Abstract
Previous studies have identified the association between cartilage endplate (CEP) degeneration and abnormal mechanical loading. Several studies have reported that intermittent cyclic mechanical tension (ICMT) regulates CEP degeneration via various biological processes and signaling pathways. However, the functions of microRNAs in regulating the cellular responses of CEP chondrocytes to ICMT remain to be elucidated. The current study determined the differentially expressed microRNAs in human CEP chondrocytes exposed to ICMT using microarray analysis. A total 21 significantly upregulated and 62 downregulated miRNAs were identified compared with the control. The findings were subsequently partially validated by reverse transcription-quantitative polymerase chain reaction. Potential target genes of the significantly differentially expressed miRNAs were predicted using bioinformatics analysis and were used for Gene Ontology analysis and Kyoto Encyclopedia of Genes and Genomes pathway analysis. The present study revealed that the significantly differentially expressed microRNAs were involved in various signaling pathways and biological processes that are crucial to regulating the responses of CEP chondrocytes to ICMT. The current study provided a global view of microRNA expression in CEP chondrocytes under mechanical stimulation, suggesting that microRNAs are important for regulating the mechanical response of CEP chondrocytes. Additionally, it provided a novel insight into the association between mechanical stress and the establishment and progression of intervertebral disc degeneration.
Keywords: microRNA, cyclic mechanical tension, cartilage endplate chondrocytes, cartilage endplate calcification, intervertebral disc degeneration
Introduction
Intervertebral disc degeneration (IDD) is a major contributor of low back pain (LBP) and has been recognized to be associated with various etiological factors, including aging, smoking, genetic predisposition, trauma, infection and abnormal mechanical loading (1–7). IDD is a disc cell-mediated pathological process (8). The primary cellular events in the process of IDD have been identified as disorder of extracellular matrix (ECM) metabolism in disc cells, aberrant production of matrix proteases, inflammatory cytokines and chemokines, cell senescence, apoptosis and cell death (9–12). It is of note that these cellular events have been identified to be strongly associated with a reduced nutrient supply in intervertebral disc (IVD) (13,14).
The nutrient supply of disc cells primarily depends on the diffusion of nutrient solutes from blood vessels through cartilage endplate (CEP) (15,16). However, the reduced permeability of CEP due to calcification impedes the availability of nutrients to disc cells and suppresses the clearance of metabolites. Therefore, the microenvironment of the disc becomes harsh, impairing the viability and activity of disc cells and consequently contributing to IDD (13,17). The causes of CEP calcification remain to be elucidated. The underlying molecular mechanism is not well understood. Previous studies have determined the association between CEP calcification and abnormal mechanical loading of the spine (18,19). Excessive mechanical loading has been identified to lead to the damage of chondrocytes and ECM in CEP (8). CEP chondrocytes, the basic units of CEP, have an important function in sensing and responding the mechanical stress loaded on CEP. Therefore, investigating the responses of CEP chondrocytes to mechanical loading may provide a novel insight into the mechanism of CEP calcification and the pathogenesis of IDD.
Cyclic mechanical tension (CMT) generated by the Flexcell system in vitro was widely used to simulate the mechanical strain which CEP chondrocytes undergo in vivo (20–24). The effects of CMT on the calcification of CEP chondrocytes have been discussed intensively in previous studies (20–26). It varies with the types of CMT: Intermittent CMT (ICMT, stimulation for several h each day in consecutive days) significantly upregulated the expression of calcification-associated genes, such as collagen type I, type X, osteocalcin and osteopontin and downregulated the expression of anti-calcification genes, including progressive ankylosis (ANK) gene, extracellular nucleotide phosphatase/phosphodiesterase 1 (ENPP1) and transforming growth factor-β1 (TGF-β1) leading to ICMT-induced calcification of CEP chondrocytes (21,22). Conversely, continuous CMT (CCMT, continuous stimulation for several days) upregulated the expression of progressive ankylosis (ANK) and TGF-β1 and protected CEP chondrocytes from calcification (20,23). Additionally, CMT has been identified to regulate the cartilage matrix metabolism, autophagy and cytoskeleton arrangement in CEP chondrocytes (22,24–26). In summary, the response of CEP chondrocytes to CMT was complicated, and involved in complex biological processes and signaling pathways. However, the regulatory mechanism underlying the response of CEP chondrocytes to CMT remains to be elucidated.
MicroRNAs (miRNAs) are members of the family of small non-coding RNAs and are 20–22 nucleotides in length. They bind to their target mRNAs in order to trigger degradation or to suppress their translation (27). MiRNAs have been determined to regulate various biological processes, such as cell proliferation, differentiation, apoptosis, senescence and development (27). Additionally, the involvement of miRNAs in the development and progression of various diseases has been discussed in previous studies, including carcinoma, neurodegenerative disorders, inflammatory diseases and reproductive disorders (28–34). The importance of miRNAs in musculoskeletal disorders, such as IDD, bone tumors, osteoarthritis and osteonecrosis of the femoral head, have been previously reported (35–40). However, the miRNA expression profile of CEP chondrocytes under CMT stimulation remains to be elucidated. Elucidating the altered miRNA expression profile of CEP chondrocytes under CMT stimulation helps us understand the mechanism underlying the regulatory effects of CMT on the biological processes and signaling pathways in CEP chondrocytes.
The present study aimed to determine the differentially expressed miRNAs of CEP chondrocytes under ICMT stimulation. CEP chondrocytes were isolated from human CEP specimens. Subsequently, ICMT stimulation was applied and the total RNA of CEP chondrocytes was extracted for miRNA microarray. Bioinformatics analysis was performed to determine the significantly differentially expressed miRNAs and their target genes. The signaling pathways and biological processes in which these target genes were involved were predicted. The findings of the present study identified the miRNA expression profile associated with the biological responses of CEP under ICMT stimulation, which may aid in identifying the mechanism underlying the mechanical force-induced changes of CEP. Additionally, the present study improved the clinical understanding of the pathogenesis of IDD.
Materials and methods
Ethical statement
All experimental procedures for human IVD samples were approved by the Ethical Committee of Xinqiao Hospital (Chongqing, China) and were in consistent with the Declaration of Helsinki. Signed informed consent was obtained from the participating patients.
Human CEP specimens
CEP specimens used in this study were obtained from patients who underwent posterior discectomy and a fusion procedure for IDD at the orthopedics department of Xinqiao Hospital between September 2015 and November 2015. The degenerative changes of CEP were evaluated using the method previously described by Thompson et al (41). In order to reduce the bias resulting from age and different CEP degenerative degrees, the present study selected the patients between the ages of 40 and 50. Additionally, the modic types of the selected patients were type 0, normal. The specimens from 6 patients were used in the present study. Detailed information about the 6 patients and samples is presented in Table I.
Table I.
