the model group (2.21±1.32 mg/g). These findings indicated that collagen synthesis was improved by treatment with MAE. Therefore, the wound healing effects of MAE treatment may be due to increases in collagen content.
Bacterial infections may occur at the early stages of wound healing, which may delay healing, and lead to failure of healing or wound deterioration (31). Pathogenic microorganisms delay wound healing via various mechanisms, including persistent production of inflammatory mediators, induction of tissue hypoxia, production of cytolytic enzymes and free oxygen radicals, and competitive inhibition of nutrient and oxygen levels (32). Therefore, keeping the wound clean in order to avoid infection or maintaining a good bioburden is necessary for successful wound healing. The present study identified seven compounds isolated from MAE and their bacteriostatic action on Gram-negative bacterial growth was evaluated. As the findings of the present study demonstrated, compound P6 (MAE-P6) exerted a significantly greater antibacterial effect compared with the remaining six compounds. Further investigation revealed that MAE-P6 may increase the levels of hydroxyproline in the wound healing model. Therefore, the antibacterial activity of MAE-P6 may reduce the number of pathogens growing on the skin, thus promoting wound healing.
Exposure to a pathogenic microorganism may activate neutrophils, which produce ROS and lead to oxidative stress. In burns, oxidative stress is a pathogenic factor in the inflammatory response; the excessive production of free radicals recruits inflammatory cells and may lead to endothelial dysfunction (33). However, antioxidants, such as SOD, catalase and glutathione, may eliminate free radicals and accelerate the process of burn wound healing. So it is necessary to estimate these antioxidants in granulation tissues. Significant alterations in the antioxidant profile, combined with elevated levels of MDA, which is a marker of fatty acid oxidation, may lead to impaired wound healing in rats (34). In the present study, the levels of SOD and MDA were quantified in the granulation tissue. The results demonstrated that treatment with MAE-P6 significantly increased the activity of SOD, and reduced the levels of MDA compared with in the burn model group. Notably, MAE-P6 treatment also inhibited the expression levels of the inflammatory cytokines IL-6 and TNF-α.
The surface of burn wounds may be anoxic or hypoxic; both conditions limit the growth of ulcerated or injured tissues. Therefore, treatment that may improve the microcirculation of the burn wound may also improve partial ischemia and hypoxia, and promote healing. VEGFA has previously been described as an important stimulator of angiogenesis and vasopermeability, and is produced by macrophages in the hypoxic burn environment, in order to stimulate the migration and proliferation of endothelial cells (35,36). In the present study, the increase in VEGF and MVD levels coincided with a faster decrease of the wound area (Fig. 4), thus suggesting the importance of angiogenic stimulation by MAE-P6 in the overall acceleration of wound healing.
The possible signaling molecules were also investigated in the present study, in order to determine the potential regulatory targets associated with MAE-P6. The phosphorylation of Akt, which is considered a crucial node of the phosphoinositide 3-kinase-Akt pathway, may be regarded as a cell survival factor via the inhibition of apoptosis and downstream NF-κB, resulting in the inhibition of inflammation and oxidative stress (37,38). The findings of the present study revealed that the post-burn reduction in p-Akt was accompanied by an increased release of IL-6 and TNF-α, and an increase in the expression levels of NF-κB. Conversely, MAE-P6 administration led to increased expression levels of p-Akt and reduced NF-κB expression levels. Inhibition of Akt with LY294002 attenuated the expression of p-Akt and increased the expression of NF-κB. Therefore, it may be hypothesized that the Akt/NF-κB signaling pathway contributes to MAE-P6-induced inflammatory regulation in burn wounds. Furthermore, inhibition of Akt with LY294002 also inhibited the expression of VEGFA, whereas rats treated with MAE-P6 only exhibited increased expression of VEGFA.
In conclusion, to the best of our knowledge, the present study was the first to demonstrate the beneficial effects of MAE on burn wound healing via its antibacterial, antioxidative and anti-inflammatory activities. It is possible that MAE-P6 functions via the Akt/NF-κB signaling pathway to regulate the release of inflammatory cytokines and free radicals. MAE-P6 may also regulate angiogenesis and vasopermeability via the Akt/VEGFA pathway. The findings of the present study suggested that MAE-P6 has multi-target mechanisms for improving burn wound healing, and may provide useful information for the development of burn wound healing treatments.
