Abstract
The Janus kinase (JAK)1 and JAK2 inhibitor, ruxolitinib, and the active form of vitamin D (calcitriol) were previously reported to possess anticancer effects in breast cancer. The present study investigated the combined effects of ruxolitinib and calcitriol on an estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-positive, breast cancer cell line. The ER and HER2-positive MCF7-HER18 breast cancer cell line was used to investigate the combination effect of ruxolitinib and calcitriol. A bromodeoxyuridine (BrdU) assay was used to investigate cell growth inhibition. The synergism of this combination therapy was examined using the Chou-Talalay method. Cell cycle analysis was performed by flow cytometry, and apoptosis was evaluated by flow cytometry following Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining. Alterations in protein expression levels were analyzed by western blotting. The BrdU assay indicated that combination treatment using ruxolitinib and calcitriol produced a synergistic anti-proliferative effect in MCF7-HER18 breast cancer cells. Annexin V-FITC/PI staining and cell cycle analysis identified a synergistic increase in apoptosis and sub-G1 arrest in the presence of ruxolitinib and calcitriol. Western blot analysis revealed that these synergistic effects of ruxolitinib and calcitriol were associated with reduced protein levels of JAK2, phosphorylated JAK2, c-Myc proto oncogene protein, cyclin-D1, apoptosis regulator Bcl-2 and Bcl-2-like protein 1, and with increased levels of caspase-3 and Bcl-2-associated agonist of cell death proteins. The results of the present study demonstrated the synergistic anticancer effects of ruxolitinib and calcitriol in ER and HER2-positive MCF7-HER18 breast cancer cells. Based on these findings, ruxolitinib and calcitriol may have potential as a combination therapy for patients with ER and HER2-positive breast cancer.
Keywords: breast neoplasm, ruxolitinib, calcitriol, synergism, apoptosis
Introduction
In 2016, breast cancer was the most common type of cancer and the second leading cause of cancer mortality in women worldwide (1). To facilitate the selection of an appropriate therapeutic strategy, patients with breast cancer may be subdivided into four molecular subtypes, which are determined by hormone receptor and human epidermal growth factor receptor 2 (HER2) status (2,3). The luminal B subtype includes 10–15% of breast cancer cases and is defined as estrogen receptor (ER)-positive and HER2-positive (4,5). Clinically, this subtype has an aggressive course and the current therapeutic strategy for metastatic or locally advanced luminal B breast cancer includes cytotoxic chemotherapy, hormone therapy and targeted monoclonal antibody therapy (6). However, novel therapeutic approaches are required to overcome the limitations of the current therapeutic strategy; these include a relatively low chemosensitivity, compared with the triple negative or HER2 subtypes (7,8), and a lack of responsiveness or resistance to monoclonal antibody treatment (9,10).
The Janus kinase (JAK) pathway serves multiple roles in regulating oncogenesis and cancer cell progression, and has represented a potentially attractive therapeutic target in various solid tumors (11–13). Numerous JAK pathway inhibitors have been developed, including ruxolitinib, a JAK1 and JAK2 inhibitor that has been approved for use in primary myelofibrosis and polycythemia vera (14,15). In breast cancer, previous in vitro studies have demonstrated the anticancer effects of JAK inhibitors, including ruxolitinib (16–18), and the potential application of ruxolitinib for the treatment of patients with metastatic or locally advanced breast cancer is under evaluation in ongoing clinical trials.
In addition to its conventional role in the regulation of calcium homeostasis, vitamin D has important biological functions in cell differentiation, apoptosis and cell cycle modulation; these activities are mediated by its binding to the vitamin D receptor (VDR) (19,20). Clinically, a number of reports have suggested an association between the vitamin D status of a patient and their breast cancer prognosis (21,22). Calcitriol (1,25-dihydroxyvitamin D) is the biologically active form of vitamin D and previous in vitro studies have identified anticancer effects of calcitriol in various malignancies, including breast cancer (23–28).
Despite the number of previous preclinical studies into the anticancer effects of ruxolitinib and calcitriol in breast cancer, the therapeutic benefits of these treatments for breast cancer have not been established in clinical settings. Therefore, based on the reported anticancer effects of ruxolitinib and calcitriol individually, it was hypothesized that there may be a synergistic anticancer effect of combination therapy using ruxolitinib and calcitriol in breast cancer. The present study aimed to investigate the combined treatment effects of ruxolitinib and calcitriol in the ER-positive HER2-positive luminal B subtype of breast cancer.
