Abstract
Lyme disease, caused by the bacterial spirochete Borrelia burgdorferi, is a tick-borne zoonosis. Lyme neuroborreliosis is a principal manifestation of Lyme disease and its pathogenesis remains incompletely understood. Recent studies have demonstrated that Borrelia burgdorferi lipoproteins caused similar inflammatory effects as exhibited in Lyme neuroborreliosis. Basic membrane protein A (BmpA) is one of the dominant lipoproteins in the Borrelia burgdorferi membrane. In addition, nuclear factor κ-B (NF-κB) modulates the regulation of gene transcription associated with immunity and inflammation; however, in unstimulated cells, NF-κB is combined with the inhibitor of NF-κB (IκB-β). Therefore, it was hypothesized that NF-κB may be associated with BmpA-induced inflammation and the occurrence of Lyme neuroborreliosis. Therefore, the aim of the present study was to investigate the role that NF-κB serves in the signaling pathway of rBmpA-induced inflammatory chemokines. The present study measured the expression levels of NF-κB, IκB-β and inflammatory chemokines following recombinant BmpA (rBmpA) stimulation of murine microglia BV2 cells. Following stimulation with rBmpA, concentrations of pro-inflammatory cytokines including C-X-C motif chemokine 2, C-C motif chemokine (CCL) 5 and CCL22 were determined by ELISA analysis. Reverse transcription-quantitative polymerase chain reaction and western blotting were used to detect the expression levels of NF-κB p65 and IκB-β. The data demonstrated that concentrations of these chemokines in cell supernatants increased significantly following rBmpA stimulation. NF-κB was overexpressed, but IκB-β expression was significantly decreased. In conclusion, these results suggested that NF-κB serves an important stimulatory role in the signaling pathway of rBmpA-induced inflammatory chemokines in BV2 cells.
Keywords: Lyme neuroborreliosis, Borrelia burgdorferi, nuclear factor κ-B, recombinant basic membrane protein A, BV2 microglia, pathogenesis
Introduction
Lyme disease (LD) is a multisystem inflammatory, tick-borne disease resulting from infection with Borrelia burgdorferi (1,2). LD is present in >80 countries, including China. A total of ~3,000,000 cases of LD are reported each year worldwide, representing an incidence of ~0.111% (2,3). LD seriously affects human health, in addition to economic development. As a result, this widespread disease has been given attention regarding its prevention and treatment by the World Health Organization (3). Lyme neuroborreliosis (LNB) is a principal manifestation of LD and is caused by the inflammatory effects of the spirochete B. burgdorferi on the nervous system. LNB causes extensive neurological damage and encephalitic memory impairment, even leading to dementia and personality disorders. Given the high disability rate caused by the disease, researchers and clinicians are giving more concentrated attention to LNB (4,5); however, its pathogenesis remains incompletely understood. It is generally accepted that LNB is caused by an autoimmune response triggered by molecular mimicry (6,7).
Microglial cells, the resident macrophage cells within the central nervous system (CNS), are important in initiating an immune response to microbial products (8). As a previous study demonstrated, CNS damage and inflammation may activate microglia (9). Reactive microglia produce various cytokines and chemokines causing an acute inflammatory reaction, which leads to neuronal damage and apoptosis. Microglia cells are hypersensitive to CNS damage. These cells multiply rapidly, begin to express major histocompatibility complex proteins, migrate and subsequently differentiate into phagocytes that secrete cytokines and other toxic substances (10,11).
Previous studies have suggested that B. burgdorferi basic membrane protein A (BmpA), one of the primary B. burgdorferi pathogenic substances, exhibits a potent pro-inflammatory effect (11–13). In the authors' previous study, an Escherichia coli expression system was established and purified recombinant BmpA (rBmpA) was successfully obtained. When stimulated with rBmpA, murine microglia BV2 cells produced pro-inflammatory chemokines, including C-X-C motif chemokine 2 (CXCL2), C-C motif chemokine (CCL) 5 and CCL22, causing inflammation and damage in mice (13). However, the signal transduction mechanism involved is unclear (13).
