Supplemental Digital Content is available in the text.
Abstract
Background:
Surgical treatment of lower extremity lymphedema (LEL) remains challenging. Application of the superior-edge-of-the-knee incision method for lymphaticovenular anastomosis (LVA) is reported to have a strong therapeutic effect in patients with LEL because lymph-to-venous flow at the anastomosis is enhanced by knee joint movement during normal walking. We investigated whether a single LVA created by this method is adequate for early LEL.
Methods:
The study involved 10 patients with LEL characterized by stage 2 or 3 leg dermal backflow and treated by a single LVA at the thigh via the superior-edge-of-the-knee incision method. The lymphatic vessel and direction of flow were assessed intraoperatively, and reduction in lymphedema volume was assessed postoperatively.
Results:
Use of our incision method yielded a single anastomosis in all patients with stage 2 leg dermal backflow and in all patients with stage 3 leg dermal backflow. The lymphatic vessel was 0.65 ± 0.08 mm in diameter (0.65 ± 0.09 and 0.65 ± 0.09 mm, respectively; P = 1.000). No venous reflux occurred in any patient. Mean follow-up was 7.70 ± 3.30 months (9.60 ± 3.29 and 5.80 ± 2.17 months, respectively; P = 0.068). Mean reduction in the LEL index was 20.160 ± 9.892 (22.651 ± 12.272 and 17.668 ± 7.353, respectively; P = 0.462).
Conclusion:
A single LVA created by the superior-edge-of-the-knee incision method can be expected to have a strong therapeutic effect in patients with stage 2 or 3 leg dermal backflow.
Lymphaticovenular anastomosis (LVA) is widely recognized as an effective, minimally invasive treatment for refractory lymphedema.1–8 In cases of lower extremity lymphedema (LEL), multiple LVAs are strongly recommended because improvement in the edema, including reduction in the stiffness and size of the affected limb, is often limited to the area around the anastomosis.
Unfortunately, the mid- to late postoperative period is not a risk-free period, and some LVAs lose their therapeutic effect.9 The risk of occlusion of the anastomosed vessel is increased when the lymphatic vessel had sclerosed, resulting in poor lymph flow because lymphatic vessels with degenerated smooth muscle cells are inefficient in pushing lymph into the anastomosed vein.10,11 It is the resulting stagnation of fluid that leads to occlusion of the LVA. Multiple LVAs serve as a safety net to circumvent the risk of occlusion; some of the anastomoses will remain patent.
The superior-edge-of-the-knee incision method is a novel means of facilitating LVA that has an optimum therapeutic effect on LEL. We reported this method in the series of LVA surgeries for 30 patients: all 15 patients in whom the superior-edge-of-the-knee incision method was utilized with traditional LVAs revealed postoperative volume reduction, but only 8 of 15 patients in whom traditional LVAs were utilized revealed postoperative volume reduction.8 Continuous and strong flow of lymph at the superior-edge-of-the-knee incision site in the distal medial thigh, promoted by knee joint movement during normal walking, is thought to prevent mid-term and late vessel occlusion. Moreover, reduction in the stiffness and size of the affected limb has been remarkable at the groin, thigh, and lower leg in patients treated by the superior-edge-of-the-knee incision method. Although we had been treating LEL by creating several, usually 4–8, LVAs, for the therapeutic effect in the whole limb areas with reduced risk of total occlusions,6–8 the superior-edge-of-the-knee incision method can allow creation of fewer anastomoses. We conducted a retrospective study to determine whether a single LVA is indeed adequate for early LEL.
PATIENTS AND METHODS
The study included 10 patients with International Society of Lymphology stage 2 secondary LEL who, between February 2015 and March 2016, underwent preoperative indocyanine green (ICG) lymphography for the evaluation of LEL and in whom stage 2 or 3 leg dermal backflow was observed.12,13 A single LVA was created by means of the superior-edge-of-the-knee incision method in each of these patients.
All patients included in the study had received compression therapy with elastic stockings, and all suffered from lymphedema refractory to conservative therapy. No patient with International Society of Lymphology stage 1 or 3 lymphedema and no patient in whom multiple LVAs had been created was included in the study. The study was conducted under approval from the St. Marianna University School of Medicine ethics committee.
LVA Performed by the Superior-Edge-of-the-Knee Incision Method
LVA was performed by the superior-edge-of-the-knee incision method, as described previously.8 The superior-edge-of-the-knee incision site in the distal medial thigh was identified by the intersection of a transverse line drawn at the superior edge of the patella and a longitudinal line drawn along the medial axis of the distal thigh with the patient in the supine position (Fig. 1). From the point of intersection, a posteriorly directed 2.5-cm-long transverse incision was made. The incision site was set regardless of ICG lymphography findings.