Materials and methods
Materials
Ruxolitinib and calcitriol were purchased from Selleck Chemicals (Houston, TX, USA). The final concentrations were achieved by diluting the stock solutions with RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and all solutions were prepared immediately prior to use. The antibody raised against VDR (cat. no. SC-1008) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies against caspase-3 (cat. no. 9662S), apoptosis regulator Bcl-2 (Bcl-2; cat. no. 2876S), Bcl-2-like protein 1 (Bcl-xL; cat. no. 2762S), Bcl-2-associated agonist of cell death (BAD; cat. no. 9292S), cyclin-D1 (cat. no. 2922S), JAK2 (cat. no. 3230S), phosphorylated JAK2 (p-JAK2; cat. no. 3776S), c-Myc proto-oncogene protein (c-Myc; cat. no. 13987S) and β-actin (cat. no. 4967S) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cells and cell culture
ER and HER2-positive MCF7-HER18 human breast cancer cells were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (Corning Life Sciences B.V., Amsterdam, Netherlands), 100 units/ml penicillin and 100 µg/ml streptomycin. All cells were cultured in a humidified incubator containing 5% CO2 at 37°C.
Bromodeoxyuridine (BrdU) assay of cell proliferation
The quantification of cell proliferation was based on the measurement of BrdU incorporation during DNA synthesis. This assay was performed according to the manufacturer's protocol (Cell Proliferation ELISA BrdU, cat. no. 11647229001, Roche Diagnostics GmbH, Mannheim Germany). In brief, MCF7-HER18 cells (1×104/well) were seeded in triplicate into 96-well plates and allowed to grow overnight, prior to treatment with various concentrations of ruxolitinib alone (5, 10, 15, 20, 25 and 30 µM), calcitriol alone (3, 6, 9, 12, 15 and 18 µM), or a combination of ruxolitinib and calcitriol (ratio 5:3) for 72 h at room temperature. The cells were subsequently treated with BrdU labeling solution for 2 h. The culture medium was removed, the cells were fixed using a fixative solution (3.7% formaldehyde in PBS) for 30 min at room temperature and the DNA was denatured. Cells were incubated with the Anti-BrdU-POD solution for 90 min and antibody conjugates were removed in three washing cycles with 1× PBS. Following washing, the tetra-methyl-benzidine substrate was added and incubated in the dark for 15 min at room temperature. Absorbance was quantified within 30 min at dual wavelengths of 450 and 540 nm using microplate reader (VersaMax, Molecular Devices, LLC, Sunnyvale, CA, USA).
Isobologram analysis of the interaction between ruxolitinib and calcitriol
The synergistic effects of ruxolitinib and calcitriol were examined using the Chou-Talalay combination index (CI) method (29,30). The resultant CI values reflect the potential interactions between two drugs, where a CI <1 indicates a synergistic effect, CI=1 indicates an additive effect and a CI >1 indicates antagonism. The mean values of three independent experiments were used. The combination index analysis was performed using CompuSyn software version 2.0.1 (ComboSyn, Inc., Paramus, NJ, USA).
Cell cycle analysis
MCF7-HER18 cells were seeded in six-well plates (1×105 cells/well). Following incubation for 24 h, the cells were treated with the test reagents (20 µM ruxolitinib, 12 µM calcitriol, or a combination of 20 µM ruxolitinib and 12 µM calcitriol). Standard growth medium was used for the negative control. At 72 h, the cells were harvested, washed with PBS, and fixed in 70% ethanol at 4°C for 24 h. The cells were incubated with PBS containing 100 µg/ml RNase A and 100 µg/ml propidium iodide (PI) for 30 min at room temperature in the dark. Cell cycle analysis was performed using a Navios flow cytometer and Kaluza software version 1.3 (Beckman Coulter, Inc., Brea, CA, USA).
Apoptosis assay
The apoptotic status of MCF7-HER18 cells was evaluated by flow cytometry using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (SouthernBiotech, Birmingham, AL, USA) and PI staining, according to the manufacturer's protocol. A total of 1×106 cells/ml were incubated with the test reagents (20 µM ruxolitinib, 12 µM calcitriol, or a combination of 20 µM ruxolitinib and 12 µM calcitriol). Standard growth medium was used as the negative control. At 72 h, the cells were washed with PBS and suspended in binding buffer containing 0.01 M HEPES, 140 mM NaCl and 2.5 mM CaCl2 at a final concentration of 1×106 cells/ml. The cell suspension (100 µl containing 105 cells) was incubated with 5 µl Annexin-FITC and 5 µl PI at room temperature for 15 min in the dark. Following this incubation, 400 µl binding buffer was added and the cells were analyzed by flow cytometry using a Navios flow cytometer with Kaluza software version 1.3 (Beckman Coulter, Inc.).
Western blot analysis
MCF7-HER18 cells were seeded in six-well plates (1×106 cells/well). Following incubation for 24 h, the cells were treated with the test reagents (20 µM ruxolitinib, 12 µM calcitriol, or a combination of 20 µM ruxolitinib and 12 µM calcitriol). Standard growth medium was used for the negative control. At 72 h, the cells were lysed using ionic detergent protein extraction buffer (PRO-PREP™, iNtrON Biotechnology, Suwon, Korea) containing phosphatase (1/100 ml lysis buffer, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and protease inhibitors (1/100 ml lysis buffer, Sigma-Aldrich; Merck KGaA). The protein concentration was determined using the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA). A total of 20 µg of proteins were resolved by 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes. The membranes were blocked for 1 h at room temperature using 5% skim milk in TBS-Tween-20. Membranes were probed overnight at 4°C with the indicated 1:1,000 diluted primary antibodies (All primary antibodies obtained from Cell Signaling Technology, Inc.), prior to washing and incubating with the anti-rabbit immunoglobulin G horseradish peroxidase-linked antibody (1:1,000; cat. no. 7074, Cell Signaling Technology, Inc.) for 1 h at room temperature. The membranes were developed and the protein signals were detected using enhanced chemiluminescence western blotting detection reagents (GE Healthcare Life Sciences, Little Chalfont, UK). β-actin was used as a loading control in all western blotting analyses and the results were obtained using densitometry by MultiGauge software version 2.0 (FUJIFILM Corporation, Tokyo, Japan).
