Abstract
Graft-versus-host disease (GVHD) is the major cause of non-relapse morbidity and mortality after allogeneic stem cell transplant (allo-SCT). Prevention and treatment of GVHD remains inadequate and commonly leads to end-organ dysfunction and opportunistic infection. The role of IL-17 and IL-22 in GVHD remains uncertain, due to an apparent lack of lineage fidelity and variable and contextually determined protective and pathogenic effects. We demonstrate that donor T-cell-derived IL-22 significantly exacerbates cutaneous chronic GVHD, and that IL-22 is produced by highly inflammatory donor CD4+ T-cells post-transplant. IL-22 and IL-17A derive from both independent and overlapping lineages, defined as Th22 and IL-22+Th17 cells. Donor Th22 and IL-22+Th17 share a similar IL-6-dependent developmental pathway and whilst Th22 arise independently of the IL-22+Th17 lineage, IL-17 signaling to donor Th22 directly promotes their development in allo-SCT. Importantly, while both IL-22 and IL-17 mediate skin GVHD, Th17-induced chronic GVHD can be attenuated by IL-22 inhibition in preclinical systems. In the clinic, high levels of both IL-17A and IL-22 expression are present in the skin of GVHD patients after allo-SCT. Together, these data demonstrate a key role for donor-derived IL-22 in chronic skin GVHD and confirm parallel but symbiotic developmental pathways of Th22 and Th17 differentiation.
Introduction
A significant and increasing proportion (10–15%) of patients with hematological cancers undergo stem cell transplantation (SCT), meaning it remains an important curative therapeutic option. The success of allogeneic SCT (allo-SCT) is limited by the development of graft-versus-host disease (GVHD) whereby donor T-cells mount an immune response to disparate recipient alloantigens expressed within host tissues. GVHD can manifest acutely or chronically, resulting in significant and widespread tissue damage occurring primarily within the gastrointestinal tract, skin, lung and liver.1 Despite advances in immunosuppressive therapies, the incidence of GVHD remains high (>50%).2,3 Cutaneous manifestations including lichenoid and sclerodermatous GVHD are common late after allo-SCT with limited treatment options beyond protracted steroid therapy.3–5 Chronic GVHD thus has a significant detrimental impact on patient quality-of-life,3 meaning new therapeutic strategies are urgently needed.
Donor T-cells play a central role in GVHD; depletion of T-cells from the donor graft prevents GVHD, but is associated with increased rates of relapse and infective complications which negate any beneficial effect.6 Cytokines are critical mediators of inflammatory processes after allo-SCT and interleukin-22 (IL-22) has been shown to play a particularly complex role in GVHD.7 IL-22 is a member of the IL-10 cytokine superfamily expressed by both the adaptive (CD4+ and CD8+ T-cells) and innate immune system (γδ T, NKT and innate lymphoid cells (ILCs) including NKs, ILC3 and lymphoid tissue inducer (LTi) cells).8–10 The IL-22 receptor shares one subunit with the IL-10 receptor (IL-10RB2), which forms a heterodimer with the IL-22Rα subunit, expressed almost exclusively by non-hematopoietic tissue.9,10 The IL-22Rα is expressed in epithelia, keratinocytes and fibroblasts such that IL-22 provides an important conduit between the immune system and organ surfaces during infection and tissue injury.8,9 In adaptive immunity, IL-22 is often linked with IL-17 expression and indeed is commonly considered as a “Th17” cytokine. However, more recent studies have demonstrated IL-22 expression independent of IL-17 in multiple contexts and Th22 are increasingly recognized as a distinct T-cell differentiation program.11–14
Recipient-derived IL-22, produced by ILCs, promotes the survival and persistence of intestinal stem cells and helps maintain gut epithelial integrity.15 In contrast, others have demonstrated a pathogenic role for donor T-cell-derived IL-22 in murine models of acute GVHD and have shown that donor IL-22 does not contribute to graft-versus-leukemia effects.16,17 Given that clinical trials using IL-22-Fc are underway to treat acute gastrointestinal GVHD (clinical trials.gov, NCT02406651),18 the potential pathogenic effects of IL-22 suggests a better understanding of the cell lineages involved and their clinical relevance is urgently required. Here, we have evaluated the function of IL-22 in the pathophysiology of classic and inflammatory chronic GVHD using pre-clinical models and patient samples.
Methods
Mice
Female C57Bl/6 (WT.B6, H2b), B10.BR (B10.BR, H2k2), BALB/c (WT.BALB/c, H2d) and B6D2F1 (H2b/d) mice were purchased from the Animal Resources Center (Perth, Australia) or The Jackson Laboratory (Bar Harbor, ME, USA). IL-17Cre (B6, H2b), Rosa26eYFP (B6, H2b), IL-17RC−/− (B6, H2b), IL-22−/− (BALB/c, H2d) and IL-6−/− (B6, H2b) mice were bred and housed at QIMRB. CCR6−/− mice (B6, H2b) were bred and housed at the University of North Carolina DLAM facility. IL-22−/− (B6, H2b) were bred and housed at the University of Minnesota animal facility. IL-17RC−/− mice were provided by Amgen Inc, B6 and BALB/c IL-22−/− mice were provided by Genentech and IL-17Cre, Rosa26eYFP and Rosa26iDTR mice were provided by Dr B Stockinger (Medical Research Council NIMR, UK) and crossed to generate IL-17creRosa26eYFP and IL-17creRosa26eYFP/iDTR heterozygous mice.19 B6.IL-17eGFP/+IL-22dTom/+ (B6, H-2Db, CD45.2+) were generated by crossing Il17aeGFP (C57BL/6-Il17atm1Bcgen/J) and IL-22dTom (C57BL/6-IL22promTdtomato, generated by Dr. Scott K. Durum) reporter mice. Mice were housed in sterilized micro isolator cages and received acidified autoclaved water (pH 2.5). Animal experiments were performed in accordance with protocols approved by the local animal ethics committees.
