Abstract
Background
Transcatheter aortic valve implantation (TAVI) is evolving rapidly and is increasingly being adopted in the treatment of aortic valve disease. The goal of this study was to examine regional differences in surgical aortic valve replacement (SAVR) and TAVI across Atlantic Canada.
Methods
We identified all patients who underwent SAVR or TAVI between Jan. 1, 2010, and Dec. 31, 2014, in New Brunswick, Nova Scotia and Newfoundland and Labrador. Data obtained included patient demographic characteristics and surgical procedure details. We performed univariate descriptive analyses and calculated crude and age- and sex-adjusted incidence rates.
Results
A total of 3042 patients underwent SAVR or TAVI during the study period, 1491 in Nova Scotia, 1042 in New Brunswick and 509 in Newfoundland and Labrador. Patient demographic characteristics were similar across regions. A much higher proportion of patients in Newfoundland and Labrador (43.6%) than in Nova Scotia (4.2%) or New Brunswick (13.6%) received a mechanical versus a bioprosthetic valve. Rates of TAVI increased over the study period, with New Brunswick adopting their program before Nova Scotia (144 v. 74 procedures). Adjusted rates of all AVR procedures remained stable in Nova Scotia (40–50 per 100 000 people). Adjusted rates were lower in New Brunswick and Newfoundland and Labrador than in Nova Scotia; they increased slowly in New Brunswick over the study period.
Conclusion
Despite geographical proximity and similar patient demographic characteristics, there existed regional differences in the management of aortic valve disease within Atlantic Canada. Further study is required to determine whether the observed differences in age- and sex-adjusted rates of AVR may be explained by geographical disease-related differences, varying practice patterns or barriers in access to care.
Abstract
Contexte
Le remplacement valvulaire aortique par cathéter, une méthode en pleine évolution, est de plus en plus utilisé pour le traitement des valvulopathies aortiques. Cette étude visait à examiner les différences régionales quant au remplacement valvulaire aortique par cathéter ou par chirurgie dans les provinces de l’Atlantique.
Méthodes
Nous avons recensé tous les patients ayant subi un remplacement valvulaire aortique entre le 1er janvier 2010 et le 31 décembre 2014 au Nouveau-Brunswick, en Nouvelle-Écosse et à Terre-Neuve-et-Labrador. Nous avons recueilli des données sur les caractéristiques démographiques des patients et les interventions chirurgicales, puis nous avons réalisé une analyse descriptive univariée et avons calculé les taux d’incidence bruts et corrigés selon l’âge et le sexe.
Résultats
En tout, 3042 patients ont subi un remplacement valvulaire aortique par cathéter ou par chirurgie pendant la période à l’étude : 1491 en Nouvelle-Écosse, 1042 au Nouveau-Brunswick et 509 à Terre-Neuve-et-Labrador. Les caractéristiques démographiques des patients étaient semblables d’une région à l’autre. La proportion des patients recevant une prothèse mécanique plutôt qu’une bioprothèse était beaucoup plus élevée à Terre-Neuve-et-Labrador (43,6 %) qu’en Nouvelle-Écosse (4,2 %) ou au Nouveau-Brunswick (13,6 %). Les taux de remplacement par cathéter ont augmenté au cours de la période à l’étude; le Nouveau-Brunswick a adopté un programme à ce sujet avant la Nouvelle-Écosse (144 c. 74 interventions). Les taux corrigés pour tous les remplacements étaient stables en Nouvelle-Écosse (40–50 par 100 000 habitants); ils étaient plus faibles au Nouveau-Brunswick et à Terre-Neuve-et-Labrador, mais ont augmenté lentement au Nouveau-Brunswick pendant la période à l’étude.
Conclusion
Malgré la proximité géographique des provinces de l’Atlantique et les caractéristiques démographiques semblables des patients, il existait des différences dans la prise en charge des valvulopathies aortiques. D’autres études seront nécessaires pour déterminer si les variations dans les taux de remplacement corrigés selon l’âge et le sexe pourraient s’expliquer par des différences géographiques dans le nombre de cas, des différences dans les pratiques ou des obstacles à l’accès aux soins.