Statistical analysis
All data are expressed as the mean ± standard deviation of at least three separate experiments. The statistical comparisons between multiple groups were performed using one-way analysis of variance, followed by a Tukey's post hoc test. For all tests, P<0.05 was considered to indicate a statistically significant difference. The data were analyzed using GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
Results
VDR and JAK2 are expressed in MCF7-HER18 cell lines
Prior to evaluating the effects of ruxolitinib and calcitriol on MCF7-HER18 cells, the expression of VDR and JAK2 in this cell line was examined using western blotting. MCF7-HER18 cells expressed VDR and JAK2 proteins (Fig. 1).
Figure 1.
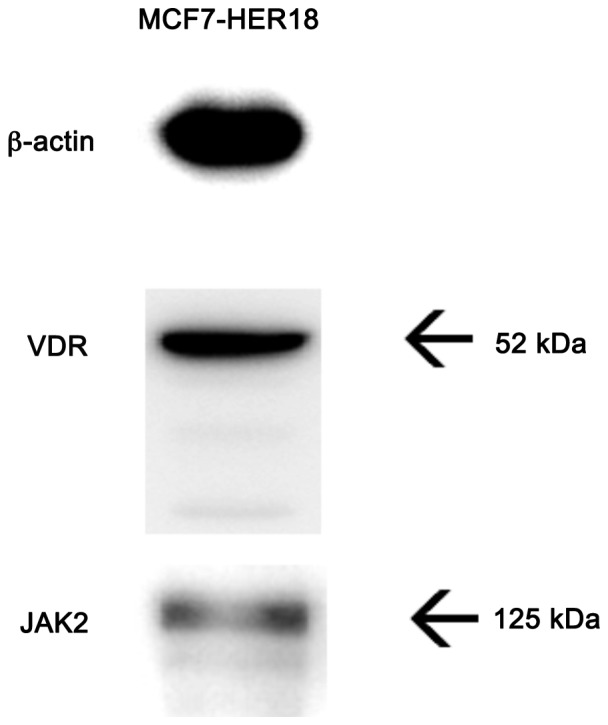
The expression of VDR and JAK2 in MCF7-HER18 breast cancer cells was demonstrated by western blot analysis. VDR, vitamin D receptor; JAK, Janus kinase; HER, human epidermal growth factor receptor.
Synergistic inhibitory effects of ruxolitinib and calcitriol on MCF7-HER18 cells
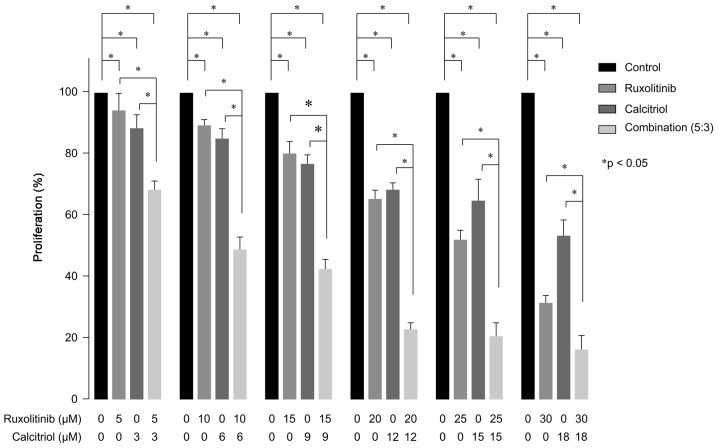
BrdU assays were performed to evaluate the inhibitory effects of ruxolitinib and calcitriol on MCF7-HER18 cell growth (Fig. 2). Compared with control cells, exposure to 5–30 µM ruxolitinib produced significant concentration-dependent cell growth inhibition in MCF-HER18 cells, with a 20% inhibitory concentration (IC20) of 14.77±1.30 µM (P=0.0359 for 5 µM; P<0.001 for 10, 15, 20, 25 and 30 µM). Additionally, MCF-HER18 cells exposed to 3–18 µM calcitriol demonstrated significant concentration-dependent growth inhibition, with an IC20 of 7.65±1.00 µM (P<0.001 in the presence of 3, 6, 9, 12, 15 or 18 µM). Investigation of the combined effects of a 5:3 ratio of ruxolitinib and calcitriol was conducted, based on these IC20 values. Combination treatment with ruxolitinib and calcitriol produced a synergistic growth inhibition effect on MCF7-HER18 at all concentrations tested and the combination index data are in Table I.
