Abstract
Objective
To determine the association between self-reported vasomotor symptoms (VMS) and obstructive sleep apnea (OSA) risk.
Methods
The STOP-BANG to evaluate OSA and Menopause Rating Scale (MRS) were administered to 2,935 women seen in the Women’s Health Clinic at Mayo Clinic in Rochester, MN between May 2015 and December 2016. Of these, 1,691 women were included in the analysis. Total MRS and VMS ratings were compared using logistic regression, with age, smoking, and body mass index (BMI) included as covariates between women at intermediate/high risk versus low risk for OSA.
Results
Total MRS scores were significantly higher in women with intermediate/high risk OSA scores versus those with low risk scores [mean (SD): 16.8 (8.0) vs 12.9 (7.0), P<0.001]. Women at intermediate/high OSA risk were older, had more education, self-reported hypertension, BMI >35 kg/m2, and were less likely to be married or employed. Self-reported severe/very severe VMS were significantly associated with intermediate/high risk vs low risk for OSA (26.6% vs 15.0%; P<0.001). After adjusting for age, BMI, and smoking status, the odds of having intermediate/high risk for OSA were 1.87 times higher for those with severe/very severe VMS compared to those with none/mild/moderate VMS (95% CI: 1.29–2.71, P<0.001). This association persisted upon subgroup analysis based on BMI <25 kg/m2 (OR 2.15; 95% CI: 1.12–4.16, P=0.022).
Conclusion
Self-reported severe/very severe VMS were associated with intermediate/high risk for obstructive sleep apnea in midlife women, even in women with BMI <25 kg/m2. However, given the limitations of the STOP-BANG tool, OSA risk may have been overestimated.
Keywords: Vasomotor symptoms, menopause, obstructive sleep apnea, sleep disturbance, hot flashes
Introduction
The menopause transition is independently associated with poor sleep1–3 and appears to be associated with increased risk for obstructive sleep apnea (OSA).4–7 In addition, up to 80% of midlife women experience hot flashes or night sweats (vasomotor symptoms-VMS).8 Although there is an association between the presence of VMS and sleep disturbances in midlife women, it may be clinically difficult to distinguish sleep disturbances directly related to menopausal symptoms from those due to an underlying primary sleep disorder such as OSA.9,10
Although OSA is more common in men than women, the risk for OSA in women increases with age, obesity, and peri- and postmenopausal status.11,12 For example, the prevalence of OSA increases from 6.5% in women in their 30s to 16% in women in their 50s.13 In a sample of peri- and postmenopausal women who experienced disturbed sleep, 53% were found to have a primary sleep disorder (OSA, restless leg syndrome or both).10 The Sleep in Midlife Women Study also found that progression through the menopausal transition was associated with increasing severity of sleep disordered breathing.14
While men with OSA tend to have loud snoring, witnessed apneas, and snort arousals, women often present with atypical symptoms such as insomnia, headache, night sweats, nocturnal enuresis, fatigue, depression, and anxiety.15–19 These differences in clinical presentation may lead to under-diagnosis of OSA in women.11 Identifying OSA is important because it is associated with significantly increased risk for coronary heart disease, hypertension, stroke, atrial fibrillation, carotid atherosclerosis, depression and death.20–27 Although OSA and VMS have both been independently associated with menopause and with cardiovascular risk, the association between VMS and OSA in midlife women is unclear (Figure 1).28 The purpose of this study was to explore the relationship between self-reported VMS and OSA risk in midlife women using commonly available questionnaires in the clinical setting.
Figure 1. Associations between vasomotor symptoms, cardiovascular risk, obstructive sleep apnea, and menopause.
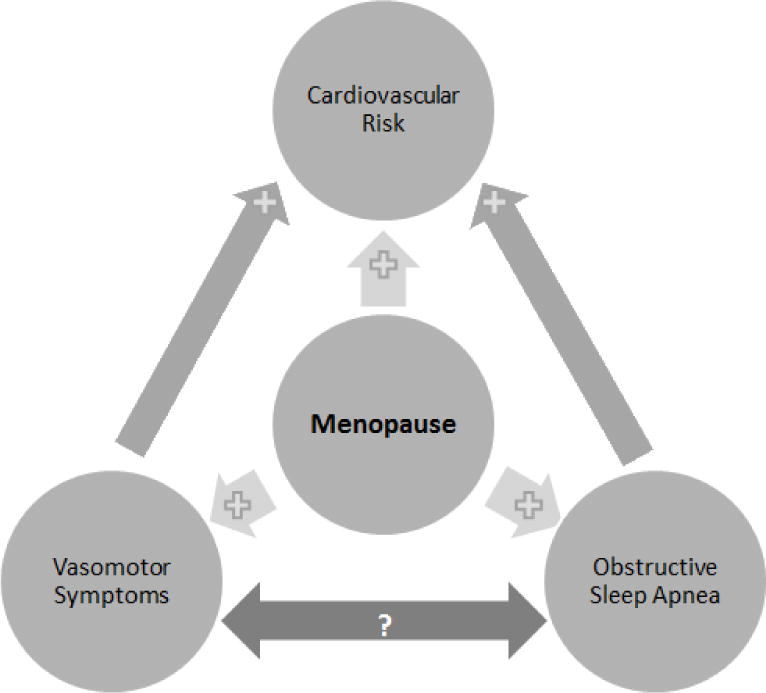
Light grey arrows between variables indicate a positive association in the direction of the plus sign, while the bidirectional dark grey arrow between vasomotor symptoms and obstructive sleep apnea indicate a potential association.
Methods
The Women’s Health Clinic (WHC) at Mayo Clinic, Rochester, MN is a subspecialty clinic that provides consultative care to women presenting with menopausal symptoms or sexual health concerns. Women seen in the WHC between May 2015 and December 2016 provided self-reported responses to the Menopause Rating Scale (MRS) and the STOP-BANG questionnaires as part of their clinical visit. Responses to these questionnaires are contained in a database called Data Registry on Experiences of Aging, Menopause and Sexuality (DREAMS). For the purposes of this study, only midlife women between the ages of 40 and 65 years were included in the analysis. Additional information about body mass index (BMI), current tobacco use, education, relationship status, employment status, race/ethnicity, menopausal status, menopausal hormone therapy use, and diagnoses of sleep disorders (using ICD codes) was obtained from the electronic medical record. Women provided written, informed consent for the use of their medical records for research purposes.
