Abstract
Background
Botulism is a rare, life-threatening paralytic illness. Equine-derived heptavalent botulinum antitoxin (HBAT), the only currently available treatment for noninfant botulism in the United States, was licensed in 2013. No reports have systematically examined safety and clinical benefit of HBAT among botulism patients.
Methods
From March 2010 through March 2013, we collected data prospectively and through medical record reviews of patients with confirmed or suspected botulism who were treated with HBAT under an expanded-access Investigational New Drug program.
Results
Among 249 HBAT-treated patients, 1 (<1%) child experienced an HBAT-related serious adverse event (hemodynamic instability characterized by bradycardia, tachycardia, and asystole); 22 (9%) patients experienced 38 nonserious adverse events reported by physicians to be HBAT related. Twelve (5%) deaths occurred; all were determined to be likely unrelated to HBAT. Among 104 (42%) patients with confirmed botulism, those treated early (≤2 days) spent fewer days in the hospital (median, 15 vs 25 days; P < .01) and intensive care (10 vs 17 days; P = .04) than those treated later. Improvements in any botulism sign/symptom were detected a median of 2.4 days and in muscle strength a median of 4.8 days after HBAT.
Conclusions
HBAT was safe and provided clinical benefit in treated patients. HBAT administration within 2 days of symptom onset was associated with shorter hospital and intensive care stays. These results highlight the importance of maintaining clinical suspicion for botulism among patients presenting with paralytic illness to facilitate early HBAT treatment before laboratory confirmation might be available. Clinical consultation and, if indicated, HBAT release, are available to clinicians 24/7 through their state health department in conjunction with CDC.
Keywords: equine-derived heptavalent botulinum antitoxin, botulism, HBAT, BAT
Botulism is a rare illness caused by toxin produced by Clostridium botulinum. is potent toxin binds irreversibly to nerve endings at neuromuscular junctions, causing varying degrees of paralysis, respiratory failure, and even death [1]. Botulinum antitoxin is the only specific therapy for botulism. It binds circulating botulinum toxin and halts illness progression, but does not reverse deficits that have already developed. Because laboratory confirmation takes time, during which neurologic deficits may progress, and because it may not confirm all botulism illnesses, antitoxin is administered empirically as soon as possible after clinical suspicion is raised. Botulism occurs sporadically each year and outbreaks may occur; botulism cases and outbreaks are public health emergencies that require a vigorous response to ensure treatment of affected persons and investigation of the source to prevent further illnesses [2]. Intentional exposures, such as in a bioterror event, could cause thousands of illnesses.
Exposure to botulinum toxin occurs through several routes, including ingestion of preformed toxin in foods, toxin produced by germinating botulinum spores in colonized wounds or the intestinal tract, and injection of high concentrations of botulinum toxin for therapeutic purposes. Infant botulism occurs due to production of toxin by C. botulinum colonizing the immature infant gastrointestinal system and is treated in the United States with BabyBIG®, a human immunoglobulin approved for infant botulism caused by toxin types A and B [3]. Noninfant botulism is treated with equine-derived antitoxin, produced by hyperimmunizing horses with botulinum toxin. There have been prior formulations of equine botulinum antitoxin available through the Centers for Disease Control and Prevention (CDC) for noninfant persons in the United States since the 1960s. In 2010, the first heptavalent formulation capable of neutralizing toxin serotypes (A, B, C, D, E, F, G), although still investigational at the time, replaced previous formulations. To ensure continued availability of antitoxin in the United States, CDC implemented an expanded-access Investigational New Drug program (“compassionate use” IND) starting in March 2010. As a secondary goal, the IND program enabled collection of data on safety and clinical benefit. In 2013, heptavalent botulinum antitoxin (HBAT, also known by its licensed name BAT) was approved by the US Food and Drug Administration (FDA) based on the Animal Efficacy Rule, under which products treating rare illnesses that are not ethical or feasible to be studied using randomized controlled trials may be approved based on efficacy demonstrated in animals [4]. Safety and clinical outcomes data on HBAT in ill patients from the IND were part of the supportive data for licensure of HBAT but have not previously been published. Data on previous antitoxin formulations are limited and HBAT is a new formulation. Post-licensure data collection by the manufacturer, Cangene Corporation, as part of its FDA-required post-marketing commitments, has not yet completed and therefore, data not available for inclusion in or as a companion to this article. We present the first report on clinical use of HBAT for suspected and confirmed botulism during the CDC IND program.