Until recently, the standard of care for aortic valve disease had been surgical aortic valve replacement (SAVR), with reported low mortality rates (1%–2%).1–3 Advances in catheter techniques have led to a less-invasive approach and the development of transcatheter aortic valve implantation (TAVI). Randomized controlled trials have shown TAVI to have outcomes as good as or superior to those of SAVR and medical therapy in selected groups of patients with aortic valve disease.3,4 These successes have led to significant changes in the clinical approach for the treatment of aortic valve disease, with increasing rates of TAVI not only for patients who are poor candidates for surgery but now also in intermediate-risk populations.3,4
Compared to SAVR, TAVI is a resource-intensive treatment with substantial associated costs in both the short and long term.5 This has meant that TAVI implementation has been gradual, and the procedure remains relatively understudied. Given the managed care model in Canada, we are in a unique position to assess regional differences in real-world practice with the adoption of TAVI and its impact on SAVR. This is particularly true in Atlantic Canada, where only 3 major regional centres are responsible for the interventional care of all aortic valve disease, in Saint John, Halifax and St. John’s. These 3 centres serve roughly 2.5 million Canadians from New Brunswick, Nova Scotia, Prince Edward Island, and Newfoundland and Labrador.
Adoption of TAVI has been tied to health care spending per capita in Europe, with the number and penetrance of TAVI procedures varying considerably between nations.6 However, the penetrance of TAVI has not been fully explored in the Canadian context. Reimbursement strategies and health care funding have been suggested to be critical factors in adopting this new technology across a large geographic area.6 Although some economic studies suggest that TAVI may be a more cost-effective solution than SAVR for some patients with severe aortic stenosis, this remains to be shown in all patient groups.7–10 Consequently, studies examining the early use of TAVI are critical to understanding resource allocation and ensuring proper access for all Canadians.
Furthermore, there are limited data examining rates of AVR procedures in Canada, and none looking specifically at the regional impact of TAVI program on patterns of aortic valve surgery. A 2004 demographic study showed that patients undergoing AVR surgery were typically more than 65 years of age and male, and that the age- and sex-adjusted incidence rates of AVR procedures in Nova Scotia (16.8/100 000 people), New Brunswick (15.8/100 000) and Prince Edward Island (15.6/100 000) were similar to those in the rest of Canada; Newfoundland and Labrador had the lowest rates in Canada (11.0/100 000).11
The goal of the present study was to characterize the real-world treatment of aortic valve disease in Atlantic Canada, with particular attention to the adoption of TAVI and its influence on patterns of use of SAVR. In doing so, we hoped to identify the features of patients in whom SAVR and TAVI are performed and to determine the prevalence of these procedures at each of the 3 cardiac surgery centres in Atlantic Canada.
Methods
The present work represents input from the Atlantic Cardiovascular Quality Initiative, comprising clinicians representing each of the 3 cardiac centres in Atlantic Canada where interventional treatment for aortic valve disease is provided: the New Brunswick Heart Centre, Saint John, the Maritime Heart Centre, Halifax, and the Health Sciences Centre, St. John’s.
Data sources
All data for this study were obtained from the institutionally established registry data at each of the 3 regional cardiac surgery centres in Atlantic Canada. For the initial phase of this study, only data on use were used, and only aggregate data were shared for the purpose of research dissemination. Each centre obtained appropriate institutional research ethics approval for the present study.
All consecutive patients who underwent AVR (SAVR or TAVI) alone or in combination with other cardiac surgical procedures between Jan. 1, 2010, and Dec. 31, 2014, were included for analysis. Procedure information included the type of valve used (mechanical or bioprosthesis) and whether additional procedures were completed (coronary artery bypass grafting, alternative valve replacement, other cardiac, noncardiac). Patient characteristics included age, sex and type of procedure performed. We defined renal insufficiency based on the Society of Thoracic Surgery (STS) risk model as a creatinine level greater than 176 mmol/L. To allow provincial procedure rates to be standardized to the age distribution of the national population, we derived all denominator data from the 2011 census administered by Statistics Canada.
