Abstract
The use of glucagon-like peptide-1 analogues, such as liraglutide, as hypoglycemic drugs has been widely employed in clinical practice. Liraglutide is reported to exert potential anti-breast cancer effects, however the specific mechanisms of this action remain unknown. In the present study, MCF-7 human breast cancer cells were cultured in vitro and treated with various concentrations of liraglutide. Cell Counting Kit-8, colony formation and flow cytometry assays were performed to determine the proliferation and apoptosis of cells following treatment. Furthermore, reverse transcription-quantitative polymerase chain reaction was employed to measure the expression level of microRNA (miRNA/miR)-27a. In addition, miR-27a mimics, inhibitors and negative controls were transfected into MCF-7 cells and the proliferation and apoptosis of cells following transfection was subsequently determined. Western blotting was performed to detect alterations in the protein expression of AMP-activated protein kinase catalytic subunit α2 (AMPKα2), proliferating cell nuclear antigen and cleaved-caspase-3 following treatments. The results demonstrated that, following treatment with liraglutide, the proliferation of MCF-7 cells was reduced and the apoptosis was increased, compared with the control group; this effect was increased with increasing concentrations of liraglutide. In addition, liraglutide treatment downregulated miR-27a expression in MCF-7 cells. While the overexpression of miR-27a promoted cell proliferation and inhibited apoptosis, knockdown of endogenous miR-27a inhibited cell proliferation and promoted apoptosis in MCF-7 cells. Furthermore, the expression of AMPKα2 protein in the group transfected with miR-27a mimics was decreased, while it was increased in MCF-7 cells transfected with miR-27a inhibitors. In conclusion, liraglutide may have a role in the inhibition of proliferation and promotion of apoptosis in MCF-7 cells. Concerning the mechanism of these effects, liraglutide may inhibit miR-27a expression, which subsequently increases the expression of AMPKα2 protein. The present study provides an experimental basis for the clinical treatment strategies of T2DM patients with breast cancer.
Keywords: type 2 diabetes, breast cancer, glucagon-like peptide-1, liraglutide, microRNA-27a
Introduction
Numerous studies have confirmed that there is a positive association between tumorigenesis and diabetes. The risk of malignancy in patients with diabetes is ~2 times higher compared with non-diabetic individuals (1,2). The incidence of breast cancer has increased rapidly; according to GLOBOCAN 2008 statistics, there were ~1.38 million new cases of breast cancer annually and ~30% of patients succumbed to tumor metastases (3). A US cohort study, after a 20-year follow up, reported that women with type 2 diabetes mellitus (T2DM) had a 17% increased risk of breast cancer compared with non-diabetic individuals (4). The choice of hypoglycemic therapy for patients with T2DM that develop tumors has an important influence on the development of the tumor. This is an important issue that has received increased interest in T2DM research.
Liraglutide, a glucagon-like peptide (GLP)-1 analogue, has been widely employed as a novel antidiabetic drug as it increases insulin secretion and improves glycemic control. Studies have demonstrated that GLP-1 exhibits various extra-pancreatic effects, including the promotion of glycogen synthesis and fat production in the liver, skeletal muscle and adipose tissue, and roles in the activation of the feeding center and cardiovascular protection (5–7). The association between GLP-1 and tumors remains unclear at present. Ligumsky et al (8) demonstrated that the selective GLP-1 receptor exists on the surface of MDA-MB-231 breast cancer cells, and a GLP-1 receptor agonist acted on the GLP-1 receptor to inhibit the proliferation and promote the apoptosis of MDA-MB-231 cells. However, it has also been reported that GLP-1 receptor agonists have the potential to increase the risk of pancreatic and thyroid cancer (9,10).
MicroRNAs (miRNAs/miRs) exist widely in organisms and are involved in the regulation of numerous physiological and pathological processes. An increasing number of studies have demonstrated that miRNAs may be involved in tumor formation by regulating the expression of tumor-associated genes (11–13). In breast cancer, miRNAs that are closely associated with metastasis are termed ‘metastamiRs’ (14). These miRNAs regulate the metastasis of breast cancer by modulating the signaling pathways associated with epithelial-mesenchymal transition and tumor metastasis (15). miR-27a is highly expressed in breast cancer, gastric cancer, pancreatic cancer and colon cancer as an oncogenic miRNA (16,17). It functions by regulating the apoptosis, cell cycle and differentiation of breast cancer cells (18,19).
Our previous study demonstrated that metformin may activate AMP-activated protein kinase (AMPK) in MCF-7 cells and downregulate the expression of miR-27a. AMPK is a key molecule in the regulation of biological energy metabolism (20). AMPK activation strongly inhibits the proliferation of various types of tumor cells and is therefore a promising antitumor target. AMPK consists of two subunits, α1 and α2. In breast cancer tissues and adjacent tissues, the expression of the AMPKα1 subunit is abundant, while the expression of AMPKα2 in breast cancer tissues is significantly lower compared with in adjacent tissues (21). Furthermore, breast epithelial carcinoma exhibits a marked reduction in AMPKα2 expression (22).
The existing literature has reported that liraglutide activates AMPK in muscle, liver and islet β-cells, exerting various biological effects (23–25). However, to the best of the authors' knowledge, whether liraglutide downregulates the expression of miR-27a and activates AMPKα2 to affect the proliferation and apoptosis of breast cancer cells is not currently clear. Therefore, the present study selected MCF-7 human breast cancer cells and aimed to perform a preliminary investigation of the effects of liraglutide on the proliferation and apoptosis of MCF-7 cells, and investigate the potential underlying mechanism.
Materials and methods
Cell culture
MCF-7 cell lines were obtained from the Cell Bank of the Type Culture Collection of Chinese Academy of Sciences (Beijing, China). Cells were cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone; GE Healthcare, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml streptomycin in humidified air at 37°C with 5% CO2. The media was replaced every 1–2 days.
Cell transfection
Briefly, 20 nM mimic (5′-UUCACAGUGGCUAAGUUCCGC-3′) or inhibitor (5′-GCGGAACUUAGCCACUGUGAA-3′) of miR-27a (Shanghai GenePharma Co., Ltd., Shanghai, China) were transfected into 6-well plates at a cell density of 1×106 cells per well using the transfection reagent Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to activate or inactivate miR-27a activity, respectively. Negative controls for mimics (5′-UUGUACUACACAAAAGUACUG-3′) and inhibitors (5′-CAGUACUUUUGUGUAGUACAA-3′) were employed. A mock group, which consisted of cells treated with Lipofectamine 2000 only, was also included. At 6 h after transfection, the transfection solution was replaced with RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. For RNA extraction and protein isolation, cells were treated for 48 h and then harvested. miRNA transfection efficiencies were determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
Detection of cell proliferation by cell counting Kit (CCK)-8 assay
MCF-7 cells were seeded in 96-well plates at 1,000 cells per well in 100 µl cell culture medium and incubated at 37°C for 24 h. All cells were divided into the following groups: Blank wells containing medium only, untreated control cells and test cells treated with different concentrations of liraglutide (10, 100, 1,000 or 10,000 nM). Cells were either treated with 10, 100, 1,000 and 10,000 nM liraglutide for 24 h or 1,000 nM liraglutide for 24, 48 and 72 h in an 5% CO2, humidified atmosphere at 37°C. Each group was established three holes. Following treatment with liraglutide or transfection with miR-27a mimics/inhibitors, 10 µl CCK-8 solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added into each well and incubated for 2 h in an 5% CO2, humidified atmosphere at 37°C, and the absorbance was detected at 450 nm. The CCK-8 assay was performed three times.
