Abstract
Thyroid carcinoma is primarily treated by surgery combined with radioactive 131iodine (131I) treatment; however, certain patients exhibit resistance to 131I treatment. Previous research indicated that nuclear factor-κB (NF-κB) was associated with resistance to 131I in cancer cells. The present study aimed to investigate the effects of NF-κB on 131I uptake and apoptosis in thyroid carcinoma cells. TPC-1 and BCPAP cell lines were employed as research models in the present study, and the expression of NF-κB was inhibited by RNA interference (RNAi). The ability of TPC-1 and BCPAP cells to uptake 131I was measured and the cell viability was detected by an MTT assay. Finally, the expression of the apoptosis-associated proteins X-linked inhibitor of apoptosis (XIAP), cellular inhibitor of apoptosis protein 1 (cIAP1) and caspase-3 in TCP-1 and BCPAP cells was determined by western blotting. Western blotting results demonstrated that the expression levels of NF-κB in TPC-1 and BCPAP cells were successfully downregulated by RNAi (P<0.05), while analysis of 131I uptake revealed no significant alterations in the 131I uptake ability of cells following RNAi (P>0.05). MTT experiments demonstrated that the inhibition of NF-κB expression in combination with radiation (131I treatment) led to a marked reduction in cell viability (P<0.05). Furthermore, western blot analysis revealed that the inhibition of NF-κB expression downregulated the expression levels of XIAP and cIAP1 (P<0.05), while the expression levels of caspase-3 were upregulated, indicating that the observed reduction in cell viability following NF-κB inhibition may be due to an increased level of apoptosis. Although NF-κB inhibition did not affect the 131I uptake of thyroid cancer cells, this inhibition may increase the apoptotic effects of radioactive 131I.
Keywords: thyroid carcinoma, nuclear factor-κB, 131iodine, cell apoptosis, RNA interference
Introduction
Differentiated thyroid carcinoma (DTC) accounts for ~86% of all thyroid cancers, which are divided into papillary thyroid and follicular thyroid type carcinomas (1). The treatment efficiency of thyroid carcinoma has been substantially improved by thyroidectomy or 131iodine (131I) radiotherapy; however, the 10-year-survival rate for certain patients was reported to be 10% (2). The particular target of radioiodine in thyroid disease is sodium iodide symporter, which is an intrinsic plasma membrane protein that mediates active iodide transport into the thyroid gland (3). Adjuvant therapy in the form of radioactive iodine is often administered as a means of reducing the risk of tumor recurrence and to facilitate future cancer surveillance (4). However, certain side effects have been reported, such as neck pain or swelling, headache and vertigo, or insomnia (5). Regardless of whether patients respond to 131I, it is difficult to obtain a satisfactory therapeutic effect with 131I treatment alone. Therefore, treatments with increased efficacy for thyroid carcinoma are required (6).
The transcription factor nuclear factor (NF)-κB was first discovered in 1986 as a nuclear factor which binds to the enhancer element of the immunoglobulin kappa light-chain of activated B cells (thereby coining the abbreviation NF-κB) (7,8). Later on, several studies demonstrated that NF-κB is a ubiquitously expressed transcription factor that mediates signal-induced expression of numerous genes involved in different biological processes, including immune responses, inflammation, cell growth and survival (9,10). NF-κB is normally sequestered in the cytoplasm as an inactive complex via physical association with inhibitory proteins, termed IκBs. In response to immune and stress stimuli, NF-κB associated proteins become activated via two major signaling pathways, the canonical and noncanonical pathways, and translocate to the nucleus to exert transcriptional functions (11–13). In recent years, in-depth research concerning thyroid carcinoma has revealed that nuclear factor-κB (NF-κB) is closely associated with the occurrence and development of thyroid carcinoma (14). Additionally, it has been reported that radiotherapy and chemotherapy may induce the production of NF-κB within thyroid carcinomas, leading to resistance (15,16). Bauerle et al (17) demonstrated that the inhibition of NF-κB activity may promote the apoptosis of thyroid carcinoma cells; however, the association between the expression of NF-κB and 131I radiation therapy in DTC remains unclear. Therefore, the present study aimed to investigate the effects of NF-κB on the uptake of 131I and apoptosis in thyroid carcinoma cells.