Patient information and the usage of cartilage endplate specimens.
| Case no. | Age | Gender | Level | Modic type | Validation type |
|---|---|---|---|---|---|
| 1 | 43 | Female | L4/L5 | 0 | Microarray |
| 2 | 45 | Male | L3/L4 | 0 | Microarray |
| 3 | 49 | Male | L5/S1 | 0 | Microarray |
| 4 | 47 | Male | L5/S1 | 0 | PCR |
| 5 | 44 | Female | L5/S1 | 0 | PCR |
| 6 | 48 | Male | L5/S1 | 0 | PCR |
CEP chondrocyte culture
CEP chondrocytes were isolated from IVD specimens according to the protocol described in previous studies (42,43). IVD specimens were placed in a sterilized culture dish and washed with phosphate buffered saline (PBS). CEP tissues were carefully separated from IVD specimens under a dissecting microscope. Subsequently, CEP tissues were minced into 1-mm3 tissue blocks followed by digestion with Dulbecco's modified Eagle's medium (DMEM)/F-12 medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 0.2% type II collagenase (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 2 h at 37°C. The cell suspension was filtered with a 70 µm cell strainer and subsequently centrifuged for 10 min at 100 × g and then re-suspended in DMEM/F12 containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). The isolated CEP chondrocytes were cultured at 37°C and 5% CO2. The medium was replaced twice a week. When the cells reached about 80% confluence, they were sub-cultivated. The cells at the second passage were used in the experiments.
ICMT treatment
Following trypsinization, CEP chondrocytes were seeded on 6-well flexible silicone rubber BioFlex plates coated with collagen type I (Flexcell International Corporation, Burlington, NC, USA) at a density of 1.0×105 cells/well. When the cells reached 80–90% confluence, ICMT at 0.5 Hz sinusoidal curve at of 10% elongation was applied for 4 h/day in 14 consecutive days using a FX-5000T Flexcell Tension Plus system (Flexcell International Corporation). The culture medium was replaced every 2 days. The cells were cultured in a humidified atmosphere at 37°C and 5% CO2 ICMT treatment. The morphology of the cells was observed and imaged using a phase contrast microscope (Olympus Corporation, Tokyo, Japan). The cells were harvested immediately after following ICMT treatment. The CEP chondrocytes from the same patients without ICMT stimulation were used as the control.
RNA isolation and microRNA microarray
CEPs isolated from 3 patients (case no. 1, 2 and 3) were treated with ICMT and then termed the ICMT samples (n=3). The CEPs from the same patient without ICMT treatment were used as the control samples (n=3). Total RNA of all samples (n=6) lysed with 1 ml TRIzol reagent (Takara Bio Inc., Otsu, Japan) were sent to Kangchen Biotech Co., Ltd. (Shanghai, China) for microRNA microarray analysis. RNA quantity and quality were determined by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc.). All samples met the quality control standards. RNA was labeled using a miRCURY Array Power Labeling kit (Exiqon, Woburn, MA, USA) and then was hybridized to a one-color miRCURY Array (Exiqon, Woburn). The slides were scanned with the Axon GenePix 4000B microarray scanner (Molecular Devices, LLC, Sunnyvale, CA, USA). After image scanning, GenePix Pro version 6.0 was used to read the raw intensity of the image. The intensity of green signal was calculated after background subtraction and four replicated spots of each probe on the same slide have been averaged. The median normalization method was used to obtain the ‘Normalized Data’, as follows: Normalized Data=(Foreground-Background)/median, the median is 50 percent quantile of microRNA intensity that is >30 in all samples after background correction. The difference in the normalized data of miRNAs between the ICMT group (n=3) and the control group (n=3) was statistically tested using a paired sample Student's t-test. The fold-change of the upregulated miRNAs was calculated using the following equation: Fold-change=ICMT normalized data/Control normalized data. The fold-change of the downregulated miRNAs was calculated as following: Fold-change=−(Control normalized data/the normalized data of the ICMT groups). The differentially expressed miRNAs were identified according to their fold-change (fold-change ≥2 or ≤-2). The significantly differentially expressed miRNAs were selected according to the following thresholds P<0.05 and fold-change ≥2 or ≤-2.
Reversed transcription-quantitative polymerase chain reaction (RT-qPCR)
A total of 10 significantly differentially expressed miRNAs with relatively high fold-changes (fold-change ≥4 or ≤-4) were selected for validation using the RT-qPCR method, including 5 upregulated miRNAs (hsa-let-7a, hsa-miR-29c, hsa-miR-142, hsa-miR-181a, hsa-miR-19b) and 5 downregulated miRNAs (hsa-miR-548q, hsa-miR-637, hsa-miR-614, hsa-miR-581, hsv1-miR-H14). RNA from the CEPs of the patients (case no. 1, 2, 3, 4, 5 and 6) was reverse transcribed using a microRNA First Strand cDNA Synthesis kit (Sangon Biotech Co., Ltd., Shanghai, China) according to the manufacturer's protocol. The reaction was performed at 37°C for 60 min followed by 85°C for 5 min. qPCR was performed in triplicate on a StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with SYBR Premix Ex Taq™ II (Takara Bio) according to the protocol provided by the manufacturer. The following thermocycling conditions were used for the PCR: 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 34 sec. PCR products were subjected to melting curve analysis. Relative expression was calculated using the 2−ΔΔCq method (44). U6 was used as the internal reference. Mean Cq values were normalized to U6. The forward primers of the miRNAs investigated in the present study were listed in Table II. The reverse primers used for the miRNAs were universal PCR primer R.
Table II.
Forward primers used in reverse transcription-quantitative polymerase chain reaction analysis.
| microRNA | Forward |
|---|---|
| hsa-miR-548q | GGCCGCTGGTGCAAAAGTAA |
| hsa-miR-637 | ACTGGGGGCTTTCGGGC |
| hsa-miR-614 | GAACGCCTGTTCTTGCCAG |
| hsv1-miR-H14 | AGTCGCACTCGTCCCTGG |
| hsa-miR-581 | GGTCTTGTGTTCTCTAGATCAG |
| hsa-miR-142-3p | GTGTAGTGTTTCCTACTTTATGGA |
| hsa-let-7a-5p | GCTGAGGTAGTAGGTTGTATAG |
| hsa-miR-181a-5p | AACATTCAACGCTGTCGGTGA |
| hsa-miR-19b-3p | GGCTGTGCAAATCCATGCAAA |
| hsa-miR-29c-3p | GCTAGCACCATTTGAAATCGGT |
Bioinformatics analysis
The present study used three popular databases MiRBase (www.mirbase.org) (45), TargetScan (www.targetscan.org) (46) and MiRanda (www.microrna.org) (47) to predict the target genes of the significantly differentially expressed miRNAs. Genes that overlapped all three databases were selected as the target genes for further function annotation analysis. The Gene Ontology analysis (GO; www.geneontology.org) was used to annotate the functions of the target genes. The Kyoto Encyclopedia of Genes and Genomes pathway analysis (KEGG; www.genome.jp/kegg) was performed to identify the enriched signaling pathways by the target genes. P<0.05 was considered to indicate statistically significant difference.
