Abstract
Cognitive outcome data are reviewed with respect to the use of magnetic-resonance guided stereotactic laser ablation (SLA) as an epilepsy surgical procedure, with comparisons drawn to traditional open resection procedures. Cognitive outcome with stereotactic laser amygdalohippocampotomy (SLAH) appears better than open resection for several functions dependent on extra-mesial temporal lobe (TL) structures, including category-related naming, verbal fluency, and object/familiar person recognition. Preliminary data suggests episodic, declarative verbal memory can decline following SLAH in the language dominant hemisphere, although early findings suggest comparable or even superior outcomes compared with open resection. The hippocampus has long been considered a central structure supporting episodic, declarative memory, with epilepsy surgical teams attempting to spare it whenever possible. However, ample data from animal and human neuroscience research suggests declarative memory deficits are greater following broader mesial TL lesions that include parahippocampal gyrus and lateral TL inputs. Therefore, employing a neurosurgical technique that restricts the surgical lesion zone holds promise for achieving a better cognitive outcome. Focal SLA lesions outside of the amygdalohippocampal complex may impair select cognitive functions, although few data have been published in such patients to date. SLA is being effectively employed with adults and children with TL or lesional epilepsies across several U.S. epilepsy centers, which may simultaneously optimize cognitive outcome while providing a curative treatment for seizures.
Keywords: epilepsy surgery, cognitive outcome, memory, naming, laser interstitial thermal therapy, LITT
Introduction
Whether smaller surgical resections improve cognitive and functional outcome compared to more traditional anterior temporal lobectomies is a major topic of debate in the field of epilepsy surgery.1 While standard anterior temporal lobectomy (ATL) retains a small advantage over selective open resection approaches for seizure control,2 research has been mixed and generally inconclusive regarding differences in cognitive outcome.1, 3 The difficulty involved in sorting out this question is related to significant variability in the functions studied and the tests used to evaluate these abilities. However, another critical variable is that most open resection approaches result in significant “collateral” damage while accessing the mesial TL structures. For example, many of these approaches disturb the temporal stem, which is traversed by critical white matter tracts, including the uncinate fasciculus (UF) and the inferior fronto-occipital fasciculus (IFOF).4 Most also affect other extra-temporal structures, such as the fusiform gyrus, basal temporal language area, temporal pole, and additional white matter tracts. The emergence of a novel laser ablation technology holds promise for a minimally invasive approach that could reduce this secondary collateral damage.
Search Criteria for Current Review
While laser interstitial thermal therapy (LiTT) has been available for several years (see Hoppe et al. for an excellent review article on this topic),5 the current review is focused on cognitive outcome observed using the more recent use of magnetic resonance-guided stereotactic laser ablation (SLA) systems (Visualase®, Neuroblate®). Pubmed was searched using the terms LiTT, SLA, or laser ablation in conjunction with several permutations of cognitive function (i.e., cognitive function, memory, language, verbal fluency, executive function) between November 2016 and July 2017. Only articles with original cognitive outcome data following epilepsy surgery were examined (including case studies). This resulted in only six publications, three of which were case studies. One additional publication6 was added that was not identified by Pubmed search terms. This article was referenced in one of the other six publications. In addition, four SLA review articles were identified.
The case studies included hypothalamic hamartoma ablation in a patient who had previously undergone a right ATL,7 a SLA procedure involving the insular cortex of a patient with post-stroke epilepsy,8 and two TLE patients with normal neuroimaging who underwent SLAH.9 The four SLA publications with group data included only one study that was prospective in nature.10 This study compared TLE patients undergoing SLAH (n=19) with those undergoing open resection procedures (n=39) on naming and object recognition tasks. One additional study compared TLE patients undergoing SLAH (n=7) to a selective amygdalohippocampectomy procedure (n=7) on a variety of clinical measures, but only had post-surgical data available for three to four patients from each patient group.6 The remaining two group level studies retrospectively examined clinical memory measures for a subset of patients who returned for follow-up evaluation and both lacked any comparison control group. Kang et al.11 presented verbal memory data available on six of 20 SLAH patients, while Jermackowicz et al.12 reported verbal and visual memory data for 20 of 23 SLAH patients. The latter study also reported on some other clinical measures as available for analysis as well. As can be seen, there remains a dearth of publications related to the cognitive outcome of SLA. Additionaly, prospective, well-controlled studies are lacking in this area.
