Abstract
Preeclampsia (PE), a life-threatening, complicated pregnancy-associated disease, has recently become a research focus in obstetrics. However, the peptidome of the amniotic fluid in PE patients has rarely been investigated. The present study used peptidomic profiling to perform a comparative analysis of human amniotic fluid between normal and PE pregnancies. Centrifugal ultrafiltration and liquid chromatography-tandem mass spectrometry (LC-MS/MS) was combined with isotopomeric dimethyl labels to gain a deeper understanding of the role of proteins and the peptidome in the onset of PE. Following ultrafiltration and LC-MS/MS, 352 peptides were identified. Of these, 23 peptides were observed to be significantly differentially expressed (6 downregulated and 17 upregulated; P<0.05). Using Gene Ontology and Blastp analyses, the functions and biological activities of these 23 peptides were identified and revealed to include autophagy, signal transduction, receptor activity, enzymatic activity and nucleic acid binding. In addition, a bibliographic search revealed that some of the identified peptides, including Titin, are crucial to the pathogenesis underlying PE. The present study identified 23 peptides expressed at significantly different levels in the amniotic fluid of PE and normal pregnancies. A comprehensive peptidome analysis is more efficient than a simple biomarker analysis at revealing deficiencies and improving the detection rate in diseases. These analyses therefore provide a substantial advantage in applications aimed at the discovery of disease-specific biomarkers.
Keywords: preeclampsia, peptidomics, amniotic fluid, liquid chromatography-tandem mass spectrometry, biomarker
Introduction
Preeclampsia (PE) is an increasingly problematic pregnancy-related disorder around the world, especially in developing countries, and it remains a major cause of maternal and fetal mortality (1). This life-threatening disease mainly manifests as new-onset hypertension and proteinuria symptoms after 20 weeks of pregnancy and is relieved after delivery, but it often has long-term effects on maternal health. As the disease progresses, many patients suffer from hypertensive disorders, cardiovascular dysfunction, and chronic renal failure in addition to gestational diabetes mellitus (GDM) (2–4). Researchers have revealed that PE can increase the risk of asymptomatic cardiac abnormalities after birth and is a specific risk factor for postpartum asymptomatic heart failure (5). The exploration of the molecular mechanisms underlying PE have become the focus of a substantial amount of research.
Preeclampsia is a complex disease with systemic involvement. Multiple factors have been found to be related to its pathogenesis. These include hypoxia, the superficial nidation of the placenta, damage to vascular endothelial cells, and immune factors (6–8). The roles of many of these factors in the pathogenesis of PE are in the early stage of exploration, and the exact cause of PE therefore remains unknown. Scholars have come to the consensus that in PE, an effective prognosis relies on an early diagnosis. Traditional diagnostic methods include regular blood pressure measurements, determining 24-h urine protein levels, detecting placental thickness and homogeneity, obtaining a history of preeclampsia and the patient's body-mass index. However, early diagnostic efficiency is usually not unsatisfactory (9). In recent decades, to predict PE early, many researchers have applied themselves to identifying the mechanisms underlying PE. Serum markers, specific associated proteins, long non-coding RNA (lncRNA), and even circular RNA (circRNA) have been found to contribute to the onset of PE (10–13). However, all of these discoveries remain theoretical, and there is therefore a large distance between laboratory findings and clinical applications.
Proteins are important organic factors that play key roles in many physiological and pathological processes. In studying proteins, scholars have identified a new type of polymer made of amino acids that differs from protein. These are called peptides and are usually less than 10 kDa. Peptides act like proteins but are easier to acquire and transform. Performing deeper research aimed at obtaining a comprehensive understanding of all peptides or small proteins in a whole organism, collectively called the peptidome, was suggested in 2000 (14,15). In disease states, peptidome analyses have mainly been used to select biomarkers, diagnose diseases, evaluate therapeutic efficacy, and achieve early prevention. Single biomarkers are not sensitive enough for early prevention, but peptidome analysis can improve this deficiency and the rate of detection in diseases (16,17). Peptidome analysis remains an underdeveloped areas in PE patients. It therefore deserves to be more deeply explored to identify differentially expressed peptides that may be useful biomarkers for early screening in PE.
Amniotic fluid is a dynamic biological fluid that forms the living environment of a developing fetus. An analysis of the amniotic fluid can shed light on the functions of the placenta and amniotic cavity. Abnormal components in the amniotic fluid reflect the fetal and maternal state. The amniotic fluid has been used to analyze disease-specific biomarkers associated with many conditions, and the most common and most mature such application is screening for Down's syndrome (18). It is easier to obtain amniotic fluid than placental tissues during pregnancy. We therefore chose amniotic fluid for research samples in our study. In this study, we characterized the peptidome in PE using an ultracentrifugation method to obtain a basic understanding of the ranges of peptide expression. We then used liquid chromatography-tandem mass spectrometry (LC-MS/MS) to quantify the expression of peptides that were differentially up- and downregulated between normal and PE pregnancies. An analysis of the functions of these peptides may allow us to identify feasible biomarkers of PE.