The MRS assesses the presence and severity of menopausal symptoms with 11 questions, each rated on a scale of 0–4 for severity (0=none; 1=mild; 2=moderate; 3=severe; 4=very severe). In addition to total MRS score, the questions assessing hot flashes/sweating and sleep problems (difficulty falling asleep, difficulty sleeping through the night, waking up early) were assessed individually.
OSA risk was assessed with the STOP-BANG questionnaire, which consists of 8 questions with self-reported yes/no answers to Snoring, Tired, Observed apneas, Pressure (hypertension), Body mass index >35 kg/m2, and numerical responses to Age, Neck size, and categorical response to Gender. Based on studies which included both men and women, the STOP-BANG has a high sensitivity of 87% and modest specificity of 43% for detection of moderate to severe OSA, which increases the risk of false positive screening results.29 As sensitivity and specificity data for the questionnaire are not available for women, for the purposes of the current study, scores were derived according to published formulation: low risk=yes to 0–2 questions; intermediate risk=yes to 3–4 questions; high risk=yes to 5–8 questions or yes to 2 or more STOP questions and BMI > 35 kg/m2.30 Unless otherwise specified, data are presented using mean ± standard deviation for continuous variables, and frequency percentages for categorical variables. The intermediate and high risk groups were combined to compare to the low risk group for a binary variable indicating OSA risk (yes versus no).
The answers to the individual MRS questions pertaining to hot flashes/sweating and sleep problems were grouped to produce binary variables: moderate/severe/very severe versus none/mild; severe/very severe versus none/mild/moderate. Univariate logistic regression was then performed with OSA risk as the dependent variable, and demographics or MRS measures as the explanatory variable. Multivariable logistic regression was used to assess the association between OSA risk and MRS measures after adjusting for age, smoking and BMI. All statistical tests were two-sided and the threshold statistical significance was set at p<0.05. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Of the 2,935 women between the ages of 40 and 65 years seen in consultation in WHC at Mayo Clinic in Rochester, MN between May 2015 and December 2016, 1,691 completed both the STOP-BANG and MRS questionnaires and provided consent for the use of their medical records for research. Of those, 147 (8.7%) had STOP-BANG scores representing high risk for OSA, 274 (16.2%) had intermediate risk scores, and 1,270 (75.1%) had low risk scores. Together, the intermediate and high risk categories added up to 24.9% of the women. Women with intermediate/high OSA risk scores were more likely to be older, have a higher BMI, and have self-reported hypertension. They were more likely to have education beyond high school, and were less likely to be married or to be employed. Women in the intermediate/high risk OSA group were more likely to be postmenopausal compared to those in the low risk group. The use of menopausal hormone therapy was similar across all STOP-BANG score groups (Tables 1 and 2).
Table 1. Participant Demographics by OSA risk group based on STOP-BANG scores and BMI>35kg/m2.
Summary of demographics for the 1,691 women in the study cohort from May 2015 through December 2016.
| Total (N=1691) |
Low (N=1270) |
Intermediate (N=274) |
High (N=147) |
p-valuea | Intermediate/High (N=421) |
p-valueb | |
|---|---|---|---|---|---|---|---|
| Age, Mean (SD) | 53.3 (6.1) | 52.9 (6.2) | 55.8 (4.8) | 52.3 (6.5) | <0.001 | 54.6 (5.7) | <0.001 |
| BMI, Mean (SD) | 26.7 (6.0) | 25.4 (4.9) | 28.4 (6.0) | 35.7 (6.4) | <0.001 | 31.0 (7.1) | <0.001 |
| Race | 0.35 | 0.12 | |||||
| White | 1576 (93.2%) | 1181 (93.0%) | 258 (94.2%) | 137 (93.2%) | 395 (93.8%) | ||
| Black or African American | 18 (1.1%) | 10 (0.8%) | 4 (1.5%) | 4 (2.7%) | 8 (1.9%) | ||
| Asian | 33 (2.0%) | 29 (2.3%) | 3 (1.1%) | 1 (0.7%) | 4 (1.0%) | ||
| Other | 28 (1.7%) | 23 (1.8%) | 3 (1.1%) | 2 (1.4%) | 5 (1.2%) | ||
| Unknown/Choose Not to Disclose | 36 (2.1%) | 27 (2.1%) | 6 (2.2%) | 3 (2.0%) | 9 (2.2%) | ||
| Menopausal Status | |||||||
| Missing | 1259 | 946 | 200 | 113 | 0.002 | 313 | 0.002 |
| Premenopausal | 44 (10.2%) | 42 (13.0%) | 1 (1.4%) | 1 (2.9%) | 2 (1.9%) | ||
| Perimenopausal | 62 (14.4%) | 51 (15.7%) | 6 (8.1%) | 5 (14.7%) | 11 (10.2%) | ||
| Postmenopausal | 293 (67.8%) | 208 (64.2%) | 63 (85.1%) | 22 (64.7%) | 85 (78.7%) | ||
| Unknown Menopause Status | 33 (7.6%) | 23 (7.1%) | 4 (5.4%) | 6 (17.7%) | 10 (9.3%) | ||
| Menopausal Hormone Therapy | 0.31 | 0.17 | |||||
| Missing | 236 | 187 | 30 | 19 | 49 | ||
| No | 1030 (70.8%) | 777 (71.8%) | 163 (66.8%) | 90 (70.3%) | 253 (68.0%) | ||
| Yes | 425 (29.2%) | 306 (28.3%) | 81 (33.2%) | 38 (29.7%) | 119 (32.0%) | ||
| Marital Status | 0.004 | 0.021 | |||||