Statistical analysis
Data were presented as the mean ± standard error of the mean. Data from the RT-qPCR were statistically tested using Kruskal-Wallis non-parametric analysis and Mann-Whitney U post-hoc tests as previously described (48). The remaining data were analyzed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism version 6 (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Morphological changes of CEP chondrocytes subjected to ICMT
The morphology of CEP chondrocytes subjected to ICMT changed from a polygonal morphology into a spindle morphology. Additionally, ICMT induced an orderly alignment of CEP chondrocytes with a certain direction (Fig. 1).
Figure 1.
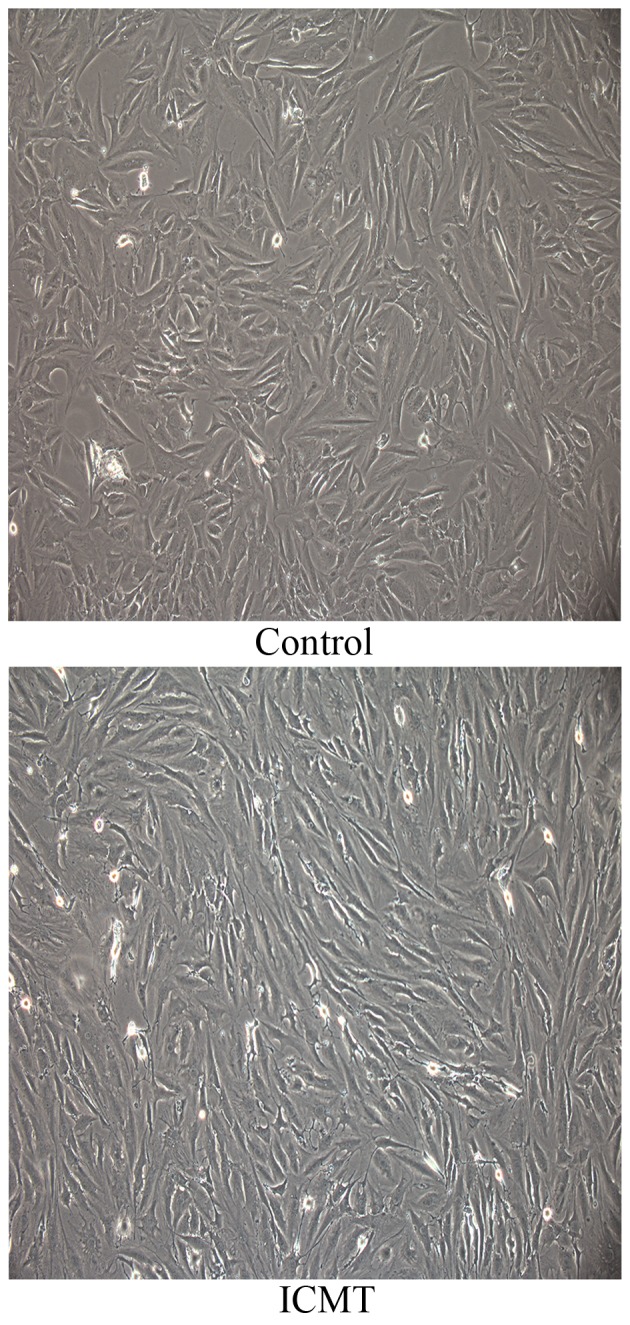
Morphology of CEP chondrocytes with ICMT stimulation. Magnification, ×200. CEP chondrocytes without ICMT stimulation had a polygonal morphology with a random distribution. CEP chondrocytes exposed to ICMT had a spindle morphology, additionally they had orderly alignment with a certain direction. CEP, cartilage endplate; ICMT, intermittent cyclic tension.
Differentially expressed miRNAs of CEP chondrocytes induced by ICMT
All differentially expressed miRNAs were filtered according to their fold-changes (fold-change ≥2 or ≤-2). There were 86 upregulated miRNAs and 188 downregulated miRNAs compared with the control. From these, 83 miRNAs (21 upregulated and 62 downregulated) were statistically significant (P<0.05; Tables III and IV). To validate the microarray results, 10 significantly differentially expressed miRNAs with high fold-changes (fold-change ≥4 or ≤-4), including 5 upregulated miRNAs (hsa-let-7a, hsa-miR-29c, hsa-miR-142, hsa-miR-181a, hsa-miR-19b) and 5 downregulated miRNAs (hsa-miR-548q, hsa-miR-637, hsa-miR-614, hsa-miR-581, hsv1-miR-H14) were selected as representative miRNAs for RT-qPCR assays. With the exception of has-miR-614, these miRNAs were significantly regulated by ICMT, which was consistent with the microarray results (Fig. 2).
Table III.
Significantly upregulated microRNAs in cartilage endplate chondrocytes under cyclic mechanical tension.
| ID | Name | Fold-change | P-value |
|---|---|---|---|
| 42,865 | hsa-miR-181a-5p | 15.84 | 3.29×10−2 |
| 10,947 | hsa-miR-142-3p | 14.65 | 2.29×10−2 |
| 11,041 | hsa-miR-29c-3p | 10.53 | 2.00×10−2 |
| 10,998 | hsa-miR-19b-3p | 10.07 | 3.11×10−2 |
| 11,013 | hsa-miR-181a-3p | 9.22 | 4.65×10−3 |
| 4610 | hsa-miR-126-3p | 8.47 | 1.94×10−2 |
| 9938 | hsa-let-7i-5p | 6.00 | 3.82×10−2 |
| 145,693 | hsa-miR-92a-3p | 5.19 | 4.85×10−2 |
| 10,990 | hsa-miR-196a-5p | 5.04 | 8.43×10−3 |
| 17,506 | hsa-miR-24-3p | 4.90 | 1.11×10−2 |
| 32,884 | hsa-miR-342-3p | 4.76 | 2.17×10−2 |
| 147,162 | hsa-let-7a-5p | 4.44 | 3.52×10−2 |
| 19,588 | hsa-miR-17-3p | 3.63 | 2.25×10−2 |
| 146,049 | hsa-miR-28-5p | 3.15 | 2.17×10−2 |
| 33,902 | hsa-miR-128-3p | 2.88 | 1.30×10−2 |
| 10,925 | hsa-miR-10b-5p | 2.64 | 3.42×10−2 |
| 17,935 | hsa-miR-101-5p | 2.52 | 2.21×10−2 |
| 42,783 | hsa-miR-197-3p | 2.47 | 9.19×10−2 |
| 42,829 | hsa-miR-127-3p | 2.25 | 1.96×10−2 |
| 168,925 | hsa-miR-1273g-3p | 2.18 | 1.72×10−2 |
| 169,385 | hsa-miR-4500 | 2.02 | 3.12×10−2 |
Table IV.