The nuclear factor κ-B (NF-κB) is A protein complex which was found to specifically bind to the κB sequence (GGGACTTTCC) of the immunoglobulin K enhancer and regulated the expression of target genes (14–16). NF-κB has received widespread interest from researchers for its crucial role in the immune system. It has been demonstrated in unstimulated cells that inhibitor of NF-κB (IκB-β) combines with NF-κB and maintains NF-κB an inactive state. The stimuli that cause NF-κB activation target IκB-β for degradation via a phosphorylation-dependent ubiquitination process (16,17). With the degradation of IκB-β, NF-κB is activated, entering the cell nucleus where it encounters a promoter with DNA binding sites for NF-κB (17,18). The activation of target gene transcription induces the expression of specific mRNAs, and the production of cytokines and chemokines, thereby regulating the activation, multiplication, infiltration, chemotaxis and secretion of immune cells (18–20). As rBmpA stimulates the production of CXCL and CCL chemokines in microglia, it was hypothesized that NF-κB may be closely associated with rBmpA-induced chemokine production in BV2 cells (13).
Based on the conclusions of the authors' previous study demonstrating that rBmpA stimulates the production of inflammatory chemokines in BV2 cells, the present study continued to investigate the signal transduction mechanism of LNB (13). Additionally, the present study investigated the role of NF-κB in the signaling pathway of rBmpA-induced inflammatory chemokines and provides a scientific basis for the prevention and treatment of LBN.
Materials and methods
rBmpA preparation
Recombinant BmpA proteins were produced in E. coli using the bacterial expression vector pGEX-6P1 (GE Healthcare, Chicago, IL, USA). Expression, purification and enzymatic cleavage of the glutathione S-transferase fusion proteins were performed as previously described (14,15).
Cell culture and groups
BV2 cells (Kunming Medical University Biological Engineer Center, Kunming, China) were cultured in Dulbecco's modified Eagle's medium (DMEM)/high glucose with 5% cattle serum (CS; HyClone, Logan, UT, USA), 1% penicillin/streptomycin (Sangon Biotech Co., Ltd., Shanghai, China) at 37°C with 5% CO2. BV2 cells were seeded into a 6-well plate at a concentration of 3×105 cells/ml. Supernatants were discarded until the cells fully adhered to the plate. Cells were divided into three groups (negative control group, positive control group and experimental group), and were stimulated with 2 ml/well PBS with 5% CS-DMEM medium, 1 µg/ml lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and 20 µg/ml rBmpA, respectively. Radioimmunoprecipitation assay buffer (high; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was used to lyse the cells and cell lysates were collected at 12, 24 and 48 h following stimulation.
ELISA analysis
Culture supernatants were collected at 6, 12 and 24 h following stimulation with rBmpA and were analyzed by ELISA. Mouse CXCL2 (MIP-2), mouse CCL22 (MDC) and mouse CCL5 (RANTES) ELISA kits (RayBiotech, Inc., Norcross, GA, USA; cat. nos. P10889, O88430 and P30882) was used following manufacturer's protocol. A Bio-Rad microplate reader (Model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to measure the absorbance at 450 nm.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Harvested cells were lysed with RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China) and RNA was extracted, following the manufacturer's protocol. Total RNA was reverse transcribed to cDNA. The RT-qPCR was performed using the real-time PCR System Cfx-Connect (Bio-Rad Laboratories, Inc.). The reaction conditions of the RT-qPCR were as follows: Denaturation at 95°C for 30 min, annealing at 58°C for 1 h and extension at 65°C for 5 sec. The total volume used in the PCR was 25 µl, including 2 µl cDNA, 12.5 µl SYBR (Takara Biotechnology, Co., Ltd.), 8.5 µl dH2O, 1 µl forward primer and 1 µl reverse primer (Tsingke Biotech Co., Ltd., Kunming, China). The sequences of the specific primers used to amplify NF-κB p65 and GAPDH were as follows: NF-κB p65 forward, 5′-GCTACACAGAGGCCATTGAA-3′ and reverse, 5′-TCCCGGAGTTCATCTATGTG-3′; IκB-β forward, 5′-GGGAACGTCAGTCTGTACCA-3′ and reverse, 5′-GCACCATCGCTCTCTGTTTT-3′; GAPDH forward, 5′-TCCCAGAGCTGAACGGGAAG-3′ and reverse, 5′-TCAGTGGGCCCTCAGATGC-3′. The gene expression level was calculated using the method of 2−ΔΔCq (21–23).