METHODS
The CDC, working with state and local health departments, provides clinical consultation and antitoxin release 24 hours a day for all noninfant patients who may have botulism in the United States (antitoxin for treatment of infant botulism is available by contacting the California Department of Public Health). During the IND period of March 2010 through March 2013 and for 4 patients treated in 2008–2009, CDC provided investigational HBAT for treatment of patients under a CDC Institutional Review Board–approved protocol. The protocol contained information about HBAT, including case report forms (CRFs), instructions on obtaining informed consent, HBAT administration, and monitoring and reporting of adverse events (AEs), including serious adverse events (SAEs). Skin sensitivity testing was optional. The HBAT regimen for adults was a single dose of 1 vial whereas the pediatric dose was weight-based; the infant dose during the early phase of the IND was 20% of the adult dose. HBAT is formulated to meet a minimum potency level for each antitoxin type expressed as units based on mouse neutralization assay: A (4500 U), B (3300 U), C (3000 U), D (600 U), E (5100 U), F (3000 U), G (600 U) [5]. The half-life of one vial of HBAT ranges from 7.51 hours to 34.20 hours depending on the antitoxin serotype [5].
Definitions and Data Collection
Baseline clinical information was collected verbally at the time of HBAT consultation and, in keeping with HBAT investigational status, physicians were required to comply with the protocol and complete CRFs assessing AEs from HBAT through written reports to CDC. CRF data were obtained soon after HBAT administration and upon patient discharge. CDC systematically contacted hospital staff during patients’ hospitalizations to enhance CRF completion. Medical records were obtained from hospitals to abstract: (1) dates of symptom onset and improvement, hospital admissions, transfers, and discharges and dates of tracheostomy placement; (2) durations of mechanical ventilation and hospitalization in intensive care settings; and (3) clinical deficits at time of acute care discharge and location to which a patient was discharged.
AEs were defined as any untoward medical occurrence related to HBAT per FDA’s IND safety reporting regulations [6]. Physicians reported AEs related to HBAT by completing and returning CRFs that inquired about (1) occurrence or absence of AEs including fever, chills/rigors, rash, urticaria, edema, urinary retention, anaphylaxis, serum sickness, and other reactions; (2) AE duration and timing; (3) any treatment of AEs; and (4) sequelae from AEs. SAEs, including serious unexpected suspected adverse reactions, were defined per FDA IND safety reporting regulations [6]. Information on patient clinical outcomes (eg, survival) was obtained through patient discharge from acute hospitalization and inpatient rehabilitation. Because equine antitoxins carry a risk of hypersensitivity including anaphylaxis and serum sickness, skin testing results and the occurrence of AEs to HBAT administration were evaluated when data on skin sensitivity testing were available. Results of laboratory testing were obtained directly from public health reference laboratories. We used the Council of State and Territorial Epidemiologists definition of confirmed botulism: illness clinically-compatible with botulism and that is laboratory-confirmed or epidemiologically-linked to a laboratory-confirmed case [7]. For analysis of duration of hospitalization, intensive care unit (ICU) stay, and mechanical ventilation, early treatment was defined as HBAT administration within 2 days of symptom onset and later treatment as >2 days from symptom onset.
Data Analysis
Safety was assessed among all HBAT-treated patients, irrespective of botulism confirmation. SAEs, including all deaths reported by physicians regardless of botulism confirmation status or physician-reported association with HBAT, were assessed by the CDC principal investigator for relatedness to HBAT by reviewing patients’ medical records and correspondence with physicians. To assess clinical benefit of HBAT, only confirmed botulism cases were further analyzed. Median durations of hospitalization, ICU stay, and mechanical ventilation were calculated for patients treated early (≤2 days of symptom onset) and those treated later (>2 days from symptom onset). Differences were assessed by Wilcoxon rank-sum test. Patients who died during acute care were excluded to avoid biasing calculations of median durations of hospitalization, ICU stay, and mechanical ventilation. Kaplan-Meier curves with log-rank test were generated to compare durations of hospitalization, ICU stay, and mechanical ventilation between patients treated early and those treated later. The percentage of botulism-confirmed patients who died was compared between patients treated with HBAT early and those treated later, with difference assessed by Fisher exact test.
Timing of HBAT administration from symptom onset and time to first documented improvement in any signs or symptoms of botulism (including extraocular palsy, ptosis, pupillary signs, impaired gag reflex, blurred vision, diplopia, dysphagia, slurred speech, subjective strength improvement) was assessed by simple linear regression. Patients with reported improvement before HBAT administration were excluded from linear regression analysis. All statistical analyses were done using SAS version 9.3 (SAS Institute). Two-sided P values <.05 were considered statistically significant.