Indications for transcatheter aortic valve implantation
In all regions, a multidisciplinary TAVI team was created that included members from cardiac surgery, cardiology, interventional radiology, anesthesia and nursing. The cases of all patients considered for TAVI were reviewed by at least a surgeon and a cardiologist to determine suitability. The procedure was offered to patients who were believed to be at higher risk based on clinical criteria, supported by standardized scores (STS score [surgical (> 8) or intermediate (4–8)], EuroSCORE and Frailty Index).12 A final decision to proceed to TAVI was vetted in all cases by the multidisciplinary team. Investigations to determine suitability included cardiac catheterization, contrast computed tomographic angiography of the arterial anatomy and appropriate echocardiography investigation. The preferred approach was transfemoral, with alternative approaches considered only when TAVI was not feasible transfemorally.
Statistical analysis
We conducted univariate descriptive analysis on all variables. We determined counts and proportions for all variables. Variables with a nonnormal distribution are reported as median and interquartile range. We calculated crude rates of isolated SAVR, TAVI and SAVR in conjunction with other procedures and adjusted them for age and sex distribution using the 2011 Canadian population; they are expressed as the number of cases per 100 000 people. We performed all analyses using SAS statistical software version 9.4 (SAS Institute).
Results
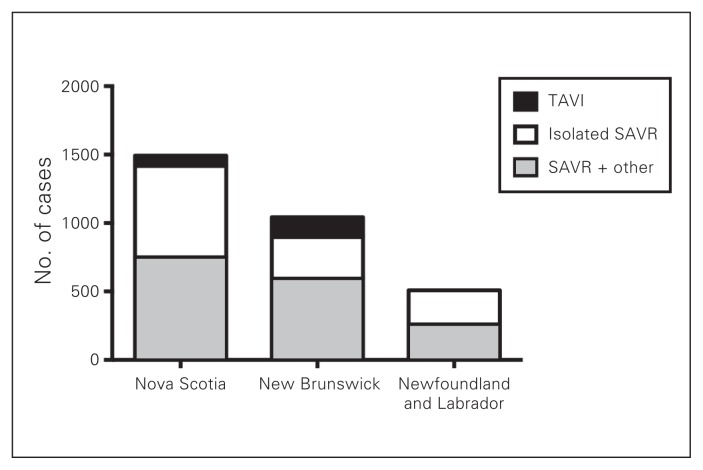
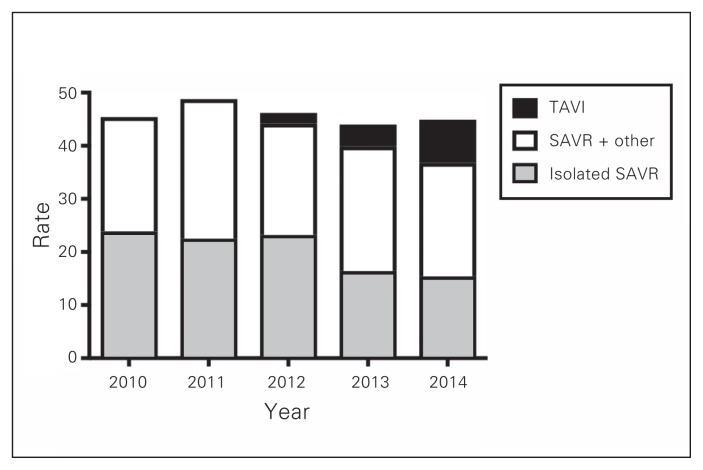
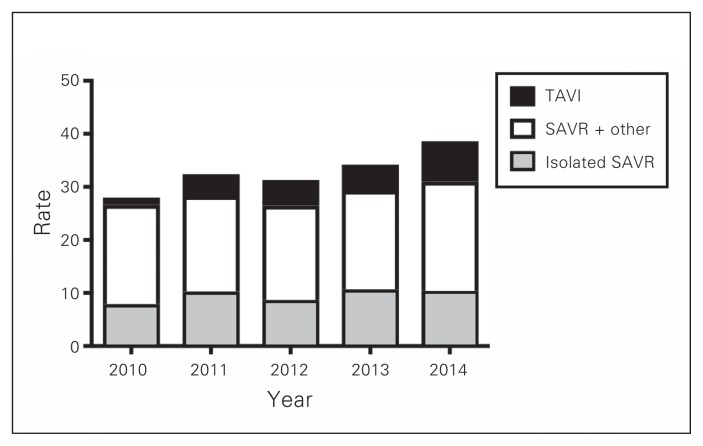
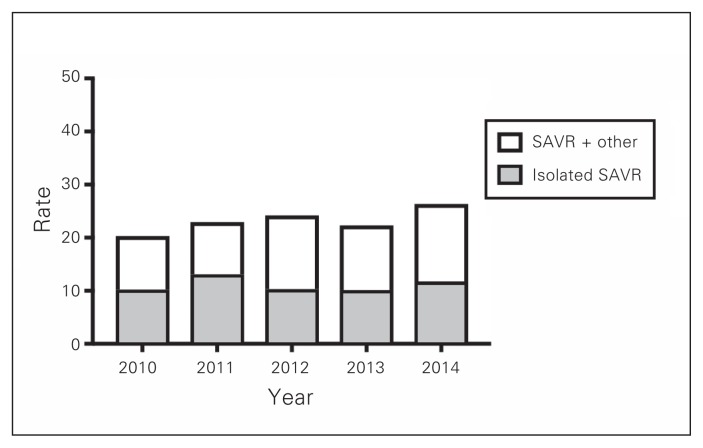