Because no studies with children came up in Pubmed with the specified search terms, this search was redone with specific terms for children. This combination of terms led to an additional four publications involving children, although none of these included formal cognitive data. Therefore, this review does not cover cognitive outcome of SLA in children, apart from a brief mention of the successful application of SLA to treat hypothalamic hamartoma.
Potential for Improved Cognitive Outcome with Laser Ablation
Object Recognition and Naming Outcome
Drane and colleagues10 have demonstrated superior cognitive outcome for select functions with SLAH when prospectively compared to a variety of open resection approaches, including both standard and selective procedures. Naming of man-made objects and/or famous persons declined in 21 of 22 TLE patients undergoing language dominant open resection, while no significant decline on these tasks was experienced by any dominant SLAH patient (n=10). Similarly, 11 of 17 TLE patients undergoing right (nondominant) open resection experienced significant decline in their ability to recognize famous persons, while no TLE patients undergoing SLAH experienced any decline on this task (n=9). Duffau and colleagues demonstrated in a series of tumor resection patients that even a subtle restriction in naming response speed following surgery predicts poor functional recovery when gauged by a successful return to gainful employment.13 Busch and colleagues recently examined man-made object naming in a large cohort of open resection epilepsy surgery patients, demonstrating that naming decline is a significant problem which they believe still lacks adequate attention by the epilepsy community (significant declines occurred in greater than 40% of left TL resections and 5% of right TL resections).14 Overall, initial data suggests SLAH is superior to a variety of open resection surgical approaches for avoiding cognitive decline on tasks likely supported by extra-mesial TL regions. The findings of Drane et al.10 with visual confrontational naming ability have been confirmed in two additional publications,9, 12 and this group has more recently presented data on the first 40 patients to undergo SLAH at Emory University, with still no significant decline on any of the naming and recognition measures examined.15
Episodic Memory Outcome
Very few data have been published describing laser ablation and memory function, and some of the reports have presented contradictory results. Differences across studies are likely due to the very small sample sizes in existing memory studies, procedural differences with SLA (e.g., potential variability in the extent of ablation and/or number of laser device passes in some cases), and variability in statistical analysis (e.g., reliable change methodology versus standard deviation change), test selection, timing of post-ablation assessment, and patient cohorts. For example, one small case series involved only TLE patients with normal neuroimaging who were assessed in the early stages of recovery (i.e., two patients examined clinically approximately 3-4 months following surgery),9 while another study only examined older patients, some of whom had significant comorbid medical issues.6 Most of these studies described the results of basic clinical care, with some groups only providing post-surgical testing to those patients with clinical complaints. This practice often introduces bias, with good outcome patients not returning for clinical follow-up.
Three studies have reported outcome on a verbal contextual memory task (i.e., story recall, Logical Memory subtest from the 4th edition of the Wechsler Memory Scale),16 with results appearing somewhat mixed at least from a surface level review. Kang et al.11 indicated that none of six patients (5 dominant/1non-dominant) declined on this task using reliable change indices. In contrast, Jermakowicz et al.12 reported that a significant decline on this task was observed for the learning portion of this task at the group level. Of note, however, when exploring outcome on an individual level, only three of 20 patients (15%) actually declined on this task (2/10 dominant, 1/10 nondominant). Finally, Waseem et al.,6 found that one of four older TLE patients declined on the learning portion of this task (and one improved) following language dominant SLAH, while none of these four patients declined with regards to delayed recall. Across all three studies, only three of 19 (15.8%) language dominant hemisphere patients declined following SLAH on this contextual learning task. These numbers compare favorably to historical data involving memory outcome with language dominant TLE patients undergoing open resection procedures, where memory decline often occurs in 30 to 50% of such patients.17-20
Kang et al.11 reported that three of six (50%) patients declined on the learning portion of a verbal list-learning task that provides semantic cues (i.e., 2nd edition of the California Verbal Learning Test),21 and that two patients also exhibited decline on their subsequent recall of this information. In contrast, Waseem et al.6 indicated that 2 of 4 SLAH patients improved significantly on the delayed recall portion of a complex list-learning task (Rey Auditory Verbal Learning Test),22 while only one patient experienced decline. Jermakowicz et al.12 did not report on a list-learning task in their study.
Dredla et al.9 provide cognitive outcome information for two PET positive, MRI-negative patients who underwent SLAH. Both patients had adult onset of seizures, placing them at a high risk of decline, with one of the patients having a history of encephalitis, raising the possibility of bilateral disease. Both patients experienced significant decline on verbal and visual memory measures, although no declines in naming, verbal fluency, or other assessed cognitive functions were present. Of note, however, both patients were assessed during the early stages of recovery (i.e., 3 to 4 months), and neither returned for subsequent follow-up. These researchers reported that they had performed over 20 SLAH procedures, with most patients reporting no cognitive complaints, although formal cognitive testing was not undertaken.