Significantly downregulated microRNAs in cartilage endplate chondrocytes under cyclic mechanical tension.
| ID | Name | Fold-change | P-value |
|---|---|---|---|
| 145,750 | hsa-miR-614 | −15.33 | 1.40×10−2 |
| 147,815 | hsv1-miR-H14-5p | −7.84 | 1.47×10−2 |
| 14,962 | hsa-miR-581 | −5.81 | 5.64×10−2 |
| 146,148 | hsa-miR-548q | −5.60 | 4.05×10−2 |
| 169,118 | hsa-miR-5009-3p | −5.55 | 4.19×10−2 |
| 148,677 | hsa-miR-637 | −5.29 | 1.54×10−2 |
| 147,623 | hsa-miR-4304 | −5.17 | 1.33×10−2 |
| 168,895 | hsa-miR-548ag | −4.40 | 1.58×10−2 |
| 46,606 | hsa-miR-1288-3p | −4.15 | 3.34×10−2 |
| 17,402 | ebv-miR-BART2-5p | −4.12 | 3.10×10−2 |
| 168,667 | hsa-miR-4999-3p | −3.87 | 1.65×10−2 |
| 45,775 | hsa-miR-1279 | −3.84 | 2.98×10−2 |
| 148,140 | hsa-miR-181d-3p | −3.84 | 1.75×10−2 |
| 169,131 | hsa-miR-4724-3p | −3.82 | 3.26×10−2 |
| 46,235 | hsa-miR-524-5p | −3.79 | 8.34×10−3 |
| 42,493 | hsa-miR-892b | −3.58 | 1.87×10−2 |
| 169,281 | hsa-miR-4752 | −3.27 | 4.72×10−2 |
| 169,084 | hsa-miR-5708 | −3.17 | 3.51×10−3 |
| 147,866 | hsa-miR-3134 | −3.12 | 4.62×10−2 |
| 42,786 | hsa-miR-188-3p | −3.11 | 2.14×10−2 |
| 11,154 | hsa-miR-517c-3p | −3.07 | 4.48×10−2 |
| 148,474 | hsa-miR-3622a-5p | −3.00 | 2.71×10−2 |
| 169,318 | hsa-miR-4649-3p | −2.95 | 3.40×10−2 |
| 169,164 | hsa-miR-4705 | −2.92 | 2.64×10−2 |
| 42,773 | ebv-miR-BART17-3p | −2.90 | 4.87×10−2 |
| 17,851 | hsa-miR-200c-5p | −2.88 | 3.22×10−2 |
| 168,698 | hsa-miR-3127-3p | −2.73 | 4.35×10−2 |
| 168,886 | hsa-miR-4745-5p | −2.71 | 2.72×10−2 |
| 148,215 | hsa-miR-3591-3p | −2.71 | 3.65×10−2 |
| 147,979 | hsa-miR-3150a-3p | −2.68 | 1.33×10−2 |
| 46,634 | hsa-miR-1281 | −2.65 | 4.57×10−2 |
| 148,102 | hsa-miR-3619-5p | −2.60 | 4.67×10−2 |
| 147,977 | hsa-miR-3190-3p | −2.48 | 1.02×10−2 |
| 46,800 | hsa-miR-1224-3p | −2.40 | 3.47×10−4 |
| 168,745 | hsa-miR-4667-3p | −2.40 | 3.02×10−2 |
| 146,006 | hsa-miR-670-5p | −2.40 | 3.03×10−2 |
| 169,080 | hsa-miR-4684-5p | −2.36 | 2.83×10−2 |
| 146,042 | hsv1-miR-H8-3p | −2.33 | 4.63×10−2 |
| 42,613 | ebv-miR-BART19-5p | −2.33 | 3.63×10−2 |
| 46,363 | hsa-miR-1272 | −2.32 | 2.82×10−2 |
| 46,556 | hsa-miR-623 | −2.28 | 2.30×10−2 |
| 46,705 | hsa-miR-548k | −2.28 | 5.75×10−3 |
| 17,336 | hsa-miR-618 | −2.28 | 2.53×10−3 |
| 146,169 | mcv-miR-M1-3p | −2.22 | 4.99×10−2 |
| 46,210 | hsa-miR-1249-3p | −2.20 | 2.25×10−2 |
| 21,498 | hsa-miR-654-3p | −2.19 | 3.65×10−2 |
| 169,166 | hsa-miR-4683 | −2.17 | 1.80×10−2 |
| 17,306 | ebv-miR-BART12 | −2.15 | 2.81×10−2 |
| 42,584 | hsa-miR-432-3p | −2.15 | 2.51×10−3 |
| 168,904 | hsa-miR-4473 | −2.12 | 5.84×10−3 |
| 17,299 | hcmv-miR-UL22A-3p | −2.09 | 4.27×10−2 |
| 148,063 | hsa-miR-3713 | −2.09 | 2.82×10−2 |
| 169,038 | hsa-miR-488-3p | −2.09 | 3.31×10−3 |
| 168,603 | hsa-miR-4664-5p | −2.07 | 2.58×10−3 |
| 148,398 | hsa-miR-3908 | −2.07 | 1.93×10−3 |
| 147,837 | hsa-miR-3119 | −2.07 | 4.05×10−2 |
| 168,850 | hsa-miR-3191-5p | −2.07 | 2.24×10−2 |
| 169,185 | hsa-miR-5187-3p | −2.07 | 2.71×10−3 |
| 46,408 | hsa-miR-1322 | −2.06 | 1.04×10−2 |
| 42,457 | hsa-miR-323a-5p | −2.05 | 1.68×10−2 |
| 168,841 | hsa-miR-5588-3p | −2.04 | 9.37×10−3 |
| 148,643 | hsa-miR-642a-5p | −2.04 | 3.38×10−2 |
Figure 2.