During the 5-year study period, a total of 3042 patients underwent AVR in Atlantic Canada: 1491 in Nova Scotia, 1042 in New Brunswick and 509 in Newfoundland and Labrador. The number of patients who underwent TAVI, isolated SAVR and SAVR in conjunction with other procedures is illustrated in Fig. 1. The distribution of procedures among TAVI, isolated SAVR and SAVR plus other procedure in the 3 provinces is shown in Fig. 2, Fig. 3 and Fig. 4. Most interventions were surgical, with a small proportion of patients undergoing TAVI. In both New Brunswick and Nova Scotia, TAVI numbers increased yearly; Newfoundland and Labrador did not start its TAVI program until 2016.
Fig. 1.
Total volumes of aortic valve interventions across Nova Scotia, New Brunswick, and Newfoundland and Labrador from Jan. 1, 2010, to Dec. 31, 2014. SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation.
Fig. 2.
Age- and sex-adjusted rates of aortic valve interventions per 100 000 people in Nova Scotia. SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation.
Fig. 3.
Age- and sex-adjusted rates of aortic valve interventions per 100 000 people in New Brunswick. SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation.
Fig. 4.
Age- and sex-adjusted rates of aortic valve interventions per 100 000 people in Newfoundland and Labrador. SAVR = surgical aortic valve replacement.
Patient characteristics and prosthetic choice
In each region, most patients undergoing AVR were aged 65 years or more and male, and a substantial number had a history of coronary artery disease. Patient characteristics are shown in Table 1 (Nova Scotia), Table 2 (New Brunswick) and Table 3 (Newfoundland and Labrador). Patients who underwent TAVI were older than those who underwent SAVR, which suggests higher predicted surgical risk. EuroSCORE values were available for Nova Scotia only: isolated SAVR had the lowest median risk score, at 2.9 (interquartile range [IQR] 1.5–6.5), and TAVI had the highest, at 8.2 (IQR 4.2–11.4).
Table 1.