Western blot analysis
Total protein quantitation analysis was used as the internal control for quantitative fluorescent western blot analysis (24–27). Cells were lysed in radioimmunoprecipitation lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) with 1 mM phenylmethylsulfonyl fluoride (Beyotime Institute of Biotechnology), and quantified using a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology). Proteins were separated on 10% TGX FastCast acrylamide gels (Bio-Rad Laboratories, Inc.) with a loading mass of 20 µg total protein, then subsequently transferred to a polyvinylidene difluoride (PVDF) membranes (0.2 µm; Bio-Rad Laboratories, Inc.) using Trans-Blot Turbo Transfer System (p65, 15 V in 4-min protocol; IκB-β, 15 V in 4-min protocol; Bio-Rad Laboratories, Inc.) with Trans-Blot Turbo Midi Transfer Packs (Bio-Rad Laboratories, Inc.). The PVDF membranes were blocked with 5% skim milk at 37°C for 2 h. Blots were incubated with specific primary antibodies of NF-κB p65 (cat. no. ab16502; 1:2,000; Abcam, Cambridge, UK) or IκB-β (cat. no. ab7574; 1:1,000; Abcam) overnight at 4°C, and subsequently incubated with goat anti-rabbit secondary antibodies (cat. no. BS13278; p65 1:10,000; Nanjing Bioworld Biotech Co., Ltd., Nanjing, China; cat. no. ab150077; IκB-β; 1:3,000; Abcam) at room temperature for 2 h. The blots were washed three times between the primary and secondary antibody incubations. The immunoreactive bands were imaged using an infrared imaging system (Bio-Rad Laboratories, Inc.). ChemiDoc™ XRS+Systems with Image Lab™ software (Bio-Rad Laboratories, Inc.) was used to analyze the western blot results of NF-κB p65 and IκB-β.
Statistical analysis
The results are expressed as the mean ± standard error of the mean and were analyzed using the GraphPad Prism 6 software package (GraphPad Software, Inc., La Jolla, CA, USA) (26). Two-way analysis of variance was used to calculate the P-values, multiple comparison between the groups was performed using the Student-Newman-Keuls method. P<0.05 was considered to indicate a statistically significant difference.
