Abstract
PURPOSE
To examine whether herpes zoster antigen (also called varicellazoster virus antigen) was detectable in temporal artery biopsies taken from individuals with giant cell arteritis (GCA).
DESIGN
Retrospective comparative case series.
METHODS
Sections of formalin-fixed paraffin-embedded temporal arteries were examined first by hematoxylin-eosin (H&E) staining to establish the diagnosis of GCA. Adjacent sections of the same biopsy were then examined by immunohistochemistry, using 2 different monoclonal antibodies against a major antigen of varicella-zoster virus called gE. Pathologic specimens were obtained from patients cared for at the University of Iowa and Washington University in St. Louis ophthalmology clinics.
RESULTS
The study included biopsies from 25 patients with symptoms of GCA as well as positive H&E pathology and 25 patients with symptoms compatible with GCA but negative H&E pathology. Among the GCA-positive group, 3 patients had positive staining for herpes zoster antigen. Among the GCA-negative group, herpes zoster antigen was not detected in any biopsy. In both groups of patients, false-positive staining for herpes zoster antigen was detected in the presence of calcifications in the arteries. False-positive staining was also detected on some extra-arterial skeletal muscle and erythrocytes.
CONCLUSION
Herpes zoster antigen was detected in 3 of 25 temporal arteries from patients with biopsy-proven GCA. One of the 3 positive cases was noteworthy because the patient had had herpes zoster ophthalmicus diagnosed 3 weeks before the onset of GCA symptoms. False-positive staining for herpes zoster antigen was detected on several temporal artery biopsies.
Giant cell arteritis (GCA) is an inflammatory process usually involving the temporal artery.1–3 The most feared complication is blindness secondary to inflammation in the nearby posterior ciliary arteries with subsequent ischemic optic neuropathy.4 GCA predominantly affects people over the age of 55 years, women more than men. In one epidemiologic analysis carried out in the United Kingdom, the prevalence of GCA was estimated to be 250 per 100 000 individuals 55 years and over.5 The etiology of GCA remains obscure, although the disease is often linked with polymyalgia rheumatic.6,7 There appears to be a genetic component because GCA is reported to be more common in individuals of Northern European than Southern European ancestry, although this point has been questioned.8
The pathogenesis of GCA is considered to be a vasculitis of the superficial temporal arteries secondary to an inflammatory process against an as-yet-unknown antigen or set of antigens.9 Other arteries of the head and neck may also be involved. The disease was once called Horton’s disease.10 The temporal arteries are innervated by branches of autonomic ganglia and the trigeminal ganglia.11 All of these ganglia can contain latent varicella-zoster virus (VZV).12 Because the trigeminal ganglion is the single most common site of clinical herpes zoster (shingles), causing herpes zoster ophthalmicus, the question has arisen whether GCA is a vasculitis driven by deposition of herpes zoster antigen in the temporal artery.13 A recent analysis found herpes zoster antigen in 74% of temporal artery biopsies obtained from patients with previously diagnosed GCA.14 Because of that extremely high value, we sought to determine whether temporal artery biopsies for GCA from our 2 institutions had a similarly high rate of positivity for herpes zoster antigen.
METHODS
STUDY DESIGN
We performed a retrospective search of the ophthalmic pathology databases at the University of Iowa and Washington University in St. Louis for temporal artery biopsies between January 2014 and April 2017. The study adhered to the Declaration of Helsinki and was compliant with the Health Insurance Portability and Accountability Act. The study protocol was approved by the institutional review boards of both universities. The methods for processing each temporal artery (TA) biopsy are illustrated in Figure 1; the protocol is also described in more detail.15 First, numerous hematoxylin-eosin (H&E)-stained slides of each temporal artery sample were examined by an ophthalmic pathologist for histologic evidence of GCA. For this study, a total of 25 patients with GCA histopathology on their TA biopsy were included. Sections immediately adjacent to sections with convincing evidence of GCA were examined for the presence of zoster antigen by immunohistochemistry (IHC). Altogether, at least 10 sections for every centimeter of the TA biopsy were examined, including skip areas.16 As controls, we included slides from TA biopsies from 25 patients who had symptoms compatible with GCA but no evidence of GCA histopathology when their slides were examined. Again, at least 10 sections were examined by the VZV IHC test for every centimeter of the TA biopsy. Results were then reviewed by 2 virologists at the University of Iowa and subsequently by 2 ophthalmologists at either location.
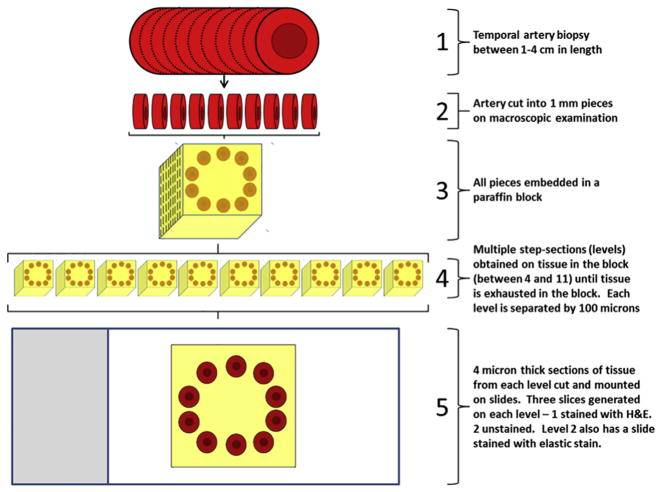
FIGURE 1.