Cell preparation for transplantation
For murine stem cell mobilized grafts, recombinant human Granulocyte Colony Stimulating Factor (G-CSF) was given subcutaneously to donors at 10μg/dose/animal (4–6 days)20 and splenocytes isolated prior to transplant. T-cell depletion was performed by anti-CD4 (RL172.4), anti-CD8 (TIB211), and anti-Thy1.2 (HO-13-4) treatment, followed by rabbit complement.21 Cell suspensions contained less than 1% viable CD3+ T-cells. For bone marrow (BM) transplantation, donor T-cell depleted (TCD) BM cells were isolated as described previously.22,23 Total T-cells were isolated using T-cell columns (Cedarlane Laboratories). Following column purification, the sample was antibody depleted using anti-mouse B220 and anti-mouse CD25 conjugated with PE (eBioscience), and magnetic bead isolation was performed using anti-PE beads (Miltenyi Biotec).23
In vitro Th17 differentiation: Naïve CD4+ T-cells were isolated from WT.B6 spleens using CD4+ columns (Cedarlane Laboratories) followed by sort purification (CD4+CD62LhiCD25−). Purified T-cells were stimulated with plate-bound anti-CD3 (3μg/ml, eBioscience) and anti-CD28 (5μg/ml, eBioscience) in the presence of TGF-β (2ng/mL, Peprotech), IL-6 (30ng/mL, Peprotech), TNF-α (20ng/mL, Peprotech), IL-1β (10ng/mL, Peprotech), anti-IL-2 antibody (10μg/mL, BioXCell), and anti-IFN-γ (10μg/mL, BioXCell) for 2 days. On day 3, cells were replenished with the same cytokines and antibodies and cultured for another 2 days. On day 5, cells were replated into low cluster 24-well plates in the presence of TGF-β1: 2ng/mL, IL-6: 25ng/mL, anti-IL-2: 10μg/mL, anti-IFN-γ: 10μg/mL for 2 days. Cells were re-stimulated on d7 with plate-bound anti-CD3 and anti-CD28 mAbs with TGF-β: 2ng/mL, IL-6: 30ng/mL, TNF-α: 20ng/mL, IL-1β: 10ng/mL, anti-IL-2: 10μg/mL, anti-IFN-γ: 10μg/mL, IL-23 (R&D Systems): 15ng/mL. On d9, cells were again removed from CD3 and CD28 stimulation and cultured in low cluster 24-well plates in the presence of 2ng/mL TGF-β1, 30ng/mL IL-6, 20ng/mL TNF-α, 10ng/mL IL-1β, 10μg/mL anti-IL-2, 10μg/mL anti-IFN-γ, 15ng/mL IL-23. On d10 cells were re-fed with: TGF-β1: 2ng/mL, IL-6: 25ng/mL, IL-23: 15ng/mL, IL-1β: 10ng/mL, anti-IL-2: 10μg/mL, anti-IFN-γ: 10μg/mL. On d12, IL-17 cytokine was assessed by stimulating 1×107 cells with 50ng/mL Phorbol 12-myristate 13-acetate (PMA), 500ng/mL ionomycin and 3μg/mL Brefeldin A (Sigma-Aldrich) for 6h.
Stem cell transplantation
For murine stem cell mobilized grafts, B6D2F1 recipients received 10×106 G-CSF-treated B6-derived splenocytes (d0) after 1100 cGy total-body irradiation (TBI) split over 2 doses (d-1). B6 recipients received 25×106 G-CSF-treated WT (BALB/c) or BALB/c.IL-22−/− derived splenocytes (d0) after 1000 cGy TBI. Non-GVHD control groups were injected with TCD grafts. For iDTR-based cell depletions, mice were injected with 250ng diphtheria toxin (DT) on d4–6 post-transplant. B10.BR recipients received 10×106 B6-derived bone marrow (BM) after 2 ip doses of 120mg/kg Cyclophosphamide (d-3 & d-2) and 830 cGy TBI. Grafts were supplemented with or without 8×104 T-cells from either WT (B6) or B6.IL-22−/ − mice where indicated. B6D2F1 recipients of grafts containing in vitro polarized T-cells received 3×106 TCD BM and 4×106 Th17 cells or total T-cells (d0) after 950 cGy TBI (d-1).
GVHD monitoring and pulmonary function tests
In all in vivo transplant systems, mice were regularly monitored and weights recorded each week. For pulmonary function assessment, anesthetized mice were weighed, un-blinded animals were intubated, and lung function assessed by whole body plethysmography using the Flexivent system (SCIREQ) and analyzed using the Flexivent software (version 7.3). B6 and B6D2F1 recipients of G-CSF mobilized grafts were monitored daily and systemic overall GVHD assessed weekly using an established cumulative scoring system.24 Cutaneous GVHD component: 0: normal; 0.5: scaling of paws/tail/ears, 1: areas of alopecia, skin thickening, 2: obvious ulceration, areas of denuded skin. B6D2F1 recipients of in vitro polarized T-cells were scored at least twice a week for GVHD symptoms using an established cumulative scoring system.25 Cutaneous GVHD Score: 0: No ulcers or alopecia; 1: Skin ulcers with alopecia less than 1cm2 in area; 2: 1–2 cm2 in area; 3: Greater than 2cm2 in area; an additional score of 0.3 was added if mice had ulcers or scaling on paws, tail, or ears.
Patients
Patients were enrolled as part of an observational study tracking hematopoietic reconstitution after allo-SCT to treat hematological malignancies. Recipients received either a myeloablative conditioning regimen consisting of cyclophosphamide at 60mg/kg/day for two days and 12Gy of fractionated TBI given over three days or a reduced intensity conditioning regimen consisting of fludarabine at 25mg/m2/day for 5 days (d-7 to d-3) and melphalan at 120mg/m2 on d-2. All patients received a T-cell-replete G-CSF-mobilized peripheral blood stem cell graft from HLA (A, B, C, DR, DQ) matched donors. GVHD prophylaxis consisted of Cyclosporine A (5mg/kg d-1 to d1 and thereafter 3mg/kg daily with subsequent adjustment to target a therapeutic range of 140–250 ng/mL) and methotrexate on d1 (15mg/m2), 3, 6 and 11 (10mg/m2). Ethics approval was obtained from both QIMR Berghofer and the Royal Brisbane and Women’s Hospital Human Ethics Committees with written informed consent obtained from participating patients.
4–6 mm punch biopsies of the skin were obtained from patients with cutaneous manifestations of GVHD >100 days post allogeneic stem cell transplant. Half of the biopsied tissue was evaluated for GVHD by a clinical pathologist. The other half of the biopsy was flash frozen in liquid nitrogen and stored at −80°C. mRNA control skin tissue was obtained from the posterior iliac crest from patients undergoing autologous stem cell transplant at the time of bone marrow evaluation. The Ct method was used to calculate fold induction relative to levels found in control samples. All patients provided informed written consent and studies were approved by the University of North Carolina Institutional Review Board and Office of Human Research Ethics.