Researchers from Emory University have presented preliminary data suggesting episodic memory outcome is better in TLE patients undergoing SLAH as compared to comparable open resection patients.15, 23 Only 3 of 20 (15%) prospectively studied SLAH patients showed any significant decline on clinical memory measures (i.e., Rey Auditory Verbal Learning Test & Visual Reproduction from the Wechsler Scale) at 6-month follow-up using reliable change methodology. This percentage is again much smaller than the historical rate of decline for dominant TLE open resections in the research literature.18-20 Only two of 13 language dominant SLAH cases (15.4%) demonstrated significant decline on the RAVLT in this series. Both of these patients had normal imaging, although one of them returned to her baseline level of function by 1-year follow-up. 15 More outcome data is needed with TLE patients with normal neuroimaging and language dominant onset, and epilepsy programs should likely take a cautious approach to such patients when memory appears generally intact (i.e., alternative surgical strategies such as repetitive neurostimulation [RNS] could be considered as an initial treatment option).
Given that initial findings across studies suggest a rate of declarative verbal memory decline following SLAH that is less than commonly cited figures for open resections,18 these results suggest that declarative verbal memory decline is worse when broader damage is sustained to the TL that extends beyond the amygdalohippocampal complex in language dominant patients. Such findings could be seen as consistent with several studies in the animal literature related to memory decline, which also indicate broader mesial TL regions must be involved to affect episodic memory.24-26 On a related theme, researchers from Emory University have reported significant verbal memory declines in two patients undergoing SLA of cavernous malformations located in the white matter of the left fusiform gyrus of the language dominant hemisphere.27 The SLA procedure was restricted to the cavernous malformations and the hemosiderin ring around each lesion with complete sparing of all mesial TL structures. However, both patients experienced greater verbal memory decline than the majority of their SLAH cases, whose ablations involved the hippocampus and amygdala only. It seems possible that these fusiform gyrus ablations may have removed structures providing input to mesial TL regions, and that such damage may have been more disruptive to memory functioning than limited ablations of the mesial TL structures only. Others have suggested a role for lateral aspects of the TL in memory, and have noted that disturbing inputs to the memory system likely affect the overall performance of this system.28-30 Of note, it would not be expected that verbal memory would have reorganized away from mesial TL structures in these individuals, as their lesions were in the lateral TL region. Taken together, such data suggests the presumed role of the hippocampus in memory may be more complex than currently taught in most clinical settings, and that we may need to rethink our long held beliefs about the structure-function of possible memory circuits and what needs to be preserved during TL surgery. Although clinical epilepsy programs often focus on sparing the hippocampus, memory experts in the neuroscience community have indicated that broader regions of the TL play critical roles in memory.24, 25, 31 Research with SLA appears to be confirming the results of many animal and human studies which suggest an important contribution of the parahippocampal gyrus, and which have demonstrated that broader lesions are more destructive than smaller, hippocampal-based insult. In fact, even in Scoville and Milner's seminal paper involving H.M.,32 the authors wrote that H.M.'s memory deficits could have resulted from injury to the hippocampus, the parahippocampal gyrus, or perhaps both.
There have been two case studies involving SLA, both of which reported declines in verbal memory functioning. One of these involved using SLA to successfully treat post-stroke epilepsy arising from the insula of a patient's language dominant cerebral hemisphere.8 Post-surgical neuropsychological testing 8 months after testing revealed declines of at least 1 SD in complex list learning and retention of this information (CVLT-2), semantic fluency, and complex auditory comprehension, but stable functioning on verbal contextual learning (WMS-4 Logical Memory), visual memory measures, naming ability, and most remaining cognitive constructs. Improvements were observed on a few measures as well. The other case involved treating a hypothalamic hamartoma with SLA in a patient who had previously undergone a right ATL.7 The researchers indicated that the patient's ablation damaged the bilateral medial mammillary bodies (greater damage on the side contralateral to the ATL) and the adjacent left thalamus. The patient exhibited a severe amnestic syndrome during post-surgical follow-up approximately 8 months after surgery. Given that the latter complication occurred in a patient with hypothalamic hamartoma, it should be noted that Wilfong and Curry have reported on successful use of SLA for treating these lesions in a series of 14 pediatric patients (12 patients became seizure free).33 They report that their patients did not experience the typical complications associated with an open resection approach to hypothalamic hamartoma (e.g., hemiparesis, hormonal dysfunction, severe memory declines), although they did not report any results of formal cognitive testing. Overall, coupled with the aforementioned ablations of cavernous malformations,27 it is clear that patients can be harmed cognitively by SLA if damage is done to critical brain regions or networks, and that the presence of dual pathology and the results of prior surgical interventions need to be considered when assessing associated procedural risks.