Characteristics of patients undergoing aortic valve surgery in Nova Scotia between Jan. 1, 2010, and Dec. 31, 2014
| Characteristic | Procedure; no. (%) of patients* n = 1491 |
||
|---|---|---|---|
| Isolated SAVR n = 668 |
TAVI n = 74 |
SAVR + other n = 749 |
|
| Age, yr | |||
| 20–49 | 37 (5.5) | 0 (0.0) | 71 (9.5) |
| 50–64 | 191 (28.6) | 2 (2.7) | 183 (24.4) |
| 65–74 | 213 (31.9) | 8 (10.8) | 237 (31.6) |
| ≥ 75 | 227 (34.0) | 64 (86.5) | 258 (34.4) |
| Age, mean ± SD, yr | 69 ± 8.3 | 79 ± 4.8 | 68 ± 8.1 |
| Male sex | 435 (65.1) | 46 (62.2) | 560 (74.8) |
| Diagnosis | |||
| Coronary artery disease | 174 (26.0) | 50 (67.6) | 507 (67.7) |
| Chronic renal failure | 43 (6.4) | 2 (2.7) | 59 (7.9) |
| Diabetes | 213 (31.9) | 18 (24.3) | 222 (29.6) |
| NYHA class III heart failure | 307 (46.0) | 41 (55.4) | 276 (36.8) |
| NYHA class IV heart failure | 104 (15.6) | 18 (24.3) | 150 (20.0) |
| Valve type | |||
| Bioprosthesis | 651 (97.4) | 74 (100.0) | 704 (94.0) |
| Mechanical | 17 (2.5) | 0 (0.0) | 45 (6.0) |
| EuroSCORE, median (Q1–Q3) | 2.9 (1.5–6.5) | 8.2 (4.2–11.4) | 8.0 (4.4–17.0) |
NYHR = New York Heart Association; SAVR = surgical aortic valve replacement; SD = standard deviation; TAVI = transcatheter aortic valve implantation.
Except where noted otherwise.
Table 2.
Characteristics of patients undergoing aortic valve surgery in New Brunswick between Jan. 1, 2010, and Dec. 31, 2014
| Characteristic | Procedure; no. (%) of patients* n = 1042 |
||
|---|---|---|---|
| Isolated SAVR n = 304 |
TAVI n = 144 |
SAVR + other n = 594 |
|
| Age, yr | |||
| 20–49 | 28 (9.2) | 0 (0.0) | 43 (7.2) |
| 50–64 | 90 (29.6) | 4 (2.8) | 167 (28.1) |
| 65–74 | 102 (33.6) | 27 (18.8) | 198 (33.3) |
| ≥ 75 | 84 (27.6) | 113 (78.5) | 186 (31.3) |
| Age, mean ± SD, yr | 67 ± 9.1 | 77 ± 5.3 | 69 ± 8.7 |
| Male sex | 189 (62.2) | 69 (47.9) | 427 (71.9) |
| Diagnosis | |||
| Coronary artery disease | 39 (12.8) | 94 (67.6)† | 382 (64.3) |
| Chronic renal failure | 8 (2.6) | 10 (6.9) | 31 (5.2) |
| Diabetes | 73 (24.0) | 49 (34.0) | 171 (28.8) |
| NYHA class III heart failure | 92 (30.3) | 73 (50.7) | 142 (23.9) |
| NYHA class IV heart failure | 62 (20.4) | 40 (27.8) | 235 (39.6) |
| Valve type | |||
| Bioprosthesis | 256 (84.2) | 144 (100.0) | 499 (84.0) |
| Mechanical | 48 (15.8) | 0 (0.0) | 94 (15.8) |
| Homologous | 0 (0.0) | 0 (0.0) | 1 (0.2) |
NYHR = New York Heart Association; SAVR = surgical aortic valve replacement; SD = standard deviation; TAVI = transcatheter aortic valve implantation.
Except where noted otherwise.
n = 139.
Table 3.
Characteristics of patients undergoing aortic valve surgery in Newfoundland and Labrador between Jan. 1, 2010, and Dec. 31, 2014
| Characteristic | Procedure; no. (%) of patients* n = 509 |
|
|---|---|---|
| Isolated SAVR n = 249 |
SAVR + other n = 260 |
|
| Age, yr | ||
| 20–49 | 27 (10.8) | 19 (7.3) |
| 50–64 | 86 (34.5) | 74 (28.5) |
| 65–74 | 85 (34.1) | 94 (36.2) |
| ≥ 75 | 51 (20.5) | 73 (28.1) |
| Age, mean ± SD, yr | 63 ± 10.1 | 65 ± 9.0 |
| Male sex | 162 (65.1) | 195 (75.0) |
| Diagnosis | ||
| Coronary artery disease | 26 (10.4) | 204 (78.5) |
| Chronic renal failure | 20 (8.0) | 32 (12.3) |
| Diabetes | 71 (28.5) | 91 (35.0) |
| NYHA class III heart failure | 54 (21.7) | 45 (17.3) |
| NYHA class IV heart failure | 19 (7.6) | 16 (6.2) |
| Valve type | ||
| Bioprosthesis | 125 (50.2) | 153 (58.8) |
| Mechanical | 122 (49.0) | 100 (38.5) |
| Unknown | 2 (0.8) | 7 (2.7) |
NYHR = New York Heart Association; SAVR = surgical aortic valve replacement; SD = standard deviation.