Monoclonal Antibodies and flow cytometry
Antibodies were purchased from Biolegend: anti-mouse CD3 (145-2C11), CD4 (GK1.5), CD8α (53–6.7), CD90.2 (30-H12), IL-17A (TC11-18H10), IFNγ (XMG1.2), TNF (MP6-XT22), IL-22 (poly5164), anti-human CD3 (UCHT1), CD4 (RPA-T4), CD8α (RPA-T8), TNF (MAb11), IL-17A (BL168), IFNγ (45.B3), or eBioscience: anti-mouse GM-CSF (MP1-22E9). Rat anti-mouse IL-6R mAb (MR16-1, Chugai Pharmaceutical Co) or Rat IgG (Sigma-Aldrich) was given intraperitoneally at 500μg/dose on d-1 and d3 post SCT.26 In IL-22 in vivo blocking studies, mice were injected intraperitoneally with either 16mg/kg of control IgG or anti-IL-22 antibody (provided by Pfizer) once a week for four weeks (wk0 – wk3). After transplant, cells were isolated by mechanical disruption and treated with lysis buffer to remove contaminating erythrocytes. For intracellular cytokine staining, cells were cultured with PMA (5μg/mL) and Ionomycin (50μg/mL) for 4h with Brefeldin A (BioLegend). Cells were surface-labeled and processed for intracellular staining, cytokines were assessed via cytofix/cytoperm kit (BD Biosciences). All samples were acquired on BD LSR Fortessa (BD Biosciences) using BD FACSDiva (v7.0) and analyzed with FlowJo (v9.7).
Gene expression analysis
4mm skin bunch biopsies were taken from affected areas of mice or 4–6 mm punch biopsies from affected areas from patients and homogenized using lysing Matrix D tubes (MP Biomedicals, Santa Ana, CA) and FastPrep Homogenizer (MP Biomedicals). RNA was isolated from skin homogenates using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s recommendations. RNA was run through the Qiagen clean-up kit and cDNA synthesized using the Superscript III cDNA Synthesis Kit (Invitrogen). Equal amounts of cDNA were analyzed in triplicate for each transcript by real-time quantitative PCR using Taqman universal PCR mastermix (Applied Biosystems, Foster City, CA) and the Applied Biosystems’ ABI QuantStudio 6 real-time PCR system. The expression level of each gene was standardized to the housekeeping gene, Gapdh for mouse studies and 18S for human studies, and the Ct method was used to calculate fold induction relative to levels found in control samples.
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. Unpaired two-tailed Mann-Whitney tests were used to evaluate differences in cytokine expression and cell frequencies, ANOVA was used when comparisons were made between three or more groups. One-sample t tests were used to compare cytokine expression by qPCR in clinical samples. Data are mean ±SEM unless otherwise stated and p<0.05 considered significant.
Results
Donor derived IL-22 promotes cutaneous chronic GVHD in allo-SCT recipients
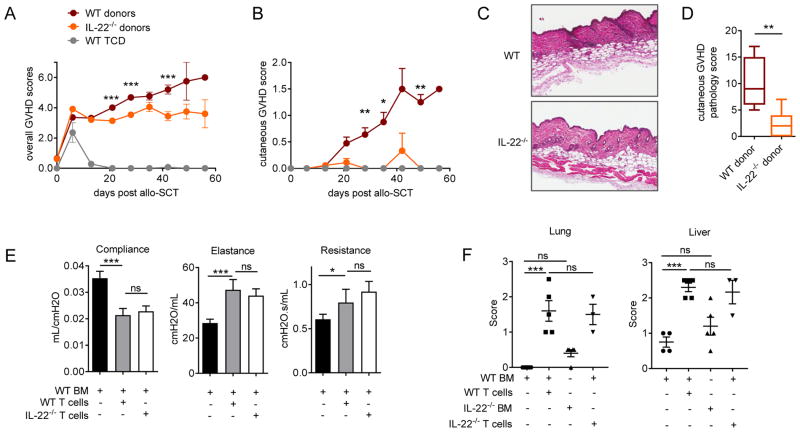
Given the contrasting roles reported for IL-22 in inflammation and acute GVHD,8,15–17 we utilized allo-SCT models whereby chronic GVHD was directed to an MHC disparity in WT and IL-22−/− recipients. Since IL-22 is known to contribute to inflammatory skin disease pathology,11,27–29 we utilized a murine model in which cutaneous chronic GVHD is induced by WT T-cells,20 and examined the effect of IL-22 deficiency in the donor graft. Here we observed a significant reduction in overall GVHD clinical scores and a striking reduction specifically in cutaneous GVHD scores in the absence of donor IL-22 (Figure 1A–B). Semi-quantitative histopathology of skin 35 days after transplant also demonstrated significantly reduced cellular infiltration and inflammation equating to a reduction in cutaneous GVHD pathology in these mice (Figure 1 C–D). IL-22 is also reported to have a dual role in airway inflammation, whereby IL-22 expression in the lung suppresses antigen-induced eosinophilic infiltration and airway inflammation30–32, however IL-22 can also exacerbate asthma development in response to subcutaneous antigen sensitization.33,34 Therefore we utilized a model of chronic GVHD that closely models the features of bronchiolitis obliterans (BO), resulting in alloantigen induced epithelial injury, fibroproliferation, impaired pulmonary function and lung pathology (Figure 1E–F).35 Lung function studies in mice 60 days after transplant demonstrated clear impairment in lung compliance, and increased elastance and resistance in the presence of donor WT T-cells (Figure 1E), however IL-22 deficiency in donor T-cells did not significantly alter these functional manifestations of BO. Chronic liver and lung GVHD were evident in semi-quantitative histopathology assessments of tissue derived from recipients of T-cell replete grafts, however no significant differences were observed between recipients of either WT or IL-22−/− T-cells (Figure 1F). These data suggest that donor-derived IL-22 plays a relatively specific role in promoting chronic GVHD in the skin late after allo-SCT.
Figure 1. Donor derived IL-22 drives cutaneous GVHD in allo-SCT recipients.
(A–D) 25×106 G-CSF mobilised BALB/c.WT or BALB/c.IL-22−/− splenocytes transplanted into lethally-irradiated allogeneic B6 recipients. (A) Overall GVHD clinical scores (WT & IL-22−/− donors, n = 49 mice/group, ***p<0.001) and (B) cutaneous GVHD are shown (WT & IL-22−/− donors, n = 24 mice/group, *p< 0.05, **p< 0.01). (C) Representative images and (D) quantitative histopathology scores of skin GVHD in haemotoxylin and eosin stained sections collected 35 days post-transplant (original magnification ×200, n = 5–7 mice/group, **p< 0.01). (E–F) 10×106 T-cell depleted BM and 8×104 purified T-cells derived from WT.B6 or IL-22−/− B6 mice were transplanted into lethally irradiated allogeneic B10.BR recipients. (E) Pulmonary function of recipient mice 60 days post-transplant (n=3–11 mice/group, *p< 0.05, ***p<0.001). (F) Quantitative histopathology scores of liver and lung GVHD target organs assessed in haemotoxylin and eosin stained sections collected 60 days post-transplant (n=3–5 mice/group, *p< 0.05, ***p<0.001).