Materials and methods
Sample collection
This study was permitted by the Human Research Ethics Committee of Nanjing Medical University as well as the Nanjing Maternal and Child Health Hospital and the Obstetrics and Gynecology Hospital Affiliated to Nanjing Medical University.
Samples of amniotic fluid were obtained from women with normal and PE pregnancies at 34–38 weeks of gestation at Nanjing Maternal and Child Health Hospital, Obstetrics and Gynecology Hospital Affiliated to Nanjing Medical University, China, during January 14 and March 27. The pregnant participants were fully informed regarding the aims and scope of the study and freely signed informed consent forms. Preeclampsia patients were diagnosed as first appearance of systolic blood pressure ≥140 mmHg and (or) diastolic blood pressure ≥90 mmHg, companied with urine protein quantification ≥300 mg per 24 h after 20 weeks of gestation. Samples in our study were severe cases that blood pressure more than 160/110 mmHg, proteinuria ≥5,000 mg/24 h, headache or visual impairment, abnormal liver function with or without right upper quadrant pain, and platelet less than 100×109/l. Women suffered from chronic hypertension, acute or chronic heart disease, nephropathy, diabetes mellitus or other maternal autoimmune diseases before pregnant were not included in our research. All amniotic fluids were collected during cesarean sections and placed on ice while en route to the laboratory. The samples of amniotic fluid were centrifuged at 2,000 g for 20 min at 4°C to remove cell debris. The supernatant was then mixed with protease inhibitors (Complete mini EDTA-free; Roche, Mannheim, Germany) and stored at −80°C until used.
Ultrafiltration and LC-MS/MS
The amniotic fluid samples were centrifuged (12,000 g, 4°C, 20 min), and the supernatant was transferred into a new centrifuge tube and mixed with acetonitrile (20% v/v). The mixture was then vortexed and incubated at room temperature for approximately 25 min (19). Molecular weight cut-off (MWCO) filters (10,000 Da; Millipore, Billerica, MA, USA) were washed with H2O (0.5 ml) before they were used, and the processed samples were centrifuged through the filters according to the manufacturer's recommendations. The concentrations of the peptides in each of the samples were determined using the bicinchonic acid method (BCA) (Pierce Biotechnology, Inc., Rockford, IL, USA). The filtrates were then concentrated and lyophilized.
LC-MS/MS is commonly used as a method for identifying peptides or low molecular weight proteins according to their detection sensitivity and throughput. We labeled the peptides derived from each sample with isotopomeric dimethyl labels, which was based on the inter-reaction of primary amines with deuterated and 13C-labeled formaldehyde to generate a Schiff base rapidly reduced by the addition of cyanoborohydride (20,21). The labeled samples were simultaneously detected using LC-MS/MS based on their abundance, which was reflected by mass differences in the dimethyl labels.
The lyophilized samples were dissolved in 0.1% formic acid, filtered through a 0.45-µm membrane and then set aside. We chose an LC Packings C18 trap column (Acclaim PepMap100, 75 µm × 20 mm) to perform reverse-phase chromatography and separated the peptides using an Ultimate 3000 nano-LC system (Dionex) with a linear gradient in liquid phase containing (A) 0.1% formic acid dissolved in water and (B) 0.1% formic acid dissolved in acetonitrile (22,23). After the samples were transferred into the MALDI TOF/TOF (Ultraflextreme; Bruker Daltonics, Bremen, Germany) instrument under the positive ion mode, the mass spectral analysis strategy went as follows: full scan analysis was operated over the m/z range 600–5,000 at 2 spectra/s, with the capillary voltage and cone voltage were maintained at 3.9 kV and 40 V.
For MS analysis, each position (10 random positions on each sample) was irradiated with 200 laser pulses, and 2000 single-shot spectra were collected. Mass Lynx™ software (v.4.1, Waters Corp., Milford, MA, USA) was used to analyze the protein databases using the MS/MS spectral data for the protein ID. The human taxonomy was searched for protein IDs in the NCBI and Matrix Science databases (http://www.matrixscience.com/, ©2016). The MS/MS data were searched using the Mascot database (http://www.matrixscience.com, ©2016).
Bioinformatics
Each peptide isoelectric point (pI) was calculated using an online pI/Mw tool (http://web.expasy.org/compute_pi/, ©2017). A GO analysis was then performed to investigate whether the identified peptide precursors favored any compartments or protein classes. The peptides were classified according to both their cellular components and their molecular functions using annotations obtained from the UniProt Database (http://www.uniprot.org/, ©2002–2017, last modified January 10, 2017).