| Married | 1407 (83.2%) | 1076 (84.7%) | 226 (82.5%) | 105 (71.4%) | 331 (78.6%) | ||
| Committed Relationship | 7 (0.4%) | 6 (0.5%) | 1 (0.4%) | 0 (0.0%) | 1 (0.2%) | ||
| Single | 121 (7.2%) | 83 (6.5%) | 18 (6.6%) | 20 (13.6%) | 38 (9.0%) | ||
| Separated | 8 (0.5%) | 3 (0.2%) | 2 (0.7%) | 3 (2.0%) | 5 (1.2%) | ||
| Divorced | 115 (6.8%) | 79 (6.2%) | 23 (8.4%) | 13 (8.8%) | 36 (8.6%) | ||
| Widowed | 18 (1.1%) | 13 (1.0%) | 2 (0.7%) | 3 (2.0%) | 5 (1.2%) | ||
| Unknown | 15 (0.9%) | 10 (0.8%) | 2 (0.7%) | 3 (2.0%) | 5 (1.2%) | ||
| Education | <0.001 | <0.001 | |||||
| High school graduate/GED or lower | 123 (7.7%) | 81 (67.1%) | 17 (6.7%) | 25 (17.7%) | 42 (10.6%) | ||
| Some College or 2 year degree | 446 (27.5%) | 311 (15.6%) | 85 (33.2%) | 50 (35.5%) | 135 (34.0%) | ||
| 4-year college graduate | 543 (33.5%) | 432 (9.5%) | 79 (30.9%) | 32 (22.7%) | 111 (28.0%) | ||
| Post graduate studies | 507 (31.3%) | 398 (7.8%) | 75 (29.3%) | 34 (24.1%) | 109 (27.4%) | ||
| Employment Status | <0.001 | <0.001 | |||||
| Employed | 1071 (65.3%) | 829 (67.1%) | 160 (61.3%) | 82 (57.7%) | 242 (60.0%) | ||
| Full time homemaker | 229 (14.0%) | 193 (15.6%) | 24 (9.2%) | 12 (8.5%) | 36 (8.9%) | ||
| Retired | 174 (10.6%) | 118 (15.6%) | 37 (14.2%) | 19 (13.4%) | 56 (13.9%) | ||
| Other | 165 (10.1%) | 96 (9.5%) | 40 (15.3%) | 29 (20.4%) | 69 (17.1%) | ||
| How often do you have a drink containing alcohol | 0.052 | 0.20 | |||||
| Never | 272 (18.0%) | 193 (17.2%) | 45 (18.0%) | 34 (24.6%) | 79 (20.4%) | ||
| ≤ 1 per month | 336 (22.3%) | 240 (21.4%) | 56 (22.4%) | 40 (29.0%) | 96 (24.7%) | ||
| 2–4 per month | 362 (24.0%) | 281 (25.1%) | 55 (22.0%) | 26 (18.8%) | 81 (20.9%) | ||
| 2–3 per week | 324 (21.5%) | 248 (22.1%) | 50 (20.0%) | 26 (18.8%) | 76 (19.6%) | ||
| 4+ per month | 215 (14.3%) | 159 (14.2%) | 44 (17.6%) | 12 (8.7%) | 56 (14.4%) | ||
| Smoking Status | 0.007 | 0.010 | |||||
| Never | 1210 (73.9%) | 932 (75.8%) | 183 (69.3%) | 95 (66.4%) | 278 (68.3%) | ||
| Former | 351 (21.4%) | 246 (20.0%) | 70 (26.5%) | 35 (24.5%) | 105 (25.8%) | ||
| Current | 76 (4.7%) | 52 (4.2%) | 11 (4.2%) | 13 (9.1%) | 24 (5.9%) |
p-value comparing Low vs Intermediate vs High
p-value comparing Low vs Intermediate/High
Table 2. Responses to components of the STOP-BANG questionnaire by OSA risk defined by STOP-BANG scores and BMI>35 kg/m2.
Summary of study cohort responses to the STOP-BANG questionnaire by low versus intermediate/high OSA risk groups.
| Total (N=1691) |
Low (N=1270) |
Intermediate (N=274) |
High (N=147) |
Intermediate/High (N=421) |
|
|---|---|---|---|---|---|
| STOP BANG total | |||||
| Mean (SD) | 1.9 (1.2) | 1.3 (0.7) | 3.2 (0.4) | 4.3 (0.9) | 3.6 (0.8) |
| Median | 2.0 | 1.0 | 3.0 | 4.0 | 3.0 |
| Snore loudly | |||||
| Missing | 8 | 6 | 1 | 1 | 2 |
| No | 1237 (73.5%) | 1109 (87.7%) | 95 (34.8%) | 33 (22.6%) | 128 (30.5%) |
| Yes | 446 (26.5%) | 155 (12.3%) | 178 (65.2%) | 113 (77.4%) | 291 (69.5%) |
| Tired, fatigued, sleepy | |||||
| Missing | 9 | 7 | 2 | 0 | 2 |
| No | 868 (51.6%) | 773 (61.2%) | 78 (28.7%) | 17 (11.6%) | 95 (22.7%) |
| Yes | 814 (48.4%) | 490 (38.8%) | 194 (71.3%) | 130 (88.4%) | 324 (77.3%) |
| Observed stop breathing or choking/gasping during sleep | |||||
| Missing | 5 | 0 | 1 | 4 | 5 |
| No | 1547 (91.8%) | 1246 (98.1%) | 212 (77.7%) | 89 (62.2%) | 301 (72.4%) |
| Yes | 139 (8.2%) | 24 (1.9%) | 61 (22.3%) | 54 (37.8%) | 115 (27.6%) |
| Hypertensiona | |||||
| Missing | 5 | 4 | 0 | 1 | 1 |
| No | 1425 (84.5%) | 1181 (93.3%) | 171 (62.4%) | 73 (50.0%) | 244 (58.1%) |
| Yes | 261 (15.5%) | 85 (6.7%) | 103 (37.6%) | 73 (50.0%) | 176 (41.9%) |
| Body mass index > 35 kg/m2 | |||||
| Missing | 332 | 260 | 68 | 4 | 72 |
| No | 1066 (78.4%) | 923 (91.4%) | 138 (67.0%) | 5 (3.5%) | 143 (41.0%) |
| Yes | 293 (21.6%) | 87 (8.6%) | 68 (33.0%) | 138 (96.5%) | 206 (59.0%) |
| Age > 50 | |||||
| No | 545 (32.2%) | 463 (36.5%) | 25 (9.1%) | 57 (38.8%) | 82 (19.5%) |
| Yes | 1146 (67.8%) | 807 (63.5%) | 249 (90.9%) | 90 (61.2%) | 339 (80.5%) |
| Neck size (≥16 inches/41cm) | |||||
| Missing | 343 | 205 | 76 | 62 | 138 |
| No | 1300 (96.4%) | 1061 (99.6%) | 181 (91.4%) | 58 (68.2%) | 239 (84.5%) |
| Yes | 48 (3.6%) | 4 (0.4%) | 17 (8.6%) | 27 (31.8%) | 44 (15.5%) |
| Gender | |||||
| Female | 1691 (100.0%) | 1270 (100.0%) | 274 (100.0%) | 147 (100.0%) | 421 (100.0%) |
Hypertension as self-reported on STOP-BANG
Total MRS scores were significantly higher in women at intermediate/high risk for OSA compared to those at low risk [mean (SD): 16.8 (8.0) vs 12.9 (7.0), P<0.001] (Table 3). After adjusting for age, BMI, smoking status, and self-reported hypertension, the odds of having an intermediate/high risk score for OSA are 1.34 times higher for women with moderate/severe/very severe VMS compared to those with none/mild VMS (95% CI: 0.99–1.81, P=0.063) and 1.87 times higher for women with severe/very severe hot flashes compared to those with none/mild/moderate VMS (95% CI: 1.29–2.71, p<0.001) (Table 4).