GVHD results in pro-inflammatory donor Th22 and IL-22+Th17 differentiation
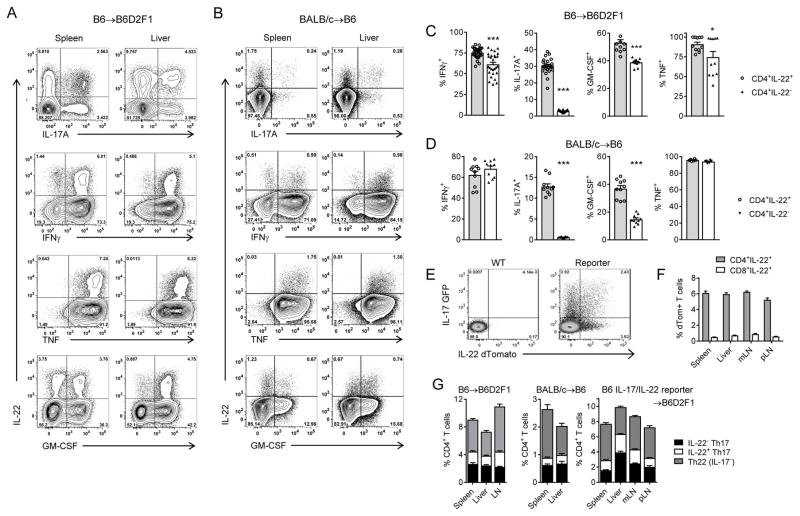
We next assessed T-cell differentiation after transplant by cytokine expression within donor T-cells in multiple models of GVHD. In both B6 → B6D2F1 and BALB/c → B6 systems, we observed IL-22 expression almost exclusively by CD4+ T-cells, a minority of which co-expressed IL-17A (Figure 2A–B). In addition, a large proportion of IL-22+ cells also co-expressed the pro-inflammatory cytokines TNF, IFNγ and GM-CSF after transplant, all known hallmarks of pathogenic T-cell differentiation.36–39 Comparisons between CD4+IL-22+ and CD4+IL-22− T-cells demonstrated significantly higher frequencies of IFNγ, IL-17A and GM-CSF expression in IL-22 expressing CD4+ T-cells (Figure 2C–D). To explore the relationship between IL-17A and IL-22 further, and to exclude potential artefacts that may arise from in vitro re-stimulation, we utilized donor grafts derived from B6.IL-17eGFP/+IL-22dTom/+ reporter mice. Here we found that unlike IL-17A, IL-22 production is indeed restricted to CD4+ T-cells after allo-SCT (Figure 2E–F). In all transplant systems tested, three distinct populations were clearly apparent in CD4+ T-cells including IL-22+IL-17A−, IL-22−IL-17A+ and IL-22+IL-17A+ subsets, which we defined as Th22, IL-22+Th17 and Th17 respectively (Figure 2G).
Figure 2. Allo-SCT induces pro-inflammatory donor Th22 and IL-22+Th17 differentiation.
(A,C) 10×106 B6 G-CSF mobilised, splenocytes transplanted into lethally-irradiated allogeneic B6D2F1 recipients. (B, D) 25×106 BALB/c G-CSF mobilised, splenocytes transplanted into lethally-irradiated allogeneic WT.B6 recipients. (A) Representative flow cytometry analysis of cytokine expression in CD4+ T-cells isolated d7 post-transplant (B6→B6D2F1) from spleen and liver after in vitro restimulation. (B) Representative flow cytometry analysis of cytokine expression in CD4+ T-cells isolated d7 post-transplant (BALB/c→B6) from spleen and liver after in vitro restimulation. (C) Frequency of cytokine co-expression by CD4+ T-cells isolated d7 post-transplant after in vitro restimulation (B6→B6D2F1, 5–17 mice/group, ***p<0.001). (D) Frequency of cytokine co-expression by CD4+ T-cells isolated d7 post-transplant after in vitro restimulation (BALB/c→B6, 6–10 mice/group, ***p<0.001). (E–F) 10×106 G-CSF mobilised, B6.IL-17eGFP/+IL-22dTom/+ splenocytes transplanted into lethally-irradiated allogeneic B6D2F1 recipients. (E) Representative flow cytometry analysis d7 post-transplant and (F) frequencies of IL-22dTom+ cells within the CD4+ and CD8+ T-cell subsets in spleen, liver, mesenteric lymph nodes (mLN) and peripheral lymph nodes (pLN) (5 mice/group). (G) Th22, Th17 and IL-22+Th17 frequencies d7 post-transplant (B6→B6D2F1: 15 mice/group, BALB/c →B6: 10 mice/group, B6.IL-17eGFP/+IL-22dTom/+ →B6D2F1: 5 mice/group).