Experimental methods. Temporal biopsy samples from 1 to 4 cm in length (1) were cut into 1-mm pieces on macroscopic examination (2). All sections were embedded in a paraffin block (3) and multiple step-sections were cut from the block until all tissue was exhausted (4). Then, 4-μm-thick sections from each level were cut and mounted on slides (5). Three slides were generated on each level. One of these slides was stained with hematoxylin-eosin (H&E) and 2 slides were retained as unstained reserves. The reserve slides were subsequently used in the IHC assays.
IMMUNOHISTOCHEMISTRY ASSAYS FOR HERPES ZOSTER ANTIGEN
For the virology studies, each slide containing multiple paraffin-embedded artery sections was deparaffinized by washing 3 times for 5 minutes in xylenes and 3 times for 5 minutes in ethanol and rinsing 1 time in water. Epitope retrieval was then performed in a microwave oven by heating slides to 95 C for 5 minutes, allowing slides to cool for 5 minutes, reheating to 95 C for 5 minutes, and allowing them to cool. The slide was washed 2 times for 5 minutes in phosphate-buffered saline (PBS)/0.025% Triton X-100, followed by blocking for 2 hours at room temperature in PBS with 10% normal goat serum and 1% bovine serum albumin. Each primary antibody was incubated on the slide at 4 C for 16 hours. The slide was washed 3 times for 5 minutes in PBS with 0.025% Triton X-100 and quenched by adding 3% H2O2 for 15 minutes at room temperature, followed by washing 3 times for 5 minutes in PBS/Triton X-100. Biotinylated goat anti-mouse secondary antibody (Jackson Laboratory #115-066-012, Bar Harbor, Maine, USA) was diluted 1:1000 in PBS/1% bovine serum albumin (BSA) and added to the slide for 1 hour at room temperature, followed by washing 3 times for 5 minutes with PBS/Triton X-100. Streptavidin conjugated to horseradish peroxidase (Jackson Laboratory #016-030-084) was diluted 1:250 in PBS/1% BSA and added to the slide for 30 minutes at room temperature. The slide was washed in PBS/Triton X-100 3 times for 5 minutes and diaminobenzidene peroxidase substrate (Vector Laboratory #SK-4100, Burlingame, California, USA) was added for 2–10 minutes. Each slide was then rinsed under running water for 10 minutes. Tissue sections on the slides were viewed initially with a Nikon Eclipse Ti-S microscope (Melville, New York, USA), under a wet mount. This protocol, using a droplet of water to improve the refractive index, gives the images a bluish tint. Other images were obtained with an Olympus BX61 microscope (Center Valley, Pennsylvania, USA) without addition of water for refraction (dry mount); these images lack the bluish tint.
PRIMARY ANTIBODY REAGENTS
For a primary antibody, 3 reagents were used: Anti-VZV gE monoclonal antibody (MAb) 3B3 diluted 1:1000 in PBS with 1% BSA (murine IgG2a MAb produced in the C. Grose laboratory); anti-VZV gE MAb (murine IgG1 MAb from Santa Cruz #sc-56995, Dallas, Texas, USA) diluted 1:500 in PBS with 1% BSA, and anti-reovirus MAb (murine IgG2a MAb from Developmental Studies Hybridoma Bank #10G10, Iowa City, Iowa, USA) diluted to 2.5 μg/mL in PBS with 1% BSA. The Santa Cruz anti-gE MAb reagent is the same reagent as used in prior IHC studies of temporal artery biopsies; its specifications have been extensively described previously.14 Murine MAb 3B3 against VZV gE protein has been used extensively to detect VZV antigens in human tissues, both by immunofluorescence and IHC. This MAb has previously detected VZV antigens in human skin, lung, and brain tissues.17,18 These tissues included VZV-infected human skin xenografts in the severe combined immunodeficient mouse model of VZV infection.19 The linear epitope of this MAb has been defined and the affinity of binding of the MAb to its epitope has been calculated.20,21 Numerous assays have documented an absence of nonspecific binding of MAb 3B3 to uninfected cells. The anti-reovirus antibody was an isotype-matched control for MAb 3B3; an anti-reovirus antibody was selected because there is no known persistent human infection with this virus.22
STATISTICAL ANALYSIS
Prior studies reported that >70% of TA biopsies from patients with GCA exhibited a positive test for herpes zoster antigen.14 Based on a defined formula for power analysis, we calculated that a sample size of 12 TA biopsies from 12 different patients with GCA would be the minimum sample size to confirm that high percentage.23 Our test group included 25 patients.
RESULTS
ANALYSIS OF TEMPORAL ARTERY BIOPSIES FROM PATIENTS WITH GIANT CELL ARTERITIS DIAGNOSIS
Records of the ocular pathology laboratories at the University of Iowa and Washington University in St. Louis were screened for TA biopsies read as positive for GCA. We selected slides from 17 patients seen at the University of Iowa and 8 patients seen at Washington University, all of whom were diagnosed as GCA based on both clinical signs and H&E pathologic diagnosis of a TA biopsy (Supplemental Table 1; Supplemental Material at AJO. com). For the zoster-specific IHC test, each slide contained a section of tissue immediately adjacent to the section of tissue on an H&E slide with histopathology compatible with GCA; the same slide also contained 10–40 additional sections, depending on the length of the TA specimen removed from the patient. Of the 25 sets of slides of TA biopsies, slides from 3 patients were positive by herpes zoster–specific IHC assay with either primary anti-VZV MAb (Figure 2). Images are presented after viewing by both the Nikon microscope (wet mount) and the Olympus microscope (dry mount). The locations of the positive immunolabeling for zoster antigen in the 3 arteries are described in Figure 2. We observed no differences in staining patterns between the 2 MAb reagents directed against the VZV gE antigen.