Except where noted otherwise.
Overall, 2606 patients (85.7%) received a bioprosthetic valve. However, large regional differences in the use of bioprostheses were observed, with Nova Scotia using the highest proportion (95.8%), and Newfoundland and Labrador (54.6%), the lowest. Most patients aged 65 years or more received bioprostheses (94.3% in Nova Scotia, 83.1% in New Brunswick and 97.7% in Newfoundland and Labrador).
Age- and sex-adjusted procedure rate
The age- and sex-adjusted rates of aortic valve surgery in Nova Scotia are shown in Fig. 2. Overall rates appeared stable during the study period, peaking at 48.8/100 000 in 2011. Rates of TAVI increased over the study period, whereas rates of isolated SAVR decreased. Overall, Nova Scotia appeared to have highest rates of aortic valve intervention among the Atlantic provinces.
In contrast, in New Brunswick, adjusted rates of all aortic valve interventions increased yearly, peaking at 38.6/100 000 in 2014 (Fig. 3). Unlike in Nova Scotia, there was no apparent decrease in rates of isolated SAVR. Rates of TAVI increased during the study period, whereas rates of isolated SAVR and SAVR plus other procedures largely remained unchanged. Rates of isolated SAVR and SAVR plus other procedures were higher in Nova Scotia than in New Brunswick, whereas rates of TAVI were similar between the 2 provinces.
In Newfoundland and Labrador, adjusted rates of aortic valve procedures increased between 2010 and 2014, peaking at 26.4/100 000 in 2014 (Fig. 4). In general, rates of any aortic valve intervention were lower in Newfoundland and Labrador than in Nova Scotia or New Brunswick over the study period.
Discussion
In this study of rates of use of isolated SAVR, TAVI and SAVR plus other procedures across 3 regional centres in Atlantic Canada in 2010–2014, we found that aortic valve interventions were common, with nearly 600 procedures performed yearly. The overall unadjusted rates of aortic valve procedures were 35.2/100 000 in New Brunswick, 32.4/100 000 in Nova Scotia and 19.8/100 000 in Newfoundland and Labrador.
Overall, TAVI rates increased over the study period. New Brunswick was first to start a TAVI program, in 2010, and, as such, provides the largest Atlantic Canadian experience with TAVI, accounting for 66% of procedures. Not unexpectedly, TAVI was more likely to be performed in older patients with generally higher surgical risks. Although in general patient demographic characteristics appeared similar between geographic regions, patients in Newfoundland and Labrador were slightly younger than those in Nova Scotia and New Brunswick. This suggests that access for older patients (aged 65 yr or older) with aortic valve disease may be significantly different between regions; however, this has yet to be proven. One could also speculate that access in Newfoundland and Labrador may have increased as of 2016, when the TAVI program was started in that province.
We observed substantial differences between regions in the proportion of patients who received biological versus mechanical valves. In Nova Scotia, less than 5% of all valves implanted were mechanical, whereas in Newfoundland and Labrador, 44% of valves were mechanical. These differences cannot be explained simply on the basis of patient age, with a slightly older population in Nova Scotia than in the 2 other provinces. The practice pattern seen in Nova Scotia was well established before the adoption of TAVI, in 2012, with previously published rates of bioprosthesis use greater than 80%.13 In addition, there has been a general change worldwide in practice favouring bioprostheses, with which repeat surgery may be less of a concern when TAVI is an option.14,15 In fact, the differences we observed represent important practice variations that are unique to the philosophy and interpretation of the evidence by the clinician group practising at each institution. It should be noted that these observations are not intended to determine which centre is performing AVR more appropriately. Furthermore, these practice variations make it difficult to compare provinces in terms of the demographic characteristics of patients in whom SAVR and TAVI are performed.