Materials and methods
Cell culture
The TPC-1 and BCPAP human papillary thyroid carcinoma cell lines were obtained from the Chinese Academy of Sciences (Shanghai, China). Dulbecco's modified Eagle's medium was obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and was supplemented with 5% fetal bovine serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 1% (w/v) penicillin (final concentration: 100 IU/ml) and streptomycin (100 IU/ml), and 1 mU/ml thyroid-stimulating hormone. Penicillin and streptomycin were purchased from Sigma-Aldrich (Merck KGaA). The conditions of cell culture were 37°C and 5% CO2 (18).
Cell transfection and RNA interference (RNAi)
According to the mRNA sequence of the NF-κB gene of humans (GenBank accession number, X61498), the sequence of RNAi was designed. The mRNA sequences were selected with GC contents of 50% and lengths of 29 bp; the aforementioned sequences and the mRNA of other genes in the Human Genome Database (19) were analyzed with NCBI-BLAST (20) (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to exclude the presence of homologous sequences; an NF-κB-RNAi sequence in accordance with the conditions was selected, some of the base sequences were mutated randomly, following exclusion of the homologous sequences with other genes and finally the sequences were selected as the negative control scramble-RNAi. Sequences are presented in Table I, which were synthesized by Shanghai Shenggong Biology Engineering Technology Service, Ltd. (Shanghai, China).
Table I.
Nucleic acid sequences for RNAi.
| RNAi | Sequence |
|---|---|
| NF-κB | 5′-CTTCCTTGTCTTCCACCAGAGGGTAATAG-3′ |
| Scramble | 5′-TCTCGTCTTATCTCCGGACCAGAGAGTAT-3′ |
RNAi, RNA interference; NF-κB, nuclear factor-κB.
Once thawed, the TPC-1 and BCPAP cells were cultured to the logarithmic growth phase and digested with trypsin for counting. The cells were diluted with fresh medium and were inoculated into a 96-well plate at a density of 5×104/well for 24 h at 37°C. Targeted RNA (final concentration 50 mM) or control scramble RNA (50 mM) was transfected into the cells using a Liposome INTERFER in Transfection kit (Polyplus-transfection SA, Illkirch, France) according to the manufacturer's protocol. After 6 h, medium was replaced with normal culture medium containing serum. After 48 h continuous incubation, cells were collected for further analysis.
131I uptake experiments
To determine whether the inhibition of NF-κB expression affects the 131I uptake of thyroid carcinoma cells, TCP-1 and BCPAP cells were cultured to the logarithmic growth phase and were seeded into a 6-well plate (5×105/well). Into each well, 1 ml fresh culture medium containing Na131I (37 kBq) (Sigma-Aldrich; Merck KGaA) was added. After 1 h, the culture medium was removed and the cells were collected and treated with trypsin following two washes with PBS. Cell radiation was measured using a γ-ray counter. The effect of NF-κB expression on the uptake of 131I was investigated (21).