Colony formation assay
Cells were seeded into 6-well plates (500 cells/well) and incubated at 37°C in an environment with 5% CO2 for 2 days. Subsequently, cells were treated with 1,000 nM liraglutide for 48 h. The medium was refreshed with medium containing 10% FBS once every three days, and the colony formation was observed after 14 days. The number of colonies of more than 50 cells was counted using the inverted fluorescent microscope (IX51-A12PH; Olympus Corporation, Tokyo, Japan). Image magnification, ×10. To identify colonies, 0.1% crystal violet staining was used for 20 min at 37°C (Beyotime Institute of Biotechnology, Haimen, China) and representative photographs were captured. Each experiment was performed in triplicate.
Cell apoptosis detection by flow cytometry
Cells were digested with 0.25% trypsin for 2 min and a single-cell suspension was prepared. Cells were subsequently seeded into 12-well plates (5×104 cells/well) and incubated at 37°C in an environment with 5% CO2 overnight. Following treatment with 1,000 nM liraglutide 48 h or transfection with miR-27a mimic/inhibitor 24 h, the medium was transferred to a centrifuge tube (containing apoptotic or necrotic cells). PBS was used to wash the adherent cells, followed by digestion with 0.25% trypsin to prepare a single-cell suspension. Subsequently, the suspension was transferred to a centrifuge tube and centrifuged at 800 × g for 5 min at 4°C. Subsequently, cell suspension (100 µl) was transferred to a 1.5 ml Eppendorf tube, and 5 µl annexin V and 5 µl propidium iodide was added, according to the protocol of FITC Annexin V Apoptosis Detection kit I (BD Biosciences, Franklin Lakes, NJ, USA). After 15 min incubation at room temperature in the dark, flow cytometry was performed to analyze the changes in cell apoptosis using FACS Aria I (BD Biosciences, Franklin Lakes, NJ, USA).
AMPK small interfering (si)RNA transfection
AMPKα2 siRNA (5′-GAGAAGCAGAAGCACGACGTT-3′) and scrambled control (5′-UUCUCCGAACGUGUCACGUTT-3′) were purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA). Cells were incubated at 37°C and 5% CO2 until they reached a confluency of 70%. Subsequently, all transient transfections were performed with 100 nM AMPKα2 siRNA or scrambled control using Lipofectamine 2000, according to the manufacturer's protocol. After 6 h, the transfection mixtures were replaced with RPMI-1640 with 10% FBS. Cells were harvested at 48 h post-transfection.
RT-qPCR
Following treatment with 0, 10, 100 and 1,000 nM liraglutide for 48 h or transfection with miR-27a mimics/inhibitors, total miRNA from MCF-7 cells was extracted with an PureLink™ miRNA Isolation kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The expression of miR-27a was measured using a mirVana™ qRT-PCR miRNA Detection kit (Invitrogen; Thermo Fisher Scientific, Inc.). The stemloop-RT primer of miR-27a is 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGTGGA-3′. The thermocycling parameters of reverse transcriptase are 25°C for 10 min, 42°C for 1 h and 85°C for 5 min. U6 small nuclear RNA was used for the normalization of relative abundance of miR-27a. The primer of miR-27a is 5′-TTCACAGTGGCTAAGTTCCGC-3′ and universal primer R is 5′-CCAGTGCAGGGTCCGAGGT-3′. The primer of U6 is 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and 5′-ACGCTTCACGAATTTGCGTGTC-3′. A total of 100 ng cDNA product amplified in the reverse transcription step above was added to a 20 µl reaction volume using the following amplification program: 95°C for 5 min, followed by 40 amplification cycles of denaturation at 95°C for 10 sec, annealing at 62°C for 20 sec, and elongation at 72°C for 10 sec. For AMPKα2 mRNA expression analysis, which was performed following treatment with 0, 10, 100 and 1,000 nM liraglutide for 48 h, transfection with miR-27a mimics/inhibitors or transfection with or without miR-27a mimics followed by treatment with 1,000 nM liraglutide for 48 h. Total RNA was extracted using TRIzol™ LS Reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and the reverse-transcription reactions were performed using a TransScript First-Strand cDNA Synthesis SuperMix (Transgene Bio, Inc., Peking, China). The thermocycling parameters of reverse transcriptase are 25°C for 10 min, 42°C for 1 h and 85°C for 5 min. qPCR amplification was performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd., Dalian, China). A total of 100 ng of the cDNA product amplified in the reverse transcription step above was added to a 20 µl reaction volume using the following thermocycling parameters: 95°C for 5 min, followed by 40 amplification cycles of denaturation at 95°C for 10 sec, annealing at 58°C for 20 sec, and elongation at 72°C for 10 sec. The primers of AMPKα2 are forwards, 5′-GCCAAGAAGCAAATGAGAATG-3′, and reverse, 5′-GACACAACGCAAACTCCTGA-3′. The primers of GAPDH are forwards, 5′-ACCACAGTCCATGCCATCAC-3′, and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′. The expression levels of the target genes were normalized to GAPDH. Standard PCR samples were analyzed with a Bio-Rad iQ™ 5 thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Melting curves were generated for each RT-qPCR to verify the specific amplification products and primer dimers of each PCR reaction. All qPCR reactions were performed in triplicate. miR-27a and AMPKα2 expression levels were normalized using the comparative Cq method (26), and relative fold changes were calculated by the 2−ΔΔCq equation.