RESULTS
A total of 249 persons aged 10 days–88 years (median, 46 years) were treated with HBAT (Table 1). Of these, 17 (7%) were children (median, 6 years; range, 10 days–17 years). None of the 249 treated patients were pregnant or breastfeeding. Botulism was laboratory or epidemiologically confirmed for 104 (42%) patients. Confirmed cases were caused by toxin types A (74%), Ab (1%), B (7%), E (7%), F (4%), and indeterminate type (8%) with exposure occurring via all naturally occurring transmission routes.
Table 1.
Demographic Characteristics of Patients Treated With Heptavalent Botulinum Antitoxina
| Characteristic | All Patients Treated With HBAT (n = 249) | HBAT-Treated Patients With Confirmed Botulism (n = 104) |
|---|---|---|
| Median age (range) | 46 y (10 d–88 y) | 43 y (10 d–83 y) |
| Age group, y | ||
| 0 to <1b | 1 (<1%) | 1 (1%) |
| 1–9 | 8 (3%) | 1 (1%) |
| 10–17 | 8 (3%) | 4 (4%) |
| 18–29 | 35 (14%) | 21 (20%) |
| 30–39 | 46 (19%) | 21 (20%) |
| 40–49 | 42 (17%) | 24 (23%) |
| 50–59 | 49 (20%) | 14 (14%) |
| 60–69 | 36 (15%) | 11 (11%) |
| 70–79 | 17 (7%) | 6 (6%) |
| ≥80 | 7 (3%) | 1 (1%) |
| Sex | ||
| Male | 178 (72%) | 84 (81%) |
| Female | 71 (29%) | 20 (19%) |
| Ethnicity | ||
| Hispanic/Latino | 90 (36%) | 38 (37%) |
| Not Hispanic/Latino | 110 (44%) | 56 (54%) |
| Not reported | 49 (20%) | 10 (10%) |
| Race | ||
| White | 139 (56%) | 73 (70%) |
| Alaska Native | 14 (6%) | 8 (8%) |
| African American | 14 (6%) | 3 (3%) |
| Asian | 12 (5%) | 5 (5%) |
| Other | 6 (2%) | 4 (4%) |
| Native American | 3 (1%) | 3 (3%) |
| Not reported | 61 (25%) | 8 (8%) |
Abbreviation: HBAT, heptavalent botulinum antitoxin.
The majority of patients were treated during the expanded access Investigational New Drug period from 2010–2013; 4 patients were treated 2008–2009. Most patients were treated in the United States while 5 patients were treated in Mexico.
Infant botulism type F illness treated with HBAT because the licensed product for infant botulism treatment, BabyBIG® does not contain Anti-F activity.
Safety of Heptavalent Botulinum Antitoxin
Among 249 patients, 23 (9%) experienced at least 1 AE reported by physicians as HBAT-related: 22 (9%) patients experienced 38 nonserious AEs and 1 patient experienced an HBAT-related SAE (Table 2). No patients experienced anaphylaxis. The single case of serum sickness occurred in a 64-year-old man, which occurred 11 days after HBAT administration and physician-reported as mild, self-limited serum sickness characterized by myalgia and arthralgia treated with ibuprofen; the principal investigator also determined it as not serious [8].
Table 2.
Number of Physician-Reported Adverse Events Related to Treatment With Heptavalent Botulinum Antitoxin
| Adverse Event | Events Among Adult Patients (n = 232), No. (%) | Events Among Pediatric Patients (n = 17), No. (%) | No Events Among All Patients (N = 249), No. (%) |
|---|---|---|---|
| Nonserious adverse event | |||
| Fever | 8 (3%) | 1 (6%) | 9 (4%) |
| Rash | 4 (2%) | 0 | 4 (2%) |
| Chills | 3 (1%) | 0 | 3 (1%) |
| Agitation/anxiety | 2 (1%) | 1 (6%) | 3 (1%) |
| Edema | 2 (1%) | 0 | 2 (1%) |
| Hypertension/increased blood pressure | 2 (1%) | 0 | 2 (1%) |
| Nausea | 2 (1%) | 0 | 2 (1%) |
| Mild serum sicknessa | 1 (<1%) | 0 | 1 (<1%) |
| Otherb | 11 (5%) | 1 (6%) | 12 (5%) |
| Total No. of nonserious adverse events | 35 | 3 | 38 |
| Serious adverse event | |||
| Hemodynamic instability (bradycardia, tachycardia, asystole)c | 0 | 1 (6%) | 1 (<1%) |
| Anaphylactic reaction | 0 | 0 | 0 |
| Total No. of serious adverse events | 0 | 1 | 1 |
| Total number of adverse events | 35 | 4 | 39 |
Reported as “self-limited serum sickness” 11 days after heptavalent botulinum antitoxin administration.
Bronchospasm, chest pressure, diaphoresis, erythema, increased respiratory rate, “jitteriness,” leukocytosis, mild hypotension, tachycardia, urinary retention, and vomiting were each reported once each among adults and a complaint of “hurting all over” during infusion was reported for 1 pediatric patient.