Figure 2.
Cell proliferation evaluation using the BrdU assay in the presence of the indicated concentrations of ruxolitinib and/or calcitriol in MCF7-HER18 cells for 72 h. The data are presented as the mean ± standard deviation. *P<0.05. BrdU, bromodeoxyuridine; HER, human epidermal growth factor receptor.
Table I.
Combination indices for ruxolitinib and calcitriol.
| Ruxolitinib (µM) | Calcitriol (µM) | Combination index (5:3) |
|---|---|---|
| 5 | 3 | 0.533 |
| 10 | 6 | 0.605 |
| 15 | 9 | 0.761 |
| 20 | 12 | 0.552 |
| 25 | 15 | 0.642 |
| 30 | 18 | 0.64 |
Ruxolitinib and calcitriol synergistically increases the number of cells in the sub-G1 phase in MCF7-HER18 cells
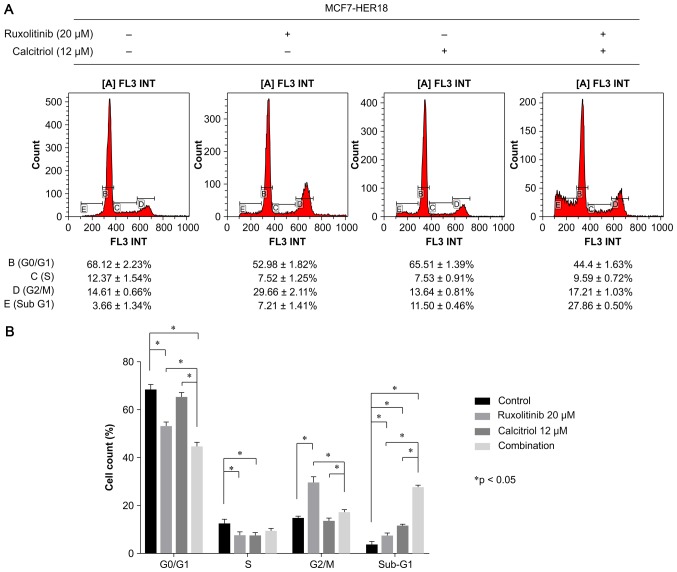
Cell cycle analysis was performed to evaluate the effects of treatment with ruxolitinib and/or calcitriol on MCF7-HER18 cell cycle distribution (Fig. 3). The results demonstrated that the proportion of cells in the sub-G1 phase was significantly increased in the presence of ruxolitinib alone (7.21±1.41%, P=0.0125) or calcitriol alone (11.5±0.46%, P<0.001), compared with control cells (3.66±1.34%). This effect was associated with a significant decrease in the percentage of cells in the G0/G1 phase in the presence of ruxolitinib alone (P<0.001), in the percentage of cells in the S phase in the presence of ruxolitinib (P=0.0038) or calcitriol (P=0.0038) alone, and a significant increase in the percentage of cells in G2/M phase in the presence of ruxolitinib alone (P<0.001). Furthermore, combination treatment with ruxolitinib and calcitriol led to a further accumulation of cells in the sub-G1 phase, compared with control cells (P<0.0001), with cells treated with ruxolitinib only (P<0.001) and with cells treated with calcitriol only (P<0.001).
Figure 3.
Cell cycle distribution of MCF7-HER18 cells treated as indicated for 72 h. (A) Cell cycle distribution was investigated using flow cytometry and (B) the data were quantified. The data are presented as the mean ± standard deviation. *P<0.05.; HER, human epidermal growth factor receptor.
Ruxolitinib and calcitriol synergistically induce apoptosis in MCF7-HER18 cells
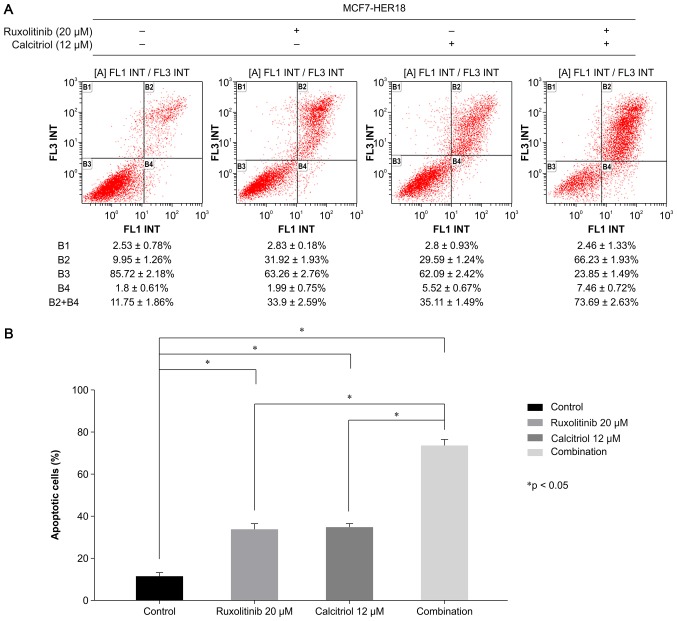
Flow cytometric analysis of Annexin V-FITC and PI double-stained cells was used to investigate the effects of ruxolitinib and calcitriol on apoptosis in MCF7-HER18 cells (Fig. 4). Compared with control cells (11.54±1.89%), those exposed to ruxolitinib or calcitriol demonstrated a significant induction of apoptosis (ruxolitinib, 33.90±1.89%, P<0.001; calcitriol, 35.11±1.49%, P<0.001). Furthermore, combination treatment with ruxolitinib and calcitriol induced significantly more apoptosis (73.69±2.63%, P<0.001) compared with what was observed in control or single agent-treated cells. These data suggested that ruxolitinib and calcitriol synergistically inhibited cell growth and promoted apoptosis in MCF7-HER18 cells.