Statistical analysis
Data from quantitative experiments were analyzed using unpaired Student's t-tests where appropriate in Statistical Program for Social Sciences (SPSS) v.22 and GraphPad Prism 5.0. The statistical significance of the results was determined using multiple comparisons followed by Student's t-tests. Significance was set at P<0.05. All data are shown as the means ± standard deviations (SDs). The heat map was generated by software HemI Version 1.0.0.
Results
Characteristics of the study population
The basic information relating to the subjects involved in our study are summarized in Table I. There was no difference in the ages, number of pregnancies, BMI and gestational week between the groups (P>0.05). Every subject delivered by caesarean section at the appropriate time, and the newborn babies were all healthy. No cases of asphyxia neonatorum were observed. There were differences between the two groups in systolic pressure, diastolic pressure, proteinuria level and neonatal weight (P<0.05).
Table I.
Comparison of characteristics between PE and normal pregnancies.
| Characteristic | PE (n=3) | Normal pregnancy (n=3) |
|---|---|---|
| Age (years) | 28.67±1.24 | 29.67±1.70 |
| Number of pregnancies | 2.67±1.26 | 2.33±0.47 |
| BMI (kg/m2) | 31.06±0.96 | 30.76±1.32 |
| Gestation week | 35.87±1.25 | 36.23±0.98 |
| Systolic pressure (mmHg) | 166.33±1.25a | 118.67±2.49 |
| Diastolic pressure (mmHg) | 84.67±2.05a | 71.33±3.40 |
| Proteinuria level (mg/24 h) | 4806.60±1518.06a | N/A |
| Mode of devliviery CS (%) | 100 | 100 |
| Birth weight (g) | 3073.33±92.86a | 3133.33±62.36 |
| Apgar score | 8.67±0.47 | 9.33±0.47 |
P<0.05 when compared with normal pregnancy group. BMI, body mass index. N/A, not applicable; PE, preeclampsia.
Peptide identification and quantitative analysis
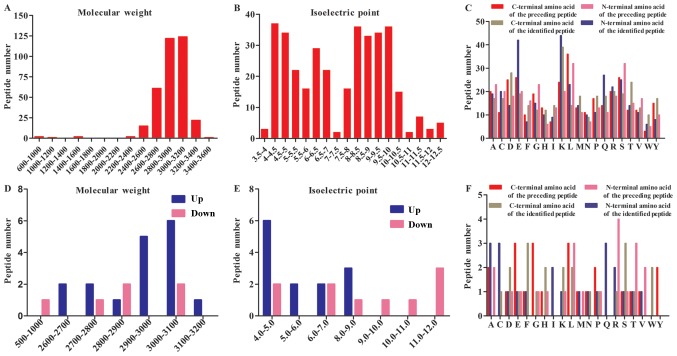
After LC-MS/MS, a total of 352 peptides were collectively detected in the human amniotic fluid samples (Fig. 1). The peptidome contained peptides spanning a wide range of molecular weight (Mw) and pI values. In addition, the analysis revealed that most of the peptides were between 2800–3200 Da in MW and between 4–6 and 8–10 in pI (Fig. 1A and B). The categories of detected peptides clustered into two general groups according to the range of acidic and basic pH. All of the Mw of the peptides analyzed using LC-MS/MS were under 3500 Da, in agreement with previous reports.
Figure 1.
General summary of the results of liquid chromatography tandem-mass spectrometry analysis of amniotic fluids. (A) Distribution of the Mw of all detected peptides. (B) Distribution of the pI of all detected peptides. (C) Distribution of the amino acids at the four cleavage sites of all detected peptides. (D) Mw of differentially expressed peptides in the amniotic fluid of PE vs. normal pregnancies. (E) pI of differentially expressed peptides. (F) Comparison of the amino acid profiles of the four cleavage sites in each of the differentially expressed peptide fragments. pI, isoelectric points; Mw, molecular weights; PE, preeclampsia.