Table 3. MRS responses by OSA risk group based on STOP-BANG scores and BMI>35kg/m2.
Summary of study cohort responses to the MRS questionnaire by low versus intermediate/high OSA risk groups.
| Total (N=1691) |
Low (N=1270) |
Intermediate (N=274) |
High (N=147) |
p-valuea | Intermediate/High (N=421) |
p-valueb | |
|---|---|---|---|---|---|---|---|
| MRS #1 - VMSc | <0.001 | <0.001 | |||||
| Mean (SD) | 1.4 (1.1) | 1.4 (1.1) | 1.4 (1.1) | 1.9 (1.3) | 1.7 (1.3) | ||
| Median (IQR) | 1.0 (1.0, 2.0) | 1.0 (0.0, 2.0) | 1.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | ||
| MRS #1 - VMSc | <0.001 | <0.001 | |||||
| 0 | 403 (23.8%) | 319 (25.1%) | 58 (21.2%) | 26 (17.7%) | 84 (20.0%) | ||
| 1 | 537 (31.8%) | 419 (33.0%) | 86 (31.4%) | 32 (21.8%) | 118 (28.0%) | ||
| 2 | 448 (26.5%) | 341 (26.9%) | 63 (23.0%) | 44 (29.9%) | 107 (25.4%) | ||
| 3 | 206 (12.2%) | 139 (10.9%) | 41 (15.0%) | 26 (17.7%) | 67 (15.9%) | ||
| 4 | 97 (5.7%) | 52 (4.1%) | 26 (9.5%) | 19 (12.9%) | 45 (10.7%) | ||
| MRS #1 - VMSc | <0.001 | <0.001 | |||||
| None/Mild | 940 (55.6%) | 738 (58.1%) | 144 (52.6%) | 58 (39.5%) | 202 (48.0%) | ||
| Moderate/Severe/Very Severe | 751 (44.4%) | 532 (41.9%) | 130 (47.4%) | 89 (60.5%) | 219 (52.0%) | ||
| MRS #1 - VMSc | <0.001 | <0.001 | |||||
| None/Mild/Moderate | 1388 (82.1%) | 1079 (85.0%) | 102 (69.4%) | 207 (75.5%) | 309 (73.4%) | ||
| Severe/Very Severe | 303 (17.9%) | 191 (15.0%) | 45 (30.6%) | 67 (24.5%) | 112 (26.6%) | ||
| MRS #3 – Sleep problems | <0.001 | ||||||
| Mean (SD) | 1.9 (1.2) | 1.8 (1.2) | 1.6 (1.2) | 1.9 (1.3) | 2.1 (1.2) | ||
| Median (IQR) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | ||
| MRS #3 – Sleep problems | <0.001 | <0.001 | |||||
| 0 | 251 (14.9%) | 203 (16.0%) | 32 (11.7%) | 16 (10.9%) | 48 (11.5%) | ||
| 1 | 412 (24.5%) | 330 (26.1%) | 56 (20.4%) | 26 (17.7%) | 82 (19.7%) | ||
| 2 | 503 (29.9%) | 387 (30.6%) | 73 (26.6%) | 43 (29.3%) | 116 (27.8%) | ||
| 3 | 342 (20.3%) | 237 (18.7%) | 68 (24.8%) | 37 (25.2%) | 105 (25.2%) | ||
| 4 | 174 (10.3%) | 108 (8.5%) | 41 (15.0%) | 25 (17.0%) | 66 (15.8%) | ||
| MRS #3 – Sleep problems | <0.001 | <0.001 | |||||
| None/Mild | 663 (39.4%) | 533 (42.1%) | 88 (32.6%) | 42 (28.6%) | 130 (31.2%) | ||
| Moderate/Severe/Very Severe | 1019 (60.6%) | 732 (57.9%) | 182 (67.4%) | 105 (71.4%) | 287 (68.8%) | ||
| MRS #3 – Sleep problems | <0.001 | <0.001 | |||||
| None/Mild/Moderate | 1166 (69.3%) | 920 (72.7%) | 161 (59.6%) | 85 (57.8%) | 246 (59.0%) | ||
| Severe/Very Severe | 516 (30.7%) | 345 (27.3%) | 109 (40.4%) | 62 (42.2%) | 171 (41.0%) | ||
| MRS Total | <0.001 | <0.001 | |||||
| Mean (SD) | 13.9 (7.5) | 12.9 (7.0) | 15.7 (7.7) | 18.9 (8.2) | 16.8 (8.0) | ||
| Median (IQR) | 13.0 (9.0, 18.0) | 12.0 (8.0, 17.0) | 15.0 (11.0, 20.0) | 18.0 (13.0 (24.0) | 16.0 (12.0, 22.0) | ||
| Sleep Diagnosis prior to visit? | <0.001 | <0.001 | |||||
| Yes | 154 (9.1%) | 57 (4.5%) | 54 (19.7%) | 43 (29.3%) | 97 (23.0%) | ||
| No | 1537 (90.9%) | 1213 (95.5%) | 220 (80.3%) | 104 (70.8%) | 324 (77.0%) | ||
| Sleep Diagnosis prior to or up to 2 years after visit? | <0.001 | <0.001 | |||||
| Yes | 225 (13.3%) | 79 (6.2%) | 77 (28.1%) | 69 (46.9%) | 146 (34.7%) | ||
| No | 1466 (86.7%) | 1191 (93.8%) | 197 (71.9%) | 78 (53.1%) | 275 (65.3%) |
p-value comparing Low vs Intermediate vs High
p-value comparing Low vs Intermediate/High
Vasomotor symptoms (VMS): hot flashes and night sweats
Table 4. Univariate and multivariable models comparing OSA risk based on STOP-BANG scores and BMI>35kg/m2by VMS severity.