Fig. 1.
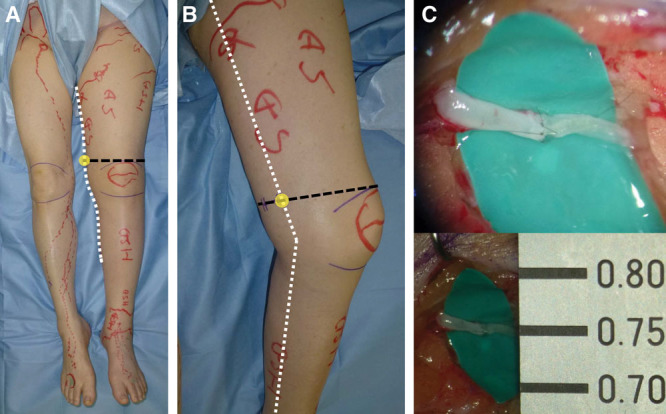
The superior-edge-of-the-knee incision method for stage 3 leg dermal backflow (A). The patient is an 81-year-old woman who had suffered left LEL for 11 years after hysterectomy. Preoperative ICG lymphography showed stardust patterns at the left ankle, lower leg, and thigh region. The correct site for the superior-edge-of-the-knee incision is easily identified, although deep lymphatic pathways cannot be detected by ICG lymphography. With the patient in the supine position, a transverse line was drawn at the upper edge of the patella (black line), and a longitudinal line was drawn along the medial axis of the distal thigh (white line) (B). The superior-edge-of-the-knee incision is a 2.5-cm linear incision that extends posteriorly from the intersection of the 2 lines (C). A lymphatic vessel of 0.75 mm in diameter and a vein of 0.95 mm in diameter were anastomosed for continuous lymph-to-venous flow of lymph at the superior-edge-of-the-knee incision site.
At the site of our incision point, several lymphatic vessels were identified over the superficial fascia in subcutaneous tissue. However, only lymphatic vessels under the superficial fascia were selected for the LVA, because it is there that strong upward propulsion of lymph through lymphatic vessels occurs due to the compression that is created between the layers of deep and superficial fascia by knee joint movement (Fig. 2). A small opening (less than 2.5-cm long) at the superficial fascia was made by dissection. The lymphatic vessel under the superficial fascia was caught with a 3-0 nylon monofilament via the small opening and cut proximally with microscissors. After the lymphatic vessel was pulled out over the superficial fascia, an LVA was created from the lymphatic vessel pulled out from under the superficial fascia and a vein running over the superficial fascia.
Fig. 2.
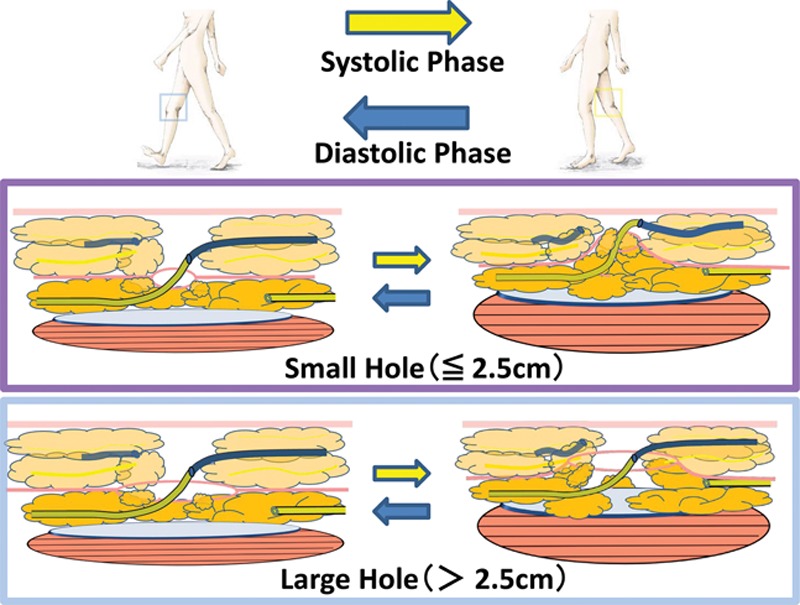
Mechanism of the pumping system achieved by anastomosis created by means of the superior-edge-of-the-knee incision method. At the superior-edge-of-the-knee incision site in the distal medial thigh, knee joint movement works as a pumping system to propel lymph through the LVA during normal walking. Soft tissues around the incision point are gently compressed during knee flexion (systolic phase) and released during knee extension (diastolic phase). Only a small opening (less than 2.5-cm long) at the superficial fascia is important to activate the pumping system at the incision point. With a large opening at the superficial fascia, the lymphatic vessels are not strongly compressed between the superficial fascia and the deep fascia. (Partially adapted from Seki Y, Yamamoto T, Yoshimatsu H, et al. The superior-edge-of-the-knee incision method in lymphaticovenular anastomosis for lower extremity lymphedema. Plast Reconstr Surg. 2015;136:665e–675e.)