Western blot analysis
Following treatment with 0, 10, 100 and 1,000 nM liraglutide for 48 h, transfection with miR-27a mimics/inhibitors, transfection with or without miR-27a mimics followed by treatment with 1,000 nM liraglutide for 48 h, or transfection with AMPKα2 siRNA followed by treatment with 1,000 nM liraglutide for 48 h, cells were lysed with Radioimmunoprecipitation assay Lysis and Extraction Buffer (Thermo Fisher Scientific, Inc.). The cytosolic extracts were prepared by centrifuging the lysates twice at 10,800 × g for 10 min at 4°C. The protein concentration in each lysate was measured by a bicinchoninic acid assay. Equal amounts of protein (10 µl, 500 ng/ml) were separated by 10% SDS-PAGE minigels. Following electrophoresis, proteins were transferred onto a nitrocellulose membrane and blocked with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in 0.1% TBS-Tween (TBST) at room temperature for 2 h. Subsequently, the membranes were incubated with a rabbit anti-AMPKα2 monoclonal antibody (1:1,000; cat. no. 2757; Cell Signaling Technology, Inc.), a rabbit anti-proliferating cell nuclear antigen (PCNA) polyclonal antibody (1:1,000; cat. no. 13110; Cell Signaling Technology, Inc.), a rabbit anti-cleaved-caspase-3 polyclonal antibody (1:1,000; cat. no. 9664; Cell Signaling Technology, Inc.), or GAPDH antibody (1:2,000; cat. no. 10494-1-AP; Protein Tech Group, Inc., Chicago, USA) overnight at 4°C. After thorough rinsing with TBST, membranes were incubated with the second antibody goat anti-rabbit immunoglobulin G (H+L)-horseradish peroxidase (1:10,000; LK2001; Sungene Biotech, Co., Ltd., Tianjin, China) for 1 h. After rinsing, chemiluminescent detection was performed using a Pierce™ ECL Western Blotting Substrate (Invitrogen; Thermo Fisher Scientific, Inc.), followed by exposing and developing X-ray film. Western blotting results were analyzed using an Image Lab 4.0 software system (Bio-Rad Laboratories, Inc.). Western blotting was performed at least three times.
Statistical analysis
Each experiment included at least three replicates. Data are presented as the mean ± standard deviation. A one-way analysis of variance was used followed by Tukey's or Dunnett's post-hoc test to analyze the differences between multiplegroups. Statistical analyses were performed using SPSS 21.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference. Graphs were produced using GraphPad Prism v5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Liraglutide inhibits the proliferation and promotes the apoptosis of MCF-7 breast cancer cells
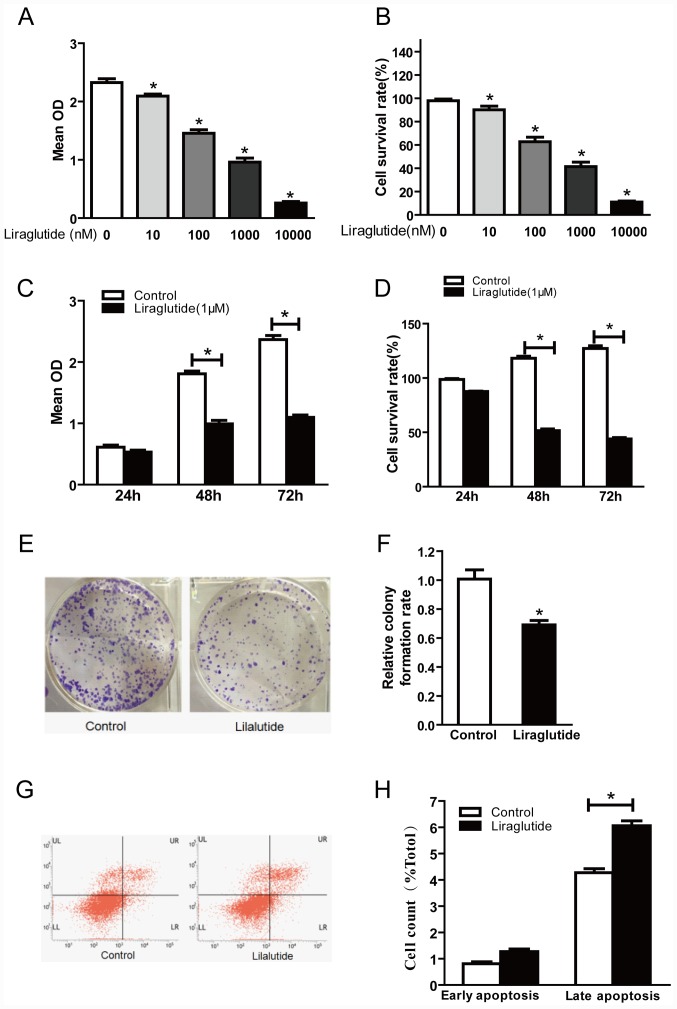
In order to investigate the effect of liraglutide on the proliferation of MCF-7 human breast cancer cells, cells were treated with 10, 100, 1,000 and 10,000 nM liraglutide for 48 h and a CCK-8 assay was performed to detect the optical density at 450 nm. Optical density was decreased in cells treated with liraglutide in a dose-dependent manner compared with the control group (Fig. 1A and B). The cell survival rate values were calculated and were 90.13, 62.80, 41.44 and 11.05%, respectively, in 10, 100, 1,000 and 10,000 nM treatment groups (Fig. 1B). These results indicated that liraglutide exhibited a marked inhibitory effect on the proliferation of MCF-7 cells in a dose-dependent manner. Further CCK-8 assays were performed to investigate whether liraglutide inhibits the proliferation of MCF-7 cells in a time-dependent manner. The results demonstrated that there was no significant difference in the proliferation between the control and liraglutide groups at 24 h, but significant differences were observed between control and liraglutide groups at 48 and 72 h time-points (Fig. 1C). The calculated cell survival rates were 86.88, 54.69 and 43.41% for liraglutide-treated cells at 24, 48 and 72 h (Fig. 1D). There was no significant difference between the control and liraglutide groups at 24 h; however, compared with the control group, the cell survival rates following liraglutide treatment were significantly decreased at 48 and 72 h time-points (Fig. 1D). These results indicated that liraglutide exhibited a marked inhibitory effect on the proliferation of MCF-7 cells in a time-dependent manner.
Figure 1.
Liraglutide inhibits the proliferation and promotes apoptosis in the MCF-7 breast cancer cell line. (A) CCK-8 assay results demonstrated that with increasing liraglutide concentration, the mean OD values were reduced. (B) Cell survival rates in each group were calculated following CCK-8 assays. (C) When 1,000 nM liraglutide was employed for 48 and 72 h, cell proliferation was markedly inhibited. (D) Cell survival rates in each group were calculated following CCK-8 assays. (E) Representative images of cell colony formation assays. (F) Quantification of cell colony formation assay results confirmed that the colony formation capacity decreased significantly following treatment with 1,000 nM liraglutide. (G) Representative flow cytometry plots in control cells or cells treated with 1,000 nM liraglutide. LR quadrant represents early apoptotic cells, and UR quadrant represents late apoptotic cells. (H) Quantified flow cytometry results demonstrated that the percentage of late apoptotic cells in the liraglutide treatment group increased significantly compared with the control group. Data are presented as the mean ± standard deviation. For parts A and B, *P<0.05 vs. 0 nM liraglutide group; for parts C, D, F and H, *P<0.05, as indicated by brackets. CCK-8, Cell Counting Kit-8; OD, optical density.
The cell colony formation assay is another laboratory technique used to determine alterations in cell proliferation. Following treatment of cells with 1,000 nM liraglutide for 48 h, colony formation assay results demonstrated that significantly fewer colonies were formed compared with the control group (69.37 vs. 100% in liraglutide and control groups, respectively; P<0.05; Fig. 1E and F). These results further confirmed that liraglutide exhibits a marked inhibitory effect on the proliferation of MCF-7 cells.