Few studies have looked specifically at rates of use at a population level with age- and sex-adjusted rates to allow comparison. In the Canadian context, Hassan and colleagues11 reviewed rates of valvular surgery from 1994/95 to 1999/2000 across all Canadian provinces but did not include TAVI. In a study investigating the adoption of TAVI across 11 European countries (2007–2011), the authors suggested that the incidence of the procedure ranged from 0.6/100 000 in Portugal to 8.9/100 000 in Germany.6 Rates of use of TAVI have continued to increase, particularly in Germany, and TAVI has become the standard of care for older populations, with this procedure now surpassing SAVR.16 Our most recent TAVI incidence rates (8.1/100 000 in Nova Scotia and 7.0/100 000 in New Brunswick) appear similar to what was seen in European countries in 2011 and, as such, may reflect the later adoption of the technology in Atlantic Canada. The growth of TAVI in both Nova Scotia and New Brunswick was likely in part tied to the funding programs that are available for the procedure. The details of the funding arrangements, including availability of additional funding should the need for TAVI increase, was not explored in the present study.
The age- and sex-adjusted incidence rates of AVR were higher in Nova Scotia (peak of 48.8/100 000) than in New Brunswick (peak of 38.6/100 000) or Newfoundland and Labrador (peak of 26.4/100 000). In contrast, TAVI rates were similar between Nova Scotia and New Brunswick. Interestingly, the introduction of TAVI did not lead to an increase in the total number of aortic valve interventions, such as has been observed in other studies.3,6 Published age- and sex-adjusted rates of aortic valve intervention in the United States approach 100/100 000.17 The non-negligible differences in rates of aortic valve interventions observed in Atlantic Canada suggest potentially important differences in access, practice patterns and/or patient characteristics that would account for these differences; however, examination of these factors was beyond the scope of the present study. Further work is needed to better understand what barriers to timely access exist to the care of patients with aortic stenosis in Atlantic Canada.
A study in Nova Scotia showed that overall demand for cardiac surgery decreased between 2001 and 2010.13 In the present study, the overall incidence rates of valvular surgery in Nova Scotia remained unchanged between 2010 and 2014, whereas the rates increased in New Brunswick and Newfoundland and Labrador, although still remaining below those of Nova Scotia. It should be noted that, for the purposes of this study, we analyzed data for all patients who received care at a particular centre as originating from that region and did not specifically try to identify out-of-region patients. Some patients may have received their care in other jurisdictions (e.g., patients from northern New Brunswick undergoing surgery in Quebec).
Strengths and limitations
The present study represents the results of an important collaborative data-sharing initiative among centres participating in the Atlantic Cardiovascular Quality Initiative. Given the centralization of care in Canada and the limited number of centres providing advanced cardiac care, this has allowed us to capture all the aortic valve procedures performed across Atlantic Canada. This also allows our observations to be generalizable. A limitation of this study is the use of 2011 census data to obtain our age- and sex-adjusted rates. Another limitation is the fact that we did not separate patients based on their province of residence when considering incidence rates of aortic valve surgery. The proportion of patients undergoing AVR outside of their home province is likely low; however, patients from Prince Edward Island may undergo the surgery at either the Nova Scotia or the New Brunswick cardiac surgery centre. Finally, we were unable to include certain variables, such as left ventricle function scores, in the analysis owing to differences in data definitions between centres. If these variables had been included, comparisons between centres may have been inaccurate.
Conclusion
Our study reflects findings from a novel procedure that is currently underresearched and that can have a large impact on an aging Canadian patient population. Examination of these data has already identified important inequalities from a resource allocation perspective and variations in practice patterns that have shifted our research efforts to better understand the reasons for our observations. Furthermore, our study represents the initial steps required to better understand real-world applications and adoption of novel procedures such as TAVI. Our work lays an important foundation for collaboration among the Atlantic regions in future research focusing on the cost-effectiveness and benefit of TAVI in Atlantic Canada.