All LVAs in this patient series were created by the same surgeon (Y.S.).
Statistical Analysis
Patients were classified into 2 groups on the basis of preoperative ICG lymphography findings: those with stage 2 leg dermal backflow (n = 5) and those with stage 3 leg dermal backflow (n = 5). Time to detection of the lymphatic vessel to be used for anastomosis, total operation time, lymphatic diameter, and postanastomosis direction of flow were evaluated intraoperatively and compared between the 2 groups. Total volume of the lymphedematous limb was evaluated on the basis of the LEL index, which was determined both preoperatively and postoperatively, and the reduction in volume was compared between the 2 groups.14 All values are reported as mean ± SD. Between-group differences were analyzed by Fisher’s exact probability test, paired Student’s t test, or Mann-Whitney U test, as appropriate. All P values were 2-sided, and statistical significance was accepted at P < 0.05. All statistical analyses were performed with JMP Pro12 software (SAS Institute, Cary, N.C.).
RESULTS
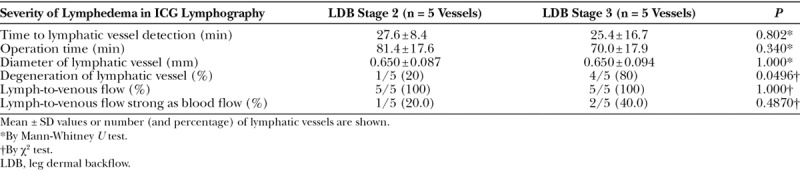
Characteristics of the study patients are shown per group in Table 1. Use of the superior-edge-of-the-knee incision method yielded a single anastomosis in each of the 5 patients with stage 2 leg dermal backflow and a single anastomosis in each of the 5 patients with stage 3 leg dermal backflow. Mean time to detection of the lymphatic vessel under the superficial fascia and to be used for anastomosis was 26.5 minutes (27.6 ± 8.4 and 25.4 ± 16.7 minutes in the stage 2 patients and stage 3 patients, respectively; P = 0.802) (Table 2). Mean operation time was 75.7 minutes (81.4 ± 17.6 and 70.0 ± 17.9 minutes for the stage 2 patients and stage 3 patients, respectively; P = 0.340). Mean diameter of the lymphatic vessel was 0.65 ± 0.08 mm (0.65 ± 0.09 and 0.65 ± 0.09 mm in the stage 2 and stage 3 patients, respectively; P = 1.000). Degeneration of lymphatic vessels were observed more frequently in stage 3 patients (20.0% and 80.0% in the stage 2 and stage 3 patients, respectively; P = 0.049). No venous reflux occurred in any patient in either group (P = 1.000). Lymph-to-venous flow as strong as blood flow was observed in 1 patient with stage 2 dermal backflow and in 2 patients with stage 3 dermal backflow (P = 0.487).
Table 1.
Characteristics of Patients, per Study Group
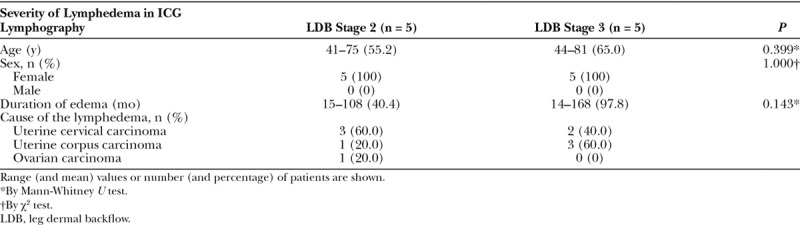
Table 2.