The effects of liraglutide on the apoptosis of MCF-7 cells were assessed by flow cytometry. The results demonstrated that treatment with 1,000 nM liraglutide for 48 h led to an increase in the percentage of apoptotic cells, compared with the control group. The percentages of early apoptotic and late apoptotic cells in the liraglutide treatment group were 1.26±0.18 and 6.06±0.32%, respectively, and the early apoptosis and late apoptosis percentages in the control group were 0.81±0.13 and 4.27±0.26%, respectively. The difference in the percentage of late apoptotic cells between the control and liraglutide treatment groups was statistically significant (P<0.05; Fig. 1G and H), while the difference in the percentage of early apoptotic cells was not significant (Fig. 1G and H). These results demonstrated that liraglutide may promote the apoptosis of MCF-7 cells.
Liraglutide inhibits miR-27a expression and upregulates AMPKα2
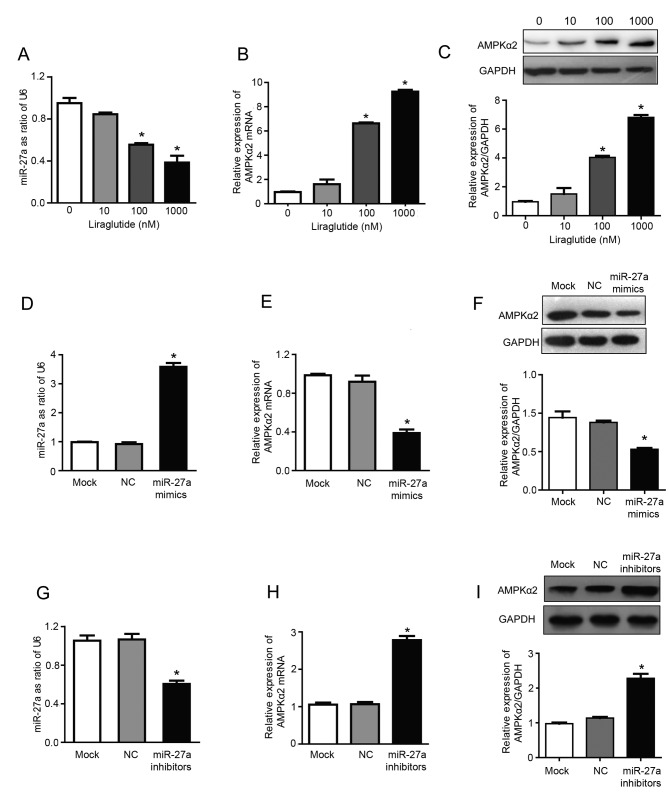
The results of RT-qPCR analysis demonstrated that treatment with 100 and 1,000 nM liraglutide significantly inhibited the expression of miR-27a and significantly increased the mRNA expression of AMPKα2, compared with untreated control cells (P<0.05; Fig. 2A and B). The expression levels of miR-27a were reduced by 15.5, 44.5 and 61.8%, respectively, in the 10, 100 and 1,000 nM treatment groups, compared with the control group (Fig. 2A). Compared with the control group, the mRNA expression of AMPKα2 was increased by 1.61, 6.63 and 9.25 times in 10, 100 and 1,000 nM treatment groups, respectively (Fig. 2B). In addition, liraglutide treatment (100 and 1,000 nM) significantly increased the expression of AMPKα2 at the protein level, compared with the control group, as determined by western blotting (P<0.05; Fig. 2C). The protein expression of AMPKα2 was 1.5, 4.0 and 6.8 times higher in the 10, 100 and 1,000 nM treatment groups, respectively, compared with the control group (Fig. 2C). These results indicated that miR-27a may promote the proliferation, and inhibit the apoptosis, of MCF-7 human breast cancer cells, and liraglutide may mediate its effects by downregulating miR-27a expression. Therefore, miR-27a may be a potential target for the prevention and treatment of breast cancer. Subsequently, experiments were performed using miR-27a mimics/inhibitors to investigate whether liraglutide may inhibit the proliferation and promote the apoptosis of MCF-7 cells by targeting miR-27a and influencing expression of AMPKα2, which has previously been validated as a target gene of miR-27a (27).
Figure 2.
Liraglutide inhibits miR-27a expression and subsequently upregulates AMPKα2. Following treatment with 10, 100 and 1,000 nM liraglutide for 48 h, RT-qPCR was performed to determine the mRNA levels of (A) miR-27a and (B) AMPKα2. (C) Western blotting was performed to measure the protein levels of AMPKα2. (D) RT-qPCR confirmed that transfection with miR-27a mimics led to the successful overexpression of miR-27a in MCF-7 cells. (E) RT-qPCR demonstrated that the mRNA levels of AMPKα2 were downregulated following transfection with miR-27a mimics. (F) Western blotting demonstrated that AMPKα2 protein expression was downregulated following transfection with miR-27a mimics. (G) RT-qPCR confirmed that transfection with miR-27a inhibitors led to the successful reduction of miR-27 levels in MCF-7 cells. (H) RT-qPCR demonstrated that AMPKα2 mRNA expression was increased following transfection with miR-27a inhibitors. (I) Western blotting demonstrated that AMPKα2 protein expression was increased following transfection with miR-27a inhibitors. Data are presented as the mean ± standard deviation. For parts A-C, *P<0.05 vs. 0 nM liraglutide; for parts D-I, *P<0.05 vs. NC group. miR, microRNA; AMPKα2, AMP-activated protein kinase catalytic subunit α2; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; NC, negative control; U6, U6 small nuclear RNA.
The present study confirmed that miR-27a exhibits a regulatory effect on the expression of AMPKα2. miR-27a mimics and inhibitors were transfected into MCF-7 cells. RT-qPCR results confirmed that transfection with miR-27a mimics was successful (Fig. 2D), and the results also demonstrated that overexpression of miR-27a led to downregulation of AMPKα2 mRNA expression by 57.77%, compared with the NC group (P<0.05; Fig. 2E). In addition, following transfection with miR-27a mimics, AMPKα2 protein expression was also downregulated by 52.55%, compared with the NC group (P<0.05; Fig. 2F). Successful transfection of miR-27a inhibitors was confirmed by RT-qPCR (Fig. 2G). Furthermore, following transfection with miR-27a inhibitors, AMPKα2 mRNA expression was increased ~2.61 times (P<0.05; Fig. 2H) and AMPKα2 protein expression was increased ~2.27 times (P<0.05; Fig. 2I) compared with the NC group. These results indicated that miR-27a may negatively regulate AMPKα2.