Analysis adjusted for age, BMI, and smoking status.
| Univariate Analysis | Multivariable Analysis (using none/mild vs Moderate/severe/very severe) | Multivariable Analysis (using none/mild/moderate vs Severe/very severe) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Intermediate/High OSA Risk (N=421) Mean (SD) | Low OSA Risk (N=1270) Mean (SD) | Odds Ratio | 95% CI | p value | Odds Ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value | |
| Age | 54.6 (6.2) | 52.9 (6.2) | 1.05 | (1.03, 1.07) | <0.001 | 1.05 | (1.03, 1.08) | <0.001 | 1.06 | (1.03, 1.08) | <0.001 |
| BMI | 31.0 (7.1) | 25.4 (4.9) | 1.17 | (1.14, 1.20) | <0.001 | 1.17 | (1.14, 1.20) | <0.001 | 1.17 | (1.14, 1.20) | <0.001 |
| Smoking Status, N (%) | |||||||||||
| Never | 278 (23.0%) | 932 (77.0%) | Reference | Reference | Reference | ||||||
| Former | 105 (29.9%) | 246 (70.1%) | 1.43 | (1.10, 1.87) | 0.42 | 1.10 | (0.77, 1.58) | 0.34 | 1.12 | (0.78, 1.61) | 0.41 |
| Current | 24 (31.6%) | 52 (68.4%) | 1.55 | (0.94, 2.56) | 0.31 | 1.88 | (0.97, 3.66) | 0.084 | 1.83 | (0.94, 3.59 | 0.11 |
| Hypertensiona, N (%) | |||||||||||
| No | 244 (17.1%) | 1181 (82.9%) | Reference | Reference | Reference | ||||||
| Yes | 176 (67.4%) | 85 (32.6%) | 10.02 | (7.47, 13.44) | <0.001 | 8.23 | (5.71, 11.85) | <0.001 | 8.34 | (5.78, 12.0) | <0.001 |
| MRS Total | 16.8 (8.0) | 12.9 (7.0) | 1.07 | (1.05, 1.09) | <0.001 | ||||||
| VMSb, N (%) | |||||||||||
| None/Mild | 202 (21.5%) | 738 (78.5%) | Reference | Reference | |||||||
| Moderate/severe/very severe | 219 (29.2%) | 532 (70.8%) | 1.54 | (1.23, 1.93) | <0.001 | 1.34 | (0.99, 1.81) | 0.063 | |||
| VMSb, N (%) | |||||||||||
| None/Mild/Moderate | 309 (22.3%) | 1079 (77.7%) | Reference | Reference | |||||||
| Severe/very severe | 112 (37.0%) | 191 (63.0%) | 2.15 | (1.64, 2.81) | <0.001 | 1.87 | (1.29, 2.71) | <0.001 | |||
Hypertension as self-reported on STOP-BANG
Vasomotor symptoms (VMS): hot flashes and night sweats
In a post-facto subgroup analysis in women with BMI <25 kg/m2, there was a significant association between severe/very severe VMS and intermediate/high risk of OSA after adjusting for age and smoking status (95% CI: 1.12–4.16, P=0.022). However, no significant association between moderate/severe/very severe VMS and intermediate/high risk of OSA was identified after adjusting for age and smoking status (Table 5).
Table 5. Univariate and multivariable models comparing OSA risk based on STOP-BANG scores and BMI>35kg/m2 by VMS severity: BMI subgroup analysis.
BMI subgroup analysis adjusted for age and smoking status.
|
BMI < 25
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariable Analysis (using none/mild vs Moderate/severe/very severe) | Multivariable Analysis (using none/mild/moderate vs Severe/very severe) | |||||||||
|
| |||||||||||
| Intermediate/High OSA Risk (N=64) Mean (SD) | Low OSA Risk (N=569) Mean (SD) | Odds Ratio | 95% CI | p value | Odds Ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value | |
| Age | 55.1 (5.1) | 53.2 (6.2) | 1.05 | (1.01, 1.10) | 0.031 | 1.05 | (1.004, 1.10) | 0.034 | 1.06 | (1.01, 1.11) | 0.024 |
| Smoking Status, N (%) | |||||||||||
| Never | 49 (10.4%) | 423 (89.6%) | Reference | Reference | Reference | ||||||
| Former | 12 (10.6%) | 101 (89.4%) | 1.03 | (0.53, 2.00) | 0.35 | 0.94 | (0.48, 1.85) | 0.47 | 0.93 | (0.47, 1.82) | 0.46 |
| Current | 1 (3.8%) | 25 (96.2%) | 0.35 | (0.05, 2.61) | 0.30 | 0.37 | (0.05, 2.83) | 0.35 | 0.36 | (0.05, 2.72) | 0.34 |
| MRS Total | 15.3 (7.9) | 12.9 (6.9) | 1.04 | (1.004, 1.08) | 0.028 | ||||||
| VMSa, N (%) | |||||||||||
| None/Mild | 36 (9.4%) | 346 (90.6%) | Reference | Reference | |||||||
| Moderate/severe/very severe | 28 (11.2%) | 223 (88.8%) | 1.12 | (0.66, 1.91) | 0.67 | 1.22 | (0.71, 2.09) | 0.47 | |||
| VMSa, N (%) | |||||||||||
| None/Mild/Moderate | 50 (9.2%) | 495 (90.8%) | Reference | Reference | |||||||
| Severe/very severe | 14 (15.9%) | 74 (84.1%) | 1.90 | (0.999, 3.62) | 0.051 | 2.15 | (1.12, 4.16) | 0.022 | |||
|
BMI ≥ 25
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariable Analysis (using none/mild vs Moderate/severe/very severe) | Multivariable Analysis (using none/mild/moderate vs Severe/very severe) | |||||||||
|
| |||||||||||
| Intermediate/High OSA Risk (N=264) Mean (SD) | Low OSA Risk (N=471) Mean (SD) | Odds Ratio | 95% CI | p value | Odds Ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value | |
| Age | 54.5 (5.8) | 53.0 (6.3) | 1.04 | (1.02, 1.07) | <0.001 | 1.05 | (1.02, 1.08) | <0.001 | 1.05 | (1.02, 1.08) | <0.001 |
| Smoking Status, N (%) | |||||||||||
| Never | 171 (33.9%) | 334 (66.1%) | Reference | Reference | Reference | ||||||
| Former | 67 (38.3%) | 108 (61.7%) | 1.21 | (0.85, 1.73) | 0.23 | 1.15 | (0.80, 1.65) | 0.14 | 1.15 | (0.80, 1.66) | 0.15 |
| Current | 21 (56.8%) | 16 (43.2%) | 2.56 | (1.30, 5.04) | 0.014 | 2.66 | (1.33, 5.31) | 0.010 | 2.62 | (1.31, 5.25) | 0.012 |
| MRS Total | 17.2 (8.2) | 12.6 (7.0) | 1.08 | (1.06, 1.11) | <0.001 | ||||||
| VMSa, N (%) | |||||||||||
| None/Mild | 120 (31.3%) | 264 (68.8%) | Reference | Reference | |||||||
| Moderate/severe/very severe | 144 (41.0%) | 207 (59.0%) | 1.59 | (1.17, 2.17) | 0.003 | 1.61 | (1.18, 2.20) | 0.003 | |||
| VMSa, N (%) | |||||||||||
| None/Mild/Moderate | 189 (32.7%) | 389 (67.3%) | Reference | Reference | |||||||
| Severe/very severe | 75 (47.8%) | 82 (52.2%) | 2.01 | (1.40, 2.90) | <0.001 | 2.03 | (1.39, 2.94) | <0.001 | |||
Vasomotor symptoms (VMS): hot flashes and night sweats
In women with intermediate/high OSA risk scores, 23% had a diagnosis of OSA by ICD codes prior to the WHC consultation, and another 11.7% received a diagnosis in the 2 years following their WHC visit (Table 3).