Intraoperative Findings, per Study Group
Changes in volume of the lymphedematous limb from the preoperative period to the postoperative period in the 2 groups are shown in Table 3. The mean follow-up time was 7.70 ± 3.30 months. The volume of the lymphedematous limb, according to the LEL index, was significantly reduced in all 10 patients postoperatively (postoperative LEL index 222.532 ± 19.390 versus preoperative LEL index 242.692 ± 19.026; P = 0.031; Fig. 3). Mean reduction in the LEL index was 20.160 ± 9.892 (22.651 ± 12.272 and 17.668 ± 7.353 in the stage 2 and 3 patients, respectively; P = 0.462). There was no statistical difference in the volume reduction between the 2 different lymphedema stage groups.
Table 3.
Follow-up and Indications of Postoperative Improvement, per Study Group
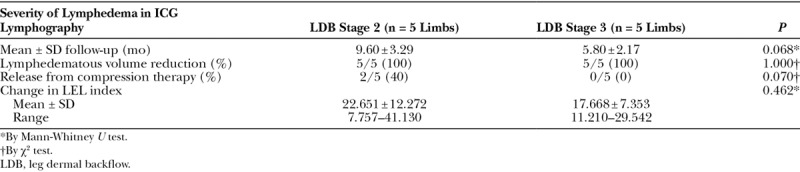
Fig. 3.
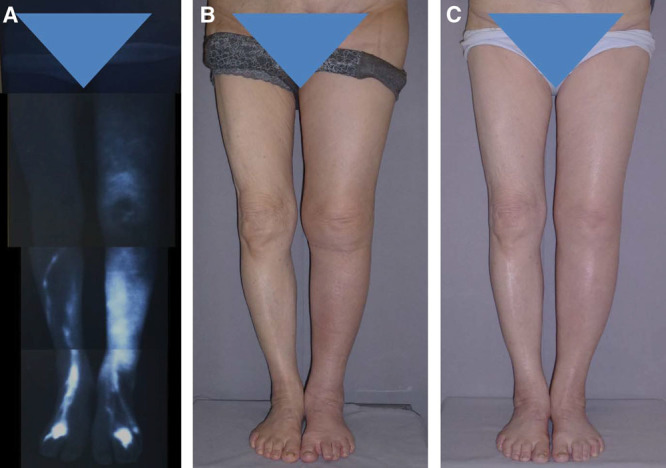
A, The patient here is the same patient shown in Figure 1. Preoperative ICG lymphography revealed stage 3 leg dermal backflow. B, Preoperative left LEL index was 248.7. C, A single LVA was created by means of the superior-edge-of-the-knee incision method. At 12 months, her left LEL index was reduced to 217.7.
Five patients underwent postoperative ICG lymphography more than 6 months after the operative procedure. Improvement was seen in 2 of the 5 patients as a reduction in the “stardust pattern” area and the appearance of new “splash patterns.” Because of markedly improved postoperative ICG lymphography findings, 2 of the patients with stage 2 leg dermal backflow were released from compression therapy, and the edema did not recur (Fig. 4). Only 1 patient needed additional LVAs for new lymphedematous swelling in the distal aspect of the ankle, swelling that emerged just after ankle trauma.
Fig. 4.
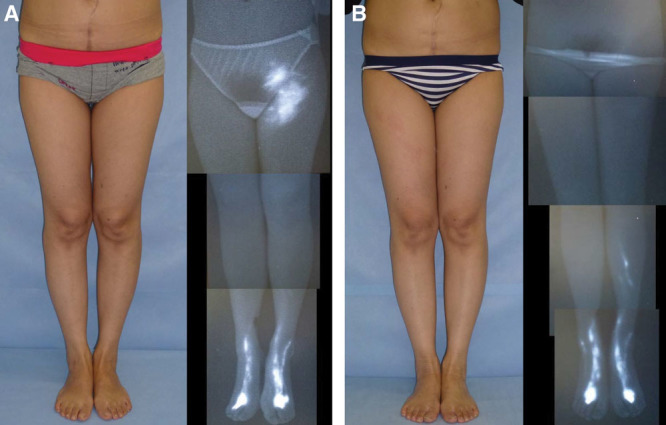
A, The patient shown here is a 41-year-old woman who had suffered left LEL for 1.3 years after undergoing a hysterectomy. Preoperatively, the right LEL index was 249.3, and ICG lymphography revealed stage 2 leg dermal backflow. B, A single LVA was created by means of the superior-edge-of-the-knee incision method. At 18 months, the right LEL index was reduced to 207.6, and ICG lymphography showed no sign of lymphedema. In fact, starting at 12 months, the postoperative ICG features appeared normal, and the patient was fully released from compression therapy at that time.