miR-27a promotes the proliferation of MCF-7 human breast cancer cells and inhibits apoptosis
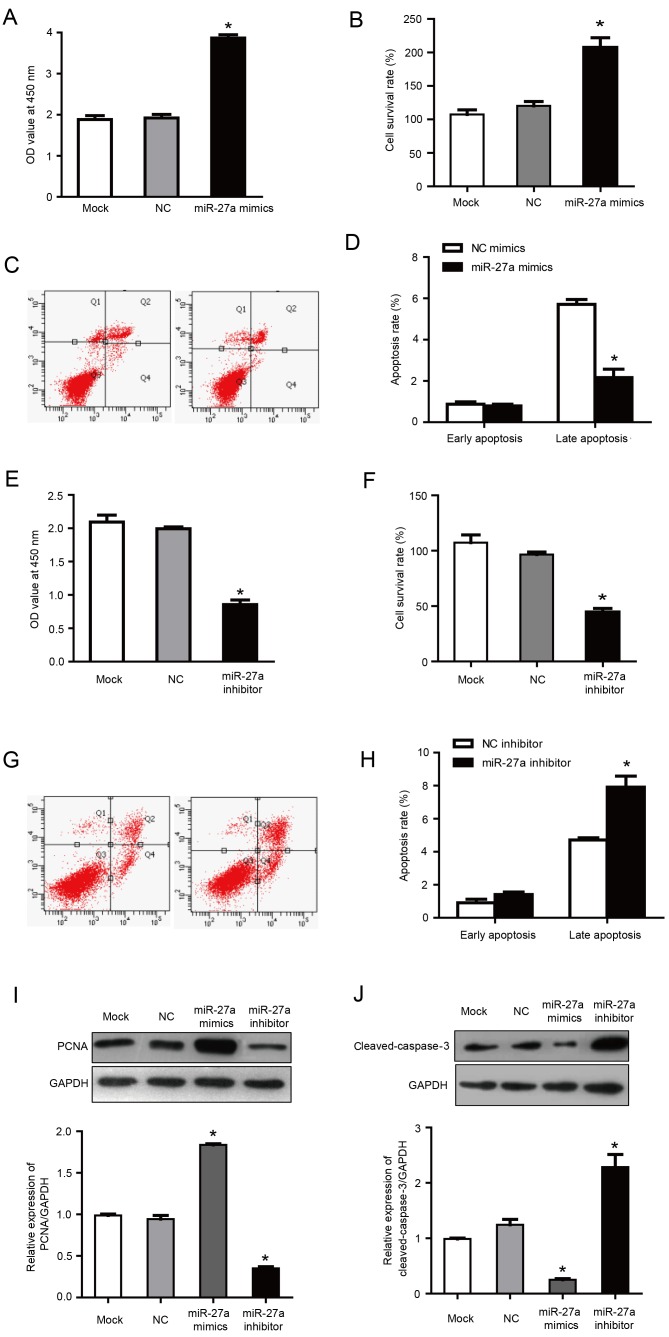
It was previously reported that miR-27a, as a carcinogenic miRNA, regulates key target genes to control the cell cycle check point to affect the growth of breast cancer cells (16). In order to investigate the role of miR-27a in the proliferation and apoptosis of MCF-7 cells, MCF-7 cells were transfected with miR-27a mimics and inhibitors. Following transfection with miR-27a mimics, the proliferation and cell survival rate was increased significantly compared with the negative control group (P<0.05; Fig. 3A and B). Furthermore, flow cytometry demonstrated that, compared with the negative control group, the percentage of late apoptotic cells was significantly reduced in the cells transfected with miR-27a mimics (2.16±0.41 vs. 5.71±0.23% in miR-27a mimics and negative control groups, respectively; P<0.05; Fig. 3C and D). Correspondingly, in cells transfected with miR-27a inhibitor, the proliferation and cell survival were significantly lower compared with the negative control group (P<0.05; Fig. 3E and F), and the late apoptosis percentage was increased significantly compared with the negative control group (6.91±0.27 vs. 4.71±0.23% in miR-27a inhibitor and negative control groups, respectively; P<0.05; Fig. 3G and H). There was no statistical difference between the NC group and the mock group. These results indicated that miR-27a promoted the proliferation and inhibited the apoptosis of MCF-7 cells.
Figure 3.
miR-27a overexpression promotes the proliferation and inhibits the apoptosis of MCF-7 human breast cancer cells. (A) CCK-8 assays demonstrated that cells transfected with miR-27a mimics exhibited enhanced proliferation. (B) Cell survival rates were calculated following CCK-8 assays. (C) Representative flow cytometry plots following transfection of MCF-7 cells with NC mimics or miR-27a mimics. The UR quadrant was considered to indicate late apoptotic cells and the LR quadrant was considered to indicate early apoptotic cells (D) Quantified flow cytometry results demonstrated that the percentage of late apoptotic cells was significantly reduced following transfection of cells with miR-27a mimics. (E) CCK-8 assays demonstrated that cells transfected with miR-27a inhibitors exhibited reduced proliferation. (F) Cell survival rates were calculated following CCK-8 assays. (G) Representative flow cytometry plots following transfection of MCF-7 cells with NC inhibitors or miR-27a inhibitors. The UR quadrant was considered to indicate late apoptotic cells and the LR quadrant was considered to indicate early apoptotic cells. (H) Quantified flow cytometry results demonstrated that the percentage of late apoptotic cells was significantly increased following transfection of cells with miR-27a inhibitors. (I) Western blotting demonstrated that transfection with miR-27a mimics led to a significant increase in the protein expression of PCNA, while miR-27a inhibitors significantly reduced PCNA protein expression. (J) Western blotting demonstrated that transfection with miR-27a mimics significantly reduced cleaved-caspase-3 expression, while miR-27a inhibitors significantly increased cleaved-caspase-3 protein levels. Data are presented as the mean ± standard deviation. *P<0.05 vs. NC group. miR, microRNA; CCK-8, Cell Counting Kit-8; NC, negative control; PCNA, proliferating cell nuclear antigen; OD, optical density; UR, upper right; LR, lower right.
PCNA is only expressed in normal proliferating cells and tumor cells. It has an important role in the initiation of cell proliferation and is a good indicator of the cell proliferation. In a pre-test, no significant differences were observed between negative control mimics and negative control inhibitor groups and the mock group (data not shown). Therefore, although separate mimics and inhibitor negative controls were used for all aforementioned transfection results, negative control mimics were selected as a common control for the western blotting results presented in Fig. 3I and J. Previous studies have also included the use of a common control for mimics and inhibitor experiments (28,29).
Western blotting demonstrated that the expression of PCNA was increased in cells transfected with miR-27a mimics, and was increased by ~2 times compared with the NC group (P<0.05; Fig. 3I). Furthermore, PCNA expression was reduced in the group transfected with miR-27a inhibitors, with an expression of ~43.22% of the NC group (P<0.05; Fig. 3I). These results further demonstrated that miR-27a may inhibit the proliferation of MCF-7 human breast cancer cells.
Caspase-3 is a key apoptotic factor in the caspase family. Western blotting demonstrated that the expression of cleaved-caspase-3 was decreased in cells transfected with miR-27a mimics, with an expression of ~28.56% of the NC group (P<0.05; Fig. 3J). Correspondingly, following transfection with miR-27a inhibitor, the cleaved-caspase-3 expression was ~2.1 times higher compared with the NC group (P<0.05; Fig. 3J). These results further indicated that miR-27a may promote the apoptosis of MCF-7 cells.