A 10-year-old child experienced hemodynamic instability characterized by bradycardia, tachycardia, and asystole.
One HBAT-related SAE occurred in a 10-year-old boy weighing 29 kg who was reported to have experienced bradycardia leading to asystole approximately 90 minutes after initiation of HBAT infusion; the lowest observed heart rate immediately before asystole was 10–15 beats per minute [bpm]). Pediatric administration instructions at the time recommended starting the infusion at a rate of 0.1–0.5 mL/min for at least 30 minutes, then increasing the rate to 0.2–1 mL/min for the subsequent 30 minutes and thereafter. The treating physician reported an initial infusion rate of 0.1 mL/min for 30 minutes. The infusion rate thereafter was not reported, including during the time at which the patient experienced bradycardia and asystole. The administration was halted when asystole occurred, epinephrine was administered, and chest compressions performed. Administration was restarted after a pause of approximately 5 minutes. At this time the patient was tachycardic (140 bpm), possibly in consequence of receiving epinephrine, but regained normal cardiac rhythm. No other abnormalities were observed until approximately 30 minutes after the infusion had been restarted, when the patient abruptly experienced a second bradycardic episode (30–40 bpm) and HBAT administration was altogether stopped; an estimated 73% of the intended dose (40 ml of 55 ml) was administered overall.
A total of 12 deaths (5%) were reported; none were related to HBAT by either physician report or by CDC principal investigator review (Table 3). Characteristics of patients who died and cause of death as determined by CDC principal investigator review are listed in Table 3.
Table 3.
Characteristics of Fatal Cases of Botulism Among Patients Treated With Heptavalent Botulinum Antitoxin (n = 12)
| Age/Sex | Toxin Typea | Cause of Deathb | Death related to HBAT?c | Days From Symptom Onset to Death | Days From Symptom Onset to HBAT Administration | Days From HBAT Administration to Death |
|---|---|---|---|---|---|---|
| 27/M | Ab | Aspiration leading to cardiopulmonary arrest | No | 41d | 22 | 19 |
| 43/M | A | Acute respiratory distress syndrome due to pneumonia | No | 179d | 4 | 175 |
| 64/M | A | Respiratory failure and metastatic prostate cancer | No | 48d | 3 | 45 |
| 83/M | A | Myocardial infarction | No | 45 | 17 | 28 |
| 64/M | F | Unknown cause of death; cardiopulmonary arrest possibly due to respiratory failure because of known pulmonary problems | No | 70 | 18 | 52 |
| 77/M | A | Unknown cause; death occurred >70 d after hospital transfer | No | 99 | 5 | 94 |
| 57/M | A | Unknown cause of death; cardiopulmonary arrest due to undetermined reasons occurred following nonspecific complaints 1 d following prolonged hospitalization | No | 48 | 3 | 45 |
| 82/F | NC | Aspiration pneumonia | No | 11 | 8 | 3 |
| 88/F | NC | Respiratory failure and complications from Guillain-Barré syndrome and underlying medical problems | No | 10 | 3 | 7 |
| 61/M | NC | Myocardial infarction due to triple vessel coronary artery disease | No | 8 | 6 | 2 |
| 85/M | NC | Sepsis, bacteremia, possible endocarditis | No | 10 | 5 | 5 |
| 49/M | NC | Septic shock and respiratory failure due to pneumonia | No | 19e | 9e | 10 |
Abbreviation: HBAT, heptavalent botulinum antitoxin; NC, botulism not confirmed.
Toxin type is indicated for only those illnesses confirmed to be botulism. Illnesses not confirmed to be botulism (ie, “NC”) may have been cases of botulism that could not be confirmed or they may have been illnesses other than botulism.
Cause of death determined through CDC principal investigator review of chart and sometimes, discussion with treating physician.
Relatedness to HBAT was assessed by CDC principal investigator through review of medical records and, when possible, discussion with physicians.
Death occurred among botulism-confirmed patients during acute inpatient hospitalization.
Onset date not reported; hospital admission date used to calculate this duration in lieu of onset date.
Most reported, HBAT-related AEs were nonserious and included fever (n = 9 [4%]), rash (n = 4 [2%]), and chills (n = 3 [1%]) (Table 2) and resolved with medications such as acetaminophen, diphenhydramine, and methylprednisolone. Although skin sensitivity testing was not required, it was conducted for 33 patients; all but 1 had negative skin test results and no resulting allergic reaction with HBAT administration. The 1 patient with a positive skin test prior to HBAT treatment experienced facial swelling and facial and truncal rash 1 day after HBAT treatment and nausea, chest pressure, and “jitteriness” 2 days later. Information is not available on whether the patient was pretreated for allergy before HBAT administration. The patient recovered without sequelae after treatment with diphenhydramine, methylprednisolone, and ondansetron.