Detection of cell viability by MTT assay
TPC-1 and BCPAP cell lines were transfected with NF-κB-RNAi or scramble-RNAi sequences and cultured to the logarithmic growth phase prior to digestion with trypsin. Following centrifugation at 500 × g for 5 min at 4°C, the supernatant was discarded and cultured with the cell suspension. A total of 6 µl cell suspension was treated with 6 µl trypan blue solution; following mixing, 10 µl solution was applied dropwise onto the cover between cell counting plate. The cells were counted under a light microscope (magnification ×40) selecting 6 random fields. These two cell groups were divided into four groups: A, scramble-RNAi group; B, scramble-RNAi + 131I group; C, NF-κB-RNAi group; and D, NF-κB-RNAi + 131I group. Groups A and C were suspended in fresh and normal culture medium; however, groups B and D were suspended in fresh culture medium containing 20 MBq/ml 131I (Institute of Isotopes Co., Ltd., Budapest, Hungary). The four groups of cell suspensions were plated in 24-well plates with 6,000 cells per well, and were cultured in a CO2 incubation box for 24 h at 37°C. To each well, 70 µl MTT solution was added and cells were cultured in a CO2 incubation box for 3 h at 37°C. Dimethyl sulfoxide was added following the removal of the supernatant and detection was performed at 570 nm (22).
Determination of the protein expression of NF-κB and apoptosis-associated proteins by western blot analysis
Cells of the four aforementioned groups were cultured for 24 h at 37°C. Following collection, the cells were pyrolyzed with radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific, Inc.) and total protein was extracted followed by quantification by Pierce bicinchoninic acid Protein Assay kit (Thermo Fisher Scientific, Inc.). Protein (20 µg/lane) was loaded into a SDS-PAGE (15% separation gel and 5% concentration gel). Proteins were transferred to polyvinylidene difluoride membranes by an electrophoretic transfer method following electrophoresis. The membrane was blocked with 5% non-fat milk powder for 1 h at 37°C; following washing with TBS with 0.1% Tween-20 (TBST), the membrane was incubated with a solution of antibodies, including anti-human NF-κB (catalogue number #8242; Cell Signaling Technology, Danvers, MA, USA; 1:1,000 dilution), anti human X-linked inhibitor of apoptosis (XIAP) (catalogue number #2045; Cell Signaling Technology; 1:1,000 dilution), anti-human cellular inhibitor of apoptosis protein 1 (cIAP1) (catalogue number #4952; Cell Signaling Technology; (1:1,000 dilution), anti-human caspase-3 (catalogue number #9662; Cell Signaling Technology; 1:1,000 dilution) and anti-human β-actin (catalogue number #4970; Cell Signaling Technology; 1:1,000 dilution) primary antibodies, at 4°C overnight. Subsequently, the membrane was washed with TBST and incubated with horseradish-peroxidase-conjugated anti-rabbit immunoglobulin G secondary antibody (catalogue number #47074; Cell Signaling Technology; 1:2,000 dilution) at room temperature for 1 h. Following washing with TBST, 3,3′-diaminoenzidine was applied to the membrane for 10 min to allow any color to develop; distilled water was added to terminate this reaction. The western blotting image was analyzed and the integral gray value was detected by a gel image-forming analysis system. The relative expression level was quantified using Image J software version 1.48 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were analyzed by SPSS 19 statistical software (IBM Corp., Armonk, NY, USA). Measurement data are presented as the mean ± standard deviation. One-way analysis of variance with Newman-Keuls multiple comparison post-hoc analysis was performed for comparison of the difference. P<0.05 was considered to indicate a statistically significant difference.
Results
Results of RNAi
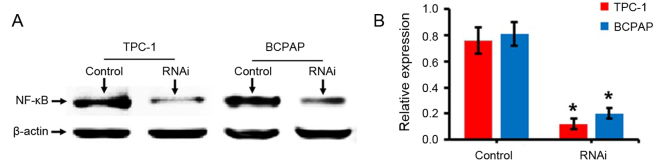
An experiment with RNAi was performed by designing specific oligonucleotide sequences. Cells transfected with the negative control scramble sequence were defined as the control. Western blotting results demonstrated that the expression levels of NF-κB (p65) protein in TPC-1 and BCPAP cells following the transfection of NF-κB-RNAi were significantly decreased compared with the control group (P<0.05; Fig. 1), indicating that the cells were successfully transfected with the NF-κB-RNAi oligonucleotide sequence.
Figure 1.