Discussion
In this large cross-sectional cohort of midlife women, total MRS scores and self-reported severe and very severe VMS ratings were significantly associated with intermediate/high risk for OSA as assessed by the highly sensitive, moderately specific STOP-BANG screening tool which may overestimate OSA risk. The nature of this association is unclear, but shared risks such as age, smoking status, and BMI do not fully account for the association because the findings persist after adjusting for these factors. Furthermore, an association between severe/very severe VMS and intermediate/high OSA risk persisted in subgroup analysis of women with BMI <25 kg/m2. This relationship most likely reflects interactions among neuronal circuits associated with temperature regulation (as might be related to vasomotor symptoms), those regulating sleep, and those controlling respiration as well as alterations in peripheral or central chemo-sensor sensitivity and their modulation by hormone status (e.g., levels of estrogen, progesterone or follicle stimulating hormone).31,32 Different patterns of VMS have been described in postmenopausal women,33 suggesting differences in neurovascular dysregulation or perhaps more general autonomic dysregulation that warrants further investigation.
Consistent with population-based data,11 about one-quarter (24.9%) of women in our cohort were at intermediate/high risk for OSA. An important finding of this study is that 65% of women in the intermediate/high risk OSA group remained without a diagnosis of OSA up to 2 years after their clinical consultation, which is also consistent with population-based data regarding the under-diagnosis of OSA in women.11 Our results underscore the importance of having a high level of clinical suspicion for the presence of OSA in menopausal women, particularly given the significant morbidity and mortality associated with untreated disease.34 For example, in a study of 207 pre- and postmenopausal women that assessed hypertension prevalence, BMI was the only factor that affected the blood pressure profile in premenopausal women, while both BMI and apnea hypopnea index affected the blood pressure profile in postmenopausal women.35 Another study of 277 midlife women concluded that OSA is underdiagnosed and independently associated with hypertension and increased arterial stiffness in perimenopausal women.36 The results of the present study suggest that the intensity of VMS might be a factor that links OSA and increased cardiovascular risk in menopausal women, thereby alerting the clinician to the need for further evaluation.
The conclusion that VMS may alert clinicians to increased cardiovascular risk is not new. Indeed, in a large population-based study involving 11,725 midlife women followed for 14 years, those reporting frequent VMS had an increased risk for incident CHD compared to those who did not, even after adjusting for multiple factors including age, menopausal status, lifestyle, BMI, diabetes and hypertension.37 Reduced flow-mediated vasodilatation was also associated with VMS in recent (<10 years from last menstrual period) and late (>10 years from last menstrual period) postmenopause phases in one study,38 but only among younger midlife women in another study.20 In the Women’s Ischemia Syndrome Evaluation (WISE) study, women with early onset VMS (first occurring before the age of 42 years) had lower flow-mediated dilatation and higher cardiovascular mortality than those who experienced later onset VMS.21 Overall, the aggregate data support an association between VMS and CHD risk,39,40 with a suggestion that night sweats more than daytime hot flashes may be associated with increases in blood pressure.41,42
The STOP-BANG self-report was used in the present study to assess OSA risk. Although in a community-based sample STOP-BANG is sensitive (87%) for detection of moderate to severe OSA in both men and women, the specificity is modest (43%) for detection of moderate to severe OSA and increases the risk of false positive screening results.29 In individuals with mid-range STOP-BANG scores (at lower risk for moderate to severe OSA), the specificity for OSA at any level is increased when combined with BMI ≥ 35 kg/m2.30 However, these studies may not be entirely applicable to the women as The Sleep Heart Health study showed that weight changes led to more significant changes in apnea hypopnea index in men than women.43 In addition, several studies have validated versions of the STOP-BANG in specific populations, including those of Chinese, Indian, and Danish descent, in whom BMI tends to be lower than in the US population.44–48 Yet, even at a lower BMI, the STOP-BANG remains a sensitive screening tool.47,48
While it is clear that menopausal hormone therapy (MHT) is helpful for VMS management and may mitigate CHD risk in younger postmenopausal women,49 the question of whether there is a role for MHT in the management of OSA or sleep disordered breathing in menopausal women is still unanswered. One study demonstrated that the prevalence of OSA was greater in postmenopausal women who did not receive MHT compared to premenopausal women and postmenopausal women on MHT.12 Estrogen and progesterone have been associated with increased sensitivity to hypercapnia and hypoxia, whereas ovariectomy has been associated with decreased sensitivity to hypoxia.50–52 One prospective randomized controlled trial investigating the impact of estrogen on sleep-related disordered breathing revealed that 3 months of unopposed transdermal estradiol was associated with decreased occurrence (P=0.047) and frequency (P=0.049) of sleep apnea in healthy postmenopausal women, but had no effect on partial upper airway obstruction.53 Further, lower estradiol levels were shown to be associated with increased OSA risk in a study of 30 depressed peri-/postmenopausal women.6 Another small study involving nine postmenopausal women showed that treatment with conjugated equine estrogens and medroxyprogesterone acetate was associated with reduced risk of sleep-related disordered breathing.54 However, given the lack of randomized controlled clinical trial data, no definitive conclusions can be made regarding the role of MHT for management of OSA or sleep-related disordered breathing.