Three adults each received 2 HBAT doses, from 4 to 34 days apart: 2 had a second episode of botulism and 1 was re-treated off-protocol due to lack of clinical improvement. No AEs were reported. An additional 3 HBAT-treated adults had a prior history of botulism for which they were treated with an older, previously available antitoxin formulation from 4 to 8 years before HBAT treatment; they also did not experience any AEs.
Clinical Outcomes and Timing of Heptavalent Botulinum Antitoxin Administration
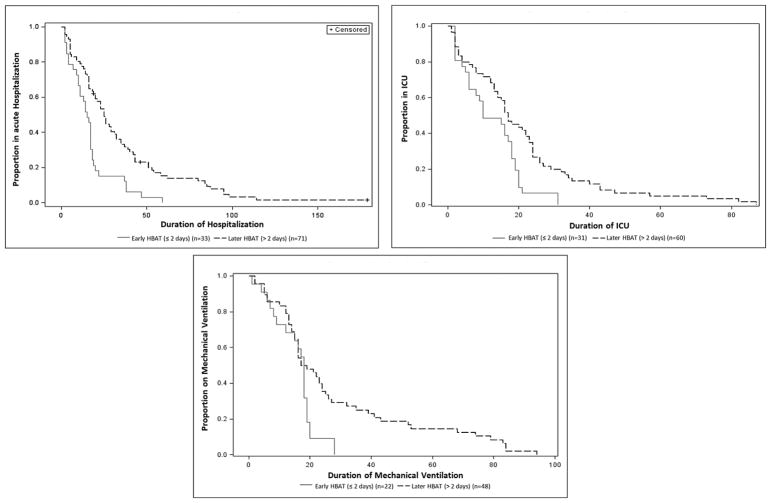
Among 104 botulism-confirmed patients, all 33 patients treated within 2 days of symptom onset (early treatment) survived while 64 of 71 (90%) patients treated later survived; this difference was not statistically significant. Early HBAT treatment was associated with statistically significant shorter hospital (median, 15 vs 25 days; P < .01) and ICU (10 vs 17 days; P = .04) stays compared with later HBAT treatment (Table 4; Figure 1). Simple linear regression indicated that improvement in any of the botulism signs or symptoms occurred 2.4 days (95% confidence interval [CI], 1.6–3.1) after HBAT administration, while strength improvement occurred 4.8 days (95% CI, 2.5–7.1) after administration, irrespective of timing of administration (regression coefficients in both models were 1.0 [95% CIs, .8–1.2 and .4–1.6]).
Table 4.
Duration of Hospitalization, Intensive Care Unit Stay, and Mechanical Ventilation Among Botulism-Confirmed Patients Treated With Heptavalent Botulinum Antitoxin
| Factor | Time From Symptom Onset to HBAT Administration, d | No. of Patients | Median Duration, d | Range, d | P Valuea |
|---|---|---|---|---|---|
| Hospitalization | ≤2 | 33 | 15 | 2–59 | <.01 |
| >2 | 68 | 25 | 2–114 | ||
|
| |||||
| ICU stay | ≤2 | 31 | 10 | 2–31 | .04 |
| >2 | 60 | 17 | 1–87 | ||
|
| |||||
| Mechanical ventilation | ≤2 | 22 | 18 | 1–28 | .13 |
| >2 | 48 | 18 | 2–94 | ||
Abbreviations: HBAT, heptavalent botulinum antitoxin; ICU, intensive care unit.
Wilcoxon rank-sum test.
Figure 1.
Kaplan-Meier curves for duration of hospitalization, intensive care unit (ICU) stay, and mechanical ventilation after heptavalent botulinum antitoxin (HBAT) among botulism-confirmed patients by timing of HBAT administration in relation to symptom onset (n=104). Three deaths during acute inpatient hospitalization were censored.
Intubation followed HBAT administration in 10 (14%) patients with median time to intubation of <1 day (range, <1 to 3 days); these patients were treated with HBAT a median of 4 days from symptom onset (range, 1–11 days). Of 104 botulism-confirmed patients, 73 (70%) required mechanical ventilation. Disposition of patients were as follows: 61 (59%) were discharged home, 32 (31%) were discharged to a long-term acute care facility, 7 (7%) were discharged to a skilled nursing facility/nursing home, and 1 (1%) left against medical advice. ree patients (3%) died during acute hospitalization. Of 73 patients who received mechanical ventilation, 54 (74%) underwent tracheostomy. Most (88, 89%) botulism-confirmed patients had residual disability upon discharge consisting of neurological deficits and/or persistent subjective weakness while 11 (11%) were reported as having no residual disability upon discharge among patients for whom data were available. The percentage of patients with residual disability at discharge did not differ significantly between patients treated with HBAT early (91%) and those treated later (88%).