Upregulation of miR-27a reverses the activation effects of liraglutide on AMPKα2
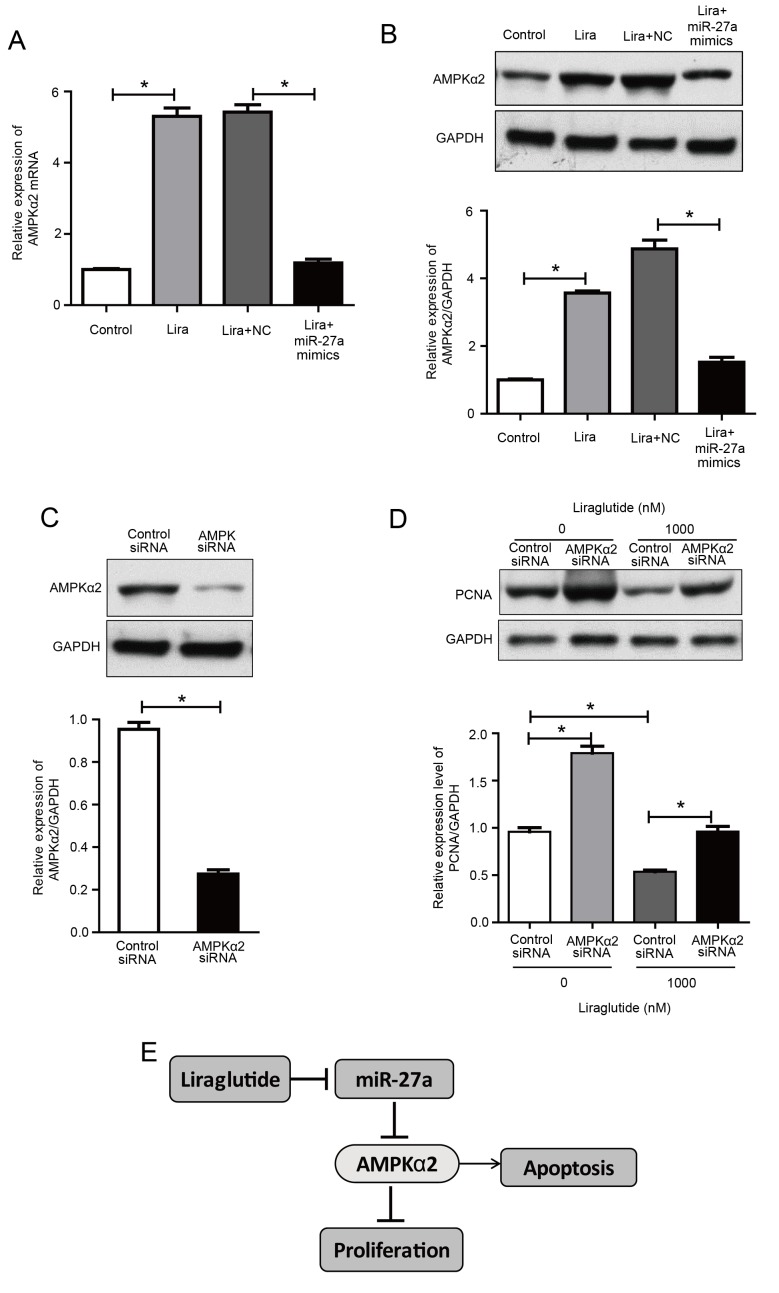
The previously discussed results indicated that the promotion of apoptosis by liraglutide may be associated with the activation of the AMPKα2 pathway, and miR-27a negatively regulates AMPKα2. Therefore, we hypothesized that the effect of liraglutide on apoptosis may be mediated via reduced miR-27a expression and activation of the AMPKα2 pathway.
The present study further investigated the effect of liraglutide in MCF-7 cells following transfection with miR-27a mimics. The results demonstrated that AMPKα2 expression was increased by liraglutide in MCF-7 cells, compared with the control group, while its expression in the liraglutide + miR-27a mimics group was decreased compared with the liraglutide + negative control mimics group (Fig. 4A and B). These results indicated that the overexpression of miR-27a potentially impaired the effect of liraglutide on apoptosis in MCF-7 cells.
Figure 4.
Upregulation of miR-27a reverses the liraglutide-induced activation of AMPKα2 and AMPKα2 siRNA inhibits liraglutide-induced inhibition of proliferation in MCF-7 cells. (A) AMPKα2 mRNA levels were measured by reverse transcription-quantitative polymerase chain reaction in MCF-7 cells transfected with or without miR-27a mimics and subsequently treated with liraglutide for 48 h. (B) AMPKα2 protein levels were measured by western blotting in MCF-7 cells transfected with or without miR-27a mimics and subsequently treated with liraglutide for 48 h. (C) MCF-7 cells were transfected with control siRNA or AMPKα2 siRNA for 48 h and western blotting was performed to measure AMPKα2 levels and confirm successful transfection. (D) MCF-7 cells were transfected with AMPKα2 siRNA and cultured with or without 1,000 nM liraglutide for 48 h. The expression of PCNA was determined by western blot analysis. GAPDH served as the loading control. (E) Summary diagram of the regulatory pathway in the present study. In breast cancer cells, liraglutide inhibits miR-27a expression and subsequently activates the AMPK pathway, thereby leading to the induction of cell apoptosis. Data are presented as the mean ± standard deviation. *P<0.05, as indicated by brackets. miR, microRNA; AMPKα2, AMP-activated protein kinase catalytic subunit α2; siRNA, small interfering RNA; PCNA, proliferating cell nuclear antigen; NC, negative control; Lira, liraglutide.
AMPKα2 knockdown reduces the inhibitory effect of liraglutide on MCF-7 cells
To determine whether AMPK is responsible for the inhibition of proliferation induced by liraglutide, MCF-7 cells were transfected with AMPKα2 siRNA. As demonstrated in Fig. 4C, cells exhibited a marked decrease in AMPKα2 protein expression, compared with the control siRNA group. With or without liraglutide treatment, knockdown of AMPKα2 increased the protein expression of PCNA, compared with cells transfected with control siRNA, indicating that proliferation was increased following AMPKα2 knockdown (Fig. 4D). These results indicate that AMPK activation may be essential for the inhibitory effect of liraglutide on the proliferation of MCF-7 cells. These results demonstrated that liraglutide-induced inhibition of proliferation may be associated with the AMPK pathway. Taken together, the results of the current study indicate that liraglutide, potentially through the miR-27a/AMPKα2 pathway, may promote apoptosis in MCF-7 cells (Fig. 4E).
Discussion
Breast cancer is among the most common types of malignant tumors that affect women, according to statistics from developed countries in North America and Europe (30). Several studies have reported that breast cancer incidence and mortality risk are closely associated with metabolic disease (31–33). The present study reported that liraglutide, which is widely employed for hypoglycemic treatment, also exhibited an inhibitory effect on the proliferation of MCF-7 cells and promoted apoptosis. These effects may be associated with the inhibition of miR-27a expression by liraglutide, and miR-27a negatively regulates AMPKα2 expression.