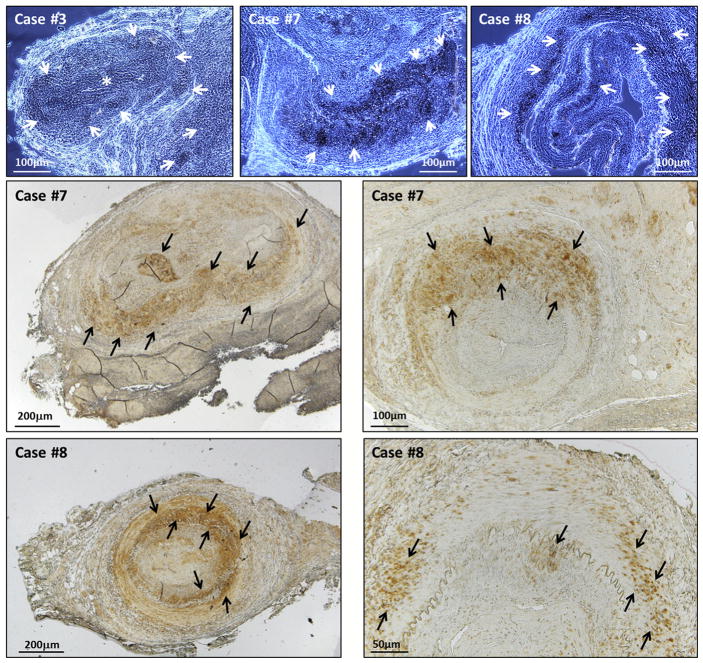
FIGURE 2.
Varicella-zoster virus (VZV) positivity in temporal artery biopsies. Examples of immunohistochemistry-positive results from 3 giant cell arteritis (GCA)-positive patients. The top row includes samples examined by wet mount; the center and bottom rows contain samples examined by dry mount at 2 magnifications. Zoster antigen was detected with monoclonal antibody 3B3 against major zoster protein E (gE) and appears as darker stained areas indicated by white arrows in the top row (wet mounts) and by black arrows in the center and bottom rows (dry mounts). Case #3 shows VZV-positive staining located in a branch artery off the main vessel (asterisk). Case #7 (center row) shows VZV-positive staining located mainly in the medial layer. Case #8 shows VZV-positive staining located in the intimal, medial and adventitial layers. Scale bars are shown in each image.
ANALYSIS OF TEMPORAL ARTERY BIOPSIES FROM PATIENTS WITH NO EVIDENCE OF HERPES ZOSTER ANTIGEN
We screened slides of TA biopsies from 25 patients who underwent a biopsy because of symptoms and signs of GCA but were found to lack evidence of GCA by H&E histopathology. All these patients were seen in Iowa (Supplemental Table 1). Each of these temporal arteries also had been sectioned at multiple sites, as illustrated in Figure 1. When slides containing these multiple TA sections were subjected to zoster-specific IHC testing by either of the 2 anti-VZV MAbs, none was positive. Representative examples of VZV-negative IHC tests from the GCA-positive (22 cases) and the GCA-negative (25 cases) groups are shown in Figure 3. The resolution of the wet mount when viewed in real time under the microscope was excellent; the same clarity was not always captured by the photograph of the wet mount. Both anti-VZV antibodies gave the same negative results in these IHC assays. A representative H&E example of inflammation characteristic of GCA (in a VZV-negative biopsy) included lymphocytes and histiocytes in the intimal, medial, and adventitial layers, as well as a disrupted internal elastic lamina. We also screened 1 biopsy of a vein adjacent to the temporal artery as an additional specificity control test; the zoster-specific IHC test was negative (not shown). We did find, however, that other slides in this group had a false-positive herpes zoster IHC test because of calcifications (see sections below).
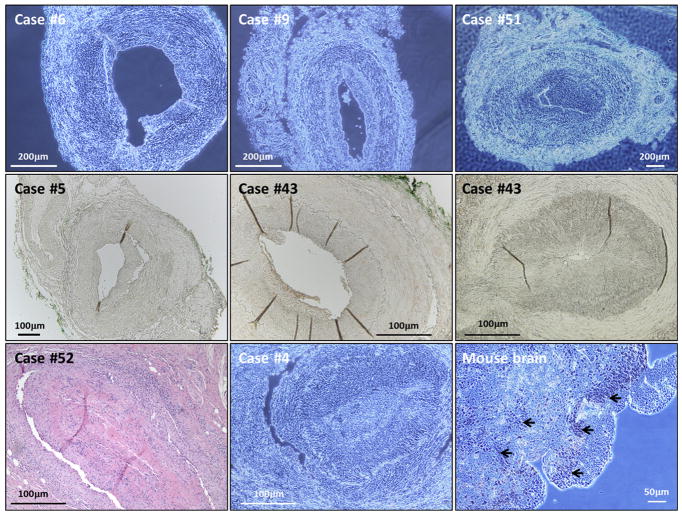
FIGURE 3.