Figure 4.
Flow cytometry analysis of Annexin-FITC/PI stained MCF7-HER18 cells was performed. Following exposure to the indicated treatments for 72 h. (A) B1 quadrant (Annexin−, PI+) represents necrotic cells, B2 quadrant (Annexin+, PI+) represents late apoptotic cells, B3 quadrant (Annexin−, PI−) represents viable cells, and B4 quadrant (Annexin+, PI−) represents early apoptotic cells. (B) The graph shows the proportions of early and late apoptotic cells following 72 h of treatment as indicated. The data are presented as the mean ± standard deviation. *P<0.05. FITC, fluorescein isothiocyanate; PI, propidium iodide; HER, human epidermal growth factor receptor.
Ruxolitinib and calcitriol inhibit cell growth by downregulating the expression of proteins within JAK2 and apoptosis-associated pathways
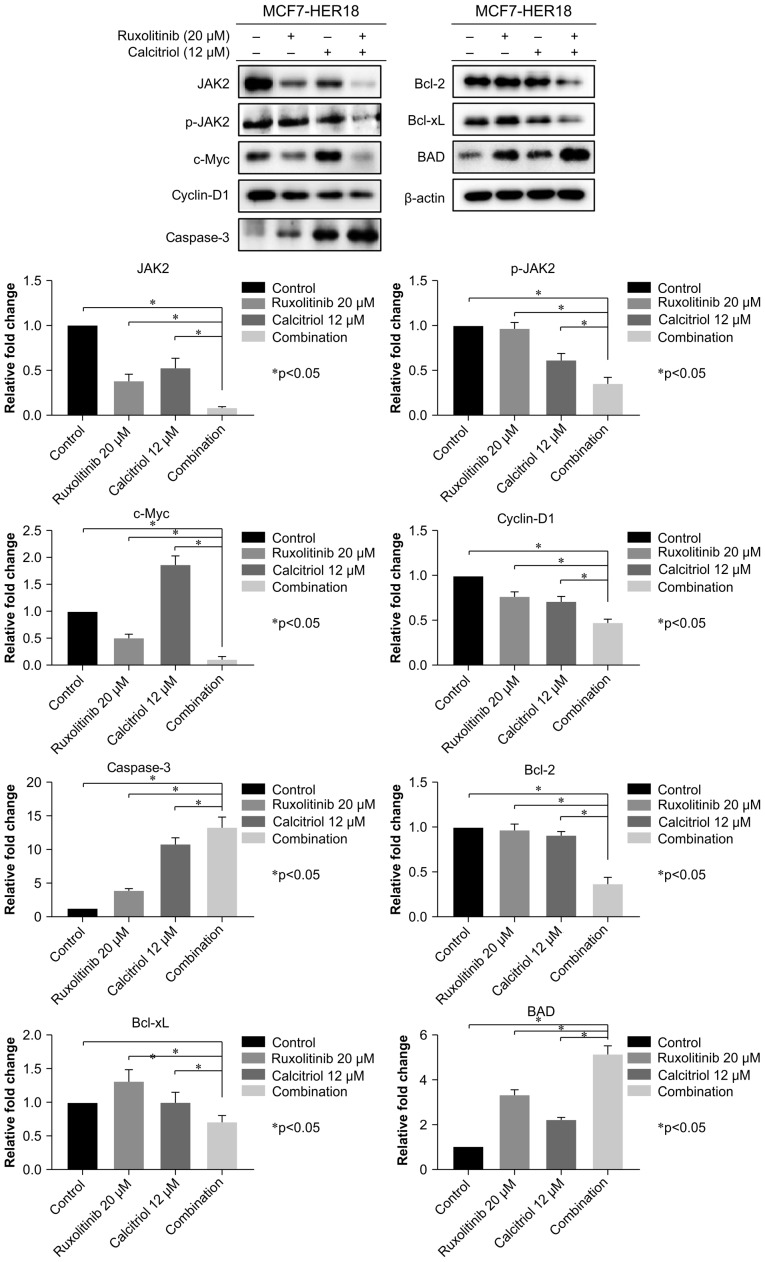
c-Myc and cyclin-D1 are known downstream targets of the JAK2 pathway, which is an important regulator of cell growth, proliferation and apoptosis (31,32). To investigate the molecular mechanism underlying the effects of combined ruxolitinib and calcitriol, western blotting was performed to evaluate the expression levels of proteins associated with downstream JAK2 and apoptosis-associated pathways in MCF7-HER18 cells; these included JAK2, p-JAK2, c-Myc, cyclin-D1, caspase-3, Bcl-2, Bcl-xL and BAD. These results demonstrated that combination treatment with ruxolitinib and calcitriol significantly decreased the expression of JAK2, p-JAK2, c-Myc, cyclin-D1, Bcl-2 and Bcl-xL, while increasing the expression of caspase-3 and BAD, compared with control cells or those exposed to ruxolitinib or calcitriol alone (Fig. 5).