Detection of the NF-κB expression by western blotting. (A) Representative western blot bands for NF-κB following transfection of TPC-1 and BCPAP thyroid carcinoma cells with negative control scramble- or NF-κB-RNAi. (B) Quantified results for western blot analysis to confirm successful knockdown of NF-κB. *P<0.05 vs. control. NF-κB, nuclear factor- κB; RNAi, RNA interference; control, cells transfected with negative control scramble-RNAi.
Effects of NF-κB expression on thyroid carcinoma cell uptake of 131I
The effects of NF-κB expression on the 131I uptake of thyroid carcinoma cells were evaluated by an 131I uptake experiment. The results demonstrated that the uptake of 131I by TPC-1 and BCPAP cells was not significantly altered following the inhibition of NF-κB expression using RNAi, compared with the cells transfected with scramble RNAi (P>0.05; Fig. 2).
Figure 2.
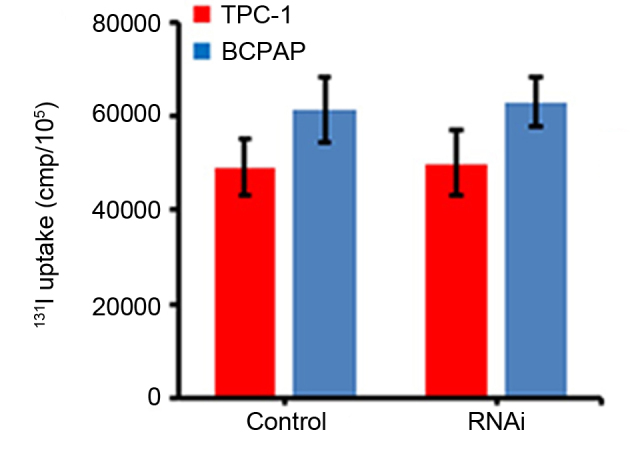
Effects of inhibition of NF-κB expression on thyroid carcinoma cell uptake of 131I. TCP-1 and BCPAP thyroid carcinoma cells were transfected with negative control scramble- or NF-κB-RNAi and the cellular uptake of 131I was subsequently measured. NF-κB, nuclear factor-κB; RNAi, RNA interference; 131I, 131iodine; control, cells transfected with negative control scramble-RNAi.
Detection of alterations in cell viability by MTT assays
The viability of TPC-1 and BCPAP cells was detected via an MTT assay following various treatments. As presented in Fig. 3, the radioactive effects of 131I (group B) or interference and inhibition of NF-κB expression (group C) decreased the survival rate of cells, compared with the control group (group A; P<0.05). Furthermore, when NF-κB inhibition and 131I radiation was combined (group D), the survival rate of thyroid carcinoma cells was markedly lower compared with groups B and C (Fig. 3).
Figure 3.
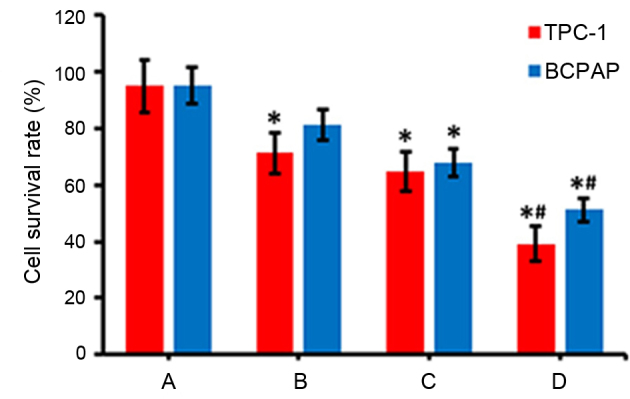
Detection of the viability of cells by MTT assays. TPC-1 and BCPAP thyroid carcinoma cells were transfected with negative control scramble- or NF-κB-RNAi with or without treatment with 131I, and MTT assays were subsequently performed to determine the effects on cell viability. Group A represents cells transfected with negative control scramble-RNAi only, group B represents cells transfected with negative control scramble-RNAi and treated with 131I, group C represents cells transfected with NF-κB-RNAi only and group D represents cells transfected with NF-κB-RNAi and treated with 131I. *P<0.05 vs. group A; #P<0.05 vs. group B and group C. NF-κB, nuclear factor-κB; RNAi, RNA interference; 131I, 131iodine.