Absence of varicella-zoster virus (VZV)-specific immunohistochemistry staining in temporal artery biopsies. Representative examples of VZV-negative immunohistochemistry results from the temporal arteries of 6 patients (Top row, wet mounts; Center row, dry mounts). No zoster antigen was detected after probing with anti-gE antibody in the 6 examples. Case #43 is shown in duplicate because the 2 panels show negative results with both anti-VZV antibodies. In the bottom row, the left panel is a representative hematoxylin-eosin stain to demonstrate abundant inflammation typical of giant cell arteritis (GCA). Control experiments with anti-reovirus antibody are included in the middle and right panels of bottom row. Anti-reovirus antibody was incubated with a GCA-positive TA sample (Case #4) as a negative control (Middle panel; wet mount). Anti-reovirus antibody was incubated on a sample of reovirus-infected mouse brain as a positive control for the antibody; black arrows designate sites of reovirus infection with positive staining (right, wet mount). Scale bars are shown in each image.
ISOTYPE-MATCHED CONTROL ANTIVIRAL MONOCLONAL ANTIBODY
We performed several control IHC experiments. In this control for primary antibody specificity, we included an IHC assay in which we substituted an isotype-matched anti-reovirus MAb (mouse IgG2a) for our anti-VZV antibody (mouse IgG2a). Because reovirus is not a human pathogen, reovirus antigen is never found in human artery tissues. We first tested the antibody on formalin-fixed paraffin-embedded samples of mouse brain infected with reovirus, in order to determine the dilution of antibody that would easily detect reovirus antigen under our IHC assay conditions (Figure 3, Bottom row). We found concentrations between 1.0 and 2.5 μg/mL of antibody-immunolabeled reovirus antigens in mouse brain. Subsequently, we tested temporal artery biopsies from 10 patients who had clinical features of GCA but negative histopathology. All 10 sets of slides were negative for both zoster antigen and reovirus antigen (Figure 3, Bottom row).
FALSE-POSITIVE HERPES ZOSTER IMMUNOLABELING WHEN CALCIFICATIONS ARE PRESENT IN ARTERY
While examining slides from both the GCA-positive group and the GCA-negative group listed above, we observed that our zoster-specific IHC assay appeared to give a false-positive result in the presence of temporal artery calcifications. In an additional set of control experiments, we confirmed this observation by systematically comparing sections in adjacent slides of a temporal artery known to have calcifications by its characteristic appearance after H&E staining with the locations of apparent positivity after the zoster-specific IHC test (Figure 4, Top panels). The pattern of zoster-specific staining overlapped with the areas that were calcified, usually along the internal elastic lamina. In some arteries with abundant calcifications, the tissue had a ripped appearance because it was difficult to cut. The nonspecific attachment of the MAb probe to calcified tissue was seen after immunolabeling with either anti-VZV MAb 3B3 or the MAb from Santa Cruz. In order to confirm that these sites of attachment did not also contain zoster antigen, we performed IHC testing without the primary anti-VZV antibody. Again, there was false-positive staining (Figure 4, Bottom left). Finally, we repeated the IHC testing with the anti-reovirus antibody on TA specimens known to contain calcium deposits; again, the result was a false-positive (data not shown). Thus, these control experiments indicated that false-positive staining of calcified tissue was owing to attachment of either primary or secondary antibody to the calcifications themselves. The H&E specimens from 3 patients in Figure 2 were reexamined to assure that calcifications did not account for the VZV-positive IHC results. Altogether, we observed calcifications in 25% of the temporal artery biopsies investigated in this study. Finally, in a few specimens we observed false-positive staining as an edge effect in the adventitia (Figure 4, Bottom right).
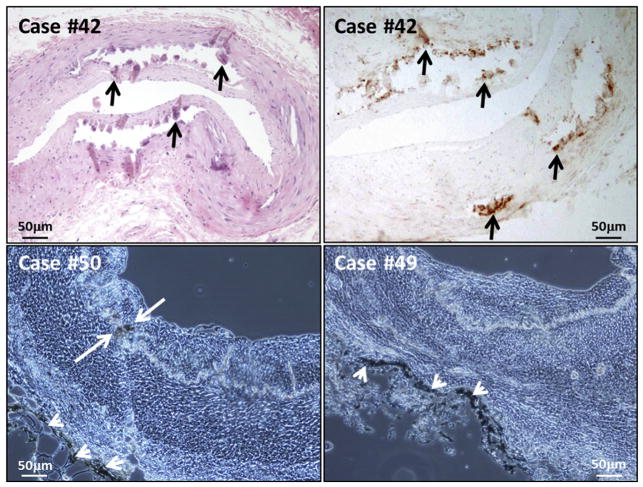
FIGURE 4.
False-positive immunohistochemistry: Calcifications and edge effect. The samples were selected from Case #42 (Top row), Case #50 (Bottom left), and Case #49 (Bottom right). Top panels were photographed as dry mounts and bottom panels are wet mounts. (Top left) Hematoxylin-eosin stain of a temporal artery, with calcifications demarcated by black arrows; (Top right) immunohistochemistry (IHC) stain with MAb 3B3, to illustrate attachment of MAb, indicated by black arrows, to similar calcified regions of the artery shown at top left. Areas of attachment of MAb to calcifications in the wet mounts are demarcated by white arrows in bottom panels. (Bottom left) False-positive attachment to calcification when primary antibody was omitted but secondary antibody and color reagents were retained in the IHC assay. (Bottom right) False-positive attachment of secondary antibody and color reagents (without primary antibody) along the edge of a temporal artery section in IHC assay, near the adventitia, often called “edge effect” (also seen in bottom left panel, at white arrowheads in both panels). Scale bars are shown in each image.