Figure 5.
Levels of JAK2, p-JAK2, c-Myc, cyclin-D1, caspase 3, Bcl-2, Bcl-xL, BAD and β-actin proteins in MCF7-HER18 cells were determined by western blot analysis following the indicated 72 h treatments. The data are presented as the mean ± standard deviation. *P<0.05. JAK, Janus kinase; Bcl-2, apoptosis regulator Bcl-2; Bcl-xL, Bcl-2-like protein 1; BAD, Bcl-2-associated agonist of cell death; Myc, Myc proto oncogene protein HER, human epidermal growth factor receptor.
Discussion
A number of malignant diseases, including breast cancer, may be associated with two or more oncogenic signal transduction pathways, rather than with one specific oncogenic pathway. Therefore, recent studies have focused on the development of combination therapies using anticancer agents in order to inhibit multiple oncogenic pathways more effectively, compared with conventional single-agent treatment. In breast cancer, previous preclinical studies have reported the anticancer effects of ruxolitinib and calcitriol, used individually. The present study investigated whether these compounds produced synergistic effects in a breast cancer cell line.
The results of the present study demonstrated significant concentration-dependent antiproliferative effects of ruxolitinib or calcitriol in the ER and HER2-positive MCF7-HER18 breast cancer cell line. Notably, whereas the effective concentration of ruxolitinib that produced a significant antiproliferative effect was relatively consistent with previously published studies, the effective concentration of calcitriol was relatively high in the present study, compared with previous studies. In a study by Tavallai et al (17), the effective treatment dose of ruxolitinib in triple negative breast cancer cells (SUM149) and luminal B breast cancer cells (BT474) was in the range 0.5–2.5 µM, which was similar to the 5–30 µM identified in the present study. However, the study of Yuan et al (33) reported an effective calcitriol treatment dose of 1–100 nM in a luminal A breast cancer cell line (MCF7); this was decreased compared with the 3–18 µM identified in the present study. This difference may reflect the different ER and HER2 status of the breast cancer cell lines employed in these studies. Segovia-Mendoza et al (34) reported that VDR expression, which is an important determinant of responsiveness to calcitriol, was relatively lower in HER2-positive breast cancer cells compared with HER2-negative breast cancer cells. Compared with the study of Yuan et al (33), which used a HER2-negative breast cancer cell line (MCF7), the higher effective treatment concentration of calcitriol in the present study was possibly associated with the HER2-positive status of the MCF7-HER18 breast cancer cell line.
In the present study, the synergistic inhibition of breast cancer cell growth was primarily due to increased apoptosis. The JAK pathway transmits information from extracellular chemical signals to the nucleus, resulting in DNA transcription and the expression of genes involved in proliferation, differentiation and apoptosis (13). Previous reports have indicated that, when combined with other anticancer drugs, ruxolitinib and calcitriol produce synergistic antiproliferative and anticancer effects via the JAK2-associated downstream pathway. Tavallai et al (17) reported that combination treatment with ruxolitinib and mono-methyl fumarate decreased the expression of the anti-apoptotic signaling protein Bcl-xL, and increased that of the pro-apoptotic signaling protein BAD. Furthermore, Ju et al (35) reported that a combination of ruxolitinib and navitoclax produced synergistic anti-proliferative and pro-apoptotic effects that were associated with decreased expression of c-Myc and Bcl-xL, and increased expression of apoptosis regulator BAX. Additionally, Segovia-Mendoza et al (36) reported that a combination of calcitriol and lapatinib produced a synergistic increase in apoptosis in triple negative breast cancer cells via increased activity of caspase-3. The results of the present study demonstrated synergistic antiproliferative and pro-apoptotic effects of ruxolitinib and calcitriol in MCF7-HER18 breast cancer cells via decreased expression of c-Myc, cyclin-D1, Bcl-2 and Bcl-xL, and increased expression of caspase-3 and BAD.