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study and acquired the data, which C. McGuire, A. Yip, C. Adams, K. Melvin, A. Hassan and J.-F. Légaré analyzed. C. McGuire, A. Yip, C. Adams, A. Hassan and J.-F. Légaré wrote the article, which all authors reviewed and approved for publication.
References
- 1.Lung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014;30:962–70. doi: 10.1016/j.cjca.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Quality-based procedures clinical handbook for aortic valve disease. Cardiac Care Network of Ontario & Ministry of Health and Long-Term Care; 2016. [accessed 2018 Feb 27]. Available: www.health.gov.on.ca/en/pro/programs/ecfa/docs/hb_aortic_valve_disease.pdf. [Google Scholar]
- 3.Hahn RT, Pibarot P, Steward WJ, et al. Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis. J Am Coll Cardiol. 2013;61:2514–21. doi: 10.1016/j.jacc.2013.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 5.Neragi-Miandoab S, Michler RE. A review of the most relevant complications of transcatheter aortic valve implantation. ISRN Cardiol. 2013 May 12; doi: 10.1155/2013/956252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mylotte D, Osnabrugge RLJ, Windecker S, et al. Transcatheter aortic valve replacement in Europe. J Am Coll Cardiol. 2013;62:210–9. doi: 10.1016/j.jacc.2013.03.074. [DOI] [PubMed] [Google Scholar]
- 7.Fairbairn TA, Mead DM, Hulme C, et al. The cost-effectiveness of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis at high operative risk. Heart. 2013;99:914–20. doi: 10.1136/heartjnl-2013-303722. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds MR, Magnuso EA, Lei Y, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis. J Am Coll Cardiol. 2012;60:2683–92. doi: 10.1016/j.jacc.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Howard-Hancock RL, Feindel CM, Rodes-Cabau J, et al. Cost effectiveness of transcatheter aortic valve replacement compared to medical management in inoperable patients with severe aortic stenosis: Canadian analysis based on the PARTNER Trial Cohort B findings. J Med Econ. 2013;16:566–74. doi: 10.3111/13696998.2013.770747. [DOI] [PubMed] [Google Scholar]
- 10.Rosato S, Santini F, Barbanti M, et al. Transcatheter aortic valve implantation compared with surgical aortic valve replacement in low-risk patients. Circ Cardiovasc Interv. 2016;9:e003326. doi: 10.1161/CIRCINTERVENTIONS.115.003326. [DOI] [PubMed] [Google Scholar]
- 11.Hassan A, Newman AM, Gong Y, et al. Canadian Cardiovascular Outcomes Research Team. Use of valve surgery in Canada. Can J Cardiol. 2004;20:149–54. [PubMed] [Google Scholar]
- 12.Piérard L. Transcatheter aortic valve implantation: indications. Euro Soc Cardiol. 2016 Jan 12;14 doi: 10.1016/j.ejcts.2008.04.039. [DOI] [Google Scholar]
- 13.Buth KJ, Gainer RA, Legare JF, et al. The changing face of cardiac surgery: practice patterns and outcomes 2001–2010. Can J Cardiol. 2014;30:224–30. doi: 10.1016/j.cjca.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Kalavrousiotis D, Li D, Buth KJ, et al. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) is not appropriate for withholding surgery in high-risk patients with aortic stenosis: a retrospective cohort study. J Cardiothorac Surg. 2009;4:32. doi: 10.1186/1749-8090-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JM, O’Brien SM, Wu C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: change in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Eggebrecht H, Mehta RH. Transcatheter aortic valve implantation (TAVI) in Germany 2008–2014: On its way to standard therapy for aortic valve stenosis in the elderly? EuroIntervention. 2016;11:1029–33. doi: 10.4244/EIJY15M09_11. [DOI] [PubMed] [Google Scholar]
- 17.Barreto-Filho JA, Wang Y, Dodson JA, et al. Trends in aortic valve replacement for elderly patients in the United States, 1999–2011. JAMA. 2013;310:2078–84. doi: 10.1001/jama.2013.282437. [DOI] [PMC free article] [PubMed] [Google Scholar]