Th22 arise independently of the Th17 lineage but IL-17 promotes Th22 differentiation
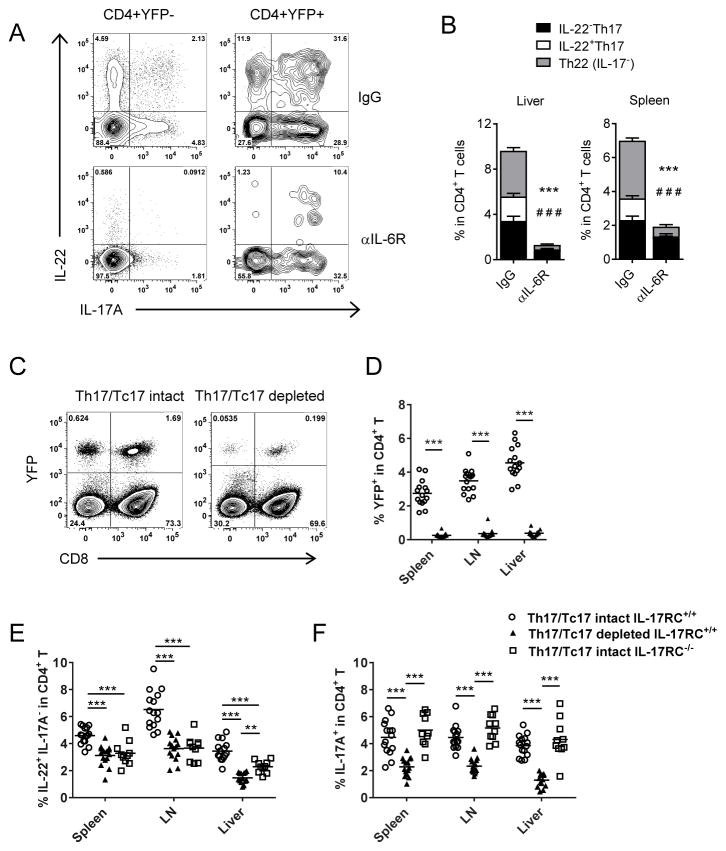
Cytokine plasticity is a well-characterized feature of IL-17 producing T-cells.19,40 We therefore utilized an IL-17 ‘fate-mapping’ reporter system (B6.IL-17creRosa26eYFP) to determine whether the Th22 subset identified post-transplant arose independently of the Th17 differentiation pathway. In this system, YFP is permanently expressed in IL-17A producing cells regardless of ongoing IL-17A gene expression.19 Using G-CSF mobilized stem cell grafts from B6.IL-17crerosa26eYFP mice, we examined IL-22, IL-17A and IL-17A-induced YFP expression after short-term re-stimulation by PMA/Ionomycin and intracellular cytokine staining (Figure 3A).40 Again, a distinct IL-22+YFP− CD4+ T-cell population was clearly observed in these analyses, in parallel to that seen in direct cytokine reporter mice (Figure 2E). Whilst Th22 and Th17 are known to have distinct differentiation pathways (e.g. differential requirements for TGFβ), both populations are reported to be dependent upon IL-6.8,10,41 We have previously demonstrated systemic IL-6 dysregulation in response to transplant conditioning,26,42 which we have shown drives ‘type-17’ polarization in mice. Like IL-17A, IL-22 induction was highly dependent upon IL-6 (Figure 3A–B). Whilst the IL-22R is exclusively expressed on non-hematopoietic cells, T-cells commonly express the receptors required for IL-17A recognition (IL-17RA/IL-17RC heterodimer). To assess the influence of IL-17A cytokine on Th22 development we utilized an IL-17 ‘fate-mapping’ reporter-deleter model (B6.IL-17creRosa26eYFP/iDTR), which enables specific deletion of IL-17A+ cells as they arise, following diphtheria toxin administration (Figure 3C–D).40 When both donor Th17 and CD8+IL-17+ T-cells (Tc17) were depleted after allo-SCT, Th22 frequencies were significantly reduced (Figure 3E). Similarly, donor Th22 frequencies were also diminished in the absence of IL-17A signaling within T-cells, demonstrating IL-17A directly acts to promote and/or maintain Th22 differentiation after allo-SCT. This effect was specific to Th22, since Th17 frequencies remained unchanged in the absence of IL-17 signaling (Figure 3F). Together these data demonstrate there are multiple donor T-cell sources of IL-22 (Th22 and Th17), and both IL-6 and IL-17 are key cytokines in Th22 development after transplant.
Figure 3. Th22 differentiation is promoted by IL-6 and IL-17A in allo-SCT.
(A–B) Th22 development and lineage fidelity was assessed in lethally irradiated allogeneic mice transplanted with G-CSF mobilized grafts (B6.IL-17CreRosa26eYFP→B6D2F1) in the presence (αIL-6R) or absence (IgG) of IL-6 blockade. (A) Representative flow cytometry analysis and (B) frequencies of IL-17A and IL-22 expressing splenic CD4+ T-cells in the absence of IL-6R signalling d7 post-transplant (n= 8–10 mice/group, ***p<0.001 Th22 (IL-17−) treated vs untreated, # # #p<0.001 IL-22+Th17 treated vs untreated. (C–F) 5×106 TCD BM and 2×106 T-cells isolated from IL-17CreRosa26eYFP, IL-17CreRosa26eYFP/iDTR or IL-17RC−/− mice were transplanted into lethally-irradiated allogeniec B6D2F1 recipients. (C) Representative flow cytometry analyses and (D) frequency of YFP+CD4+ T-cells d7 post-transplant after DT treatment. Frequency of (E) Th22 (IL-22+IL-17−) and (F) IL-17+ cells in CD4+ T-cell compartment d7 post-transplant in the presence and absence of Th17/Tc17 and IL-17RC signalling (n=10 mice/group; **p< 0.01, ***p<0.001).
Donor Th17 derived IL-22 drives skin GVHD late post-transplant
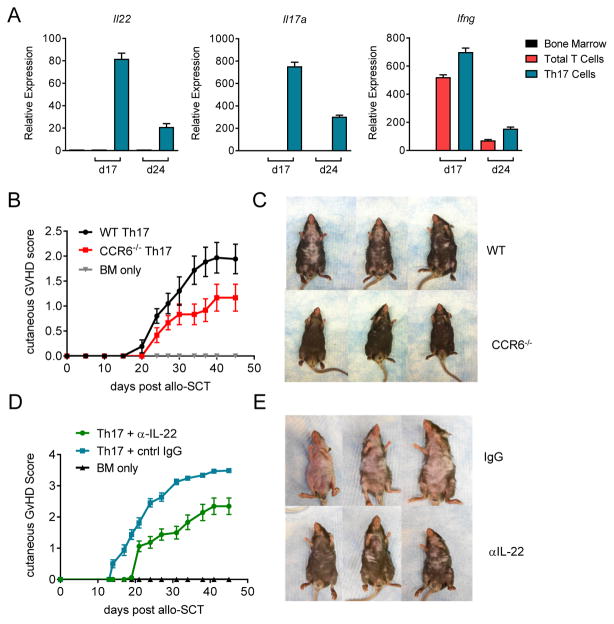
We have previously demonstrated that in vitro polarized Th17 cells can significantly exacerbate skin GVHD22 and mRNA-seq data indicates that these cells share a similar transcriptome profile with Th17 cells in the skin of patients with inflammatory chronic GVHD (Serody and Coghill unpublished). Thus, we revisited this system to examine the role of IL-22 in this process. To assess Th17 cytokine profiles post-transplant, we compared Il22, Il17 and Ifng gene expression by quantitative PCR in murine skin 17 and 21 days after transplantation of grafts containing Th17 cells (Figure 4A). Here we detected high mRNA expression of all three cytokines in comparison to unpolarized T-cells in which only IFNγ encoding mRNA was detected. Since CCR6 expression is important in both IL-17+CD4+ and IL-22+CD4+ T-cell migration and is reported to be required in IL-22 dependent models of psoriasis,13,14,43 we examined the capacity of CCR6 deficient Th17 polarized cells to induce GVHD. Here we found that whilst no differences were observed in mortality (data not shown), CCR6−/− Th17 induced significantly less cutaneous GVHD than WT counterparts following allotransplantation (>d37 p<0.01; Figure 4B–C). This cutaneous GVHD manifested last after transplant (beyond day 21) with lichenoid features and alopecia, most consistent with chronic disease. To confirm that this pathology was indeed IL-22 dependent, we next assessed the specific capacity of Th17-derived IL-22 to induce GVHD by comparing the effects of IL-22 neutralization after allo-SCT. Mice transplanted with Th17 cells were treated for three weeks with IL-22 blocking mAb, which resulted in a significant reduction in skin GVHD (>d17 p<0.01; Figure 4D–E). However, anti-IL-22 mAb did not improve the systemic manifestations of late inflammatory chronic GVHD with no difference in overall survival between control and anti-IL-22 mAb treated animals (data not shown).