DISCUSSION
Treatment by means of LVA remains challenging in cases of LEL versus upper extremity lymphedema.15 The difficulty arises from the effect of gravity: lymphatic vessels in the lower extremities must propel lymphatic fluid against gravity. Once degeneration of smooth muscle cells of the lymphatic vessels sets in,10,11,16,17 the edema worsen progressively as the function of the smooth muscle weakens. In this situation, traditional LVA has a limited effect because deteriorating lymphatic vessels cannot efficiently propel lymph into the anastomosed vein incorporated into the LVA.
An LVA created by means of the superior-edge-of-the-knee incision method has a strong therapeutic effect in patients with LEL because knee joint movement is enlisted as an alternative power source to propel lymph into the anastomosed vein.8 In patients in whom the method has been applied for the treatment of LEL, there has been no exacerbation of the edema in the affected limb after the surgery.
Anatomically, the lymphatic vessels differ in location from patient to patient,7,18 because the lymphedematous swelling progresses on an individual basis. This is why detection of lymphatic vessels during traditional LVA surgery is always challenging for microsurgeons. However, the fixed incision site that characterizes the superior-edge-of-the-knee incision method fully resolves the problem of detecting lymphatic vessels in the thigh region.
The effect of the superior-edge-of-the-knee incision method usually begins as decreased stiffness and decreased knee circumference, and this effect spreads out over time, medially to laterally, up to the groin region and down to the upper edge of the ankle. The effect of the superior-edge-of-the-knee incision method does not extend over the ankle; therefore, lymphedema distal to the ankle region necessitates additional LVA around the ankle for local improvement.
Multiple LVAs are highly recommended as treatment for LEL.6–8 The procedure requires several microsurgeons to operate simultaneously and the use of several operating microscopes. Because LVA itself is a difficult surgical procedure, there is a shortage of microsurgeons skilled enough to treat the numerous LEL patients that exist. To overcome this problem, we try to minimize the LVA surgery so that each microsurgeon can take on more patients without compromising the clinical results.
We conducted this study not only to confirm the effectiveness of the superior-edge-of-the-knee incision method for treatment of LEL but also to clarify which stages of LEL can be treated by a single LVA via the superior-edge-of-the-knee incision method. The clinical effect of the superior-edge-of-the-knee incision method becomes apparent much more quickly than does that of traditional LVA; early improvement in the lymphedema, including reduced stiffness, reduced associated skin redness, and a decrease in the size of the affected limb, can be observed around the knee in most patients within a week after the superior-edge-of-the-knee incision method has been applied. (see video, Supplemental Digital Content 1, which depicts early effects of the superior-edge-of-the-knee incision method that allows for lymphatic drainage, http://links.lww.com/PRSGO/A669). However, improvement extending as far as the upper and lower regions requires time, much like the improvement that occurs with traditional LVA. Therefore, it is possible that the LEL will progress to the ankle before the superior-edge-of-the-knee incision-based anastomosis takes effect there. Surgeons should consider whether patients with stage 3 dermal backflow in the leg and a stardust patterns in the lower leg region are candidates for additional LVA around the ankle if their lymphedema progresses over the ankle before the single anastomosis takes effect there.
Video Graphic 1.
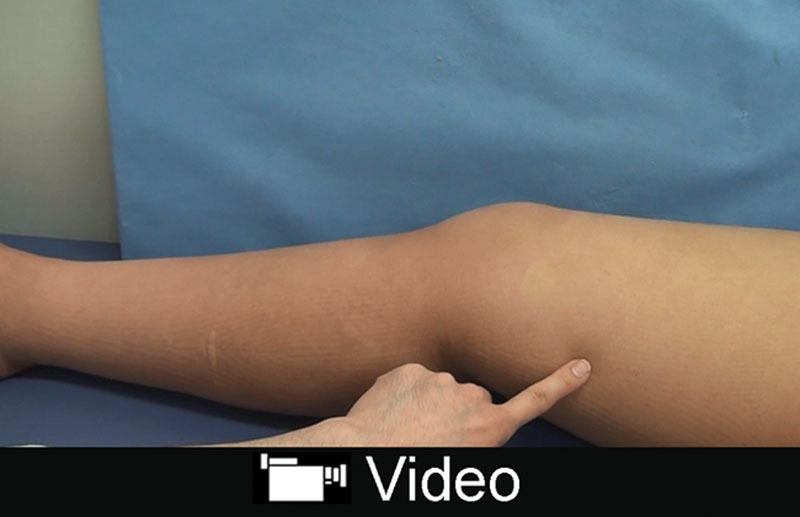
See video, Supplemental Digital Content 1, which shows the preoperative and postoperative lymphatic drainage to the LVA at the superior-edge-of-the-knee incision site. Before the surgery, lymphedema-associated skin redness and stiffness persisted. By postoperative day 6, the lymphedema-associated skin redness and stiffness had disappeared as a result of restored lymphatic drainage. This phenomenon in lymphatic drainage was not observed at other LVA site, http://links.lww.com/PRSGO/A669.