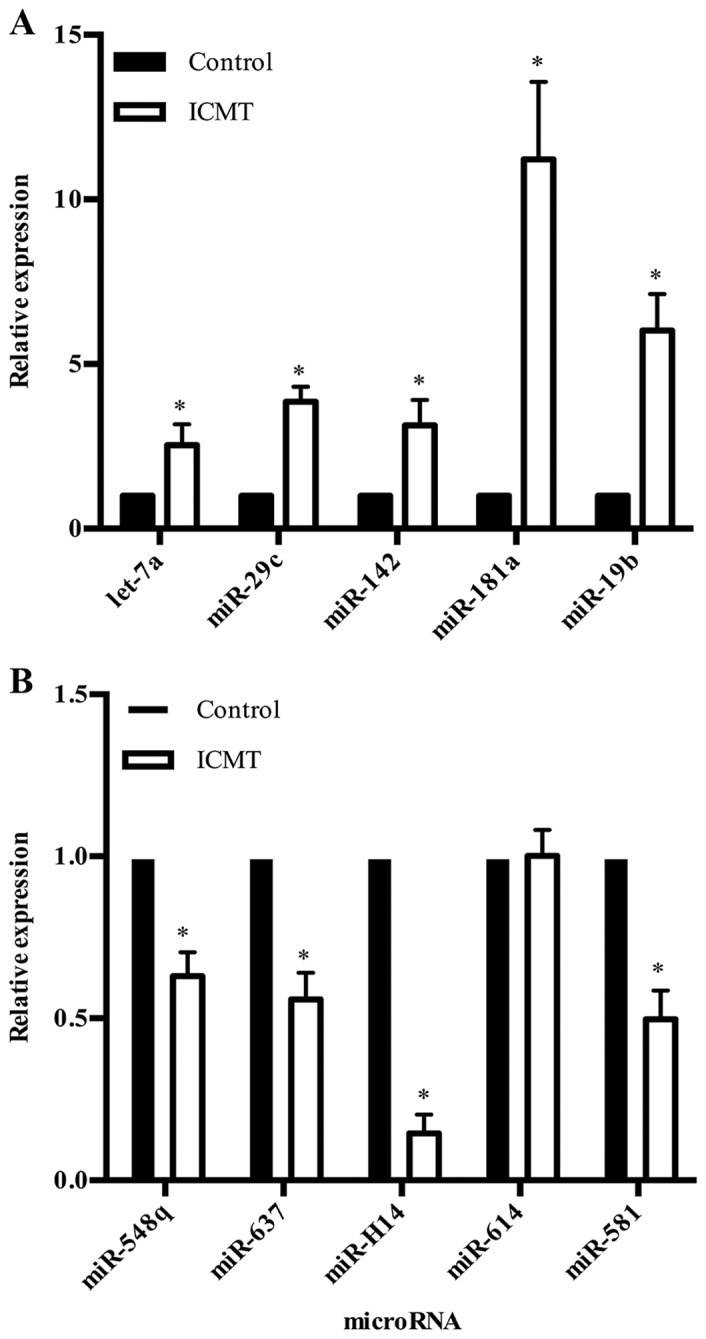
Validation of the representative microRNAs by reverse transcription-quantitative polymerase chain reaction. (A) Upregulated and (B) downregulated microRNAs. *P<0.05 vs. control. ICMT, intermittent cyclic tension; miR, microRNA.
KEGG pathway analysis
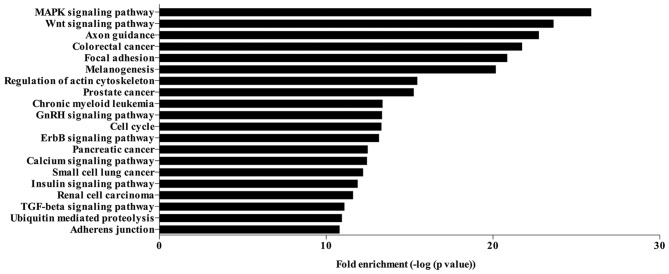
The target genes of the 83 significantly differentially expressed miRNAs were identified based on the overlapping of 3 databases (MiRBase, MiRanda and TargetScan). A total of 1,836 genes were identified to be targeted by the significantly regulated miRNAs. The KEGG pathways analysis of these target genes revealed that the genes targeted by significantly differentially expressed miRNAs were enriched in various signaling pathways. The top 20 signaling pathways regulated by the significantly differentially expressed miRNAs are presented in Fig. 3, including the mitogen-activated protein kinase (MAPK) and Wnt signaling pathways, focal adhesion, regulation of actin cytoskeleton, GnRH signaling pathway, cell cycle, ErbB signaling pathway, insulin signaling pathway, TGF-β signaling pathway and adherens junction.
Figure 3.
Kyoto Encyclopedia of Genes and Genomes pathway analysis based on the genes targeted by the significantly differentially expressed microRNAs. Top 20 signaling pathways enriched by the target genes of the significantly differentially expressed microRNAs. MAPK, mitogen-activated protein kinase.
GO analysis
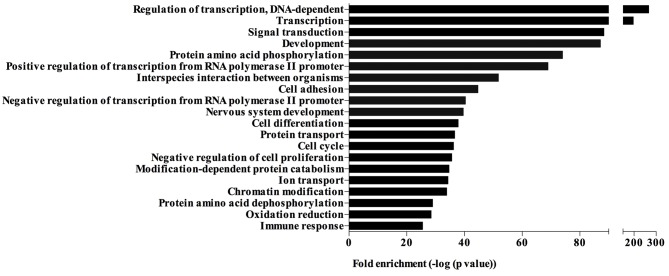
The significantly differentially expressed miRNAs were involved in different biological processes, including regulation of transcription, signal transduction, development, cell adhesion, cell cycle, negative regulation of cell proliferation and chromatin modification (Fig. 4). The top 20 biological processes enriched by the target genes of significantly differentially expressed miRNAs are presented in Fig. 4.
Figure 4.
Gene Ontology analysis based on the genes targeted by the significantly differentially expressed microRNAs. Top 20 biological processes enriched by the target genes of the significantly differentially expressed microRNAs.
Discussion
Previous studies have reported that ICMT markedly induced the calcification of CEP chondrocytes (21,22). Furthermore, CMT has been identified to regulate the matrix metabolism, autophagy and cytoskeleton arrangement of CEP chondrocytes through MAPK and Wnt/β-catenin pathways (22,24–26). Briefly, CMT is involved in the regulation of complex biological processes and signaling pathways of CEP chondrocytes. It is of note that CEP calcification is a major pathological characteristic of degenerative discs, as it impedes the availability of nutrients to disc cells and suppresses the clearance of metabolites (13,17). The presence of disc cell autophagy and the disturbance of the balance between matrix anabolism and catabolism in human degenerative disc cells has also been identified as causal factor of IDD (49–52). Therefore, mechanical tension is a widely recognized as a primary contributor of IDD. However, the mechanism underlying the effect of ICMT on the viability and function of CEP chondrocytes remains to be fully elucidated. The present study determined that 83 miRNAs were significantly differentially expressed in human CEP chondrocytes in response to ICMT. Additionally, GO and KEGG pathway analyses revealed that the differentially expressed miRNAs were widely involved in the regulation of various cellular processes and signaling pathways of human CEP chondrocytes, suggesting that they are crucial intermediators of the effects of ICMT on the viability and function of CEP chondrocytes. More importantly, the present study suggests that miRNA dysregulation has an important function in the establishment and progression of IDD through mediation of the effect of mechanical loadings on the structural and functional homeostasis of IVDs.