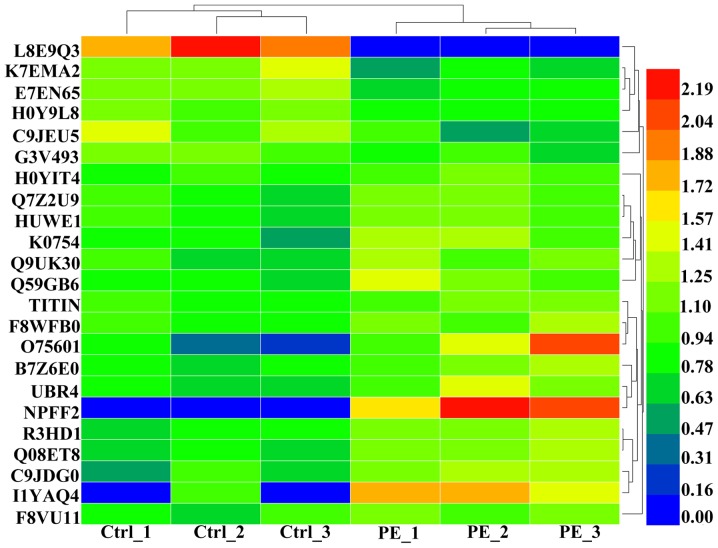
Among the 352 detected peptides, 23 were found to be differentially expressed (6 were downregulated, and 17 were upregulated) (P≤0.05) between PE and normal pregnancies. Table II lists these 23 peptides and their respective precursor proteins. Further analyses revealed that most of the upregulated peptides were distributed between 2900–3100 Da in Mw and that they were predominantly acidic in pI, whereas the corresponding distributions of the downregulated peptides were more average (Fig. 1D and E). A heat map of the six individuals is shown to clarify these differences in expression (Fig. 2).
Table II.
Characteristics of the peptides that were differentially expressed peptides between PE and normal pregnancies.
| Sequence | Protein ID | Fragment | Protein names | Cellular component | Function | Fold change | P-value |
|---|---|---|---|---|---|---|---|
| Peptides downregulated in amniotic fluid of pregnancy with PE | |||||||
| KEDEKAPQLMASY | L8E9Q3 | 61–85 | Alternative protein | Unknown | Unknown | −∞ | 7.48×10−05 |
| DLYLCQWNIIGW | CNKSR2 | ||||||
| AAWGQTVLLLHAL | K7EMA2 | 29–53 | Beclin-1 | Nucleus | Autophagy | 0.5585 | 1.38×10−02 |
| ANKMGLKFQRMD | |||||||
| CGIADFLSTYQTKVDKDLQSLEDIL | C9JEU5 | 49–73 | Fibrinogen gamma chain | Fibrinogen complex | Platelet activation; protein polymerization; signal transduction | 0.5711 | 4.19×10−02 |
| CLGKACG | E7EN65 | 704–710 | Elastin | Extracelluar region | Extracellular | 0.6332 | 4.42×10−03 |
| IDHYCKHSEEIKDNCRNWKPTCDVF | G3V493 | 33–57 | Protein SIX6OS1 | unknown | Unknown | 0.7276 | 2.44×10−02 |
| FMLKPGDLLYFPRGTIHQADTPAGL | H0Y9L8 | 70–94 | Bifunctional lysine-specific demethylase and histidyl-hydroxylase MINA | Cytoplasm; nucleus | Unknown | 0.7587 | 8.28×10−03 |
| Peptides upregulated in amniotic fluid of pregnancy with PE | |||||||
| VDGKCCKECKCEH NFYDEYFLWKNK | H0YIT4 | 95–119 | Protein kinase C-binding protein NELL2 | Unknown | Unknown | 1.1962 | 4.96×10−02 |
| CEKIGKIYEMRMM MDFNGNNRGYAF | Q7Z2U9 | 76–100 | A1CF protein | Nucleus | Nucleic acid binding; nucleotide binding | 1.2911 | 4.31×10−02 |
| EEKAGKESDEKEQEQDKDRELQQAE | F8VU11 | 824–848 | Pre-mRNA-processing factor 40 homolog B | Unknown | Unknown | 1.2913 | 2.49×10−02 |
| IKITWFANDREIKESSKHRMSFVES | Q8WZ42 | 5256–5280 | Titin | Cytoplasm; nucleus | Kinase, Transferase, Serine/threonine-protein kinase | 1.3004 | 1.12×10−02 |
| QELEKTLEESKEMDIKRKENKGNDT | Q7Z6Z7 | 1698–1722 | E3 ubiquitin-protein ligase HUWE1 | Cytoplasm; nucleus | protein ubiquitination | 1.3191 | 1.19×10−02 |
| DEAWKSFLENPLTAATKAMMSINGD | F8WFB0 | 30–54 | Grainyhead-like protein 1 homolog | Unknown | Unknown | 1.3407 | 3.43×10−02 |
| RSRESRLPQSVRHH LIISHFSSKDF | Q9UK30 | 26–50 | Ras-GRF2 | Unknown | Unknown | 1.3864 | 4.47×10−02 |
| MDENFISRAFATMGETVMSVKIIRN | B7Z6E0 | 14–38 | cDNA FLJ52201, mRNA | Nucleus | Nucleic acid binding; nucleotide binding | 1.4922 | 1.01×10−02 |
| PSISAAQNALKKQINSVNKFKLRTS | Q15032 | 1060–1084 | R3H domain-containing protein 1 | Nucleus | Poly(A) RNA binding | 1.4973 | 2.78×10−03 |
| QGEDKAKLMRAYMQEPLFVEFADCC | Q59GB6 | 93–117 | Thyroid hormone receptor interactor | Membrane | Receptor | 1.5141 | 2.93×10−02 |
| ASNPSLQEKKESSSALTESSGHLDH | O94854 | 24–48 | Uncharacterized protein KIAA0754 | Membrane | Unknown | 1.5746 | 3.18×10−02 |
| SENELLELFEKMMEDMNLNEDKKAP | C9JDG0 | 118–142 | Deleted | Unknown | Unknown | 1.6752 | 1.22×10−02 |
| AAQSEGGPKSRDSKRPVATRGAKEH | Q08ET8 | 18–42 | WRAP53 | Unknown | Unknown | 1.7077 | 1.75×10−03 |