Results
rBmpA significantly stimulates the production of the inflammatory cytokines CXCL2, CCL5 and CCL22 in BV2 cells
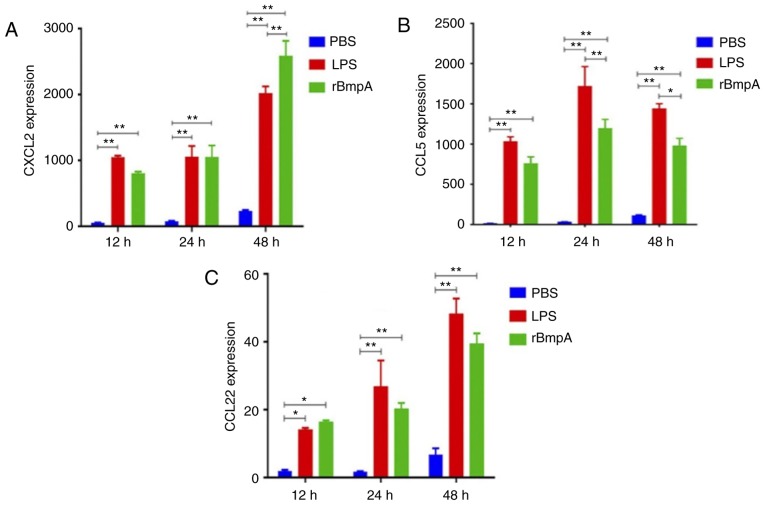
To determine whether rBmpA induces the production of pro-inflammatory chemokines in BV2 cells, analysis of CXCL2, CCL5 and CCL22 concentrations in the cell supernatants was assayed by ELISA. The results demonstrated that rBmpA significantly increased CXCL2, CCL5 and CCL22 expression at all time points tested compared with the PBS only group (P<0.05; Fig. 1). The expression of the three chemokines demonstrated an increasing trend between 12 and 48 h, except for a slight decline in CCL5 between 24 and 48 h (Fig. 1B).
Figure 1.
Analysis of the expression of inflammatory cytokines in BV2 cells. (A) CXCL2, (B) CCL5 and (C) CCL22 expression following stimulation with 20 µg/ml rBmpA for different time periods (12, 24 and 48 h). The experimental results exhibited are representative of three independent tests. Each value represents the mean ± standard error of the mean. *P<0.05; **P<0.01. rBmpA, recombinant basic membrane protein A; LPS, lipopolysaccharide; CXCL2, C-X-C motif chemokine 2; CCL, C-C motif chemokine.
rBmpA stimulation induces an increase in NF-κB and decrease in IκB-bmRNA in BV2 cells
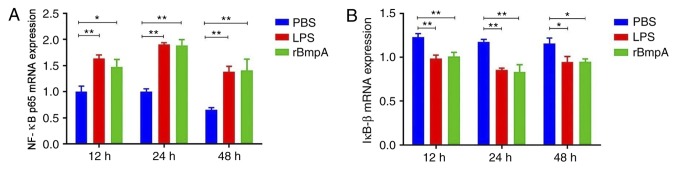
rBmpA stimulation significantly increased CXCL2, CCL5 and CCL22 expression compared with the PBS control (P<0.05). Therefore, the rBmpA-induced nuclear translocation of NF-κB p65 and degradation of cytosolic IκB-β was investigated. RT-qPCR was used to analyze the expression of NF-κB p65 and IκB-β mRNA. As exhibited in Fig. 2, the expression of NF-κB mRNA in the group treated with rBmpA was significantly increased (P<0.01) compared with the PBS-only control at 12, 24 and 48 h and rBmpA treatment significantly decreased the intracellular levels of IκB-β (P<0.05).
Figure 2.
Time-dependent effects of rBmpA on the expression of NF-κB p65 and IκB-β mRNA at 12–24 h. BV2 microglia were stimulated by 20 mg/ml rBmpA for 48 h. The levels of (A) NF-κB p65 and (B) IκB-β mRNA were determined by reverse transcription-quantitative polymerase chain reaction. Each value represents the mean ± standard error of the mean. n=3. *P<0.05; **P<0.01. NF-κB, nuclear factor κ-B; rBmpA, recombinant basic membrane protein A; IκB-β. inhibitor of NF-κB; LPS, lipopolysaccharide.