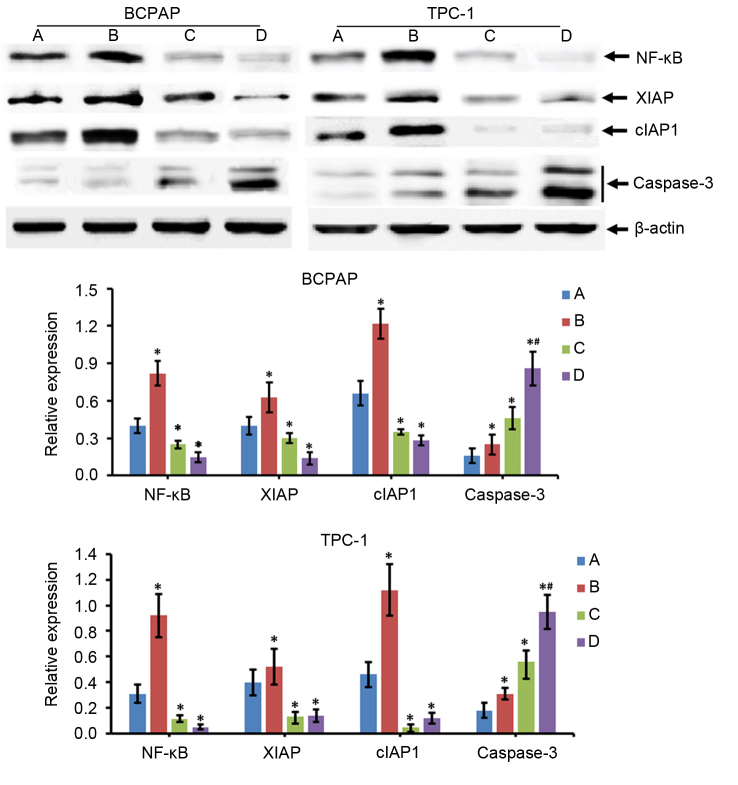
Expression of apoptosis-associated proteins in thyroid carcinoma cells
Alterations in the expression of apoptosis-associated proteins were determined by western blotting. The results of the present study revealed that compared with the control group (group A), the expression levels of NF-κB, XIAP, cIAP1 and caspase-3 in the 131I treatment group (group B) were significantly increased (P<0.05; Fig. 4). However, the protein expression levels of NF-κB, XIAP and cIAP1 were decreased in the NF-κB interference group (group C) compared with in group A (P<0.05), while levels of caspase-3 were increased (P<0.05; Fig. 4). The results for the 131I treatment + NF-κB interference group (group D) were similar to those in group C. In addition, compared with group C, caspase-3 expression levels were increased significantly in group D (P<0.05), but the alterations in NF-κB, XIAP and cIAP1 expression levels between groups C and D were not significantly different (P>0.05; Fig. 4).
Figure 4.
Alterations in the protein expression levels of apoptosis-associated proteins in thyroid carcinoma cells. TPC-1 and BCPAP thyroid carcinoma cells were transfected with negative control scramble- or NF-κB-RNAi with or without treatment with 131I, and western blotting was performed to measure the protein expression of certain apoptosis-associated proteins. Group A represents cells transfected with negative control scramble-RNAi only, group B represents cells transfected with negative control scramble-RNAi and treated with 131I, group C represents cells transfected with NF-κB-RNAi only and group D represents cells transfected with NF-κB-RNAi and treated with 131I. *P<0.05 vs. A group; #P<0.05 vs. group B and group C. NF-κB, nuclear factor-κB; RNAi, RNA interference; 131I, 131iodine; XIAP, X-linked inhibitor of apoptosis; cIAP1, cellular inhibitor of apoptosis protein 1.