Broader Cognitive Outcome
While broader outcomes are still lacking for SLA, preliminary data from Emory University related to their cavernous malformation patient series suggests that focal decline may occur when lesions are made in any brain regions continuing to play a functional role in critical neural networks.27 For example, some patients exhibited focal declines in aspects of naming and verbal fluency when lesions were made in more lateral TL regions (e.g., fusiform gyrus, temporal pole).
Nevertheless, such patients often showed sparing of aspects of these functions in a manner not previously reported (e.g., naming and semantic fluency declined for one category of object but improved for another). This is likely due to the focal nature of ablations that can be made with laser technology, which may affect limited portions of white matter bundles while sparing other sections. Greater preservation of even partial white matter tracts resulting from the exquisitely focal lesions made with this procedure may allow greater recovery of function over time. Significant improvement in broad, general cognitive functions in such patients has also been observed, likely resulting from improved neural processing in regions distant from the focal ablation. For example, TLE patients experiencing excellent seizure control often experience improvements in general attention, processing speed, and executive functions, a finding that can also be observed following open resections.34
Summary and Future Directions
Preliminary data suggest MR-guided SLA may offer a significantly better cognitive outcome than open resection in many circumstances,10, 11 presumably because this procedure allows for focal tissue ablation with minimal collateral damage. Initial research has mostly focused on TLE patients undergoing SLAH, and suggests that functions more dependent on extra-mesial TL structures are better preserved, including naming, verbal fluency, and object and person recognition. Improved declarative verbal memory outcome also appears promising with SLAH, with initial reports suggesting comparable or lessor rates of memory decline than observed with open resection surgical techniques.11, 12, 15 Nevertheless, verbal memory declines have been reported in TLE samples, and may be more likely to occur in patients with normal neuroimaging results.9 Limited cognitive outcome data are available from SLA of epileptic foci related to focal lesions (e,g., cavernous malformation, hypothalamic hamartoma) or less common seizure onset zones (e.g., insular cortex onset), and there is virtually no cognitive data from patients with SLA procedures performed on other extra-temporal brain regions. As can be seen from the case studies and presentations reviewed,7, 8, 27 significant deficits can occur from SLA when functional brain networks are disrupted by ablation. However, open resection techniques are likely to be equally or more destructive in such cases as broader brain regions will be affected. Given the capacity of SLA to reach brain regions that are difficult to access with open resection procedures, and its ability to create highly focal damage zones, it will often be a tool to be considered in these difficult cases. Studying the effects of such small surgical lesions also provides a unique opportunity to study structure-function brain relationships.
Whether or not SLA proves to be consistently superior to open resection techniques for sparing cognitive function, its use in clinical practice will ultimately depend heavily on its ability to control seizures. Just as selective approaches to open resection have been shown to produce slightly lower rates of seizure freedom than standard ATL,35 SLA will likely fall short in this area as well given the more limited extent of tissue destruction. Nevertheless, early results of seizure outcome with SLA suggest that it may approach the reported rates of seizure freedom following open resection in patients with mesial temporal sclerosis (MTS).36 Given the potential benefits of better preserved cognitive function, reduced time of recovery, and the minimally-invasive nature of the procedure, SLA will be seen as an advantageous approach by many patients. Additionally, if a patient fails to become seizure free following SLA, they can still elect to undergo an open resection procedure.
Future research is needed to confirm preliminary cognitive outcome results with SLA, to explore the patients most at risk for cognitive decline (e.g., normal MRI, intact cognitive function), to evaluate a full range of cognitive abilities, and to insure that open resection and SLA samples are equivalent across many relevant variables, such as age of seizure onset, baseline level of function, seizure duration, seizure freedom, and lesion status (e.g., MTS, non-lesional, dual-pathology). It will also be important to investigate outcome in terms of mood/psychiatric variables, functional status, and quality of life. Greater use of neuroimaging and electrophysiological techniques is needed to predict which patients are most likely to benefit from SLA, and to improve targeting of seizure onset zones. Moreover, traditional neuropsychological testing does not include many cognitive constructs potentially of great relevance in understanding the functional limitations of most epilepsy patients. This includes the category-specific naming and fluency deficits that have been demonstrated in recent years,37 as well as object and face recognition deficits. Limited research suggests that the importance of right temporal lobe function is under-appreciated, with potential functions supported by this region including aspects of emotional processing, theory of mind, social cognition, feeling of knowing/familiarity, and face processing.38 The true value of SLA will only be recognized as these subtle yet important aspects of cognitive processing are more fully appreciated by the epilepsy community. Many still underestimate the effects of cognitive dysfunction resulting from epilepsy surgery on long-term functional status.