rBmpA stimulation induces an increase in NF-κB p65 and the degradation of IκB-bproteins in BV2 cells
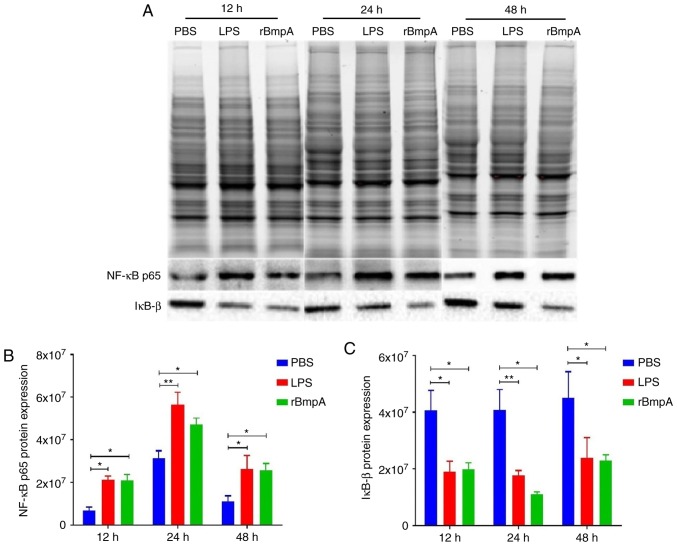
Western blot analysis was used to detect the protein expression levels of NF-κB p65 and IκB-β in BV2 cells. As exhibited in Fig. 3, following stimulation with rBmpA for 12, 24 and 48 h, NF-κB p65 protein expression was significantly increased (P<0.05) and the protein level of IκB-β was significantly decreased (P<0.05) in the rBmpA treatment group compared with the PBS-only control. Increased expression levels of NF-κB p65 protein were observed in the rBmpA and LPS treatment groups. rBmpA significantly decreased the intracellular levels of IκB-β protein compared with the PBS control (P<0.05).
Figure 3.
Total protein quantification was used to analyze the protein expression of NF-κB p65 and IκB-β in BV2 cells. The cells were stimulated with 20 µg/ml rBmpA or 1 µg/ml LPS at different time points (12, 24 and 48 h). (A) The total protein was measured by SDS-PAGE using a Stain-Free Gel. The expression of (B) NF-κB p65 and (C) IκB-β, exhibited as the mean ± standard error of the mean. n=3. *P<0.05; **P<0.01. NF-κB, nuclear factor κ-B; rBmpA, recombinant basic membrane protein A; IκB-β inhibitor of NF-κB; LPS, lipopolysaccharide.
Discussion
NF-κB is a nuclear transcription factor which regulates the immune response, stress response and apoptosis (28). Upregulation of NF-κB has been demonstrated in various tumors, pulmonary disease and hepatic disease. Previous in vitro studies have suggested that NF-κB is a key signal transduction molecule in the downstream pathway of Toll-like receptors (TLRs) (29,30). Once the TLRs are stimulated by various pathogenic factors, the degradation of IκB-β is initiated and NF-κB is free to enter the cell nucleus, where NF-κB combines with the IκB motif controlling the transcription of a number of cytokines, including tumor necrosis factor-α (TNF-α), CXCL13 and interleukin (IL)-6. Studies have demonstrated that NF-κB is a transducer of various common inflammatory signaling pathway reactions (27–30). Therefore, methods of inhibiting NF-κB signaling have potential therapeutic applications in inflammatory diseases. At present, a number of anti-inflammatory drugs and anti-rheumatic drugs, including corticosteroids and aspirin, have been confirmed to be inhibitors of NF-κB activation (31,32).
It has been reported that the B. burgdorferi bmpA/B gene operon exhibits the most marked upregulation in mouse and human joints (33). BmpA possesses stimulatory activity through functional domains that trigger the inflammatory response. For example, the activated NF-κB kinase signalling pathway in articular synovial cells was demonstrated to produce pro-inflammatory cytokines including TNF-α and IL-1β, triggering prostaglandin E2 receptor EP4 subtype (PGE-2) (15,34). Rasley et al (35) confirmed that B. burgdorferi was an important stimulus that induced microglia to produce IL-6, TNF-α and PGE-2, which was associated with increased expression of NF-κB, TLR2 and cluster of differentiation 14 in BV2 cells. By activating signaling molecules, B. burgdorferi stimulated immune cells to produce multiple inflammatory substances (35). Further investigation by Sun et al (36) suggested that substance P was able to prompt B. burgdorferi-induced NF-κB activation by upregulating NF-κB subunit p65, causing a significant increase in the production of inflammatory cytokines (36). NF-κB serves an important role in the inflammatory cytokine signaling pathway of immune cells. However, rBmpA and NF-κB associated pathogenesis in LNB, to the best of the authors' knowledge, has not been reported in the literature. A recent report indicated that rBmpA induced activation of BV2 cells with a concentration-dependent secretion of inflammatory chemokines (13). Therefore, rBmpA may be associated with the inflammatory chemokines produced by BV2 cells and the occurrence of LNB. However, the precise mechanism of the signal transduction pathway remains uncertain.