GLP-1 exerts its function primarily through binding to its receptor. GLP-1 receptors are not exclusive to the pancreas, and are also present in the gastrointestinal tract, brain, heart, aorta, kidney, lung, peripheral nervous system and other tissues and organs (34). In addition to a role in regulating blood glucose, GLP-1 also exhibits numerous extra-hypoglycemic effects. Liraglutide, a GLP-1 analogue, has 97% homology with GLP-1 in the human body and regulates blood glucose through various mechanisms. It has been reported that sustained GLP-1 receptor activation may increase the incidence of colorectal cancer in patients with T2DM that exhibit hyperinsulinemia (35). However, a meta-analysis demonstrated that the use of liraglutide did not increase the incidence of acute pancreatitis and pancreatic cancer in patients with T2DM (36). Ligumsky et al (8) reported that exenatide, a GLP-1 receptor agonist, led to sustained activation of the GLP-1 receptor, which subsequently led to the inhibition of proliferation and promotion of apoptosis in MDA-MB-231 cells through the cyclic AMP pathway.
MCF-7 is the most commonly employed breast cancer cell line in research, and is estrogen receptor positive with a high proliferation rate. The results of the current study demonstrated that liraglutide effectively inhibited the proliferation of MCF-7 cells in a concentration- and time-dependent manner. Furthermore, colony formation assay and flow cytometry results also confirmed that liraglutide inhibited the proliferation of MCF-7 cells and promoted cell apoptosis.
AMPK is an important kinase that regulates energy homeostasis and also a key protein involved in various cellular signaling pathways, including those involved in the regulation of the cell cycle and apoptosis. A recent study reported that liraglutide activated AMPK in the muscle cells of mice, thereby regulating muscle cell translocation of glucose transporter 4 (37). Furthermore, Miao et al (24) demonstrated that liraglutide promoted the proliferation of insulin-producing β cells through the AMPK/mechanistic target of rapamycin kinase (mTOR) signaling pathway, while Ben-Shlomo et al (38) reported that liraglutide inhibited the formation of fatty liver via the AMPK pathway. The present study also indicated that liraglutide activates AMPK. Therefore, liraglutide-induced inhibition of proliferation and apoptosis promotion in MCF-7 cells may be associated with the activation of AMPK.
miRNAs exhibit their biological roles through specific recognition of the 3′untranslated regions within target genes and through complementary base-pairing to inhibit target gene expression. miR-27, which includes miR-27a and miR-27b, has important biological functions. As an oncogenic miRNA, it has been reported to be highly expressed in breast, gastric, pancreatic and colon cancer. It has been reported that miR-27a regulates cell growth and differentiation, and is implicated in drug resistance, dose-dependently. Furthermore, miR-27a may promote the metastasis of cancer cells through induction of epithelial-mesenchymal transition. miR-27a was also reported to be involved in cell apoptosis, cell cycle checkpoints and metabolism (39). The results of the present study also confirmed that miR-27a exhibited an important role in inhibiting proliferation and promoting apoptosis in MCF-7 cells. Therefore, miR-27a may be employed as an effective target for the prevention and treatment of breast cancer. However, miR-27a inhibitor is not yet suitable for clinical application. The present study employed liraglutide, which is widely used in the clinic, to interfere with MCF-7 human breast cancer cells, and the results demonstrated that liraglutide inhibited miR-27a expression, which may provide a novel treatment option for the prevention and control of breast cancer by targeting miR-27a.
Our previous study verified that AMPKα2 was a target gene of miR-27a. AMPKα2 is one of the catalytic subunits of AMPK and its expression in MCF-7 human breast cancer cells is suppressed, as it functions as a tumor suppressor (27). Fox et al (21) investigated AMPKα2 expression among tumor samples, non-tumorous adjacent (ADJ) breast epithelial tissue and normal epithelial tissue samples, and the results demonstrated that AMPKα2 protein expression was reduced by 27% in tumor samples compared with the patient-matched ADJ samples and by 37% compared with normal epithelial tissue samples. Further experiments indicated that AMPKα2 arrested the cell cycle through cyclin D1 and reduced protein biosynthesis through the mTOR pathway. Furthermore, the same study reported that AMPKα2 may act directly on P53, resulting in MCF-7 cell apoptosis. The results of the present study demonstrated that liraglutide inhibited the proliferation and promoted the apoptosis of MCF-7 cells, which may occur via inhibition of miR-27a expression and subsequent upregulation of the miR-27a target gene, AMPKα2.
In conclusion, the present study demonstrated that the hypoglycemic drug liraglutide inhibited the proliferation and promoted the apoptosis of MCF-7 cells, exhibiting potential anti-breast cancer effects. In addition, the results demonstrated that liraglutide may function as an miR-27a inhibitor, thereby potentially providing a novel method for the clinical prevention and treatment of breast cancer. It has been previously reported that certain antidiabetic drugs, including insulin and sulfonylureas antidiabetic drugs, may affect the levels of insulin, inflammatory factors and insulin-like growth factor-1, thus increasing tumor risk in patients with T2DM (40,41). The current study may provide a basis for the selection of hypoglycemic agents in patients with T2DM, particularly in patients with breast cancer. However, various issues are associated with the present study. For example, the results demonstrated that 100 nM liraglutide inhibited miR-27a expression in MCF-7 cells, while 10 nM liraglutide did not lead to a statistically significant decline. In addition, further investigation is required to determine whether 100 nM liraglutide may lead to any side effects prior to clinical application. In conclusion, the current study may provide a novel direction for investigating the roles of GLP-1 besides its glucose-lowering effects.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 81400784) and the Natural Science Foundation of Tianjin (grant no. 16JCYBJC26800).