Multisite studies designed to evaluate multifaceted outcome parameters are needed. While a randomized comparison to various open resection options would be ideal, recruitment for such a study will likely be difficult given the current availability of SLA. While SLA is unlikely to completely supplant open resection due to the broad neural networks that often underlie seizure onset, this procedure appears to be gaining a firm foothold among the surgical options for seizure management, and may well become the first choice for individuals concerned about their long-term functional outcome who are deemed appropriate candidates based upon their seizure onset.
Figure 1.
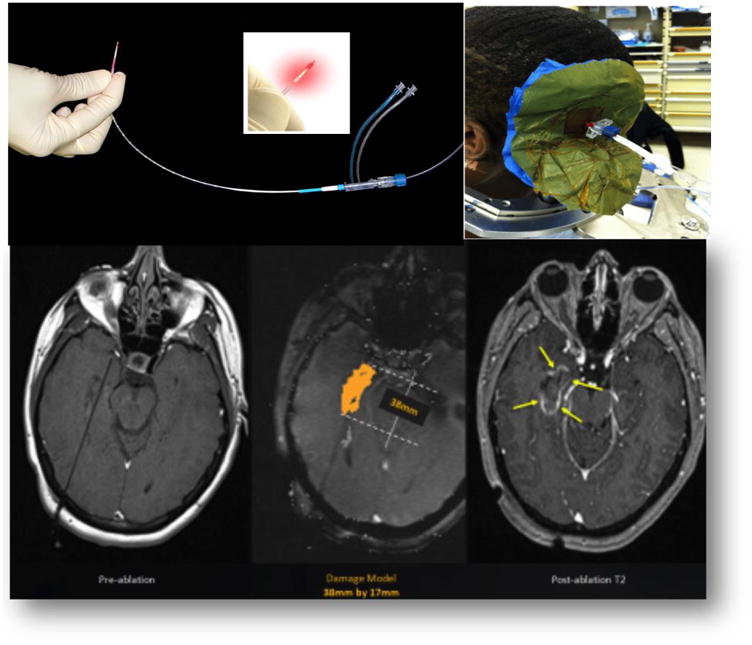
Depiction of the Optical Fiber, the Ablation Process, and Pre- and Post-Ablation MRI Images in an Axial Plane Demonstrating the Focal Nature of the Damage Zone.
Figure 2.
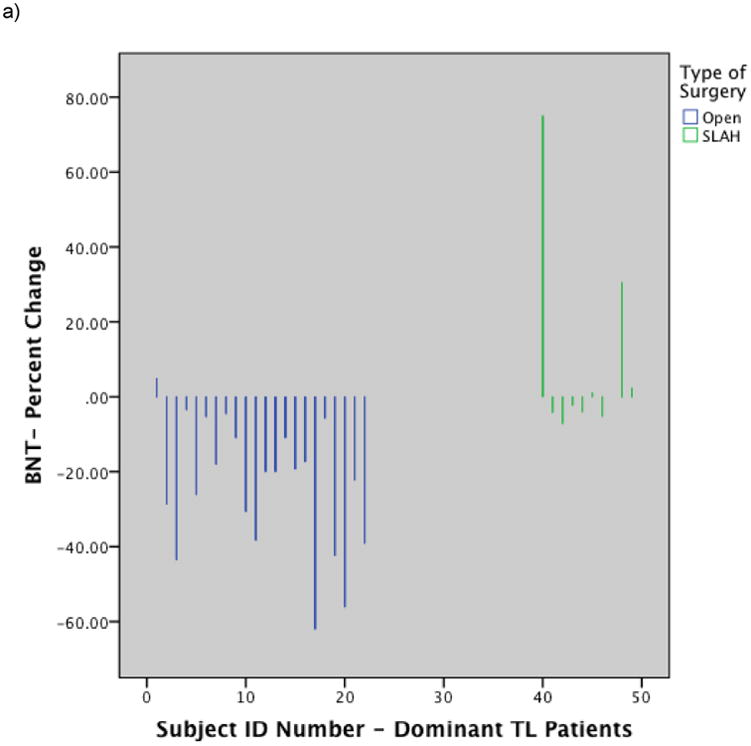
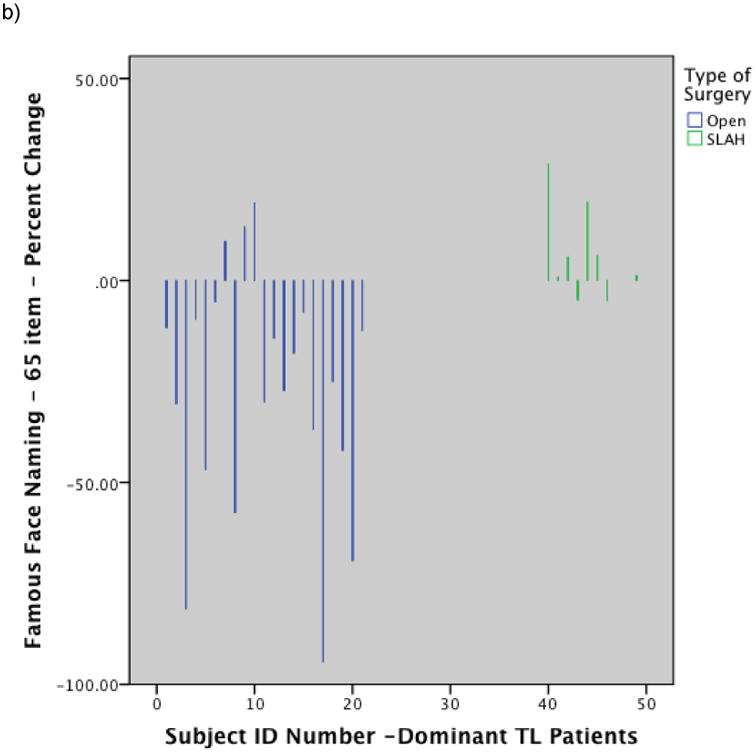