No studies, to the best of the authors' knowledge, have reported definite anticancer effects of ruxolitinib or calcitriol in patients with breast cancer in a clinical setting. To date, a number of clinical trials (https://clinicaltrials.gov/ct2/results?cond=BREAST+CANCER&term=RUXOLITINIB&cntry=&state=&city=&dist) have investigated the clinical benefits of ruxolitinib in metastatic or locally advanced breast cancer, as a single agent and in combination with other well-established anticancer agents, including capecitabine or trastuzumab. Unfortunately, none of the studies have successfully reported clinical benefits of ruxolitinib or calcitriol in breast cancer; two studies were terminated as there was no therapeutic effect of ruxolitinib (NCT01562873) (https://clinicaltrials.gov/ct2/show/NCT01562873?term=RUXOLITINIB&cond=BREAST+CANCER&rank=2); (NCT02120417) (https://clinicaltrials.gov/ct2/show/NCT02120417?term=RUXOLITINIB&cond=BREAST+CANCER&rank=3), and other studies are in progress. Based on the results of the present study, it is suggested that a future clinical trial be conducted to explore the possible therapeutic potential of combination treatment with ruxolitinib and calcitriol in breast cancer.
In conclusion, the present study demonstrated the synergistic anticancer effects of ruxolitinib and calcitriol in ER and HER2-positive MCF7-HER18 breast cancer cells. This indicated that combination therapy with ruxolitinib and calcitriol may provide a novel and effective treatment option for patients with ER and HER2-positive breast cancer. Further animal studies are required prior to confirmation of this result in a clinical setting.
Acknowledgements
The present study was supported by the Medical Research Center, Catholic University of Korea, St. Vincent's Hospital (Suwon, Korea).
Glossary
Abbreviations
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- BrdU
bromodeoxyuridine
- FITC
fluorescein isothiocyanate
- PI
propidium iodide
- JAK
Janus kinase
- VDR
vitamin D receptor
- CI
combination index
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets; Proc Natl Acad Sci USA; 2003; pp. 8418–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel members Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russnes HG, Lingjærde OC, Børresen-Dale AL, Caldas C. Breast cancer molecular stratification: From intrinsic subtypes to integrative clusters. Am J Pathol. 2017;187:2152–2162. doi: 10.1016/j.ajpath.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 6.Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011;13:221. doi: 10.1186/bcr2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo C, Ahn JH, Jung KH, Kim SB, Kim HH, Shin HJ, Ahn SH, Son BH, Gong G. Impact of immunohistochemistry-based molecular subtype on chemosensitivity and survival in patients with breast cancer following neoadjuvant chemotherapy. J Breast Cancer. 2012;15:203–210. doi: 10.4048/jbc.2012.15.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liedtke C, Packeisen J, Hess KR, Vogt U, Kiesel L, Kersting C, Korsching E, Brandt B, Buerger H. Systematic analysis of in vitro chemosensitivity and mib-1 expression in molecular breast cancer subtypes. Eur J Cancer. 2012;48:2066–2074. doi: 10.1016/j.ejca.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Valabrega G, Montemurro F, Sarotto I, Petrelli A, Rubini P, Tacchetti C, Aglietta M, Comoglio PM, Giordano S. TGFalpha expression impairs trastuzumab-induced HER2 downregulation. Oncogene. 2005;24:3002–3010. doi: 10.1038/sj.onc.1208478. [DOI] [PubMed] [Google Scholar]
- 10.Wilks ST. Potential of overcoming resistance to HER2-targeted therapies through the PI3K/Akt/mTOR pathway. Breast. 2015;24:548–555. doi: 10.1016/j.breast.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Sai K, Wang S, Balasubramaniyan V, Conrad C, Lang FF, Aldape K, Szymanski S, Fokt I, Dasgupta A, Madden T, et al. Induction of cell-cycle arrest and apoptosis in glioblastoma stem-like cells by WP1193, a novel small molecule inhibitor of the JAK2/STAT3 pathway. J Neurooncol. 2012;107:487–501. doi: 10.1007/s11060-011-0786-z. [DOI] [PubMed] [Google Scholar]
- 12.Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signaling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer. 2015;113:365–371. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna P, Chua PJ, Bay BH, Baeg GH. The JAK/STAT signaling cascade in gastric carcinoma (Review) Int J Oncol. 2015;47:1617–1626. doi: 10.3892/ijo.2015.3160. [DOI] [PubMed] [Google Scholar]