Discussion
Thyroid carcinoma is a common type of malignant tumor in humans and in recent years, due alterations in human lifestyle and living environments, the incidence of this disease has been increasing annually. Research concerning the molecular biology of thyroid carcinoma has indicated that combining thyroidectomy with radioactive 131I may improve the efficacy of treatment for this disease. Additionally, β-ray therapy and radiotherapy in vitro have exhibited lethal effects on cancer cells (23); however, certain patients with thyroid carcinoma have been reported to exhibit resistance to 131I radiation (24). A study reported that NF-κB was associated with the occurrence, development and treatment, as well as the resistance, of various types of malignant tumor, such as thyroid carcinoma (25). Researchers have also demonstrated that the inhibition of NF-κB activity may induce cancer cell apoptosis (26). In the present study, the use of RNAi technology to inhibit the expression of NF-κB was investigated, followed by analysis of the effects of NF-κB on the 131I uptake by thyroid carcinoma cells. In addition, the levels of apoptosis were investigated. The results of the present study revealed that decreased expression levels of NF-κB exhibited no significant effects on the ability of thyroid carcinoma cells to uptake 131I; however, NF-κB inhibition may enhance the function of 131I-induced apoptosis of cancer cells.
Various studies have demonstrated that NF-κB may inhibit the apoptosis of various types of cancer cells as NF-κB serves as a nuclear factor that regulates the expression of various cell apoptosis-inhibiting genes at the transcriptional level, including XIAP, cIAP1and B-cell lymphoma-extra-large (27–29). XIAP and cIAP1 belong to the family of inhibitor of apoptosis (IAP) proteins, which constitute a highly conserved family of endogenous anti-apoptotic factors that suppress apoptosis by inhibiting caspase activity (30). IAP family proteins within the mitochondrial pathway are able to bind to caspase-9 precursors and interfere with their processing. Additionally, the caspase activation and recruitment domain of the IAP family protein structure binds to apoptotic peptidase-activating factor 1 to interfere with the activation of caspases (31). Finally, XIAP is reported to inhibit the activation of caspase-3 and caspase-7 via the baculovirus inhibitor of apoptosis protein repeat domain to inhibit apoptosis (32). In the present study, the protein expression of XIAP, cIAP1 and caspase-3 in cells was detected by western blotting following various treatments, and the results demonstrated that the inhibition of NF-κB was associated with decreased expression levels of XIAP and cIAP, and increased expression levels of caspase-3, compared with the control group. However, although 131I exposure increased the expression levels of the anti-apoptotic genes NF-κB, XIAP and cIAP, cell viability was also reduced following 131I exposure, indicating that other factors may be involved, which may include microRNA-100 and retinoblastoma 1 serine phosphates from human chromosome 3, as reported by a previous study (33). In addition, although the inhibition of NF-κB mediated by RNAi markedly suppressed the protein expression of XIAP and cIAP, and increased caspase-3 expression, NF-κB inhibition did not reduce cell viability compared with control group, which may be due to the function of other factors that may compensate the effect of NF-κB inhibition on cell proliferation, including certain cell cycle proteins, retinoblastoma protein or cyclin-dependent kinases (34).
The balance between apoptosis-inducing effects and anti-apoptotic effects of cells is important for normal cell function; however, when this balance is disrupted, cells may become cancerous. NF-κB is reported to increase the transcriptional level of the anti-apoptotic genes, which may promote the malignant transformation of cells (35). Furthermore, NF-κB has been demonstrated to regulate cyclin D1 and promote cell proliferation, and also promote the expression of the proto-oncogene, H-ras, leading to the promotion of cell carcinogenesis (36). In the course of cancer treatment, the activity of NF-κB has been reported to be closely associated with the resistance of cancer cells to radiotherapy and chemotherapy. Therefore, the effects of radiotherapy and chemotherapy treatments may be improved by inhibiting the activity of NF-κB in a particular manner (37).