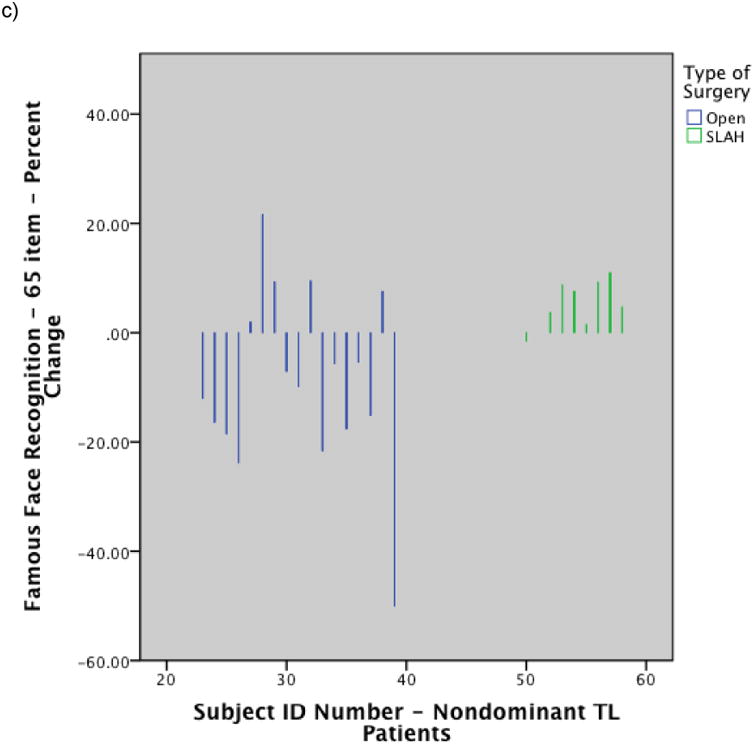
Histograms showing individual level performance change by type of surgery: a) Change in BNT raw score for dominant temporal lobe cases by surgery type; b) Change in Famous Face naming performance on modified Iowa Famous Faces Test for dominant temporal lobe cases by surgery type; c) Change in Famous Face recognition performance on modified Iowa Famous Faces Test for nondominant temporal lobe cases by surgery type. Note. SLAH = stereotactic laser amygdalohippocampotomy; BNT = Boston Naming Test; TL = Temporal Lobe. Reprinted from Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia 2015;56:101-113.
Highlights.
Preliminary studies suggest that stereotactic laser ablation allows for a reduction in collateral damage during neurosurgical procedures targeting the amygdalar-hippocampal complex as compared to standard open resection surgical procedures for the control of epilepsy.