- 14.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, Harrison CN, Pane F, Zachee P, Mesa R, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372:426–435. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavallai M, Booth L, Roberts JL, McGuire WP, Poklepovic A, Dent P. Ruxolitinib synergizes with DMF to kill via BIM+BAD-induced mitochondrial dysfunction and via reduced SOD2/TRX expression and ROS. Oncotarget. 2016;7:17290–17300. doi: 10.18632/oncotarget.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavallai M, Booth L, Roberts JL, Poklepovic A, Dent P. Rationally repurposing ruxolitinib (Jakafi (®)) as a solid tumor therapeutic. Front Oncol. 2016;6:142. doi: 10.3389/fonc.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Seo NJ, Jeong SJ, Park Y, Jung DB, Koh W, Lee HJ, Lee EO, Ahn KS, Ahn KS, et al. Oral administration of penta-O-galloyl-β-D-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 inhibition. Carcinogenesis. 2011;32:804–811. doi: 10.1093/carcin/bgr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange TS, Singh RK, Kim KK, Zou Y, Kalkunte SS, Sholler GL, Swamy N, Brard L. Anti-proliferative and pro-apoptotic properties of 3-bromoacetoxy calcidiol in high-risk neuroblastoma. Chem Biol Drug Des. 2007;70:302–310. doi: 10.1111/j.1747-0285.2007.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashid SF, Moore JS, Walker E, Driver PM, Engel J, Edwards CE, Brown G, Uskokovic MR, Campbell MJ. Synergistic growth inhibition of prostate cancer cells by 1 alpha,25 dihydroxyvitamin D(3) and its 19-nor-hexafluoride analogs in combination with either sodium butyrate or trichostatin A. Oncogene. 2001;20:1860–1872. doi: 10.1038/sj.onc.1204269. [DOI] [PubMed] [Google Scholar]
- 21.Yao S, Kwan ML, Ergas IJ, Roh JM, Cheng TD, Hong CC, McCann SE, Tang L, Davis W, Liu S, et al. Association of serum level of vitamin D at diagnosis with breast cancer survival: A case-cohort analysis in the pathways study. JAMA Oncol. 2017;3:351–357. doi: 10.1001/jamaoncol.2016.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose AA, Elser C, Ennis M, Goodwin PJ. Blood levels of vitamin D and early stage breast cancer prognosis: A systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141:331–339. doi: 10.1007/s10549-013-2713-9. [DOI] [PubMed] [Google Scholar]
- 23.Wu G, Fan RS, Li W, Ko TC, Brattain MG. Modulation of cell cycle control by vitamin D3 and its analogue, EB1089, in human breast cancer cells. Oncogene. 1997;15:1555–1563. doi: 10.1038/sj.onc.1201329. [DOI] [PubMed] [Google Scholar]
- 24.Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J. Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol. 2001;15:1370–1380. doi: 10.1210/mend.15.8.0673. [DOI] [PubMed] [Google Scholar]
- 25.Verlinden L, Verstuyf A, Convents R, Marcelis S, Van Camp M, Bouillon R. Action of 1,25(OH)2D3 on the cell cycle genes, cyclin D1, p21 and p27 in MCF-7 cells. Mol Cell Endocrinol. 1998;142:57–65. doi: 10.1016/S0303-7207(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 26.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367–376. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Narvaez CJ, Welsh J. Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem. 2001;276:9101–9107. doi: 10.1074/jbc.M006876200. [DOI] [PubMed] [Google Scholar]
- 28.Weitsman GE, Ravid A, Liberman UA, Koren R. Vitamin D enhances caspase-dependent and independent TNF-induced breast cancer cell death: The role of reactive oxygen species. Ann N Y Acad Sci. 2003;1010:437–440. doi: 10.1196/annals.1299.079. [DOI] [PubMed] [Google Scholar]
- 29.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 30.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 31.Acosta JJ, Muñoz RM, González L, Subtil-Rodríguez A, Dominguez-Caceres MA, García-Martínez JM, Calcabrini A, Lazaro-Trueba I, Martín-Pérez J. Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol Endocrinol. 2003;17:2268–2282. doi: 10.1210/me.2002-0422. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Chang YC, Liu CL, Chang KJ, Guo IC. Leptin-induced growth of human ZR-75-1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast Cancer Res Treat. 2006;98:121–132. doi: 10.1007/s10549-005-9139-y. [DOI] [PubMed] [Google Scholar]
- 33.Yuan L, Jiang R, Yang Y, Ding S, Deng H. 1,25-Dihydroxyvitamin D3 inhibits growth of the breast cancer cell line MCF-7 and downregulates cytochrome P4501B1 through the COX-2/PGE2 pathway. Oncol Rep. 2012;28:2131–2137. doi: 10.3892/or.2012.2031. [DOI] [PubMed] [Google Scholar]
- 34.Segovia-Mendoza M, Díaz L, González-González ME, Martínez-Reza I, García-Quiroz J, Prado-Garcia H, Ibarra-Sánchez MJ, Esparza-López J, Larrea F, García-Becerra R. Calcitriol and its analogues enhance the antiproliferative activity of gefitinib in breast cancer cells. J Steroid Biochem Mol Biol. 2015;148:122–131. doi: 10.1016/j.jsbmb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Ju W, Zhang M, Wilson KM, Petrus MN, Bamford RN, Zhang X, Guha R, Ferrer M, Thomas CJ, Waldmann TA. Augmented efficacy of brentuximab vedotin combined with ruxolitinib and/or Navitoclax in a murine model of human Hodgkin's lymphoma; Proc Natl Acad Sci USA; 2016; pp. 1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segovia-Mendoza M, Díaz L, Prado-Garcia H, Reginato MJ, Larrea F, García-Becerra R. The addition of calcitriol or its synthetic analog EB1089 to lapatinib and neratinib treatment inhibits cell growth and promotes apoptosis in breast cancer cells. Am J Cancer Res. 2017;7:1486–1500. [PMC free article] [PubMed] [Google Scholar]