In conclusion, in the present study, the expression of NF-κB in thyroid carcinoma cells was inhibited by RNAi the effects of NF-κB, and the results demonstrated that although NF-κB inhibition did not alter the uptake of 131I by DTC cells, the inhibition of NF-κB expression may enhance 131I-induced apoptosis of DTC cells. These results may provide a theoretical basis for improving the efficacy of radioactive 131I in the treatment of thyroid carcinoma and reducing the effective therapeutic dose of 131I in the future.
References
- 1.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Londero SC, Krogdahl A, Bastholt L, Overgaard J, Trolle W, Pedersen HB, Bentzen J, Schytte S, Christiansen P, Godballe C, Danish Thyroid Cancer Group Papillary thyroid microcarcinoma in Denmark 1996–2008: A national study of epidemiology and clinical significance. Thyroid. 2013;23:1159–1164. doi: 10.1089/thy.2012.0595. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y. Internal radiation therapy: A neglected aspect of nuclear medicine in the molecular era. J Biomed Res. 2015;29:345–355. doi: 10.7555/JBR.29.20140069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pryma DA, Mandel SJ. Radioiodine therapy for thyroid cancer in the era of risk stratification and alternative targeted therapies. J Nucl Med. 2014;55:1485–1491. doi: 10.2967/jnumed.113.131508. [DOI] [PubMed] [Google Scholar]
- 5.Lu L, Shan F, Li W, Lu H. Short-term side effects after radioiodine treatment in patients with differentiated thyroid cancer. Biomed Res Int. 2016;2016:4376720. doi: 10.1155/2016/4376720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hombach-Klonisch S, Natarajan S, Thanasupawat T, Medapati M, Pathak A, Ghavami S, Klonisch T. Mechanisms of therapeutic resistance in cancer (stem) cells with emphasis on thyroid cancer cells. Front Endocrinol (Lausanne) 2014;5:37. doi: 10.3389/fendo.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 8.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-X. [DOI] [PubMed] [Google Scholar]
- 9.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 10.Hayden MS, Ghosh S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Sun SC. NF-kappaB in inflammation and renal diseases. Cell Biosci. 2015;5:63. doi: 10.1186/s13578-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wertz IE, Dixit VM. Signaling to NF-kappaB: Regulation by ubiquitination. Cold Spring Harb Perspect Biol. 2010;2:a003350. doi: 10.1101/cshperspect.a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 14.Pozdeyev N, Berlinberg A, Zhou Q, Wuensch K, Shibata H, Wood WM, Haugen BR. Targeting the NF-κB Pathway as a combination therapy for advanced thyroid cancer. PLos One. 2015;10:e0134901. doi: 10.1371/journal.pone.0134901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Sethi G. Targeting transcription factor NF-kappa B to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805:167–180. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Bai L, Chen WJ, Xu SL. The NF-kappa B activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Tar. 2010;14:45–55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauerle KT, Schweppe RE, Haugen BR. Inhibition of nuclear factor-kappa B differentially affects thyroid cancer cell growth, apoptosis, and invasion. Mol Cancer. 2010;9:117. doi: 10.1186/1476-4598-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phan T, Yu XM, Kunnimalaiyaan M, Chen H. Antiproliferative effect of chrysin on anaplastic thyroid cancer. J Surg Res. 2011;170:84–88. doi: 10.1016/j.jss.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letovsky SI, Cottingham RW, Porter CJ, Li PW. GDB: The human genome database. Nucleic Acids Res. 1998;26:94–99. doi: 10.1093/nar/26.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: Architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flux GD, Haq M, Chittenden SJ, Buckley S, Hindorf C, Newbold K, Harmer CL. A dose-effect correlation for radioiodine ablation in differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2010;37:270–275. doi: 10.1007/s00259-009-1261-3. [DOI] [PubMed] [Google Scholar]