In the present study, whether NF-κB was a key modulator in the inflammatory chemokines (CXCL2, CCL5 and CCL22) signaling pathway stimulated by rBmpA in BV2 cells was investigated. The results of the present study demonstrated that CXCL2, CCL5, CCL22, NF-κB mRNA and NF-κB protein increased and the protein and mRNA levels of IκB-β decreased following stimulation with rBmpA in BV2 cells. It was demonstrated that NF-κB served a key role in the signaling pathways stimulated by rBmpA in BV2 cells, resulting in the production of various inflammatory chemokines. This result increases the understanding of the pathogenesis of LNB. Future studies will investigate the key proteins in inflammatory chemokine signaling pathways stimulated by rBmpA and, most importantly, investigate whether these signaling pathways also modulate the pathogenesis of Lyme arthritis, dermatitis and carditis. The long-term aim is to investigate whether preventive and therapeutic medicines for LNB may be developed that target these proteins (37).
In conclusion, following stimulation by rBmpA, BV2 cells overexpressed NF-κB and exhibited significantly reduced expression of IκB-β in the inflammatory cytokine-signaling pathway. This result suggested that NF-κB is important in the inflammatory cytokines signaling pathways stimulated by rBmpA and may be associated with the occurrence of LNB. The present study further clarified the mechanism underlying the rBmpA-induced inflammatory chemokines signaling pathway in microglial cells, and provides a scientific basis for the prevention and treatment of LNB.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81560596 and 3156005) and the Natural Foundation of Yunnan Province (grant nos. 2012FB011, 2014FA011, 2014FB001 and 2017FE467-001).
References
- 1.Borchers AT, Keen CL, Huntley AC, Gershwin ME. Lyme disease: A rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82–115. doi: 10.1016/j.jaut.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Pal U, Wang P, Bao F, Yang X, Samanta S, Schoen R, Wormser GP, Schwartz I, Fikrig E. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med. 2008;205:133–141. doi: 10.1084/jem.20070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lantos PM, Shapiro ED, Auwaerter PG, Baker PJ, Halperin JJ, McSweegan E, Wormser GP. Unorthodox alternative therapies marketed to treat Lyme disease. Clin Inf Dis. 2015;60:1776–1782. doi: 10.1093/cid/civ186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marques AR. Lyme neuroborreliosis. Continuum (Minneap Minn) 2015;21(6 Neuroinfectious Disease):1–1744. doi: 10.1212/CON.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 6.Bremell D, Dotevall L. Oral doxycycline for Lyme neuroborreliosis with symptoms of encephalitis, myelitis, vasculitis or intracranial hypertension. Eur J Neurol. 2014;21:1162–1167. doi: 10.1111/ene.12420. [DOI] [PubMed] [Google Scholar]
- 7.Cerar T, Ogrinc K, Lotričfurlan S, Lotric-Furlan S, Kobal J, Levicnik-Stezinar S, Strle F, Ruzić-Sabljic E. Diagnostic value of cytokines and chemokines in Lyme neuroborreliosis. Clin Vaccine Immunol. 2013;20:1578–1584. doi: 10.1128/CVI.00353-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C, Cho ED, Kim HK, You S, Lee HJ, Hwang D, Lee SJ. β1-integrin-dependent migration of microglia in response to neuron-released α-synuclein. Exp Mol Med. 2014;46:e91. doi: 10.1038/emm.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Togna AR, Latina V, Trefiletti G, Guiso M, Moschini S, Togna GI. 1-Phenil-6,7-dihydroxy-isochroman inhibits inflammatory activation of microglia. Brain Res Bull. 2013;95:33–39. doi: 10.1016/j.brainresbull.