FALSE-POSITIVE HERPES ZOSTER IMMUNOLABELING OF SKELETAL MUSCLE AND BLOOD
We also observed that our zoster-specific IHC assay gave a false-positive result on skeletal tissue that surrounded a temporal artery that lacked any evidence of zoster-specific immunolabeling within an artery itself (Figure 5). Because skeletal muscle surrounds the adventitia, the immunolabeling can appear to extend into the adventitia. We found this same positive result when we used either anti-gE MAb (Figure 5, Top right). Of importance, we also found a false-positive result on skeletal muscle when we omitted both the primary and secondary antibodies and added only the color reagents in the IHC assay (Figure 5, Bottom left). VZV replicates poorly in many cell lines and is not known to be able to replicate in mature muscle tissue.24 Therefore, we concluded that reactivity with skeletal muscle was not a specific indicator of prior zoster infection.
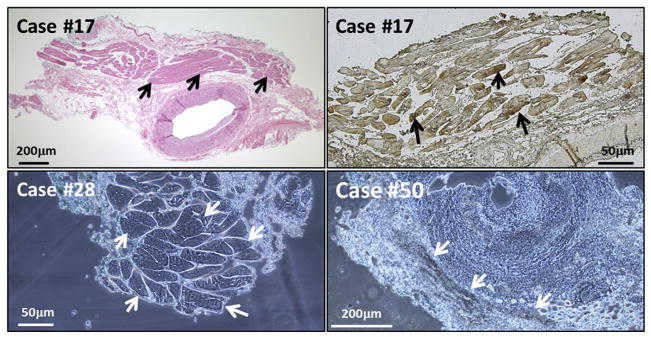
FIGURE 5.
False-positive immunohistochemistry (IHC): extra-arterial skeletal muscle and blood. Two images are included from Case #17, who was giant cell arteritis negative. (Top left) Hematoxylin-eosin stain of a temporal artery (TA), to show region with extra-arterial skeletal muscle (arrows). (Top right) False-positive IHC result when incubated with MAb 3B3 and observed with the Olympus microscope with dry mount (black arrows). There was no varicella-zoster virus–positive staining within any region of the temporal artery itself. (Bottom left) False-positive stain of skeletal muscle from Case #28. The TA specimen was incubated with only the color reagents in the IHC assay; neither primary nor secondary antibody was added. The false-positive result in the skeletal tissue is marked by white arrows. (Bottom right) False-positive attachment of anti-reovirus antibody to erythrocytes within the TA biopsy samples (white arrows). Scale bars are shown in each image.
Yet another source of false-positive staining in our VZV IHC assay was the presence of erythrocytes in the temporal artery biopsy. We observed several temporal artery biopsies that contained concentrated areas of erythrocytes within the lumen of the artery or in disrupted layers of the arterial tissue, which stained false-positive after attachment by any primary (anti-VZV or anti-reovirus) or secondary antibody in an IHC test (Figure 5, Bottom right).
HERPES ZOSTER–ASSOCIATED TEMPORAL ARTERY VASCULITIS
Perhaps the most informative case in our series was Case #3, involving a 78-year-old patient with an unusual and informative history (Figure 2). The patient first developed left trigeminal neuralgia in early June. She also had scalp tenderness and headaches. She was seen in the general ophthalmology clinic and a left temporal artery biopsy was obtained on June 30. Examination of the H&E-stained temporal artery biopsy was negative for GCA. Although she was not given systemic corticosteroid treatment, the headaches and neuralgia lessened.
During the first 2 days of December, she noticed vesicular lesions in her left hairline. She was seen in the dermatology clinic on December 3, where herpes zoster of cranial nerve V1 was diagnosed, without any ocular involvement. She received a prescription for 7 days of valacyclovir. The zoster rash abated on the antiviral treatment, without development of postherpetic neuralgia.
On December 16, she was seen in the rheumatology clinic because of worsening jaw pain. The rheumatologist noted the history concerning a negative temporal artery biopsy in June and ordered a right temporal artery biopsy, which was performed on December 19. The second biopsy was read as positive for GCA and the patient was placed on prednisone therapy. The slides adjacent to the H&E slides from June 30 and December 19 were retrieved and immunolabeled for herpes zoster antigen detection by IHC. The slide from June 30 was negative but the slide from December 19 was positive for zoster antigen (Figure 2, Top row). We also reviewed the case histories of the other 2 patients with a positive herpes zoster antigen test, but neither had a recent clinical history of herpes zoster.
DISCUSSION
The virology laboratory at the university of Iowa Children’s Hospital has had extensive experience over 3 decades with detection of viral antigens in biopsies of human tissues from patients with serious varicella and herpes zoster infections.18,25,26 The current arterial analysis was undertaken in response to the recent publication of several articles from another university center, describing the detection of herpes zoster antigen in more than 70% of TA biopsies from patients with GCA.14,27 In their first report, the group examined formalin-fixed paraffin-embedded temporal artery biopsies from 82 patients with diagnosed GCA as defined by positive histopathology.14 They examined serial sections of these biopsies and found herpes zoster antigen by IHC in slides from 74% of the patients (61/82). When they looked at adjacent sections, they found GCA histopathology in 89% of the slides. In other words, there was a statistically significant correlation between sites of positive H&E histopathology for GCA and positive IHC for zoster antigen. The herpes zoster antigen was most common in the adventitia (49%), followed by media (32%) and intima (19%). Among the set of 61 zoster-positive biopsy samples, 32 also included adjacent skeletal muscle. Of these 32 cases, zoster antigen was detected by IHC in 38% of the skeletal muscles.