Figure 4. IL-22 derived from Th17 polarised T-cells exacerbates skin GVHD.
(A–H) 5 × 106 TCD BM and 3 × 106 in vitro polarized Th17, or control T CD4+ T-cells, were transplanted into lethally irradiated B6D2F1 recipients. (A) Il22, Il17a, Ifng gene expression in murine skin d17 and d24 post transplant (9–12 mice/group/time point). (B) cutaneous GVHD scores and (C) representative images (d25) from mice transplanted with either WT or CCR6−/− in vitro polarized Th17. (D) Cutaneous GVHD scores and (E) representative images (d35) from mice treated with either control IgG or IL-22 blocking mAbs post-transplant (control IgG & αIL-22: 18–20 mice/group).
IL-22 and IL-17 are elevated in patients with cutaneous GVHD
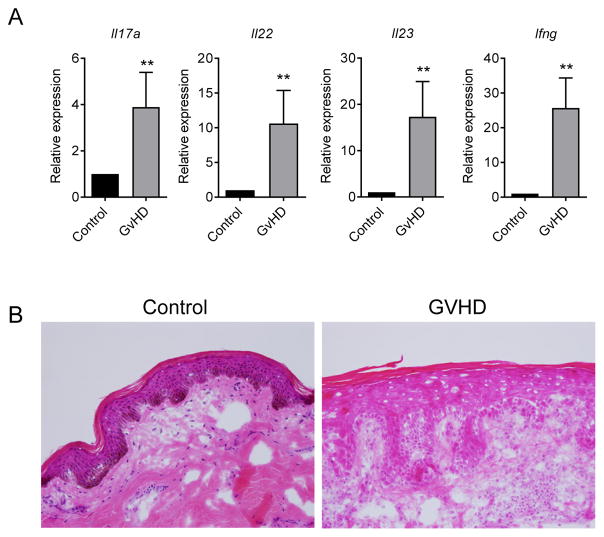
Immunosuppression during the first 3–4 months after transplant is effective in controlling most donor T-cell inflammatory cytokine production, however during the onset phase of chronic GVHD (>100 days), systemic IL-17A and IFNγ levels increase over time.42 In one study of pediatric allo-SCT recipients, a significant correlation was observed between increased Il22 gene expression in peripheral blood mononuclear cells and active cGVHD.44 Given this and our observations in murine models of chronic GVHD, we assessed plasma IL-22 levels in a clinical cohort after allo-SCT. We found that unlike IL-17A,42 systemic IL-22 protein levels remained consistently low after transplant (data not shown). To gain a better understanding of cytokine responses in GVHD target tissue, we evaluated cutaneous gene expression in patients >100 days post allo-SCT in parallel with biopsy confirmation of GVHD (Figure 5A). These data were compared to skin biopsies taken from patients after autologous transplant that do not develop GVHD (Figure 5B). We again observed a clear correlation between GVHD and elevated tissue cytokine transcript levels, particularly dysregulated IL-22, IL-17A and IFNγ. These data show striking similarities with that observed in our murine models of skin GVHD and highlight IL-22 as a potential therapeutic target for the treatment of chronic GVHD.
Figure 5. Cutaneous IL-22 cytokine levels are dysregulated in GVHD patients.
(A) Gene expression in skin explants derived from 12 allo-SCT patients with cutaneous GVHD and 8 autologous-SCT control patients. (B) Representative haemotoxylin and eosin stained images from skin explants of cutaneous GVHD and healthy autologous-SCT patient control patients (as in A).
Discussion
IL-17 and IL-22 play an important and complex role in allo-SCT; both have been described to have dual pathogenic and protective roles in GVHD that are largely dependent upon their cellular origin. Recipient-derived IL-17 and IL-22 both play early protective roles in the GI tract during GVHD, where IL-22R signaling helps maintain gut epithelial integrity15 and IL-17R signaling suppresses inflammatory responses and prevents dysbiosis in the gastrointestinal tract.45 Conversely, we and others have demonstrated that donor T-cell-derived IL-22 and particularly IL-17 are pathogenic and exacerbate GVHD.16,20,22
The contrasting roles observed for IL-22 in GVHD mirror reports in other systems that show IL-22 can be both protective and pathogenic. For example, IL-22 promotes skin wound healing and epithelial integrity, however dysregulated IL-22 expression exacerbates psoriatic skin disease.8,46 Keratinocyte responses to excess IL-22R signaling result in hyperproliferation, abnormal differentiation, epidermal hyperplasia, skin thickening and disruption,46 which are pathological features commonly observed in cutaneous GVHD. The presence of other pro-inflammatory cytokines has a significant impact on IL-22 responses, particularly IL-17, IFNγ and TNF, which synergize with IL-22 to enhance chemokine production in the skin.47,48 Indeed, both pathological and protective functions have also been identified for IL-22 in the context of damage-induced airway inflammation and these effects are regulated by the presence of IL-17.33,34 This is of particular interest since we have demonstrated that donor T-cell polarization towards an IL-17-producing phenotype in both CD4+ (Th17) and CD8+ (Tc17) subsets exacerbates GVHD after allo-SCT.20,22,40,49,50
To date, investigation of the effects of donor derived IL-22 in allo-SCT has focused on acute GVHD, with little data regarding the role of IL-22 in chronic GVHD. It is important to note that whilst clear overlap exists between the etiologies of acute and chronic GVHD, it is increasingly recognized that divergent mechanisms are also involved.51,52 A role for IL-22 in the pathophysiology of acute GVHD cannot be extrapolated to cGVHD. A single murine study has noted a correlation between lower IL-22 secretion and a reduction in cGVHD in the context of IL-12/23 blockade, without establishing any cause and effect.53 Given the complexities and the potential competing risks of targeting IL-22 early post-transplant, we therefore examined IL-22 deficiency in murine models of chronic GVHD. We observed a specific role for IL-22 in driving cutaneous chronic GVHD, which is in line with the pathogenic role of IL-22 in autoimmune skin disease.11,27–29 We identified CD4+ T-cells as the major source of donor IL-22 by both direct protein detection and cytokine reporter systems, where we observed co-expression of IL-22 with a range of pro-inflammatory cytokines including IL-17, IFNγ, GM-CSF and TNF. Importantly, we identified both Th17 and Th22 T-cell lineages contributing to IL-22 production and excluded potential cytokine plasticity to identify a definitive Th22 population in allo-SCT. Intriguingly, Bronchiolitis Obliterans (BO), a manifestation of chronic GVHD that mirrors BO after lung transplant, was not IL-22-dependent. Given that we have recently established the importance of IL-17A and Th17 cells in driving BO,38,54 this would suggest that IL-17A rather than IL-22 is the most appropriate therapeutic target for inhibition of BO after stem cell or solid organ transplant.