DISCUSSION
Since 2010, HBAT has been the only antitoxin available in the United States for treatment of noninfant botulism, capable of neutralizing toxin types (A, B, C, D, E, F, G). Our data are the first published on the safety and clinical outcomes associated with HBAT administration among patients with suspected and confirmed botulism. For most patients, HBAT was well-tolerated: 9% developed any HBAT-related AE and 1 patient (<1%) developed a related SAE. No deaths related to HBAT were reported. We found that early HBAT administration was associated with shorter hospital and ICU stays among patients with confirmed botulism. These results highlight the importance of early treatment of suspected botulism with HBAT, usually before laboratory confirmation is available. Around-the-clock clinical consultation and HBAT release are available to clinicians through their state health department in conjunction with CDC.
Mortality from botulism decreased from approximately 60% to <5% over the course of the 20th century in the United States, attributed to advances in critical care, specifically mechanical ventilation [2, 9–12]. Outbreak investigation reports, case reports, case series, and animal studies suggested that early administration of previously available botulinum antitoxins improved morbidity and mortality [12]. Our findings suggest the same is true for HBAT: we found that early HBAT administration was associated with shorter hospitalization and intensive care. Additionally, we observed no deaths among patients treated early, whereas 10% of patients who received HBAT >2 days after symptom onset died.
The observation that early HBAT administration was associated with reduced duration of hospitalization and intensive care is consistent with the mechanism of action of HBAT, neutralization of free-circulating toxin, preventing toxin from exerting its action in neuromuscular junctions and preventing further symptom progression [13, 14]. Furthermore, our findings illustrate the expected clinical benefit of HBAT is limited to halting progression of botulism and not speeding recovery. Our data show that improvements in signs and symptoms and in strengths occurred after HBAT administration. However, time to improvement of any sign or symptom (eg, improvement in cranial nerve symptoms) appears to be similar regardless of the timing of HBAT administration following symptom onset. While there is as yet no rapid point-of-care diagnostic test for botulism and even if one were to become available, HBAT should be considered upon clinical suspicion based on signs and symptoms and, if available, exposure history, as early treatment is most beneficial in preventing further disease progression [15]. Treatment should not be contingent on laboratory or other diagnostic testing (eg, electromyography) due to delay in conducting such evaluations and their limitations [15, 16]. us, HBAT should be administered as soon as possible. Even if treatment were inadvertently delayed, HBAT may still be of clinical benefit given the potential that prolonged toxemia may be present [17]. Clinicians suspecting botulism should immediately contact their state health department’s emergency telephone number for consultation and referral to CDC’s Botulism Clinical Consultation Service, which provides review of clinical presentation and, if indicated, releases an emergency shipment of HBAT for patient treatment.
Reports on botulinum antitoxin products employed in previous decades cite rates of anaphylaxis and serum sickness of 1–2% and 1–4%, respectively; rates varied with number of vials administered [18–21]. With HBAT, we observed anaphylaxis and serum sickness rates of 0% and <1%, respectively. Despeciation of HBAT involves removal of equine-derived Fc segments of the equine immunoglobulin G, resulting in purified F(ab′)2 and F(ab′)2,-related immune globulin fragments and purification [22]. Whether this contributes to lower allergenicity is uncertain. Historically, for older equine-derived botulinum antitoxin formulations, skin sensitivity testing before administration was recommended [23, 24]. Although skin testing was predictive of an allergic reaction in a single patient in our analysis, other studies suggest that the positive predictive value is low [18, 19, 25]. In clinical trials of 56 healthy adults, despite negative skin testing with horse dander immunoglobulin E and HBAT before HBAT administration, 2 subjects experienced hypersensitivity reactions including urticaria [4]. Given concerns about its accuracy, sensitivity testing, which can delay HBAT administration, is not recommended. HBAT should be administered in a setting where hypersensitivity reactions, should they occur, can be identified and treated.
The seven botulinum toxin serotypes A–G were described between 1919 and 1970. Recently, reports of new toxin types have been published, including a novel toxin subsequently shown to be hybrid type A/F fully neutralized by HBAT [26, 27], and a novel toxin identified and assembled from the published gene sequence of a C. botulinum isolate [28]. New botulinum toxins of clinical significance might be discovered. Preparedness requires careful laboratory investigation of all suspected botulism cases and research and development of new countermeasures.