To the best of our knowledge, this hypothesis generating study is the first to examine the association between VMS and OSA in midlife women. The strength of the study is the relatively large size of our cohort. As this is a cross-sectional study, causality cannot be determined. A potential limitation of this study is the lack of racial diversity in the cohort as the majority of women were white, educated, married, and employed, thus limiting the generalizability of the findings to other ethnic and socioeconomic groups. In addition, the MRS does not distinguish between daytime and nighttime symptoms, and distinguishing between the two may be helpful to identify women who are experiencing sleep disruption due to OSA and who may be more aware of nighttime VMS. The STOP-BANG questionnaire may underestimate risk for OSA in some women as the question about witnessed apneic events may not be accurate in women without bed partners, and 20% of participants did not know their neck size.
Although the STOP-BANG questionnaire has not been validated in women as a specific subpopulation, the validation studies for this tool have included large numbers of women. One study compared STOP-BANG with three other OSA screeners in a community-based sample of 4,770 individuals, 48.5% of whom were women, and found that STOP-BANG had the highest sensitivity in predicting moderate and severe sleep disordered breathing.29 Several studies have shown that the STOP-BANG tool has high sensitivity and moderate specificity and thus may overestimate OSA risk. Though sex-specific data are not available, studies involving more women than men have supported this conclusion.55–57
Additionally, VMS were self-reported and subjective rather than objectively measured and thus, subject to recall bias. We do not have specific information on which menopausal symptom caused women to seek care in the Women’s Health Clinic (e.g., VMS or sleep disturbance), which may have biased the findings. Also, we did not have access to data regarding referrals for additional testing to confirm the diagnosis of sleep disordered breathing or for treatment.
Conclusions
The results of the present study suggest that women reporting severe or very severe VMS in midlife may be at higher risk for OSA, particularly those with hypertension and obesity. Further, this risk is often unrecognized. An association between severe/very severe hot flashes and high OSA risk persisted in subgroup analysis of women with BMI <25 kg/m2 after adjustment for age and smoking status, suggesting the possibility that factors other than obesity may be contributing to this association. Given the low specificity of STOP-BANG in mixed populations of men and women and the lack of validation in women as a specific population, a critical need exists for a brief, validated screening tool that can be used for clinical assessment of women.
Both VMS and OSA are associated with cardiovascular risk,58 and increased clinical visibility of these associations is necessary to facilitate evaluation for and treatment of OSA in midlife women. It is important to note that not all sleep disturbances in midlife women relate directly to VMS, and primary sleep disorders may co-exist. Distinguishing between sleep disturbances secondary to OSA versus those predominantly related to VMS is critical in order to determine appropriate treatment in women.
Acknowledgments
Funding/support: This study was sponsored by Women’s Health Research Center, National Institutes of Health P50 AG44170, the Mayo School of Education, and the Mayo Foundation; The DREAMS database is supported by a grant to Dr. Faubion from a grateful patient.
Footnotes
Financial disclosure/conflicts of interest: S.S.F. is a consultant for Mithra Pharmaceuticals.
References
- 1.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351–358. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 3.Freeman EW, Sammel MD, Gross SA, Pien GW. Poor sleep in relation to natural menopause: a population-based 14-year follow-up of midlife women. Menopause. 2015;22(7):719–726. doi: 10.1097/GME.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 5.Ralls FM, Grigg-Damberger M. Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Curr Opin Pulm Med. 2012;18(6):568–573. doi: 10.1097/MCP.0b013e328358be05. [DOI] [PubMed] [Google Scholar]
- 6.Galvan T, Camuso J, Sullivan K, et al. Association of estradiol with sleep apnea in depressed perimenopausal and postmenopausal women: a preliminary study. Menopause. 2017;24(1):112–117. doi: 10.1097/GME.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98(3):391–397. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- 8.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96(7):1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166(12):1262–1268. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 10.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007;14(5):826–829. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 11.Kapsimalis F, Kryger M. Sleep breathing disorders in the U.S. female population. J Womens Health (Larchmt) 2009;18(8):1211–1219. doi: 10.1089/jwh.2008.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 13.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 14.Mirer AG, Young T, Palta M, Benca RM, Rasmuson A, Peppard PE. Sleep-disordered breathing and the menopausal transition among participants in the Sleep in Midlife Women Study. Menopause. 2017;24(2):157–162. doi: 10.1097/GME.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lozo T, Komnenov D, Badr MS, Mateika JH. Sex differences in sleep disordered breathing in adults. Respir Physiol Neurobiol. 2016 doi: 10.1016/j.resp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jehan S, Auguste E, Zizi F, et al. Obstructive Sleep Apnea: Women’s Perspective. J Sleep Med Disord. 2016;3(6) [PMC free article] [PubMed] [Google Scholar]
- 18.Wahner-Roedler DL, Olson EJ, Narayanan S, et al. Gender-specific differences in a patient population with obstructive sleep apnea-hypopnea syndrome. Gend Med. 2007;4(4):329–338. doi: 10.1016/s1550-8579(07)80062-3. [DOI] [PubMed] [Google Scholar]
- 19.Koo P, McCool FD, Hale L, Stone K, Eaton CB. Association of obstructive sleep apnea risk factors with nocturnal enuresis in postmenopausal women. Menopause. 2016;23(2):175–182. doi: 10.1097/GME.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurston RC, Chang Y, von Kanel R, et al. Sleep Characteristics and Carotid Atherosclerosis Among Midlife Women. Sleep. 2017;40(2) doi: 10.1093/sleep/zsw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thurston RC, Johnson BD, Shufelt CL, et al. Menopausal symptoms and cardiovascular disease mortality in the Women’s Ischemia Syndrome Evaluation (WISE) Menopause. 2017;24(2):126–132. doi: 10.1097/GME.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 23.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28(3):596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 24.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 25.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 26.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166(16):1709–1715. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 28.Muka T, Oliver-Williams C, Colpani V, et al. Association of Vasomotor and Other Menopausal Symptoms with Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(6):e0157417. doi: 10.1371/journal.pone.0157417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva GE, Vana KD, Goodwin JL, Sherrill DL, Quan SF. Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth Sleepiness Scales. J Clin Sleep Med. 2011;7(5):467–472. doi: 10.5664/JCSM.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest. 2016;149(3):631–638. doi: 10.1378/chest.15-0903. [DOI] [PubMed] [Google Scholar]