Sparing neural regions and white matter pathways in temporal lobe epilepsy surgery using stereotactic laser ablation results in preservation of language functions (particularly naming ability) and recognition of objects and famous persons.
Preliminary evidence of better episodic memory preservation in patients undergoing stereotactic laser amygdalohippocampotomy versus standard open resection surgical procedures involving the anterior temporal lobes suggests that memory networks may be broader than the current models that emphasize the primacy of the hippocampus in declarative memory.
Acknowledgments
My appreciation goes to Dr. David Loring for taking the time to provide editorial suggestions for this manuscript.
Disclosures: Dr. Drane has received three grants from the NIH/NINDS, which have supported his work in this area at both Emory University and the University of Washington (K23 NSO49100, K02 NS070960, R01NS088748). Additionally, Dr. Drane has received a research grant from Medtronic, Inc. (A1225797BFN:1056035), and is also leading the core laboratory (neuroimaging and cognitive testing) for Medtronic's multisite trial of laser ablation (Stereotactic Laser Ablation for Temporal Lobe Epilepsy [SLATE]). These grants have provided salary support for Dr. Drane and his laboratory staff, and covered the cost of neuroimaging and other study related expenses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Helmstaedter C. Cognitive outcomes of different surgical approaches in temporal lobe epilepsy. Epileptic Disorders. 2013;15:221–239. doi: 10.1684/epd.2013.0587. [DOI] [PubMed] [Google Scholar]
- 2.Wiebe SB, Girvin JP, Eliasziw M. A randomized controlled trial of surgery for temporal-lobe epilepsy. New England Journal of Medicine. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 3.Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalophippocampectomy: A study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87–95. doi: 10.1046/j.1528-1157.2002.24101.x. [DOI] [PubMed] [Google Scholar]
- 4.Sarubbo S, De Benedictis A, Maldonado IL, Basso G, Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: Anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Structure and Function. 2013;218:21–37. doi: 10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe C, Witt JA, Helmstaedter C, Gasser T, Vatter H, Elger CE. Laser interstitial thermotherapy (LiTT) in epilepsy surgery. Seizure. 2017;48:45–52. doi: 10.1016/j.seizure.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Waseem H, Osborn KE, Schoenberg MR, et al. Laser ablation therapy: An alternative treatment for medically resistant mesial temporal lobe epilepsy after age 50. Epilepsy and Behavior. 2015;51:152–157. doi: 10.1016/j.yebeh.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Zubkov S, Del Bene VA, MacAllister WS, Shepherd TM, Devinsky O. Disabling amnestic syndrome following stereotactic laser albation of a hypothalamic hamartoma in a patient with prior temporal lobectomy. Epilepsy and Behavior Case Reports. 2015;4:60–62. doi: 10.1016/j.ebcr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawasli AH, Bandt SK, Hogan RE, Werner N, Leuthardt EC. Laser ablation as treatment strategy for medically refractory dominant insular epilepsy: Therapeutic and functional considerations. Stereotactic and Functional Neurosurgery. 2014;92:397–404. doi: 10.1159/000366001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dredla BK, Lucas JA, Wharen RE, Tatum WO. Neurocognitive outcome following stereotactic laser ablation in two patients with MR-/PET+ mTLE. Epilepsy and Behavior. 2016;56:44–47. doi: 10.1016/j.yebeh.2015.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Drane DL, Loring DW, Voets NL, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56:101–113. doi: 10.1111/epi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang JY, Wu C, Tracy J, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2016;57:325–334. doi: 10.1111/epi.13284. [DOI] [PubMed] [Google Scholar]
- 12.Jermakowicz WJ, Kanner AM, Sur S, et al. Laser thermal ablation for mesiotemporal epilepsy: Analysis of ablation volumes and trajectories. Epilepsia. 2017;58:801–810. doi: 10.1111/epi.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moritz-Gasser S, Herbet G, Maldonado IL, Duffau H. Lexical access speed is significantly correlated with the return to professional activities after awake surgery for low-grade gliomas. Journal of Neurooncology. 2012;107:633–641. doi: 10.1007/s11060-011-0789-9. [DOI] [PubMed] [Google Scholar]