Acknowledgements
The present study was supported by the National Science Foundation of China (grant nos. 81470174, 81173514, 81303264, 81403134, 81403135 and 81403182) and the Xijing Research Boosting Program (grant no. XJZT14D06).
References
- 1.Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463:127–136. doi: 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Lay-flurrie K. Honey in wound care: Effects, clinical application and patient benefit. Br J Nurs. 2008;17:S30–S36. doi: 10.12968/bjon.2008.17.Sup5.29649. [DOI] [PubMed] [Google Scholar]
- 3.Riedel K, Ryssel H, Koellensperger E, Germann G, Kremer T. Pathogenesis of chronic wounds. Chirurg. 2008;79:526–534. doi: 10.1007/s00104-008-1501-2. (In German) [DOI] [PubMed] [Google Scholar]
- 4.Ghasemi Pirbalouti A. Medicinal plants used in Chaharmahal and Bakhtyari districts. Iran Herba Pol. 2009;55:34–38. [Google Scholar]
- 5.Nayak BS, Isitor G, Davis EM, Pillai GK. The evidence-based wound healing activity of Lawsonia inermis Linn. Phytother Res. 2007;21:827–831. doi: 10.1002/ptr.2181. [DOI] [PubMed] [Google Scholar]
- 6.Bahramsoltani R, Farzaei MH, Rahimi R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch Dermatol Res. 2014;306:601–617. doi: 10.1007/s00403-014-1474-6. [DOI] [PubMed] [Google Scholar]
- 7.Hou L, Shi Y, Zhai P, Le G. Antibacterial activity and in vitro anti-tumor activity of the extract of the larvae of the housefly (Musca domestica) J Ethnopharmacol. 2007;111:227–231. doi: 10.1016/j.jep.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Sherman RA. Maggot versus conservative debridement therapy for the treatment of pressure ulcers. Wound Repair Regen. 2002;4:208–214. doi: 10.1046/j.1524-475X.2002.10403.x. [DOI] [PubMed] [Google Scholar]
- 9.Dumville JC, Worthy G, Bland JM, Cullum N, Dowson C, Iglesias C, Mitchell JL, Nelson EA, Soares MO, Torgerson DJ. VenUS II team: Larval therapy for leg ulcers (VenUS II): Randomised controlled trial. BMJ. 2009;338:b773. doi: 10.1136/bmj.b773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul AG, Ahmad NW, Lee HL, Ariff AM, Saranum M, Naicker AS, Osman Z. Maggot debridement therapy with Lucilia cuprina: A comparison with conventional debridement in diabetic foot ulcers. Inter Wound J. 2009;6:39–46. doi: 10.1111/j.1742-481X.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wollina U, Liebold K, Schmidt WD, Hartmann M, Fassler D. Biosurgery supports granulation and debridement in chronic wounds-clinical data and remittance spectroscopy measurement. Int J Dermatol. 2002;41:635–639. doi: 10.1046/j.1365-4362.2002.01354.x. [DOI] [PubMed] [Google Scholar]
- 12.Huberman L, Gollop N, Mumcuoglu KY, Block C, Galun R. Antibacterial properties of whole body extracts and haemolymph of Lucilia sericata maggots. J Wound Care. 2007;16:123–127. doi: 10.12968/jowc.2007.16.3.27011. [DOI] [PubMed] [Google Scholar]
- 13.Pinheiro MA, Ferraz JB, Junior MA, Moura AD, da Costa ME, Costa FJ, Neto VF, Neto RM, Gama RA. Use of maggot therapy for treating a diabetic foot ulcer colonized by multidrug resistant bacteria in Brazil. Indian J Med Res. 2015;141:340–342. doi: 10.4103/0971-5916.156628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nigam Y, Morgan C. Does maggot therapy promote wound healing? The clinical and cellular evidence. J Eur Acad Dermatol. 2016;30:776–782. doi: 10.1111/jdv.13534. [DOI] [PubMed] [Google Scholar]
- 15.Vilcinskas A. From traditional maggot therapy to modern biosurgery. In: Vilcinskas A, editor. Insect Biotechnology. Dordrecht, Netherlands: Springer; 2011. pp. 67–755. [DOI] [Google Scholar]