IL-22 induction in CD4+ T-cells is reported to be dependent upon IL-6,8,10 which is significantly elevated early after allo-SCT.26,42 Moreover, the magnitude of IL-6 release directly correlates with the intensity of pre-transplant conditioning and the incidence of subsequent acute GVHD.49,55 To a lesser extent, IL-23 and IL-1β have also been reported to promote IL-22 expression in CD4+ T-cells, however TGFβ suppresses IL-22 production in all T-cell subsets, redirecting T-cell polarization towards Th17/Tc17 development. It has been suggested that IL-17 is required to mediate IL-22 dependent airway inflammation; Th22 differentiation persisted in the absence of IL-17 but pathogenicity was reduced.34 This is distinct from our observations in allo-SCT, where Th22 frequencies are significantly reduced when donor IL-17-producing cells are depleted or when IL-17A/F signaling responses are abrogated within donor T-cells. This dependency suggests that investigation of Th22 differentiation needs to be undertaken in the context of concurrent Th17 responses, providing the impetus for us to examine the role of IL-22 in an established adoptive transfer model of Th17-induced cutaneous GVHD. Here we observed high levels of both Il17 and Il22 gene expression in the skin of recipients of Th17 cells that persisted over time post-transplant. CCR6−/− Th17 cells failed to induce severe skin GVHD, as did Th17 cells in the presence of IL-22 neutralization. Finally, we observed a striking similarity in cytokine gene expression in samples derived from patients who developed cutaneous GVHD, suggesting comparable pathological processes during clinical GVHD.
In sum, we demonstrate that IL-22 is secreted by distinct but interacting Th22 and Th17 lineages that develop in response to IL-6 and IL-17 and mediate late-onset skin GVHD. Given the complex function of IL-22 in GVHD, therapeutic modulation will require a careful and staged approach to ensure that the benefits of IL-22R signaling early post-transplant are maintained. Our novel observations regarding the relationship between IL-22 and IL-17 suggest that an additional strategy may be to target IL-17 directly in the skin using small molecule inhibition.38 These data provide insight for rational therapeutic strategies to inhibit IL-22 secreting T-cells late after transplant, particularly for the treatment of cutaneous chronic GVHD.
Acknowledgments
This work was supported by grants from the Rio Tinto Ride to Conquer Cancer, Cancer Council Queensland, the National Health and Medical Research Council (Australia) and the National Institutes of Health (P01 CA142106 and R01 CA166794). GRH is a National Health and Medical Research Council Fellow.
Abbreviations
- GVHD
graft-versus-host disease
- SCT
stem cell transplantation
- allo-SCT
allogeneic stem cell transplant
- ILC
innate lymphoid cell
- NK
natural killer
- LTi
lymphoid tissue inducer
- G-CSF
granulocyte colony stimulating factor
- TCD
T-cell depleted
- BM
bone marrow
- TBI
total-body irradiation
- BO
Bronchiolitis obliterans
- DT
diphtheria toxin
Footnotes
Specific Contributions: KHG and HB designed, performed experiments and wrote the manuscript; KP, ANW, MO, DKR, TB, KW, FB, AV, RDK, KC, YF, TS, JC, and MZ designed and performed experiments; MWP and PSF provided essential reagents and useful discussion; ADC performed blinded histological analysis; GRH, JSS and BRB designed experiments and reviewed and edited the manuscript.
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Shlomchik WD. Graft-versus-host disease. Nature reviews. Immunology. 2007 May;7(5):340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 2.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. The New England journal of medicine. 2012 Oct 18;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Jun 1;29(16):2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai S, Arora M, Wang T, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015 Feb;21(2):266–274. doi: 10.1016/j.bbmt.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011 Apr 28;117(17):4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001 Dec 1;98(12):3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 7.Lamarthée B, Malard F, Saas P, Mohty M, Gaugler B. Interleukin-22 in Graft-Versus-Host Disease after Allogeneic Stem Cell Transplantation. Frontiers in immunology. 2016;7:148. doi: 10.3389/fimmu.2016.00148. 04/19 12/22/received 04/04/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013 Mar;252(1):116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 9.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nature reviews. Drug discovery. 2014 Jan;13(1):21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 10.Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. International immunology. 2011 Mar;23(3):159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 11.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009 Dec;119(12):3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plank MW, Kaiko GE, Maltby S, et al. Th22 Cells Form a Distinct Th Lineage from Th17 Cells In Vitro with Unique Transcriptional Properties and Tbet-Dependent Th1 Plasticity. J Immunol. 2017 Mar 01;198(5):2182–2190. doi: 10.4049/jimmunol.1601480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nature immunology. 2009 Aug;10(8):857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 14.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nature immunology. 2009 Aug;10(8):864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 15.Hanash AM, Dudakov JA, Hua G, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012 Aug 24;37(2):339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couturier M, Lamarthee B, Arbez J, et al. IL-22 deficiency in donor T cells attenuates murine acute graft-versus-host disease mortality while sparing the graft-versus-leukemia effect. Leukemia. 2013 Jul;27(7):1527–1537. doi: 10.1038/leu.2013.39. [DOI] [PubMed] [Google Scholar]
- 17.Zhao K, Zhao D, Huang D, et al. Interleukin-22 aggravates murine acute graft-versus-host disease by expanding effector T cell and reducing regulatory T cell. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2014 Sep;34(9):707–715. doi: 10.1089/jir.2013.0099. [DOI] [PubMed] [Google Scholar]
- 18.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006 Oct 1;177(7):4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 19.Hirota K, Duarte JH, Veldhoen M, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nature immunology. 2011 Mar;12(3):255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill GR, Olver SD, Kuns RD, et al. Stem cell mobilization with G-CSF induces type 17 differentiation and promotes scleroderma. Blood. 2010 Aug 5;116(5):819–828. doi: 10.1182/blood-2009-11-256495. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald KP, Rowe V, Filippich C, et al. Donor pretreatment with progenipoietin-1 is superior to granulocyte colony-stimulating factor in preventing graft-versus-host disease after allogeneic stem cell transplantation. Blood. 2003 Mar 1;101(5):2033–2042. doi: 10.1182/blood-2002-05-1529. [DOI] [PubMed] [Google Scholar]