- 22.Chen GF, Xu SH, Renko K, Derwahl M. Metformin inhibits growth of thyroid carcinoma cells, suppresses self-renewal of derived cancer stem cells, and potentiates the effect of chemotherapeutic agents. J Clin Endocr Metab. 2012;97:E510–E520. doi: 10.1210/jc.2011-1754. [DOI] [PubMed] [Google Scholar]
- 23.Verburg FA, Stokkel MP, Düren C, Verkooijen RB, Mäder U, van Isselt JW, Marlowe RJ, Smit JW, Reiners C, Luster M. No survival difference after successful (131)I ablation between patients with initially low-risk and high-risk differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2010;37:276–283. doi: 10.1007/s00259-009-1315-6. [DOI] [PubMed] [Google Scholar]
- 24.Kim WG, Ryu JS, Kim EY, Lee JH, Baek JH, Yoon JH, Hong SJ, Kim ES, Kim TY, Kim WB, Shong YK. Empiric high-dose 131-iodine therapy lacks efficacy for treated papillary thyroid cancer patients with detectable serum thyroglobulin, but negative cervical sonography and 18F-fluorodeoxyglucose positron emission tomography scan. J Clin Endocr Metab. 2010;95:1169–1173. doi: 10.1210/jc.2009-1567. [DOI] [PubMed] [Google Scholar]
- 25.Pacifico F, Leonardi A. Role of NF-kappa B in thyroid cancer. Mol Cell Endocrinol. 2010;321:29–35. doi: 10.1016/j.mce.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Lampiasi N, Azzolina A, Umezawa K, Montalto G, McCubrey JA, Cervello M. The novel NF-κB inhibitor DHMEQ synergizes with celecoxib to exert antitumor effects on human liver cancer cells by a ROS-dependent mechanism. Cancer Lett. 2012;322:35–44. doi: 10.1016/j.canlet.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Galbán S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 2010;17:54–60. doi: 10.1038/cdd.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang CY, Lin X. Regulation of NF-κB by the CARD proteins. Immunol Rev. 2012;246:141–153. doi: 10.1111/j.1600-065X.2012.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Go HS, Seo JE, Kim KC, Han SM, Kim P, Kang YS, Han SH, Shin CY, Ko KH. Valproic acid inhibits neural progenitor cell death by activation of NF-κB signaling pathway and up-regulation of Bcl-XL. J Biomed Sci. 2011;18:48. doi: 10.1186/1423-0127-18-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gyrd-Hansen M, Meier P. IAPs: From caspase inhibitors to modulators of NF-kappa B, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2979. [DOI] [PubMed] [Google Scholar]
- 31.Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci. 2010;123:3209–3214. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J. 2010;430:199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang SN, Deng BP, Zhang YY, Jiang NY. Expression of miR-100 and RBSP3 in FTC-133 cells after exposure to I-131. Nucl Med Commun. 2014;35:932–938. doi: 10.1097/MNM.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 34.Duronio RJ, Xiong Y. Signaling pathways that control cell proliferation. Cold Spring Harb Perspect Biol. 2013;5:a008904. doi: 10.1101/cshperspect.a008904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M, Sakamaki T, Casimiro MC, Willmarth NE, Quong AA, Ju X, Ojeifo J, Jiao X, Yeow WS, Katiyar S, et al. The canonical NF-kappaB pathway governs mammary tumorigenesis in transgenic mice and tumor stem cell expansion. Cancer Res. 2010;70:10464–10473. doi: 10.1158/0008-5472.CAN-10-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: Regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/MCB.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XY, Abdel-Mageed AB, Mondal D, Kandil E. The nuclear factor Kappa-B signaling pathway as a therapeutic target against thyroid cancers. Thyroid. 2013;23:209–218. doi: 10.1089/thy.2012.0237. [DOI] [PubMed] [Google Scholar]