The target genes of the significantly differentially expressed miRNAs were enriched in various biological processes according to the GO analysis. It is of note that several GO terms were associated with regulation of cell transcription, including regulation of transcription (DNA-dependent), positive regulation of transcription from RNA polymerase II promoter, negative regulation of transcription from RNA polymerase II promoter and chromatin modification. The present findings suggest that there is a regulatory effect of ICMT on the transcriptome of human CEPs. This is consistent with the findings of previous studies (21,24). ICMT has been identified to regulate the transcription of ECM-associated genes, including TGF-β gene and the genes associated with calcification, including collagen type I, type X, osteocalcin, osteopontin, ANK and ENPP1. Subsequently, the CEP chondrocytes undergo a phenotype shift from a cartilage-specific to calcification-associated, which also promotes IDD progression. Based on the current findings, miRNAs are crucial regulators of CEP chondrocyte transcription under ICMT stimulation. Therefore, it is possible that this ICMT-induced phenotype shift is mediated by miRNAs, indicating that miRNA dysregulation contributes to the initiation and progression of CEP calcification. The effect of miRNAs on the transcriptome of human CEP chondrocytes should be discussed in further studies. In addition, there were some GO terms identified to be associated with cell signal transduction, including signal transduction, protein amino acid phosphorylation, modification-dependent protein catabolism and protein amino acid dephosphorylation. Considering the essential function of signal transduction in the regulation of cell viability and function, the current findings indicate that miRNAs affect the biological responses of CEP chondrocytes to mechanical stimulation by regulation of signal transduction pathways in CEP chondrocytes. However, the function of miRNAs in regulating mechanical force-induced signal transduction remains unclear and should be investigated in future research.
The KEGG pathway analysis was used to annotate the functional roles of significantly differentially expressed miRNAs in regulating the biological responses of CEP chondrocytes to ICMT. When exposed to ICMT, human CEP chondrocytes changed from a randomly distributed polygonal morphology into a spindle morphology with a specific directional alignment, which was consistent with the findings of previous studies (20,26). Multiple mechanotransduction pathways, including focal adhesion pathway, action cytoskeleton regulation pathway and adherens junction pathway were previously identified to be responsible for these changes (53–55). The findings of the present study predicted that these signaling pathways were regulated by the significantly differentially expressed miRNAs under ICMT stimulation, suggesting that miRNAs may regulate the migration and morphology of CEP cells via these signaling pathways.
The proliferation of CEP chondrocytes is crucial for the maintenance of the number of functional cells in CEP, which is regulated by cell cycle progression. In the current study, some GO terms and KEGG pathways enriched by the target genes of miRNAs were associated with cell cycle and negative regulation of cell proliferation, suggesting that ICMT regulates cell cycle progression to affect the proliferation of CEP chondrocytes via miRNAs. ICMT has been previously reported to promote the proliferation of CEP chondrocytes (26). However, the mechanism underlying this effect had not been previously investigated. The present study investigated miRNA regulation in order to elucidate the effect of mechanical stimulation on CEP chondrocyte proliferation.
TGF-β1 is an established regulator of cell proliferation, differentiation and ECM metabolism. Previous studies have demonstrated the function of TGF-β1 in the ICMT-induced calcification of CEP chondrocytes (21,22). TGF-β1 induced the expression of ANK and ENPP1 to slow down the process of CEP calcification. However, ICMT reduced the expression of TGF-β1 and in turn reduced the expression of ANK and ENPP1 leading to calcification of the CEP cells (21,22). Additionally, TGF-β1 has been demonstrated to promote the ECM anabolism of disc cells (56). Previous studies have determined that the downregulation of collagen type II and aggrecan was accompanied by the downregulation of TGF-regulationnion of collagen type he ECM anabolis (21,26). It is of note that the present study identified an association between the significantly differentially expressed miRNAs and the TGF-β signaling pathway, indicating the roles of differentially expressed miRNAs in the pathogenesis of IDD through regulation of the matrix metabolism and calcification of CEP chondrocytes via the TGF-β signaling pathway.
The activation of Wnt pathway has been identified in human degenerative CEP specimens (26). ICMT has been identified to activate the Wnt signaling pathway in order to induce the loss of the chondrogenic phenotype of human CEP chondrocytes (26). Furthermore, the activation of the Wnt signaling pathway has been found to promote IDD by inducing nucleus pulposus (NP) cell senescence and upregulating the expression of matrix metalloproteinase-3 (MMP-3), MMP-7, MMP-9 and MMP-10 in NP cells (48). Therefore, the Wnt signaling pathway is involved in the occurrence and development of IDD. Additionally, the KEGG pathway analysis performed in the present study suggested that the Wnt signaling pathway is associated with the significantly differentially expressed miRNAs under ICMT stimulation. The function of the Wnt pathway in regulating biological responses of CEP chondrocytes under ICMT provided novel insights into the involvement of miRNA dysregulation in the pathogenesis of IDD.
The MAPK signaling pathway has been previously revealed to regulate the expression of ANK and be responsible for mechanotransduction (57–59). CMT has been previously reported to activate the p38-MAPK signaling pathway to regulate the expression of ANK in CEP chondrocytes (23), indicating the crucial role of the MAPK signaling pathway in the regulation of ICMT-induced calcification of CEP chondrocytes. According the findings of the current study, the MAPK signaling pathway may be regulated by the differentially expressed miRNA under ICMT stimulation. The mechanism underlying the regulatory effects of miRNAs on the MAPK signaling pathway will be discussed in future studies.
There are two major limitations to the current study. First one is that the sample size of miRNA microarray analysis was relatively small. Therefore, more RT-qPCR validation is required to confirm the effect of ICMT on the expression of miRNAs in human CEP chondrocytes. Secondly, further studies using dysregulated miRNAs are necessary to elucidate the effects of miRNAs on the biological functions of CEP chondrocytes in detail.
In conclusion, the present study identified the differentially expressed miRNAs of human CEP chondrocytes under ICMT stimulation. The bioinformatics analyses suggest that miRNAs are essential for the regulation of the biological responses of CEP chondrocytes to ICMT, particularly in terms of the ICMT-induced calcification of CEP chondrocytes. The present study improved the current understanding of the involvement of miRNAs in the pathogenesis of CEP calcification, suggesting the possible function of miRNA dysregulation in the pathogenesis of IDD. Determining the detailed regulatory functions of these differentially expressed miRNAs contributes to identifying novel approaches to protect CEP cells from calcification under abnormal mechanical stress and subsequently suppressing the process of IDD.
Acknowledgements
The current study was supported by the National Natural Science Foundation of China (grant nos. 81271982, 81472076 and 81572186).