| TESETKAFMAVCIETAKRYNLDDYR | Q5T4S7 | 4302–4326 | E3 ubiquitin-protein ligase UBR4 | Membrane; cytoplasm; nucleus | Ligase activity; ubiquitin-protein transferase activity; zinc ion binding | 1.7425 | 1.38×10−02 |
| NWKEEEKKKYYDAKTEDKVRVMADS | O75601 | 36–60 | Mih1/Tx protein | Nucleus | Regulation of apoptotic process | 3.1777 | 3.19×10−02 |
| QSLWEKACENQRNLNMETARISHWK | I1YAQ4 | 51–75 | LOC340970 | Intracellular | Zinc ion binding | 4.5037 | 2.83×10−02 |
| RKKQKIIKMLLIVALLFILSWLPLW | Q9Y5X5 | 369–393 | Neuropeptide FF receptor 2 | Membrane | G-protein coupled receptor, receptor, transducer | +∞ | 7.76×10−04 |
PE, preeclampsia.
Figure 2.
Heat map of differentially expressed peptides in amniotic fluid samples obtained from six individuals. Red represents higher expression, while blue represents lower or no change in expression. Ctrl, control group; normal pregnancies; PE, experimental group, pregnancies with preeclampsia.
Details and cleavage sites of endogenous peptides
LC-MS/MS identified 352 peptides, and the bioinformatics analysis showed the specificity of the four cleavage sites (Fig. 1C). Leucine (L) was the most common amino acid at the C-terminal cut site of the preceding peptide, and lysine (K) was most commonly the C-terminal amino acid of the identified peptide. Glutamic acid (E) was most commonly observed at the N-terminal of the preceding peptide, and serine (S) and tryptophan (M) were most commonly observed at the N terminus of the identified peptides.
The distribution of the cut sites in the differentially expressed peptides were also variable in the PE amniotic fluid samples. To further investigate the peptidome in these samples, we next used bioinformatics analyses to determine the amino acids at the cleavage sites of both the N- and C-termini (Fig. 1F). The results of our investigation of these four cut sites generally reflected the findings reported in endogenous proteolytic enzymes in human amniotic fluids. The results revealed that glutamic acid (E), glycine (G) and leucine (L) were the most common amino acids at the C-terminal cut site of the preceding peptide, while phenylalanine (F) and serine (S) were most commonly observed at the C-terminal of the identified peptide. However, alanine (A), cysteine (C) and glutamine (G) dominated the cleavage site at the N terminus of the identified peptide, while arginine (R) was the most common N-terminal pre-cleavage amino acid.
Subcellular localization and functional clusters of endogenous peptides
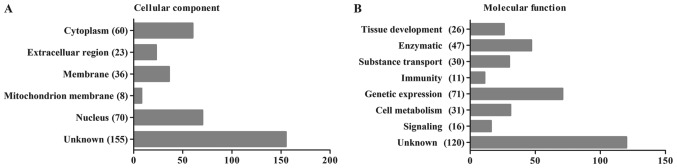
To determine whether these peptide precursors were biased toward specific protein compartments or protein classes, we combined Pathway and GO analyses to investigate the probable roles of the endogenous peptidome and the precursor proteins of peptidome components. The 352 identified peptides were categorized according to their cellular components and their molecular functions using the UniProt Database (http://www.uniprot.org/, ©2002–2017, last modified January 10, 2017). The most likely cellular component and molecular function categories are shown in Fig. 3A and B, respectively. We found that the majority of the proteins identified in the present study were cytoplasmic (17%) or nuclear (20%) proteins and that the most common functions were the regulation of gene expression (20%) and enzymatic functions (13%). Because late pregnancy amniotic fluid consists of the metabolites of a variety of both maternal and neonatal organs, the results of this analysis also demonstrated the condition of the mothers and babies.