In subsequent reports, the same group expanded their GCA and control populations.27–29 Altogether, they reassessed a total of 100 temporal artery biopsies from patients with symptoms of GCA but with previously negative H&E histopathology for GCA. Among these 100 biopsies, they found 58% to be positive for zoster antigen. In contrast to the findings of this neurology group, we found herpes zoster antigen in only 3 of 25 TA biopsies from patients with GCA. When we performed a similar analysis of TA biopsies from 25 patients with symptoms of GCA but negative histopathology, we found no sample positive for VZV antigen, using the same MAb reagent as the neurology group. In contrast, we did find false-positive IHC tests for herpes zoster antigen in the presence of calcifications in temporal arteries from both the GCA-positive and GCA-negative patients, a point that was not mentioned in prior publications.14,27 This false-positive result obviously would increase the apparent number of VZV-positive temporal artery biopsies.
Calcifications have been observed in TA biopsies by several ocular pathology laboratories, but calcifications are often not mentioned in recent publications about GCA because they are not thought to play a major role in pathogenesis of GCA.30 In one informative analysis of GCA histologic features, calcifications were found in 37% of temporal arteries when no ischemic signs were present and in 53% of temporal arteries when ischemic manifestations were documented.31 In a second, similar analysis, calcifications were found in 26% of temporal artery biopsies from patients with the clinical features of GCA but negative histopathology.32 As noted, we observed easily detectable false-positive IHC reactivity at sites of calcification; these false-positive results occurred even in IHC tests with no primary antibody. Without observing adjacent H&E-stained sections to identify sites of calcifications, we would not have been able to discriminate false-positive staining of calcifications from true-positive staining of herpes zoster antigen in areas lacking calcifications.
We also observed false-positive IHC reactivity on skeletal muscle. We are confident that attachment of VZV antibody to skeletal muscle is a false-positive result for several reasons. First of all, the prototypic alpha herpesvirus HSV-1 cannot spread through human skeletal muscle tissue. Many experiments have been performed with HSV-1, as part of protocols to determine if recombinant HSV-1 vectors could be delivered into mature adult muscle to treat human muscular dystrophies. Numerous experiments demonstrated that mature basal lamina acts as a physical barrier to HSV-1 infection of adult myofibers.33,34 HSV-1 has a much wider tropism for different tissues that VZV. Further, adenovirus does not infect and spread in adult human skeletal muscle tissue.35 The fact that HSV-1 and adenovirus cannot infect adult muscle fibers leads to a conclusion that VZV is unlikely to infect adult skeletal muscle fibers. In a prior analysis of TA biopsies, skeletal muscle was found in 32 of 36 specimens; VZV antigen was detected in skeletal muscle of 12 of 32 (38%) samples.14 In our investigation, we observed that our control antibody against reovirus antigen also attached to skeletal muscle. Based on this reanalysis of antibody binding to skeletal muscle, we conclude that VZV positivity in skeletal muscle is more likely to be a false-positive than a true-positive.
We made no attempt to detect VZV DNA in our artery biopsies for 4 reasons. First, in prior studies14 the percent of arteries positive for VZV DNA was always lower than the percent positive by IHC; and secondly, in earlier studies from other centers, 2 different virology laboratories found artery biopsy samples from 65 GCA patients to be uniformly negative for VZV DNA.36,37 As a third reason, the temporal arteries are known to be innervated by nerve fibers from ganglia that harbor latent VZV.12 Therefore, at any time a small number of temporal arteries may be positive for VZV DNA. Yet, in the absence of any VZV antigen in the artery, we do not think that VZV infection could elicit the immunopathology for which giant cell arteritis is known. As a fourth reason, we point out that the published studies have been retrospective. Therefore, all laboratories used formalin-fixed paraffin-embedded biopsies of temporal arteries. These tissues may be suboptimal for performance of polymerase chain reaction (PCR) testing.38 To perform optimal PCR testing, we would need to organize a prospective study in which the temporal artery biopsies were divided immediately after surgery. A piece that was not formalin fixed would be used instead for DNA extraction with subsequent PCR analysis.
The concept that VZV reactivation, either as herpes zoster ophthalmicus or as zoster sine herpete, can cause occasional ocular disease is not a new concept.4 There are several papers in the literature describing VZV-relatedcorneal disease, iridocyclitis, and retinitis in the absence of a clinically apparent skin eruption.39–41 In a report of 13 cases of VZV-relatedacute retinal necrosis, all occurred in the absence of a vesicular skin disease.42 The cases were diagnosed by PCR amplification of VZV DNA in vitreous and aqueous specimens. If we remove the patients with an underlying immunodeficiency, the ages of the adult patients with VZV-related retinal necrosis were 39, 48, 58, 67, and 72 years, a clear overlap with the age group most commonly associated with GCA. Thus, VZV appears able toenter any tissue in the head and neck that is innervated by nerves originating from ganglia containing latent VZV. Our Case #3, of a 78-year-old woman with clinically apparent herpes zoster ophthalmicus and GCA 3 weeks later, may provide an important confirmatory link to this association. However, in the current survey we were not able to document an association between GCA-positive histopathology and herpes zoster antigen positivity in the majority of TA biopsies from GCA patients. Therefore, we do not recommend that all patients with GCA be treated with antivirals, unless there is an immediate past history of documented herpes zoster.