Glossary
Abbreviations
- IVD
intervertebral disc
- IDD
intervertebral disc degeneration
- NP
nucleus pulposus
- AF
annulus fibrosus
- ICMT
intermittent cyclic mechanical tension
- ECM
extracellular matrix
- ANK
progressive ankylosis
- ENPP1
extracellular nucleotide phosphatase/phosphodiesterase 1
- TGF-β1
transforming growth factor-β1
- CMT
cyclic mechanical tension
- LBP
lower back pain
- CEP
cartilage endplate
- miRNA
microRNA
- MMP
matrix metalloproteinase
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
References
- 1.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):S10–S14. doi: 10.2106/00004623-200604002-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kanayama M, Togawa D, Takahashi C, Terai T, Hashimoto T. Cross-sectional magnetic resonance imaging study of lumbar disc degeneration in 200 healthy individuals. J Neurosurg Spine. 2009;11:501–507. doi: 10.3171/2009.5.SPINE08675. [DOI] [PubMed] [Google Scholar]
- 3.Cheung KM, Karppinen J, Chan D, Ho DW, Song YQ, Sham P, Cheah KS, Leong JC, Luk KD. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 2009;34:934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 4.Battie MC, Videman T, Kaprio J, Gibbons LE, Gill K, Manninen H, Saarela J, Peltonen L. The twin spine study: Contributions to a changing view of disc degeneration. Spine J. 2009;9:47–59. doi: 10.1016/j.spinee.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Vergroesen PP, Kingma I, Emanuel KS, Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH, Smit TH. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthritis Cartilage. 2015;23:1057–1070. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Nasto LA, Roughley P, Leme AS, Houghton AM, Usas A, Sowa G, Lee J, Niedernhofer L, Shapiro S, et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage. 2012;20:896–905. doi: 10.1016/j.joca.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stirling A, Worthington T, Rafiq M, Lambert PA, Elliott TS. Association between sciatica and Propionibacterium acnes. Lancet. 2001;357:2024–2025. doi: 10.1016/S0140-6736(00)05109-6. [DOI] [PubMed] [Google Scholar]
- 8.Adams MA, Roughley PJ. What is intervertebral disc degeneration and what causes it? Spine (Phila Pa 1976) 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 9.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331–341. doi: 10.1016/j.spinee.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu Y, Tian N, Huang Y, Xue E, Wang X, Xu H. Apoptosis, senescence and autophagy in rat nucleus pulposus cells: Implications for diabetic intervertebral disc degeneration. J Orthop Res. 2013;31:692–702. doi: 10.1002/jor.22289. [DOI] [PubMed] [Google Scholar]
- 12.Gruber HE, Ingram JA, Norton HJ, Hanley EN, Hanley EN., Jr Senescence in cells of the aging and degenerating intervertebral disc: Immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine (Phila Pa 1976) 2007;32:321–327. doi: 10.1097/01.brs.0000253960.57051.de. [DOI] [PubMed] [Google Scholar]
- 13.Huang YC, Urban JP, Luk KD. Intervertebral disc regeneration: Do nutrients lead the way? Nat Rev Rheumatol. 2014;10:561–566. doi: 10.1038/nrrheum.2014.91. [DOI] [PubMed] [Google Scholar]
- 14.Vo NV, Hartman RA, Patil PR, Risbud MV, Kletsas D, Iatridis JC, Hoyland JA, Le Maitre CL, Sowa GA, Kang JD. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34:1289–1306. doi: 10.1002/jor.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 16.Grunhagen T, Shirazi-Adl A, Fairbank JC, Urban JP. Intervertebral disk nutrition: A review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42:465–477. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian Q, Liang QQ, Wan C, Hou W, Li CG, Zhao YJ, Lu S, Shi Q, Wang YJ. Prolonged upright posture induces calcified hypertrophy in the cartilage end plate in rat lumbar spine. Spine (Phila Pa 1976) 2011;36:2011–2020. doi: 10.1097/BRS.0b013e3181ffde92. [DOI] [PubMed] [Google Scholar]
- 19.Peng B, Hou S, Shi Q, Jia L. The relationship between cartilage end-plate calcification and disc degeneration: An experimental study. Chin Med J (Engl) 2001;114:308–312. [PubMed] [Google Scholar]
- 20.Xu HG, Hu CJ, Wang H, Liu P, Yang XM, Zhang Y, Wang LT. Effects of mechanical strain on ANK, ENPP1 and TGF-β1 expression in rat endplate chondrocytes in vitro. Mol Med Rep. 2011;4:831–835. doi: 10.3892/mmr.2011.508. [DOI] [PubMed] [Google Scholar]
- 21.Xu HG, Zhang XH, Wang H, Liu P, Wang LT, Zuo CJ, Tong WX, Zhang XL. Intermiåttent cyclic mechanical tension-induced calcification and downregulation of ankh gene expression of end plate chondrocytes. Spine (Phila Pa 1976) 2012;37:1192–1197. doi: 10.1097/BRS.0b013e318244d989. [DOI] [PubMed] [Google Scholar]
- 22.Xu HG, Li ZR, Wang H, Liu P, Wang LT, Zuo CJ Tong WX, Zhang XL. Intermittent cyclic mechanical tension-induced down-regulation of ectonucleotide pyrophosphatase phosphodiesterase 1 gene expression is mainly dependent on TGF-β1 in end-plate chondrocytes. Orthop Surg. 2013;5:40–45. doi: 10.1111/os.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, Zhang X, Wang H, Zhang Y, Shi Y, Zhang X. Continuous cyclic mechanical tension increases ank expression in endplate chondrocytes through the TGF-β1 and p38 pathway. Eur J Histochem. 2013;57:e28. doi: 10.4081/ejh.2013.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu HG, Yu YF, Zheng Q, Zhang W, Wang CD, Zhao XY, Tong WX, Wang H, Liu P, Zhang XL. Autophagy protects end plate chondrocytes from intermittent cyclic mechanical tension induced calcification. Bone. 2014;66:232–239. doi: 10.1016/j.bone.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Xu HG, Ma MM, Zheng Q, Shen X, Wang H, Zhang SF, Xu JJ, Wang CD, Zhang XL. P120-catenin protects endplate chondrocytes from intermittent cyclic mechanical tension induced degeneration by inhibiting the expression of RhoA/ROCK-1 signaling pathway. Spine (Phila Pa 1976) 2016;41:1261–1271. doi: 10.1097/BRS.0000000000001532. [DOI] [PubMed] [Google Scholar]
- 26.Xu HG, Zheng Q, Song JX, Li J, Wang H, Liu P, Wang J, Wang CD, Zhang XL. Intermittent cyclic mechanical tension promotes endplate cartilage degeneration via canonical Wnt signaling pathway and E-cadherin/β-catenin complex cross-talk. Osteoarthritis Cartilage. 2016;24:158–168. doi: 10.1016/j.joca.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Ambros V. microRNAs: Tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/S0092-8674(01)00616-X. [DOI] [PubMed] [Google Scholar]