Figure 3.
Gene ontology and homology analysis of all 352 identified peptide precursors. (A) Cellular components of the peptide precursors. (B) Functional categorization of the peptide precursors.
We also summarized the cellular components and molecular functions of the peptides found to be differentially expressed, as shown in Table II. These peptides were mainly located in the nucleus and have been shown to play roles in gene expression, consistent with the results of our analysis of the human amniotic fluid peptidome. The different categories of the listed protein precursors demonstrated no existence of the preferential extraction from specific cellular components or with functions and the successful of the previous ultrafiltration.
Discussion
PE is a critical complication in pregnancy that has a reported morbidity rate of 2–8% among all pregnant women (24). Studies of the pathogenesis of and pathological changes that occur during PE have attracted the attention of many researchers. However, the specific factors that cause PE remain an enigma and the subject of a great deal of debate (25). It is widely accepted that early detection and intervention greatly improve perinatal outcomes in both mothers and infants. Traditional monitoring methods are performed by detecting blood pressure and urine protein levels, which exhibit wide fluctuations in response to a variety of external environmental and many other uncontrollable factors. Identifying a more reliable and valid method for detecting PE is therefore urgently needed.
Peptidome analysis is a newly emerging discipline in proteomics that sometimes outperforms proteomics in its structural simplicity, operation convenience, study universality and property stability. In a sense, the peptidome is inherited and developed from proteomics (26,27). Peptides exist everywhere in the human body, in both organs and tissues, in both cells and bodily fluids. Peptide analysis yields a more comprehensive picture of the nature and molecular functions of proteins by providing information about the synthesis, modification and degradation of proteins.
Extracting and enriching the peptides in a sample is one of the key procedures in this type of analysis. Centrifugal ultrafiltration accompanied with perfect Mw cutoff is a commonly used method in peptidom analysis (28). We used standard 1000-Da filters in our study and achieved ideal outcomes that did not include albumin and Igs, as shown in the MW and PI results (Fig. 1A and B). The products of ultrafiltration were labeled with isotopomeric dimethyl, allowing them to be immediately analyzed using LC-MS/MS. A total of 352 peptides were identified in both groups. Of these, 6 were expressed at significantly lower levels, while 17 were expressed at significantly higher levels (P≤0.05).
Peptide fragments are created when proteins are cleaved by proteases. Previous studies have indicated that the cleavage sites at the N- and C-termini of endogenous peptides are highly conserved, despite differences in the sample preparation conditions used to analyze specific serum samples across individuals. Additionally, the pattern of degradation of proteins and peptides differs substantially between diseases as a result of the specificity and activity of specific proteolytic enzymes (29,14). The cleavage sites in the peptides that were differentially expressed in our study are summarized in Fig. 1F. The probability that any one of the 20 amino acids would be located at the four cutting sites was dramatically different. Because each protease cuts proteins according to specific rules, these data reveal that a distinct set of proteases are active in the amniotic fluid of PE women. This point deserves further investigation.
Among the precursor proteins identified in our experiments, some have previously been implicated in the onset of PE. For example, titin (also known as TTN) has a MW of 4.2 MDa and is the largest known protein in the human body. Titin functions as a contractile unit in striated muscle cells, especially in cardiomyopathies (30–32). Liang et al (33) found that Titin is targeted by miR-144, which regulates proliferation and invasion in human chorionic cells via the MAPK and MMPs signaling pathways. Poor implantation of the placenta and chorionic cells, as well as insufficient uteroplacental circulation during the early stage of pregnancy, are key contributors to PE. Additionally, Farina et al (34) noted that TTN expression promoted trophoblast invasion by altering the elasticity of and reconstructing the maternal vascular system and that higher than normal levels of TTN were observed in PE. These data strongly support the notion that TTN is a disease-specific biomarker for PE.
All previous studies support the accuracy and practicability of the results reported in our study. In 2011, Araki et al (35) suggested using BLOTCHIP® analysis as a peptidomic method for biomarker discovery in PE. However, studies using peptidomic analyses in disease states have been limited to theoretical studies that lack large sample populations. Hence, this method should be studies in more detail in the future.
However, there were certain deficiencies somewhere in our study. It is necessary to include larger sample sizes to verify its effectiveness and repeatability and to exploit the advantages. Another, necessary validation experiment of these identified peptides would make our discussion more credible.
In conclusion, this study is the first to provide a peptidomic analysis of the profile of the amniotic fluid in patients with PE. The data we provide can be used to identify novel PE biomarkers. We will work hard on picking one significant peptide for functional verification in future work and exploiting the advantages associated with using peptides to prevent and more comprehensively treat diseases.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (nos. 81571444 and 81501341) and the Nanjing Medical Science and Technology Development Foundation (no. YKK15163).