Given clinical trial data on HBAT were limited to 2 phase 1 trials in 56 healthy adult subjects, our data provide the first assessment of information derived from clinical use of HBAT to treat patients with suspected or confirmed botulism. However, our investigation has several limitations. Because HBAT was provided under expanded-access IND with the primary objective of providing treatment for life-threatening disease, it was not a clinical trial and no comparison group without treatment is available. Efficacy is typically demonstrated by prospective randomized controlled clinical trials and could not be definitively drawn from clinical information collected from only HBAT-treated patients. Our data collection was dependent on physicians completing and returning CRFs. However, through our active follow-up process, the return rate of CRFs containing safety and clinical outcome information was 93%, which was historically challenging to achieve. With the exception of deaths and SAEs, nonserious AEs were physician-reported and not further investigated. Deaths that occurred after hospital discharge and completion of our follow-up may not have been reported to us for further evaluation. Other confounding factors may have contributed to clinical outcomes such as toxin exposure level, individual host factors, and progression of botulism symptoms and neurologic illness before HBAT administration, which could not be evaluated or assessed further by chart abstraction.
Despite these limitations, our findings strongly suggest that HBAT is well-tolerated for patients with suspected or confirmed botulism and that early HBAT administration is more effective than later administration. While our data were collected from patients with sporadic or outbreak-related illnesses due to unintentional exposure, it is likely that our findings would still apply in the context of an intentional mass casualty event. For the individual botulism patient properly managed in an intensive care setting, the advantages of HBAT appear to outweigh potential risks. During a mass casualty event, early HBAT treatment may help lessen the population-level strain on limited healthcare resources by reducing the duration of hospitalization and intensive care required by individual patients. However, undesirable consequences arising from the rare risk of otherwise mild HBAT-related AEs may be magnified in these situations if supportive care resources are limited. Regardless of the context in which botulism occurs, our data further support the importance of maintaining clinical suspicion for this paralytic illness to help ensure that safe and effective management is implemented in a timely manner.
Acknowledgments
We thank Ryan Fagan, MD, MPH, Kelly Jackson, MPH, Susan Maslanka, PhD, Rabahuddin Syed, MD, Hannah Kisselburgh, MPH, BSN, and Tze Li, PharmD; the physicians, nurses, and pharmacists who completed and returned data related to HBAT use in their patients to CDC; Department of Health and Human Services/Office of the Assistant Secretary for Preparedness and Response/Biomedical Advanced Research and Development Authority for their advanced development of HBAT; and Cangene Corporation, a subsidiary of Emergent BioSolutions Inc., as the manufacturer of HBAT.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplement sponsorship. is article appears as part of the supplement “Botulism,” sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript will be disclosed.
References
- 1.Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167–93. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- 2.Sobel J. Botulism. Clin Infect Dis. 2005;41:1167–73. doi: 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. [Accessed 11 October 2017];Full prescribing information: BabyBIG [botulism immune globulin intravenous (human) (BIG-IV)] Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM117160.pdf.
- 4.US Food and Drug Administration. [Accessed 11 October 2017];Approval history, letters, reviews, and related documents—BAT (botulism antitoxin heptavalent (A, B, C, D, E, F, G)—(Equine): clinical review memo. Available at: https://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm345134.htm.
- 5.US Food and Drug Administration. [Accessed 11 October 2017];Full prescribing information: BAT [botulism antitoxin heptavalent (A, B, C, D, E, F, G)–(Equine)] Available at: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-bio-gen/documents/document/ucm345147.pdf.
- 6.US Food and Drug Administration. IND safety reporting. 21 CFR §312.32. [Google Scholar]
- 7.Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS); botulism (Clostridium botulinum) 2011 case definition. [Accessed 11 October 2017]; Available at: https://wwwn.cdc.gov/nndss/conditions/botulism/case-definition/2011/
- 8.Filozov A, Kattan JA, Jitendranath L, et al. Asymmetric type F botulism with cranial nerve demyelination. Emerg Infect Dis. 2012;18:102–4. doi: 10.3201/eid1801.110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. [Accessed 11 October 2017];Botulism in the United States 1899–1996: handbook for epidemiologists, clinicians, and laboratory workers. Available at: https://www.cdc.gov/botulism/pdf/bot-manual.pdf.
- 10.Centers for Disease Control and Prevention. [Accessed 11 October 2017];Annual summaries of botulism surveillance reported to CSTE. Available at: http://www.cdc.gov/nationalsurveil-lance/botulism-surveillance.html.