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Brissette CA, Houdek HM, Floden AM, Rosenberger TA. Acetate supplementation reduces microglia activation and brain interleukin-1β levels in a rat model of Lyme neuroborreliosis. J Neuroinflammation. 2012;9:249. doi: 10.1186/1742-2094-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhlow CJ, Garcia-Monco JC, Coleman JL, Benach JL. Murine microglia are effective phagocytes for Borrelia burgdorferi. J Neuroimmunol. 2005;168:183–187. doi: 10.1016/j.jneuroim.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, Policastro PF. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun. 2008;76:5228–5237. doi: 10.1128/IAI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao H, Liu A, Cui Y, Liang Z, Li B, Bao F. Borrelia burgdorferi basic membrane protein A could induce chemokine production in murine microglia cell line BV2. Microb Pathog. 2017;111:174–181. doi: 10.1016/j.micpath.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 14.Simpson WJ, Schrumpf ME, Schwan TG. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990;28:1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Izadi H, Coleman AS, Wang P, Ma Y, Fikrig E, Anguita J, Pal U. Borrelia burgdorferi lipoprotein BmpA activates pro-inflammatory responses in human synovial cells through a protein moiety. Microbes Infect. 2008;10:1300–1308. doi: 10.1016/j.micinf.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 17.Whiteside ST, Epinat JC, Rice NR, Israël A. I kappa B epsilon, a novel member of the I kappa B family, controls RelA and cRel NF-kappa B activity. EMBO J. 1997;16:1413–1426. doi: 10.1093/emboj/16.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell S, Degitz K, Quirling M, Jilg N, Page S, Brand K. Involvement of NF-kappaB signaling in skin physiology and disease. Cell Signal. 2003;15:1–7. doi: 10.1016/S0898-6568(02)00080-3. [DOI] [PubMed] [Google Scholar]
- 19.Vinayagamoorthi R, Koner BC, Kavitha S, Nandakumar DN, Padma Priya P, Goswami K. Potentiation of humoral immune response and activation of NF-kappaB pathway in lymphocytes in experimentally induced hyperthyroid rats. Cell Immunol. 2005;238:56–60. doi: 10.1016/j.cellimm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Colleran A, Collins PE, Carmody RJ. Assessing Sites of NF-κB DNA binding using chromatin immunoprecipitation. Methods Mol Biol. 2015;1280:47–59. doi: 10.1007/978-1-4939-2422-6_4. [DOI] [PubMed] [Google Scholar]
- 21.Leal VO, Saldanha JF, Stockler-Pinto MB, Cardozo LF, Santos FR, Albuquerque AS, Leite M, Jr, Mafra D. NRF2 and NF-κB mRNA expression in chronic kidney disease: A focus on nondialysis patients. Int Urol Nephrol. 2015;47:1985–1991. doi: 10.1007/s11255-015-1135-5. [DOI] [PubMed] [Google Scholar]
- 22.Naranjo V, Ayllón N, Pérez de la Lastra JM, Galindo RC, Kocan KM, Blouin EF, Mitra R, Alberdi P, Villar M, de la Fuente J. Reciprocal regulation of NF-kB (Relish) and subolesin in the tick vector, Ixodes scapularis. PLoS One. 2013;8:e65915. doi: 10.1371/journal.pone.0065915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irvine M, Okitsu C, Hsieh CL. Q-PCR in combination with ChIP assays to detect changes in chromatin acetylation. Methods Mol Biol. 2011;791:213–223. doi: 10.1007/978-1-61779-316-5_16. [DOI] [PubMed] [Google Scholar]