References
- 1.Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: Updates in pathogenesis, definitions and guidelines. Clin J Am Soc Nephrol. 2016;11:1102–1113. doi: 10.2215/CJN.12081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 3.Drost JT, Maas AH, van Eyck J, van der Schouw YT. Preeclampsia as a female-specific risk factor for chronic hypertension. Maturitas. 2010;67:321–326. doi: 10.1016/j.maturitas.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Zhang X, Rong C, Rui C, Ji H, Qian YJ, Jia R, Sun L. Distinct DNA methylomes of human placentas between pre-eclampsia and gestational diabetes mellitus. Cell Physiol Biochem. 2014;34:1877–1889. doi: 10.1159/000366386. [DOI] [PubMed] [Google Scholar]
- 5.Ghossein-Doha C, van Neer J, Wissink B, Breetveld NM, de Windt LJ, van Dijk AP, Van Der Vlugt MJ, Janssen MC, Heidema WM, Scholten RR, Spaanderman ME. Preeclampsia, an important female specific risk factor for asymptomatic heart failure. Ultrasound Obstet. Gynecol. 2016;49:143–149. doi: 10.1002/uog.17343. [DOI] [PubMed] [Google Scholar]
- 6.Wang A, Rana S, Karumanchi SA. Preeclampsia: The role of angiogenic factors in its pathogenesis. Physiology (Bethesda) 2009;24:147–158. doi: 10.1152/physiol.00043.2008. [DOI] [PubMed] [Google Scholar]
- 7.Baumwell S, Karumanchi SA. Pre-eclampsia: Clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2007;106:C72–C81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 8.Sankaralingam S, Arenas IA, Lalu MM, Davidge ST. Preeclampsia: Current understanding of the molecular basis of vascular dysfunction. Expert Rev Mol Med. 2006;8:1–20. doi: 10.1017/S1462399406010465. [DOI] [PubMed] [Google Scholar]
- 9.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 10.Jia R, Li J, Rui C, Ji H, Ding H, Lu Y, De W, Sun L. Comparative Proteomic profile of the human umbilical cord blood exosomes between normal and preeclampsia pregnancies with high-resolution mass spectrometry. Cell Physiol Biochem. 2015;36:2299–2306. doi: 10.1159/000430193. [DOI] [PubMed] [Google Scholar]
- 11.Pillar N, Yoffe L, Hod M, Shomron N. The possible involvement of microRNAs in preeclampsia and gestational diabetes mellitus. Best Pract Res Clin Obstet Gynaecol. 2015;29:176–182. doi: 10.1016/j.bpobgyn.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YG, Yang HL, Long Y, Li WL. Circular RNA in blood corpuscles combined with plasma protein factor for early prediction of pre-eclampsia. BJOG. 2016;123:2113–2118. doi: 10.1111/1471-0528.13897. [DOI] [PubMed] [Google Scholar]
- 13.Qian Y, Lu Y, Rui C, Qian Y, Cai M, Jia R. Potential Significance of circular RNA in human placental tissue for patients with preeclampsia. Cell Physiol Biochem. 2016;39:1380–1390. doi: 10.1159/000447842. [DOI] [PubMed] [Google Scholar]
- 14.Hu L, Ye M, Zou H. Recent advances in mass spectrometry-based peptidome analysis. Expert Rev Proteomics. 2009;6:433–447. doi: 10.1586/epr.09.55. [DOI] [PubMed] [Google Scholar]
- 15.Schrader M, Schulz-Knappe P. Peptidomics technologies for human body fluids. Trends Biotechnol. 2001;19:55–60. doi: 10.1016/S0167-7799(01)01800-5. [DOI] [PubMed] [Google Scholar]
- 16.Xiang Y, Kurokawa MS, Kanke M, Takakuwa Y, Kato T. Peptidomics: Identification of pathogenic and marker peptides. Methods Mol Biol. 2010;615:259–271. doi: 10.1007/978-1-60761-535-4_20. [DOI] [PubMed] [Google Scholar]
- 17.Casalegno-Garduño R, Schmitt A, Schmitt M. Clinical peptide vaccination trials for leukemia patients. Expert Rev Vaccines. 2011;10:785–799. doi: 10.1586/erv.11.56. [DOI] [PubMed] [Google Scholar]
- 18.Tsangaris GT, Karamessinis P, Kolialexi A, Garbis SD, Antsaklis A, Mavrou A, Fountoulakis M. Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics. 2006;6:4410–4419. doi: 10.1002/pmic.200600085. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Wu LJ, Gu M, Chen YM, Zhang QJ, Li H, Cheng ZJ, Hu P, Han SP, Yu ZB, Qian LM. Peptidomic analysis of amniotic fluid for identification of putative bioactive peptides in ventricular septal defect. Cell Physiol Biochem. 2016;38:1999–2014. doi: 10.1159/000445560. [DOI] [PubMed] [Google Scholar]