- 11.Gangarosa EJ, Donadio JA, Armstrong RW, et al. Botulism in the United States, 1899–1969. Am J Epidemiol. 1971;93:93–101. doi: 10.1093/oxfordjournals.aje.a121239. [DOI] [PubMed] [Google Scholar]
- 12.Jackson KA, Mahon BE, Copeland J, et al. Botulism mortality in the USA, 1975–2009. Botulinum J. 2015;3:6–17. doi: 10.1504/TBJ.2015.078132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacket CO, Shandera WX, Mann JM, Hargrett NT, Blake PA. Equine antitoxin use and other factors that predict outcome in type A foodborne botulism. Am J Med. 1984;76:794–8. doi: 10.1016/0002-9343(84)90988-4. [DOI] [PubMed] [Google Scholar]
- 14.Kongsaengdao S, Samintarapanya K, Rusmeechan S, et al. Thai Botulism Study Group. An outbreak of botulism in Thailand: clinical manifestations and management of severe respiratory failure. Clin Infect Dis. 2006;43:1247–56. doi: 10.1086/508176. [DOI] [PubMed] [Google Scholar]
- 15.Sobel J. Diagnosis and treatment of botulism: a century later, clinical suspicion remains the cornerstone. Clin Infect Dis. 2009;48:1674–5. doi: 10.1086/599030. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler C, Inami G, Mohle-Boetani J, Vugia D. Sensitivity of mouse bioassay in clinical wound botulism. Clin Infect Dis. 2009;48:1669–73. doi: 10.1086/599029. [DOI] [PubMed] [Google Scholar]
- 17.Sheth AN, Wiersma P, Atrubin D, et al. International outbreak of severe botulism with prolonged toxemia caused by commercial carrot juice. Clin Infect Dis. 2008;47:1245–51. doi: 10.1086/592574. [DOI] [PubMed] [Google Scholar]
- 18.Schussler E, Sobel J, Hsu J, et al. Workgroup report by the joint task force involving American Academy of Allergy, Asthma & Immunology (AAAAI); Food Allergy, Anaphylaxis, Dermatology and Drug Allergy (FADDA) (Adverse Reactions to Foods Committee and Adverse Reactions to Drugs, Biologicals, and Latex Committee); and the Centers for Disease Control and Prevention Botulism Clinical Treatment Guidelines Workgroup—Allergic reactions to botulinum antitoxin: a systematic review. Clin Infect Dis. 2017;66:S65–72. doi: 10.1093/cid/cix827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black RE, Gunn RA. Hypersensitivity reactions associated with botulinal antitoxin. Am J Med. 1980;69:567–70. doi: 10.1016/0002-9343(80)90469-6. [DOI] [PubMed] [Google Scholar]
- 20.Mottate K, Yokote H, Mori S, et al. Retrospective survey to evaluate the safety and efficacy of Japanese botulinum antitoxin therapy in Japan. Toxicon. 2016;110:12–8. doi: 10.1016/j.toxicon.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Hibbs RG, Weber JT, Corwin A, et al. Experience with the use of an investigational F(ab′)2 heptavalent botulism immune globulin of equine origin during an outbreak of type E botulism in Egypt. Clin Infect Dis. 1996;23:337–40. doi: 10.1093/clinids/23.2.337. [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration. [Accessed 11 October 2017];Approval history, letters, reviews, and related documents–BAT (botulism antitoxin heptavalent (A, B, C, D, E, F, G)–(Equine): final review process validation memo. Available at: https://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm345134.htm.
- 23.Connaught Medical Research Laboratories. Botulism antitoxin trivalent (equine) types A, B, and E, package insert. 1965 [Google Scholar]
- 24.Aventis Pasteur Limited. Botulism antitoxin bivalent (equine) types A and B, package insert. 2001 [Google Scholar]
- 25.Wongtanate M, Sucharitchan N, Tantisiriwit K, et al. Signs and symptoms predictive of respiratory failure in patients with foodborne botulism in Thailand. Am J Trop Med Hyg. 2007;77:386–9. [PubMed] [Google Scholar]
- 26.Barash JR, Arnon SS. A novel strain of Clostridium botulinum that produces type B and type H botulinum toxins. J Infect Dis. 2014;209:183–91. doi: 10.1093/infdis/jit449. [DOI] [PubMed] [Google Scholar]
- 27.Maslanka SE, Luquez C, Dykes JK, et al. A novel botulinum neurotoxin, previously reported as serotype H, has a hybrid-like structure with regions of similarity to the structures of serotypes A and F and is neutralized with serotype A antitoxin. J Infect Dis. 2016;213:379–85. doi: 10.1093/infdis/jiv327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Masuyer G, Zhang J, et al. Identification and characterization of a novel botulinum neurotoxin. Nat Commun. 2017;8:14130. doi: 10.1038/ncomms14130. [DOI] [PMC free article] [PubMed] [Google Scholar]