One of our 10 study patients experienced new lymphedematous swelling in the distal aspect of the ankle. This was a patient with stage 2 leg dermal backflow, and the new swelling was seen 9 months postoperatively, despite the fact that the patient’s lymphedema had improved markedly (with a reduction of 26.971 in the LEL index at 9 months). We learned, however, that this patient had fallen and suffered possible ankle trauma just before the new lymphedema around the ankle was noted. She underwent additional LVAs around the ankle 16 months after the single LVA had been performed, and the ankle edema improved, as it had in other regions.
We can only speculate why the edema progressed around the ankle in this patient. It is possible that ankle trauma resulted in collapse of the local lymph system that could not be compensated for by the anastomosis performed by the superior-edge-of-the-knee incision method. In actuality, the strong effect of this method is seen only from the knee region up to the groin and down to upper edge of the ankle. It is also possible that the time difference between the lymphedema progression and effectiveness of the superior-edge-of-the-knee incision method was at work. ICG lymphography findings at the time of the additional LVAs strongly support the possibility of newly occurring lymphedema, because a new stardust pattern was detected around the ankle, and there was no continuity between it and the original stardust pattern in the thigh region (Fig. 5). We also think it is possible that this patient showed less or no lymphedema progression in the distal aspect of the ankle even after the fall, if multiple LVAs at the lower leg were added with the superior-edge-of-the-knee incision method in the first operation.
Fig. 5.
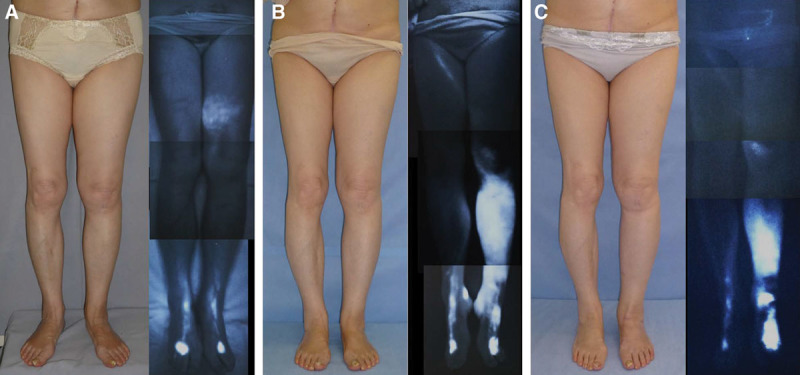
A, The patient shown here is a 71-year-old woman who had suffered left LEL for 9 years following hysterectomy. Preoperative ICG lymphography revealed stage 2 leg dermal backflow. No dermal backflow was observed around the ankle. The preoperative left LEL index was 277.7. B, A single LVA was created by means of the superior-edge-of-the-knee incision method. At 16 months, her right LEL index was reduced to 254.3. Although the lymphedema was improved at the groin, thigh, and lower leg, new lymphedematous swelling occurred around the ankle after the patient suffered a fall. Upon postoperative ICG lymphography, the stardust pattern in the thigh region had lessened, but a new stardust pattern emerged from the ankle to the lower leg. Additional LVAs were created around the ankle 16 months after the single LVA, and the edema around the ankle improved considerably. C, At 24 months after the single LVA, the LEL index was 254.1, and the edema around the ankle had improved considerably, like that at other regions. Postoperative ICG lymphography at 24 months showed reduction of stardust pattern around the thigh due to application of the superior-edge-of-the-knee incision method and around the ankle due to the creation of additional LVAs there.
ICG lymphography is a minimally invasive imaging modality that is very useful for evaluation of lymphedema and detection of lymphatic vessels.6,8,13–15 In our 10 study patients, we used ICG lymphography not for detection of lymphatic vessels but to evaluate the severity of the LEL. ICG lymphography cannot be used to visualize lymph flow more than 2 cm from the skin surface in the thigh regions.19 ICG lymphography is not absolutely necessary for detection of the lymphatic vessels in patients with early lymphedema treated by our method. This means we can avoid the use of ICG in patients who are potentially allergic to it.