- 20.Hsu JL, Huang SY, Chow NH, Chen SH. Stable-isotope dimethyl labeling for quantitative proteomics. Anal Chem. 2004;75:6843–6852. doi: 10.1021/ac0348625. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Zhao C, Liu L, Ding H, Huo R, Shi Z. Peptidome profiling of umbilical cord plasma associated with gestational diabetes-induced fetal macrosomia. J Proteomics. 2016;139:38–44. doi: 10.1016/j.jprot.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Wan J, Cui XW, Zhang J, Fu ZY, Guo XR, Sun LZ, Ji CB. Peptidome analysis of human skim milk in term and preterm milk. Biochem Biophys Res Commun. 2013;438:236–241. doi: 10.1016/j.bbrc.2013.07.068. [DOI] [PubMed] [Google Scholar]
- 23.Cui X, Li Y, Yang L, You L, Wang X, Shi C, Ji C, Guo X. Peptidome analysis of human milk from women delivering macrosomic fetuses reveals multiple means of protection for infants. Oncotarget. 2016;7:63514–63525. doi: 10.18632/oncotarget.11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Shah DM. Preeclampsia: New insights. Curr Opin Nephrol Hypertens. 2007;16:213–220. doi: 10.1097/MNH.0b013e3280d942e9. [DOI] [PubMed] [Google Scholar]
- 26.Ivanov VT, Yatskin ON. Peptidomics: A logical sequel to proteomics. Expert Rev Proteomics. 2005;2:463–473. doi: 10.1586/14789450.2.4.463. [DOI] [PubMed] [Google Scholar]
- 27.Dallas DC, Guerrero A, Parker EA, Robinson RC, Gan J, German JB, Barile D, Lebrilla CB. Current peptidomics: Applications, purification, identification, quantification, and functional analysis. Proteomics. 2015;15:1026–1038. doi: 10.1002/pmic.201400310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu RN, Fan L, Rieser MJ, El-Shourbagy TA. Recent advances in high-throughput quantitative bioanalysis by LC-MS/MS. J Pharm Biomed Anal. 2007;44:342–355. doi: 10.1016/j.jpba.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Wang F, Zhu J, Hu L, Qin H, Ye M, Zou H. Comprehensive analysis of the N and C terminus of endogenous serum peptides reveals a highly conserved cleavage site pattern derived from proteolytic enzymes. Protein Cell. 2012;3:669–674. doi: 10.1007/s13238-012-2934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gigli M, Begay RL, Morea G, Graw SL, Sinagra G, Taylor MRG, Granzier H, Mestroni L. A review of the giant protein titin in clinical molecular diagnostics of cardiomyopathies. Front Cardiovasc Med. 2016;3:21. doi: 10.3389/fcvm.2016.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindstedt S, Nishikawa K. Huxleys' missing filament: Form and function of titin in vertebrate striated muscle. Annu Rev Physiol. 2017;79:145–166. doi: 10.1146/annurev-physiol-022516-034152. [DOI] [PubMed] [Google Scholar]
- 32.Walker JS, de Tombe PP. Titin and the developing heart. Circ Res. 2004;94:860–862. doi: 10.1161/01.RES.0000126698.37440.B0. [DOI] [PubMed] [Google Scholar]
- 33.Liang Y, Lin Q, Luo F, Wu W, Yang T, Wan S. Requirement of miR-144 in CsA induced proliferation and invasion of human trophoblast cells by targeting titin. J Cell Biochem. 2014;115:690–696. doi: 10.1002/jcb.24710. [DOI] [PubMed] [Google Scholar]
- 34.Farina A, Morano D, Arcelli D, De Sanctis P, Sekizawa A, Purwosunu Y, Zucchini C, Simonazzi G, Okai T, Rizzo N. Gene expression in chorionic villous samples at 11 weeks of gestation in women who develop preeclampsia later in pregnancy: Implications for screening. Prenat Diagn. 2009;29:1038–1044. doi: 10.1002/pd.2344. [DOI] [PubMed] [Google Scholar]
- 35.Araki Y, Nonaka D, Tajima A, Maruyama M, Nitto T, Ishikawa H, Yoshitake H, Yoshida E, Kuronaka N, Asada K, et al. Quantitative peptidomic analysis by a newly developed one-step direct transfer technology without depletion of major blood proteins: Its potential utility for monitoring of pathophysiological status in pregnancy-induced hypertension. Proteomics. 2011;11:2727–2737. doi: 10.1002/pmic.201000753. [DOI] [PubMed] [Google Scholar]