- 14.Busch RM, Floden DP, Prayson B, et al. Estimating risk of word-finding problems in adults undergoing epilepsy surgery. Neurology. 2016;87:2363–2369. doi: 10.1212/WNL.0000000000003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drane DL. Minimally invasive surgical procedures: Cognitive advantages and rationale for use. American Epilepsy Society; Houston, Texas: 2016. [Google Scholar]
- 16.Wechsler D. Wechsler Memory Scale - 4th Edition (WMS-IV) technical and interpretive manual. San Antonio, Tx: Pearson; 2009. [Google Scholar]
- 17.Stroup ES, Langfit JT, Berg M, McDermott M, Pilcher W, Como P. Predicting verbal memory decline following anterior temporal lobectomy. Neurology. 2003;60:1266–1273. doi: 10.1212/01.wnl.0000058765.33878.0d. [DOI] [PubMed] [Google Scholar]
- 18.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: A systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 19.Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Annals of Neurology. 2003;54:425–432. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- 20.Baxendale S, Thompson PJ, Sander JW. Neuropsychological outcomes in epilepsy surgery patients with unilateral hippocampal sclerosis and good preoperative memory function. Epilepsia. 2013;54:131–134. doi: 10.1111/epi.12319. [DOI] [PubMed] [Google Scholar]
- 21.Delis D, Kaplan E, Kramer J, Ober B. California Verbal Learning Test - II. San Antonio, Texas: The Psychological Corporation; 2000. [Google Scholar]
- 22.Rey A. L'Examen Clinique en Psychologie. Paris: Press Universitaire de France; 1958. [Google Scholar]
- 23.Drane DL, Gross RE. Updates on cognitive outcome with stereotactic laser ablation. American Epilepsy Society; Seattle, WA: 2014. [Google Scholar]
- 24.Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. Journal of Neuroscience. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippoocampus. 2004;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]
- 26.Mishkin M. Memory in monkeys severely impaired by combined but not separate removal of the amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- 27.Drane DL, Willie JT, Loring DW, et al. Cognitive outcome of patients undergoing sterotactic laser ablation of cavernous malformations to control chronic seizures. American Epilepsy Society; Seattle, WA: 2014. Dec, 2014. [Google Scholar]
- 28.Haglund MM, Ojemann GA, Schwartz TW, Lettich E. Neuronal activity in human lateral temporal cortex during retrieval from short-term memory. The Journal of Neuroscience. 1994;14:1507–1515. doi: 10.1523/JNEUROSCI.14-03-01507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojemann GA, Creutzfeldt O, Lettich E, Haglund MM. Neuronal activity in human lateral temporal cortex related to short-term verbal memory, naming and reading. Brain. 1988;111(Pt. 6):1383–1403. doi: 10.1093/brain/111.6.1383. [DOI] [PubMed] [Google Scholar]
- 30.Fuster JM, Jervey JP. Neuronal firing in the inferotemporal cortex on performance on visual memory tasks. Journal of Neuroscience. 1982;2:361–375. doi: 10.1523/JNEUROSCI.02-03-00361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annual Review of Neuroscience. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neuropsychiatry and Clinical Neuroscience. 1957;12:103–113. doi: 10.1176/jnp.12.1.103. [DOI] [PubMed] [Google Scholar]
- 33.Wilfong AA, Curry DJ. Hypothalamic hamartomas: Optimal approach to clinical evaluation and diagnosis. Epilepsia. 2013;54:109–114. doi: 10.1111/epi.12454. [DOI] [PubMed] [Google Scholar]
- 34.Hermann B, Seidenberg M. Executive system dysfunction in temporal lobe epilepsy: Effects of nociferous cortex versus hippocampal pathology. Journal of Clinical and Experimental Neuropsychology. 1995;17:809–819. doi: 10.1080/01688639508402430. [DOI] [PubMed] [Google Scholar]
- 35.Josephson CB, Dykeman J, Fiest KM, et al. Systematic review and metaanalysis of standard vs selective temporal lobe epilepsy surery. Neurology. 2013;80:1669–1676. doi: 10.1212/WNL.0b013e3182904f82. [DOI] [PubMed] [Google Scholar]
- 36.Gross RE, Willie JT, Drane DL. The role of stereotactic laser amygdalohippocampotomy in mesial temporal lobe epilepsy. Neurosurgery Clinics of North America. 2016;27:37–50. doi: 10.1016/j.nec.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drane DL, Ojemann JG, Phatak V, et al. Famous face identification in temporal lobe epilepsy: Support for a multimodal integration model of semantic memory. Cortex. 2013;49 doi: 10.1016/j.cortex.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drane DL. Neuropsychological evaluation of the epilepsy surgical candidate. In: Barr WB, Morrison C, editors. Handbook on the neuropsychology or epilepsy. NY: Springer; 2015. [Google Scholar]