Supplementary Material
Acknowledgments
FUNDING/SUPPORT: RESEARCH ON GCA AT THE UNIVERSITY OF IOWA WAS SUPPORTED BY GRANTS FROM THE IOWA CITY VA Center for the Prevention and Treatment of Visual Loss C-9251-C RR&D and the National Shingles Foundation, New York, New York. Varicella reagents were produced during research supported by NIH grant AI89716, Bethesda, Maryland. Research at Washington University in St. Louis was supported in part by an NIH Core Grant (EY002687) and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences. Financial Disclosures: None of the authors has any financial disclosures. The authors attest that they meet the current ICMJE criteria for authorship.
References
- 1.Hayreh SS, Podhajsky PA, Zimmerman B. Occult giant cell arteritis: ocular manifestations. Am J Ophthalmol. 1998;125(4):521–526. doi: 10.1016/s0002-9394(99)80193-7. [DOI] [PubMed] [Google Scholar]
- 2.Ninan J, Lester S, Hill C. Giant cell arteritis. Best Pract Res Clin Rheumatol. 2016;30(1):169–188. doi: 10.1016/j.berh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Gay MA, Barros S, Lopez-Diaz MJ, Garcia-Porrua C, Sanchez-Andrade A, Llorca J. Giant cell arteritis: disease patterns of clinical presentation in a series of 240 patients. Medicine (Baltimore) 2005;84(5):269–276. doi: 10.1097/01.md.0000180042.42156.d1. [DOI] [PubMed] [Google Scholar]
- 4.Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125(4):509–520. doi: 10.1016/s0002-9394(99)80192-5. [DOI] [PubMed] [Google Scholar]
- 5.Yates M, Graham K, Watts RA, MacGregor AJ. The prevalence of giant cell arteritis and polymyalgia rheumatica in a UK primary care population. BMC Musculoskelet Disord. 2016;17:285. doi: 10.1186/s12891-016-1127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvarani C, Pipitone N, Versari A, Hunder GG. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol. 2012;8(9):509–521. doi: 10.1038/nrrheum.2012.97. [DOI] [PubMed] [Google Scholar]
- 7.Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014;371(1):50–57. doi: 10.1056/NEJMcp1214825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam BL, Wirthlin RS, Gonzalez A, Dubovy SR, Feuer WJ. Giant cell arteritis among Hispanic Americans. Am J Ophthalmol. 2007;143(1):161–163. doi: 10.1016/j.ajo.2006.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weyand CM, Goronzy JJ. Giant cell arteritis as an antigen-driven disease. Rheum Dis Clin North Am. 1995;21(4):1027–1039. [PubMed] [Google Scholar]
- 10.Horton BT. Temporal arteritis. Bull Tufts N Engl Med Cent. 1955;1(3):141–142. [PubMed] [Google Scholar]
- 11.Uddman R, Edvinsson L, Hara H. Axonal tracing of autonomic nerve fibers to the superficial temporal artery in the rat. Cell Tissue Res. 1989;256(3):559–565. doi: 10.1007/BF00225604. [DOI] [PubMed] [Google Scholar]
- 12.Richter ER, Dias JK, Gilbert JE, 2nd, Atherton SS. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis. 2009;200(12):1901–1906. doi: 10.1086/648474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58(1):9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilden D, White T, Khmeleva N, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015;84(19):1948–1955. doi: 10.1212/WNL.0000000000001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azari AA, Syed NA, Albert DM. Examining and processing eye specimens. Methods Mol Biol. 2014;1180:377–396. doi: 10.1007/978-1-4939-1050-2_23. [DOI] [PubMed] [Google Scholar]
- 16.Albert DM, Ruchman MC, Keltner JL. Skip areas in temporal arteritis. Arch Ophthalmol. 1976;94(12):2072–2077. doi: 10.1001/archopht.1976.03910040732006. [DOI] [PubMed] [Google Scholar]
- 17.Weigle KA, Grose C. Common expression of varicella-zoster viral glycoprotein antigens in vitro and in chickenpox and zoster vesicles. J Infect Dis. 1983;148(4):630–638. doi: 10.1093/infdis/148.4.630. [DOI] [PubMed] [Google Scholar]
- 18.Halling G, Giannini C, Britton JW, et al. Focal encephalitis following varicella-zoster virus reactivation without rash in a healthy immunized young adult. J Infect Dis. 2014;210(5):713–716. doi: 10.1093/infdis/jiu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckingham EM, Carpenter JE, Jackson W, Zerboni L, Arvin AM, Grose C. Autophagic flux without a block differentiates varicella-zoster virus infection from herpes simplex virus infection. Proc Natl Acad Sci U S A. 2015;112(1):256–261. doi: 10.1073/pnas.1417878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos RA, Padilla JA, Hatfield C, Grose C. Antigenic variation of varicella zoster virus Fc receptor gE: loss of a major B cell epitope in the ectodomain. Virology. 1998;249(1):21–31. doi: 10.1006/viro.1998.9313. [DOI] [PubMed] [Google Scholar]