- 24.Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM. Total protein analysis as a reliable loading control for quantitative fluorescent western blotting. PLoS One. 2013;8:e72457. doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.G5: ding Control for Quantitative Fluorescent Western Blotting. 2013.238: 56–605, 2097. and brain interleukin-1β lev. Plos One. 8:e72457. doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vigelsø A, Dybboe R, Hansen CN, Dela F, Helge JW, Guadalupe Grau A. GAPDH and β-actin protein decreases with aging, making stain-free technology a superior loading control in Western blotting of human skeletal muscle. J Appl Physiol (1985) 2015;118:386–394. doi: 10.1152/japplphysiol.00840.2014. [DOI] [PubMed] [Google Scholar]
- 27.Rivero-Gutiérrez B, Anzola A, Martínez-Augustin O, de Medina FS. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal Biochem. 2014;467:1–3. doi: 10.1016/j.ab.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Ruzehaji N, Avouac J, Elhai M, Frechet M, Frantz C, Ruiz B, Distler JH, Allanore Y. Combined effect of genetic background and gender in a mouse model of bleomycin-induced skin fibrosis. Arthritis Res Ther. 2015;17:145. doi: 10.1186/s13075-015-0659-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Peri S, Devarajan K, Yang DH, Knudson AG, Balachandran S. Meta-analysis Identifies NF-κB as a therapeutic target in renal cancer. PLoS One. 2013;8:e76746. doi: 10.1371/journal.pone.0076746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murqueitio MS, Ebner S, Hörtnagl P, Rakers C, Bruckner R, Henneke P, Wolber G, Santos-Sierra S. Enhanced immunostimulatory activity of in silico discovered agonists of Toll-like receptor 2 (TLR2) Biochim Biophys Acta. 2017;1861:2680–2689. doi: 10.1016/j.bbagen.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Ottonello L, Bertolotto M, Montecucco F, Bianchi G, Dallegri F. Delayed apoptosis of human monocytes exposed to immune complexes is reversed by oxaprozin: Role of the Akt/IkappaB kinase/nuclear factor kappaB pathway. Br J Pharmacol. 2009;157:294–306. doi: 10.1111/j.1476-5381.2009.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho L, Jacinto A, Matova N. The toll/NF-κB signaling pathway is required for epidermal wound repair in Drosophila; Proc Natl Acad Sci USA; 2014; pp. E5373–E5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baum E, Hue F, Barbour AG. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. MBio. 2012;3:e00434–12. doi: 10.1128/mBio.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernardino AL, Myers TA, Alvarez X, Hasegawa A, Philipp MT. Toll-like receptors: Insights into their possible role in the pathogenesis of lyme neuroborreliosis. Infect Immun. 2008;76:4385–4395. doi: 10.1128/IAI.00394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasley A, Marriott I, Halberstadt CR, Bost KL, Anguita J. Substance P augments Borrelia burgdorferi-induced prostaglandin E2 production by murine microglia. J Immunol. 2004;172:5707–5713. doi: 10.4049/jimmunol.172.9.5707. [DOI] [PubMed] [Google Scholar]
- 36.Sun J, Ramnath RD, Zhi L, Tamizhselvi R, Bhatia M. Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol. 2008;294:C1586–C1596. doi: 10.1152/ajpcell.00129.2008. [DOI] [PubMed] [Google Scholar]
- 37.Sadik CD, Hunfeld KP, Bachmann M, Kraiczy P, Eberhardt W, Brade V, Pfeilschifter J, Mühl H. Systematic analysis highlights the key role of TLR2/NF-kappaB/MAP kinase signaling for IL-8 induction by macrophage-like THP-1 cells under influence of Borrelia burgdorferi lysates. Int J of Biochem Cell Biol. 2008;40:2508–2521. doi: 10.1016/j.biocel.2008.04.014. [DOI] [PubMed] [Google Scholar]