- 16.Bohova J, Majtan J, Majtan V, Takac P. Selective antibiofilm effects of Lucilia sericata larvae secretions/excretions against wound pathogens. Evid Based Complement Alternat Med. 2014;2014:857360. doi: 10.1155/2014/857360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hultmark D. In: Techniques in Insect Immunology. Weisner A, Dunphy GB, Marmaras VJ, Morishima I, Sugumaran M, Yamakawa M, editors. SOS Publications; New Jersey: 1998. pp. 103–1075. [Google Scholar]
- 18.Mumcuoglu KY, Ingber A, Gilead L, Stessman J, Friedmann R, Schulman H, Bichucher H, Ioffe-Uspensky I, Miller J, Galun R, Raz I. Maggot therapy for the treatment of intractable wounds. Int J Dermatol. 1999;8:623–627. doi: 10.1046/j.1365-4362.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 19.Huberman L, Gollop N, Mumcuoglu KY, Block C, Galun R. Antibacterial properties of whole body extracts and haemolymph of Lucilia sericata maggots. J Wound Care. 2007;16:123–127. doi: 10.12968/jowc.2007.16.3.27011. [DOI] [PubMed] [Google Scholar]
- 20.Morellini NM, Giles NL, Rea S, Adcroft KF, Falder S, King CE, Dunlop SA, Beazley LD, West AK, Wood FM, Fear MW. Exogenous metallothionein-IIA promotes accelerated healing after a burn wound. Wound Repair Regen. 2008;16:682–690. doi: 10.1111/j.1524-475X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Chen ZW. Isolation, characterization and anti-cancer activity of SK84, a novel glycine-rich antimicrobial peptide from Drosophila virilis. Peptides. 2010;31:44–50. doi: 10.1016/j.peptides.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Sen CK. The general case for redox control of wound repair. Wound Repair Regen. 2003;11:431–438. doi: 10.1046/j.1524-475X.2003.11607.x. [DOI] [PubMed] [Google Scholar]
- 23.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan B, Akcakanat A, Holder AM, Meric-Bernstam F. Targeting the PI3-kinase/Akt/mTOR signaling pathway. Surg Oncol Clin N Am. 2013;22:641–664. doi: 10.1016/j.soc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hettiaratchy S, Dziewulski P. ABC of burns. BMJ. 2004;328:1366–1368. doi: 10.1136/bmj.328.7452.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeSanti L. Pathophysiology and current management of burn injury. Adv Skin Wound Care. 2005;18:323–332. doi: 10.1097/00129334-200507000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, Chan RK, Christy RJ, Chung KK. Burn wound healing and treatment: Review and advancements. Critical Care. 2015;19:243. doi: 10.1186/s13054-015-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinz B. The myofibroblast: Paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 31.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macri L, Clark RA. Tissue engineering for cutaneous wounds: Selecting the proper time and space for growth factors, cells and the extracellular matrix. Skin Pharmacol Physiol. 2009;22:83–93. doi: 10.1159/000178867. [DOI] [PubMed] [Google Scholar]
- 33.Richelle M, Sabatier M, Steiling H, Williamson G. Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Br J Nutr. 2006;96:227–238. doi: 10.1079/BJN20061817. [DOI] [PubMed] [Google Scholar]
- 34.Gupta A, Singh RL, Raghubir R. Antioxidant status during cutaneous wound healing in immunocompromised rats. Mol Cell Biochem. 2002;241:1–7. doi: 10.1023/A:1020804916733. [DOI] [PubMed] [Google Scholar]
- 35.Mast BA, Schultz GS. Interactions of cytokines, growth factors and proteases in acute, and chronic wounds. Wound Repair Regen. 1996;4:411–420. doi: 10.1046/j.1524-475X.1996.40404.x. [DOI] [PubMed] [Google Scholar]
- 36.Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;41:149–161. [PubMed] [Google Scholar]
- 37.Baehrecke EH. Autophagy: Dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: Autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans. 2013;41:1103–1130. doi: 10.1042/BST20130134. [DOI] [PubMed] [Google Scholar]