- 21.Grose C, Tyler S, Peters G, et al. Complete DNA sequence analyses of the first two varicella-zoster virus glycoprotein E (D150N) mutant viruses found in North America: evolution of genotypes with an accelerated cell spread phenotype. J Virol. 2004;78(13):6799–6807. doi: 10.1128/JVI.78.13.6799-6807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virgin HW, 4th, Mann MA, Fields BN, Tyler KL. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J Virol. 1991;65(12):6772–6781. doi: 10.1128/jvi.65.12.6772-6781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Seo BS. How to calculate sample size and why. Clin Orthop Surg. 2013;5(3):235–242. doi: 10.4055/cios.2013.5.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grose C. Varicella zoster virus infections:chickenpox, shingles and varicella vaccine. In: Glaser R, Jones JF, editors. Herpes-virus Infections. New York: Marcel Dekker; 1994. pp. 117–185. [Google Scholar]
- 25.Weigle KA, Grose C. Varicella pneumonitis: immunodiagnosis with a monoclonal antibody. J Pediatr. 1984;105(2):265–269. doi: 10.1016/s0022-3476(84)80126-2. [DOI] [PubMed] [Google Scholar]
- 26.Storlie J, Carpenter JE, Jackson W, Grose C. Discordant varicella-zoster virus glycoprotein C expression and localization between cultured cells and human skin vesicles. Virology. 2008;382(2):171–181. doi: 10.1016/j.virol.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilden D, White T, Khmeleva N, Boyer PJ, Nagel MA. VZV in biopsy-positive and -negative giant cell arteritis: analysis of 100+ temporal arteries. Neurol Neuroimmunol Neuroinflamm. 2016;3(2):e216. doi: 10.1212/NXI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilden D, White T, Khmeleva N, Katz BJ, Nagel MA. Blinded search for varicella zoster virus in giant cell arteritis (GCA)-positive and GCA-negative temporal arteries. J Neurol Sci. 2016;364:141–143. doi: 10.1016/j.jns.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagel MA, White T, Khmeleva N, et al. Analysis of varicella-zoster virus in temporal arteries biopsy positive and negative for giant cell arteritis. JAMA Neurol. 2015;72(11):1281–1287. doi: 10.1001/jamaneurol.2015.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordborg C, Nordborg E, Petursdottir V, Fyhr IM. Calcification of the internal elastic membrane intemporalarteries:itsrelation to age and gender. Clin Exp Rheumatol. 2001;19(5):565–568. [PubMed] [Google Scholar]
- 31.Breuer GS, Nesher R, Reinus K, Nesher G. Association between histological features in temporal artery biopsies and clinical features of patients with giant cell arteritis. Isr Med Assoc J. 2013;15(6):271–274. [PubMed] [Google Scholar]
- 32.Muratore F, Cavazza A, Boiardi L, et al. Histopathologic findings of patients with biopsy-negative giant cell arteritis compared to those without arteritis: a population-based study. Arthritis Care Res (Hoboken) 2016;68(6):865–870. doi: 10.1002/acr.22736. [DOI] [PubMed] [Google Scholar]
- 33.Huard J, Feero WG, Watkins SC, Hoffman EP, Rosenblatt DJ, Glorioso JC. The basal lamina is a physical barrier to herpes simplex virus-mediated gene delivery to mature muscle fibers. J Virol. 1996;70(11):8117–8123. doi: 10.1128/jvi.70.11.8117-8123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Deutekom JC, Floyd SS, Booth DK, et al. Implications of maturation for viral gene delivery to skeletal muscle. Neuromuscul Disord. 1998;8(3–4):135–148. doi: 10.1016/s0960-8966(98)00019-4. [DOI] [PubMed] [Google Scholar]
- 35.Acsadi G, Jani A, Massie B, et al. A differential efficiency of adenovirus-mediated in vivo gene transfer into skeletal muscle cells of different maturity. Hum Mol Genet. 1994;3(4):579–584. doi: 10.1093/hmg/3.4.579. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy PG, Grinfeld E, Esiri MM. Absence of detection of varicella-zoster virus DNA in temporal artery biopsies obtained from patients with giant cell arteritis. J Neurol Sci. 2003;215(1–2):27–29. doi: 10.1016/s0022-510x(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Pla A, Bosch-Gil JA, Echevarria-Mayo JE, et al. No detection of parvovirus B19 or herpesvirus DNA in giant cell arteritis. J Clin Virol. 2004;31(1):11–15. doi: 10.1016/j.jcv.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, He H, Yi S, Hu Q, Zhang W, Huang D. Comparison of different methods for repairing damaged DNA from buffered and unbuffered formalin-fixed tissues. Int J Legal Med. 2017;131(12):1789–1795. doi: 10.1007/s00414-017-1666-7. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M, Tanabe M, Yamada Y, Azumi A. Zoster sine herpete with bilateral ocular involvement. Am J Ophthalmol. 2000;129(6):809–810. doi: 10.1016/s0002-9394(00)00404-9. [DOI] [PubMed] [Google Scholar]
- 40.van Bijsterveld OP, Jager GV. Infectious diseases of the conjunctiva and cornea. Curr Opin Ophthalmol. 1996;7(4):65–70. doi: 10.1097/00055735-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Hu AY, Strauss EC, Holland GN, Chan MF, Yu F, Margolis TP. Late varicella-zoster virus dendriform keratitis in patients with histories of herpes zoster ophthalmicus. Am J Ophthalmol. 2010;149(2):214–220. e3. doi: 10.1016/j.ajo.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganatra JB, Chandler D, Santos C, Kuppermann B, Margolis TP. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2000;129(2):166–172. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.