- 31.Rajagopal KR, Abbrecht PH, Tellis CJ. Control of breathing in obstructive sleep apnea. Chest. 1984;85(2):174–180. doi: 10.1378/chest.85.2.174. [DOI] [PubMed] [Google Scholar]
- 32.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–227. doi: 10.1016/j.yfrne.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tepper PG, Brooks MM, Randolph JF, Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23(10):1067–1074. doi: 10.1097/GME.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joffe H, Massler A, Sharkey KM. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med. 2010;28(5):404–421. doi: 10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu HJ, Lan XF, Li QY, et al. Factors affecting blood pressure profile in pre and postmenopausal women with obstructive sleep apnea hypopnea syndrome. Sleep Breath. 2015;19(1):169–174. doi: 10.1007/s11325-014-0983-z. [DOI] [PubMed] [Google Scholar]
- 36.Pedrosa RP, Barros IML, Drager LF, et al. OSA is common and independently associated with hypertension and increased arterial stiffness in consecutive perimenopausal women. Chest. 2014;146(1):66–72. doi: 10.1378/chest.14-0097. [DOI] [PubMed] [Google Scholar]
- 37.Herber-Gast G, Brown WJ, Mishra GD. Hot flushes and night sweats are associated with coronary heart disease risk in midlife: a longitudinal study. BJOG. 2015;122(11):1560–1567. doi: 10.1111/1471-0528.13163. [DOI] [PubMed] [Google Scholar]
- 38.Silveira JS, Clapauch R, Souza M, Bouskela E. Hot flashes: emerging cardiovascular risk factors in recent and late postmenopause and their association with higher blood pressure. Menopause. 2016;23(8):846–855. doi: 10.1097/GME.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 39.Sturdee DW, Hunter MS, Maki PM, et al. The menopausal hot flush: a review. Climacteric. 2017:1–10. doi: 10.1080/13697137.2017.1306507. [DOI] [PubMed] [Google Scholar]
- 40.Franco OH, Muka T, Colpani V, et al. Vasomotor symptoms in women and cardiovascular risk markers: Systematic review and meta-analysis. Maturitas. 2015;81(3):353–361. doi: 10.1016/j.maturitas.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Tuomikoski P, Ylikorkala O, Mikkola TS. Menopausal hot flushes and vascular health. Ann Med. 2011;43(4):283–291. doi: 10.3109/07853890.2010.546364. [DOI] [PubMed] [Google Scholar]
- 42.Hitchcock CL, Elliott TG, Norman EG, Stajic V, Teede H, Prior JC. Hot flushes and night sweats differ in associations with cardiovascular markers in healthy early postmenopausal women. Menopause. 2012;19(11):1208–1214. doi: 10.1097/gme.0b013e31825541cc. [DOI] [PubMed] [Google Scholar]
- 43.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165(20):2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 44.Bille J, Bille-Hasselstrom C, Petersen CG. Translation and validation of the Stop-Bang Questionnaire for obstructive sleep apnoea into Danish. Dan Med J. 2015;62(12):A5158. [PubMed] [Google Scholar]
- 45.Luo J, Huang R, Zhong X, Xiao Y, Zhou J. STOP-Bang questionnaire is superior to Epworth sleepiness scales, Berlin questionnaire, and STOP questionnaire in screening obstructive sleep apnea hypopnea syndrome patients. Chin Med J (Engl) 2014;127(17):3065–3070. [PubMed] [Google Scholar]
- 46.Nagappa M, Liao P, Wong J, et al. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(12):e0143697. doi: 10.1371/journal.pone.0143697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasad KT, Sehgal IS, Agarwal R, Nath Aggarwal A, Behera D, Dhooria S. Assessing the likelihood of obstructive sleep apnea: a comparison of nine screening questionnaires. Sleep Breath. 2017 doi: 10.1007/s11325-017-1495-4. [DOI] [PubMed] [Google Scholar]
- 48.Tan A, Yin JD, Tan LW, van Dam RM, Cheung YY, Lee CH. Predicting obstructive sleep apnea using the STOP-Bang questionnaire in the general population. Sleep Med. 2016;27–28:66–71. doi: 10.1016/j.sleep.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 49.The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017 doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 50.Jensen D, Wolfe LA, Slatkovska L, Webb KA, Davies GA, O’Donnell DE. Effects of human pregnancy on the ventilatory chemoreflex response to carbon dioxide. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1369–1375. doi: 10.1152/ajpregu.00862.2004. [DOI] [PubMed] [Google Scholar]
- 51.Regensteiner JG, Woodard WD, Hagerman DD, et al. Combined effects of female hormones and metabolic rate on ventilatory drives in women. J Appl Physiol (1985) 1989;66(2):808–813. doi: 10.1152/jappl.1989.66.2.808. [DOI] [PubMed] [Google Scholar]
- 52.Tatsumi K, Pickett CK, Jacoby CR, Weil JV, Moore LG. Role of endogenous female hormones in hypoxic chemosensitivity. J Appl Physiol (1985) 1997;83(5):1706–1710. doi: 10.1152/jappl.1997.83.5.1706. [DOI] [PubMed] [Google Scholar]
- 53.Polo-Kantola P, Rauhala E, Helenius H, Erkkola R, Irjala K, Polo O. Breathing during sleep in menopause: a randomized, controlled, crossover trial with estrogen therapy. Obstet Gynecol. 2003;102(1):68–75. doi: 10.1016/s0029-7844(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 54.Pickett CK, Regensteiner JG, Woodard WD, Hagerman DD, Weil JV, Moore LG. Progestin and estrogen reduce sleep-disordered breathing in postmenopausal women. J Appl Physiol (1985) 1989;66(4):1656–1661. doi: 10.1152/jappl.1989.66.4.1656. [DOI] [PubMed] [Google Scholar]
- 55.Chung F, Yang Y, Brown R, Liao P. Alternative scoring models of STOP-bang questionnaire improve specificity to detect undiagnosed obstructive sleep apnea. J Clin Sleep Med. 2014;10(9):951–958. doi: 10.5664/jcsm.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vana KD, Silva GE, Goldberg R. Predictive abilities of the STOP-Bang and Epworth Sleepiness Scale in identifying sleep clinic patients at high risk for obstructive sleep apnea. Res Nurs Health. 2013;36(1):84–94. doi: 10.1002/nur.21512. [DOI] [PubMed] [Google Scholar]
- 57.Boynton G, Vahabzadeh A, Hammoud S, Ruzicka DL, Chervin RD. Validation of the STOP-BANG Questionnaire among Patients Referred for Suspected Obstructive Sleep Apnea. Journal of sleep disorders–treatment & care. 2013;2(4) doi: 10.4172/2325-9639.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biglia N, Cagnacci A, Gambacciani M, Lello S, Maffei S, Nappi RE. Vasomotor symptoms in menopause: a biomarker of cardiovascular disease risk and other chronic diseases? Climacteric. 2017:1–7. doi: 10.1080/13697137.2017.1315089. [DOI] [PubMed] [Google Scholar]