- 22.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009 Feb 5;113(6):1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014 Jun 19;123(25):3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996 Oct 15;88(8):3230–3239. [PubMed] [Google Scholar]
- 25.van Den Brink MR, Moore E, Horndasch KJ, et al. Fas-deficient lpr mice are more susceptible to graft-versus-host disease. J Immunol. 2000 Jan 01;164(1):469–480. doi: 10.4049/jimmunol.164.1.469. [DOI] [PubMed] [Google Scholar]
- 26.Tawara I, Koyama M, Liu C, et al. Interleukin-6 modulates graft-versus-host responses after experimental allogeneic bone marrow transplantation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011 Jan 1;17(1):77–88. doi: 10.1158/1078-0432.CCR-10-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. The Journal of investigative dermatology. 2010 May;130(5):1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolk K, Haugen HS, Xu W, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. Journal of molecular medicine. 2009 May;87(5):523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 29.Wolk K, Witte E, Warszawska K, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. European journal of immunology. 2009 Dec;39(12):3570–3581. doi: 10.1002/eji.200939687. [DOI] [PubMed] [Google Scholar]
- 30.Nakagome K, Imamura M, Kawahata K, et al. High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism. J Immunol. 2011 Nov 15;187(10):5077–5089. doi: 10.4049/jimmunol.1001560. [DOI] [PubMed] [Google Scholar]
- 31.Schnyder B, Lima C, Schnyder-Candrian S. Interleukin-22 is a negative regulator of the allergic response. Cytokine. 2010 May;50(2):220–227. doi: 10.1016/j.cyto.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Hirose K, Kawashima S, et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. The Journal of allergy and clinical immunology. 2011 Nov;128(5):1067–1076. e1061–1066. doi: 10.1016/j.jaci.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Besnard AG, Sabat R, Dumoutier L, et al. Dual Role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. American journal of respiratory and critical care medicine. 2011 May 01;183(9):1153–1163. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- 34.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. The Journal of experimental medicine. 2010 Jun 07;207(6):1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panoskaltsis-Mortari A, Tram KV, Price AP, Wendt CH, Blazar BR. A new murine model for bronchiolitis obliterans post-bone marrow transplant. American journal of respiratory and critical care medicine. 2007 Oct 01;176(7):713–723. doi: 10.1164/rccm.200702-335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013 Mar 28;121(13):2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gartlan KH, Varelias A, Koyama M, et al. Th17 plasticity and transition toward a pathogenic cytokine signature are regulated by cyclosporine after allogeneic SCT. Blood Advances. 2017;1(6):341–351. doi: 10.1182/bloodadvances.2016002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forcade E, Paz K, Flynn R, et al. An activated Th17-prone T cell subset involved in chronic graft-versus-host disease sensitive to pharmacological inhibition. JCI insight. 2017 Jun 15;2(12) doi: 10.1172/jci.insight.92111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Weiner J, Liu Y, et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. The Journal of experimental medicine. 2009 Jul 6;206(7):1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gartlan KH, Markey KA, Varelias A, et al. Tc17 cells are a proinflammatory, plastic lineage of pathogenic CD8+ T cells that induce GVHD without antileukemic effects. Blood. 2015 Sep 24;126(13):1609–1620. doi: 10.1182/blood-2015-01-622662. [DOI] [PubMed] [Google Scholar]
- 41.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy GA, Varelias A, Vuckovic S, et al. Addition of interleukin-6 inhibition with tocilizumab to standard graft-versus-host disease prophylaxis after allogeneic stem-cell transplantation: a phase 1/2 trial. The Lancet. Oncology. 2014 Dec;15(13):1451–1459. doi: 10.1016/S1470-2045(14)71017-4. [DOI] [PubMed] [Google Scholar]
- 43.Hedrick MN, Lonsdorf AS, Shirakawa AK, et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest. 2009 Aug;119(8):2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tumino M, Serafin V, Accordi B, et al. Interleukin-22 in the diagnosis of active chronic graft-versus-host disease in paediatric patients. Br J Haematol. 2015 Jan;168(1):142–145. doi: 10.1111/bjh.13068. [DOI] [PubMed] [Google Scholar]
- 45.Varelias A, Ormerod KL, Bunting MD, et al. Acute graft-versus-host disease is regulated by an IL-17-sensitive microbiome. Blood. 2017 Jan 30; doi: 10.1182/blood-2016-08-732628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sa SM, Valdez PA, Wu J, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. Journal of immunology. 2007 Feb 15;178(4):2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 47.Brembilla NC, Dufour AM, Alvarez M, et al. IL-22 capacitates dermal fibroblast responses to TNF in scleroderma. Annals of the rheumatic diseases. 2015 Oct 9; doi: 10.1136/annrheumdis-2015-207477. [DOI] [PubMed] [Google Scholar]
- 48.Guilloteau K, Paris I, Pedretti N, et al. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis. Journal of immunology. 2010 Mar 24; doi: 10.4049/jimmunol.0902464. [DOI] [PubMed] [Google Scholar]
- 49.Varelias A, Gartlan KH, Kreijveld E, et al. Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood. 2015 Apr 9;125(15):2435–2444. doi: 10.1182/blood-2014-07-590232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serody JS, Hill GR. The IL-17 differentiation pathway and its role in transplant outcome. Biol Blood Marrow Transplant. 2012 Jan;18(1 Suppl):S56–61. doi: 10.1016/j.bbmt.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacDonald KP, Blazar BR, Hill GR. Cytokine mediators of chronic graft-versus-host disease. J Clin Invest. 2017 Jun 30;127(7):2452–2463. doi: 10.1172/JCI90593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacDonald KP, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017 Jan 05;129(1):13–21. doi: 10.1182/blood-2016-06-686618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okamoto S, Fujiwara H, Nishimori H, et al. Anti-IL-12/23 p40 antibody attenuates experimental chronic graft-versus-host disease via suppression of IFN-gamma/IL-17-producing cells. J Immunol. 2015 Feb 01;194(3):1357–1363. doi: 10.4049/jimmunol.1400973. [DOI] [PubMed] [Google Scholar]
- 54.Alexander KA, Flynn R, Lineburg KE, et al. CSF-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J Clin Invest. 2014 Aug 26; doi: 10.1172/JCI75935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997 Oct 15;90(8):3204–3213. [PubMed] [Google Scholar]