ICG lymphography is also useful for follow-up evaluation of the effect of LVA.20,21 Improvement can be detected as reduction of stardust area with new splash patterns. Improvement in the imaging features were seen in of 2 of our 5 study patients who underwent ICG lymphography more than 6 months after the single LVA, and 2 of these patients were released from compression therapy without any recurrence of the edema.
Very early LEL in patients with stage 1 leg dermal backflow is not difficult to resolve by LVA. Satisfactory improvement is usually obtained for patients with stage 1 leg dermal backflow with a single LVA created at the distal leg region using collective lymphatic vessel or an LVA created by efferent lymphatic vessel.22 Thus, we think the superior-edge-of-the-knee incision method is not always suitable for patients with stage 1 leg dermal backflow. Our rationale is as follows: a single LVA at the top of the foot or lower leg is easily performed, it provides good results, and the operation time is relatively short; the superior-edge-of-the-knee incision method can be saved for any future exacerbation in such cases; the lymphatic vessels under the superficial fascia at the superior-edge-of-the-knee incision site might work independently to carry the main flow of lymph in cases of very early lymphedema because collateral lymph flow, which is visualized by a characteristic splash pattern upon ICG lymphography, may not yet be established in the thigh region in patients with stage 1 dermal backflow.
Degeneration of lymphatic vessels can occur even in patients with early LEL. As many as 30% of lymphatic vessels in patients with early lymphedema and a linear pattern on ICG lymphography appear contracted intraoperatively.23 This early degeneration of lymphatic vessels supports the clinical necessity of surgical treatment by LVA for early LEL; the status of lymphatic vessels is an important factor in the efficacy of LVA.
A possible drawback of the superior-edge-of-the-knee incision method is relatively long time it takes to search for the lymphatic vessels under the superficial fascia. LVA at the thigh is one of the most difficult procedures for microsurgeons to perform, and this is because it is hard to detect lymphatic vessels in the deep layer of subcutaneous tissue in the thigh region. In our study patients, detection of the lymphatic vessels under the superficial fascia layer took an average of 26.5 minutes, and total operation time for a single anastomosis achieved by the superior-edge-of-the-knee incision method was 75.7 minutes on average. This is not a short time; single LVA at the lower leg or top of the foot usually takes expert surgeons around 30–40 minutes to perform. However, the relatively long time required for anastomosis performed by the superior-edge-of-the-knee incision method does not diminish this method’s utility because the effect of the resulting single anastomosis is sufficient for most cases of early LEL. Furthermore, the total operation time required for creation of multiple LVAs is of course longer than that required for creation of the single anastomosis by the superior-edge-of-the-knee incision method.
Our study was limited by its retrospective design. A future study is needed to determine whether the lymph flow volume and condition of the lymphatic vessels correlate with the therapeutic effects in patients with LEL. In addition, we would like to improve the technique by finding other vessels, like those near the knee, in which dynamic flow of lymph through the LVA is stimulated by regular limb motions.
CONCLUSIONS
A single LVA created by the superior-edge-of-the-knee incision method is sufficient for the majority of cases of LEL characterized by stage 2 or 3 leg dermal backflow to prevent the progression of lymphedema and to improve lymphedema complaints including stiffness and enlargement of the limb. However, progression of lymphedema at the distal ankle may occur in some patients, and this will require additional LVAs around the ankle for optimum improvement.
ACKNOWLEDGMENTS
The authors thank Isao Koshima for sharing both his practical knowledge of and zeal for lymphedema treatment. The authors thank Miyuki, Hanano, and Mayoko Seki and all members of our department for their kind support during preparation of this article and also thank Tina Tajima for her meticulous English editing.
Supplementary Material
Footnotes
Presented at Plastic Surgery The Meeting 2016, Annual Meeting of the American Society of Plastic Surgeons, September 23–27, 2016, Los Angeles, Calif.