References
- 1.Noto H, Osame K, Sasazuki T, Noda M. Substantially increased risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis of epidemiologic evidence in Japan. J Diabetes Complications. 2010;24:345–353. doi: 10.1016/j.jdiacomp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Buysschaert M, Sadikot S. Diabetes and cancer: A 2013 synopsis. Diabetes Metab Syndr. 2013;7:247–250. doi: 10.1016/j.dsx.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Michels KB, Solomon CG, Hu FB, Rosner BA, Hankinson SE, Colditz GA, Manson JE. Nurses' Health Study: Type 2 diabetes and subsequent incidence of breast cancer in the Nurses' Health Study. Diabetes Care. 2003;26:1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 5.Dardevet D, Moore MC, Neal D, DiCostanzo CA, Snead W, Cherrington AD. Insulin-independent effects of GLP-1 on canine liver glucose metabolism: Duration of infusion and involvement of hepatoportal region. Am J Physiol Endocrinol Metab. 2004;287:E75–E81. doi: 10.1152/ajpendo.00035.2004. [DOI] [PubMed] [Google Scholar]
- 6.Sancho V, Trigo MV, González N, Valverde I, Malaisse WJ, Villanueva-Peñacarrillo ML. Effects of glucagon-like peptide-1 and exendins on kinase activity, glucose transport and lipid metabolism in adipocytes from normal and type-2 diabetic rats. J Mol Endocrinol. 2005;35:27–38. doi: 10.1677/jme.1.01747. [DOI] [PubMed] [Google Scholar]
- 7.Pannacciulli N, Le DS, Salbe AD, Chen K, Reiman EM, Tataranni PA, Krakoff J. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage. 2007;35:511–517. doi: 10.1016/j.neuroimage.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ligumsky H, Wolf I, Israeli S, Haimsohn M, Ferber S, Karasik A, Kaufman B, Rubinek T. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res Treat. 2012;132:449–461. doi: 10.1007/s10549-011-1585-0. [DOI] [PubMed] [Google Scholar]
- 9.Quoyer J, Longuet C, Broca C, Linck N, Costes S, Varin E, Bockaert J, Bertrand G, Dalle S. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem. 2010;285:1989–2002. doi: 10.1074/jbc.M109.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartels CL, Tsongalis GJ. MicroRNAs: Novel biomarkers for human cancer. Clin Chem. 2009;55:623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- 12.Liu T, Tang H, Lang Y, Liu M, Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;273:233–242. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Chen YJ, Xu K, Xu H, Shen XZ, Tu RQ. Circulating microRNAs as a fingerprint for endometrial endometrioid adenocarcinoma. PLoS One. 2014;9:e110767. doi: 10.1371/journal.pone.0110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samantarrai D, Dash S, Chhetri B, Mallick B. Genomic and epigenomic cross-talks in the regulatory landscape of miRNAs in breast cancer. Mol Cancer Res. 2013;11:315–328. doi: 10.1158/1541-7786.MCR-12-0649. [DOI] [PubMed] [Google Scholar]
- 15.Sun L, Fang J. Epigenetic regulation of epithelial-mesenchymal transition. Cell Mol Life Sci. 2016;73:4493–4515. doi: 10.1007/s00018-016-2303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Mertens-Talcott SU, Zhang S, Kim K, Ball J, Safe S. MicroRNA-27a indirectly regulates estrogen receptor {alpha} expression and hormone responsiveness in MCF-7 breast cancer cells. Endocrinology. 2010;151:2462–2473. doi: 10.1210/en.2009-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y, Yu S, Zhao W, Lu Z, Chen J. miR-27a regulates the growth, colony formation and migration of pancreatic cancer cells by targeting Sprouty2. Cancer Lett. 2010;298:150–158. doi: 10.1016/j.canlet.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 19.Tang W, Yu F, Yao H, Cui X, Jiao Y, Lin L, Chen J, Yin D, Song E, Liu Q. miR-27a regulates endothelial differentiation of breast cancer stem like cells. Oncogene. 2014;33:2629–2638. doi: 10.1038/onc.2013.214. [DOI] [PubMed] [Google Scholar]
- 20.Hardie DG. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MM, Phoenix KN, Kopsiaftis SG, Claffey KP. AMP-activated protein kinase alpha 2 isoform suppression in primary breast cancer alters AMPK growth control and apoptotic signaling. Genes Cancer. 2013;4:3–14. doi: 10.1177/1947601913486346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadad SM, Baker L, Quinlan PR, Robertson KE, Bray SE, Thomson G, Kellock D, Jordan LB, Purdie CA, Hardie DG, et al. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer. 2009;9:307. doi: 10.1186/1471-2407-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Ni CL, Yao Z, Chen LM, Niu WY. Liraglutide enhances glucose transporter 4 translocation via regulation of AMP-activated protein kinase signaling pathways in mouse skeletal muscle cells. Metabolism. 2014;63:1022–1030. doi: 10.1016/j.metabol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP, Shu H, Liu Y, Li CL. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013;39:71–79. doi: 10.1016/j.peptides.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, Barzilai N, Oren R, Fishman S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214–1223. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Zhao W, Zhang X, Liu J, Sun B, Tang H, Zhang H. miR-27a-mediated antiproliferative effects of metformin on the breast cancer cell line MCF-7. Oncol Rep. 2016;36:3691–3699. doi: 10.3892/or.2016.5199. [DOI] [PubMed] [Google Scholar]
- 28.Wei W, Zhang Q, Wang Z, Yan B, Feng Y, Li P. miR-219-5p inhibits proliferation and clonogenicity in chordoma cells and is associated with tumor recurrence. Oncol Lett. 2016;12:4568–4576. doi: 10.3892/ol.2016.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azumi J, Tsubota T, Sakabe T, Shiota G. miR-181a induces sorafenib resistance of hepatocellular carinoma cells through downregulation of RASSF1 expression. Cancer Sci. 2016;107:1256–1262. doi: 10.1111/cas.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prado A, Andrades P, Parada F. Recent developments in the ability to predict and modify breast cancer risk. J Plast Reconstr Aesthet Surg. 2010;63:1581–1587. doi: 10.1016/j.bjps.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 31.Schrauder MG, Fasching PA, Haberle L, Lux MP, Rauh C, Hein A, Bayer CM, Heusinger K, Hartmann A, Strehl JD, et al. Diabetes and prognosis in a breast cancer cohort. J Cancer Res Clin Oncol. 2011;137:975–983. doi: 10.1007/s00432-010-0960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juanjuan L, Wen W, Zhongfen L, Chuang C, Jing C, Yiping G, Changhua W, Dehua Y, Shengrong S. Clinical pathological characteristics of breast cancer patients with secondary diabetes after systemic therapy: A retrospective multicenter study. Tumour Biol. 2015;36:6939–6947. doi: 10.1007/s13277-015-3380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fidan-Yaylalı G, Dodurga Y, Seçme M, Elmas L. Antidiabetic exendin-4 activates apoptotic pathway and inhibits growth of breast cancer cells. Tumour Biol. 2016;37:2647–2653. doi: 10.1007/s13277-015-4104-9. [DOI] [PubMed] [Google Scholar]
- 34.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 35.Simonsen L, Pilgaard S, Orskov C, Rosenkilde MM, Hartmann B, Holst JJ, Deacon CF. Exendin-4, but not dipeptidyl peptidase IV inhibition, increases small intestinal mass in GK rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G288–G295. doi: 10.1152/ajpgi.00453.2006. [DOI] [PubMed] [Google Scholar]
- 36.Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: Acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98:271–284. doi: 10.1016/j.diabres.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Ni CL, Yao Z, Chen LM, Niu WY. Liraglutide enhances glucose transporter 4 translocation via regulation of AMP-activated protein kinase signaling pathways in mouse skeletal muscle cells. Metabolism. 2014;63:1022–1030. doi: 10.1016/j.metabol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, Barzilai N, Oren R, Fishman S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214–1223. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 39.Tang W, Zhu J, Su S, Wu W, Liu Q, Su F, Yu F. MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS One. 2012;7:e51702. doi: 10.1371/journal.pone.0051702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52:1699–1708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- 41.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: A consensus report. CA Cancer J Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]