- 28.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16:142–165. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- 30.Alexandrov PN, Dua P, Lukiw WJ. Up-Regulation of miRNA-146a in progressive, age-related inflammatory neurodegenerative disorders of the human CNS. Front Neurol. 2014;5:181. doi: 10.3389/fneur.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lv Z, Shi Q, Huang W, Xing C, Hao Y, Feng X, Yang Y, Zhang A, Kong Q, Yuki N, Wang Y. MicroRNA expression profiling in Guillain-Barre syndrome. J Neuroimmunol. 2016;301:12–15. doi: 10.1016/j.jneuroim.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Men Y, Fan Y, Shen Y, Lu L, Kallen AN. The Steroidogenic acute regulatory protein (StAR) is regulated by the H19/let-7 Axis. Endocrinology. 2016;158:402–409. doi: 10.1210/en.2016-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu L, Katsaros D, Canuto EM, Biglia N, Risch HA, Yu H. LIN-28B/let-7a/IGF-II axis molecular subtypes are associated with epithelial ovarian cancer prognosis. Gynecol Oncol. 2016;141:121–127. doi: 10.1016/j.ygyno.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Lu L, Katsaros D, Risch HA, Canuto EM, Biglia N, Yu H. MicroRNA let-7a modifies the effect of self-renewal gene HIWI on patient survival of epithelial ovarian cancer. Mol Carcinog. 2016;55:357–365. doi: 10.1002/mc.22285. [DOI] [PubMed] [Google Scholar]
- 35.Ohrt-Nissen S, Døssing KB, Rossing M, Lajer C, Vikeså J, Nielsen FC, Friis-Hansen L, Dahl B. Characterization of miRNA expression in human degenerative lumbar disks. Connect Tissue Res. 2013;54:197–203. doi: 10.3109/03008207.2013.781594. [DOI] [PubMed] [Google Scholar]
- 36.Zhao B, Yu Q, Li H, Guo X, He X. Characterization of microRNA expression profiles in patients with intervertebral disc degeneration. Int J Mol Med. 2014;33:43–50. doi: 10.3892/ijmm.2013.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan HF, Von Roemeling C, Gao HD, Zhang J, Guo CA, Yan ZQ. Analysis of altered microRNA expression profile in the reparative interface of the femoral head with osteonecrosis. Exp Mol Pathol. 2015;98:158–163. doi: 10.1016/j.yexmp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis. Nat Rev Rheumatol. 2012;8:543–552. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Qian W, Wu Z, Bian Y, Weng X. Preliminary screening of differentially expressed circulating microRNAs in patients with steroidinduced osteonecrosis of the femoral head. Mol Med Rep. 2014;10:3118–3124. doi: 10.3892/mmr.2014.2660. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Che L, Xie YK, Hu QJ, Ma CJ, Pei YJ, Wu ZG, Liu ZH, Fan LY, Wang HQ. Noncoding RNAs in human intervertebral disc degeneration: An integrated microarray study. Genom Data. 2015;5:80–81. doi: 10.1016/j.gdata.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson KJ, Dagher AP, Eckel TS, Clark M, Reinig JW. Modic changes on MR images as studied with provocative diskography: Clinical relevance-a retrospective study of 2457 disks. Radiology. 2009;250:849–855. doi: 10.1148/radiol.2503080474. [DOI] [PubMed] [Google Scholar]
- 42.Xiong CJ, Huang B, Zhou Y, Cun YP, Liu LT, Wang J, Li CQ, Pan Y, Wang H. Macrophage migration inhibitory factor inhibits the migration of cartilage end plate-derived stem cells by reacting with CD74. PLoS One. 2012;7:e43984. doi: 10.1371/journal.pone.0043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu LT, Huang B, Li CQ, Zhuang Y, Wang J, Zhou Y. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS One. 2011;6:e26285. doi: 10.1371/journal.pone.0026285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 47.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hiyama A, Sakai D, Risbud MV, Tanaka M, Arai F, Abe K, Mochida J. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62:3036–3047. doi: 10.1002/art.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye W, Xu K, Huang D, Liang A, Peng Y, Zhu W, Li C. Age-related increases of macroautophagy and chaperone-mediated autophagy in rat nucleus pulposus. Connect Tissue Res. 2011;52:472–478. doi: 10.3109/03008207.2011.564336. [DOI] [PubMed] [Google Scholar]
- 50.Jiang W, Zhang X, Hao J, Shen J, Fang J, Dong W, Wang D, Zhang X, Shui W, Luo Y, et al. SIRT1 protects against apoptosis by promoting autophagy in degenerative human disc nucleus pulposus cells. Sci Rep. 2014;4:7456. doi: 10.1038/srep07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN., Jr. Autophagy in the degenerating human intervertebral disc: In vivo molecular and morphological evidence and induction of autophagy in cultured annulus cells exposed to proinflammatory cytokines-implications for disc degeneration. Spine (Phila Pa 1976) 2015;40:773–782. doi: 10.1097/BRS.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 52.Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318–330. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Engl W, Arasi B, Yap LL, Thiery JP, Viasnoff V. Actin dynamics modulate mechanosensitive immobilization of E-cadherin at adherens junctions. Nat Cell Biol. 2014;16:587–594. doi: 10.1038/ncb2973. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y, Wang Q, Li Y, Gan Y, Li P, Li S, Zhou Y, Zhou Q. Cyclic tensile strain induces tenogenic differentiation of tendon-derived stem cells in bioreactor culture. Biomed Res Int. 2015;2015:790804. doi: 10.1155/2015/790804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamiello C, Buskermolen ABC, Baaijens FPT, Broers JLV, Bouten CVC. Heading in the Right Direction: Understanding Cellular Orientation Responses to Complex Biophysical Environments. Cell Mol Bioeng. 2016;9:12–37. doi: 10.1007/s12195-015-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine (Phila Pa 1976) 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 57.Ding L, Heying E, Nicholson N, Stroud NJ, Homandberg GA, Buckwalter JA, Guo D, Martin JA. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage. 2010;18:1509–1517. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tetsunaga T, Nishida K, Furumatsu T, Naruse K, Hirohata S, Yoshida A, Saito T, Ozaki T. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthritis Cartilage. 2011;19:222–232. doi: 10.1016/j.joca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Cailotto F, Bianchi A, Sebillaud S, Venkatesan N, Moulin D, Jouzeau JY, Netter P. Inorganic pyrophosphate generation by transforming growth factor-beta-1 is mainly dependent on ANK induction by Ras/Raf-1/extracellular signal-regulated kinase pathways in chondrocytes. Arthritis Res Ther. 2007;9:R122. doi: 10.1186/ar2330. [DOI] [PMC free article] [PubMed] [Google Scholar]