Supported by JSPS KAKENHI Grant Number JP17K17038.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by JSPS KAKENHI Grant Number JP17K17038.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Yamada Y. The studies on lymphatic venous anastomosis in lymphedema. Nagoya J Med Sci. 1969;32:1–21.. [Google Scholar]
- 2.O’Brien BM, Sykes P, Threlfall GN, et al. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg. 1977;60:197–211.. [DOI] [PubMed] [Google Scholar]
- 3.Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg. 2000;16:437–442.. [DOI] [PubMed] [Google Scholar]
- 4.Koshima I, Nanba Y, Tsutsui T, et al. Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg. J Reconstr Microsurg. 2003;19:209–215.. [DOI] [PubMed] [Google Scholar]
- 5.Granzow JW, Soderberg JM, Kaji AH, et al. Review of current surgical treatments for lymphedema. Ann Surg Oncol. 2014;21:1195–1201.. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto T, Narushima M, Yoshimatsu H, et al. Minimally invasive lymphatic supermicrosurgery (MILS): indocyanine green lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann Plast Surg. 2014;72:67–70.. [DOI] [PubMed] [Google Scholar]
- 7.Mihara M, Seki Y, Hara H, et al. Predictive lymphatic mapping: a method for mapping lymphatic channels in patients with advanced unilateral lymphedema using indocyanine green lymphography. Ann Plast Surg. 2014;72:706–710.. [DOI] [PubMed] [Google Scholar]
- 8.Seki Y, Yamamoto T, Yoshimatsu H, et al. The superior-edge-of-the-knee incision method in lymphaticovenular anastomosis for lower extremity lymphedema. Plast Reconstr Surg. 2015;136:665e–675e.. [DOI] [PubMed] [Google Scholar]
- 9.Maegawa J, Yabuki Y, Tomoeda H, et al. Outcomes of lymphaticovenous side-to-end anastomosis in peripheral lymphedema. J Vasc Surg. 2012;55:753–760.. [DOI] [PubMed] [Google Scholar]
- 10.Mihara M, Hara H, Hayashi Y, et al. Pathological steps of cancer-related lymphedema: histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One. 2012;7:e41126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto T, Yamamoto N, Yoshimatsu H, et al. Factors associated with lymphosclerosis: an analysis on 962 lymphatic vessels. Plast Reconstr Surg. 2017;140:734–741.. [DOI] [PubMed] [Google Scholar]
- 12.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. 2009 consensus document of the International Society of Lymphology. Lymphology 2009;42:51–60.. [PubMed] [Google Scholar]
- 13.Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg. 2011;128:314e–321e.. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Yamamoto N, Hayashi N, et al. Practicality of the lower extremity lymphedema index: lymphedema index versus volumetry-based evaluations for body-type-corrected lower extremity volume evaluation. Ann Plast Surg. 2016;77:115–118.. [DOI] [PubMed] [Google Scholar]
- 15.Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg. 2013;132:1305–1314.. [DOI] [PubMed] [Google Scholar]
- 16.Koshima I, Kawada S, Moriguchi T, et al. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg. 1996;97:397–405.; discussion 406. [DOI] [PubMed] [Google Scholar]
- 17.Huang GK, Hu RQ, Liu ZZ, et al. Microlymphaticovenous anastomosis in the treatment of lower limb obstructive lymphedema: analysis of 91 cases. Plast Reconstr Surg. 1985;76:671–685.. [PubMed] [Google Scholar]
- 18.Mitsumori LM, McDonald ES, Wilson GJ, et al. MR lymphangiography: how I do it. J Magn Reson Imaging. 2015;42:1465–1477.. [DOI] [PubMed] [Google Scholar]
- 19.Ogata F, Narushima M, Mihara M, et al. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg. 2007;59:180–184.. [DOI] [PubMed] [Google Scholar]
- 20.Chen WF, Zhao H, Yamamoto T, et al. Indocyanine green lymphographic evidence of surgical efficacy following microsurgical and supermicrosurgical lymphedema reconstructions. J Reconstr Microsurg. 2016;32:688–698.. [DOI] [PubMed] [Google Scholar]
- 21.Shih HB, Shakir A, Nguyen DH. Use of indocyanine green-SPY angiography for tracking lymphatic recovery after lymphaticovenous anastomosis. Ann Plast Surg. 2016;76:S232–S237.. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Yamamoto N, Yamashita M, et al. Efferent lymphatic vessel anastomosis: supermicrosurgical efferent lymphatic vessel-to-venous anastomosis for the prophylactic treatment of subclinical lymphedema. Ann Plast Surg. 2016;76:424–427.. [DOI] [PubMed] [Google Scholar]
- 23.Hara H, Mihara M, Seki Y, et al. Comparison of indocyanine green lymphographic findings with the conditions of collecting lymphatic vessels of limbs in patients with lymphedema. Plast Reconstr Surg. 2013;132:1612–1618.. [DOI] [PubMed] [Google Scholar]


