Abstract
Objective
The objective of this study was to evaluate the regulatory dynamics between stress and fatigue experienced by women during the menopausal transition (MT) and early post-menopause (EPM). Fatigue and perceived stress are commonly experienced by women during the MT and EPM. We sought to discover relationships between these symptoms and to employ these symptoms as possible markers for resilience.
Methods
Participants were drawn from the longitudinal XX Midlife Women’s Health Study. Eligible women completed questionnaires on 60+ occasions (annual health reports and monthly health diaries) (n=56 women). The total number of observations across the sample was 4224. STRAW+10 criteria were used to stage women in either in late reproductive, early or late transition, or early post-menopausal stage. Change values were generated for fatigue and stress and analyzed with a multilevel structural equation model; slopes indicate how quickly a person returns to homeostasis after a perturbation. Coupling of stress and fatigue was modeled to evaluate resilience, the notion of maintaining stability during change.
Results
Eligible women were an average 35 years old (SD=4.71), well-educated, employed, married or partnered and white. Fit indices suggested the model depicts the relationships of stress and fatigue (χ2(9 df) = 7.638, p=.57, correction factor=4.9244; RMSEA 90%CI = .000 <=.000 <=.032; CFI = 1.00). A loss in model fit across stages suggests that the four stages differed in their dynamics (χ2Δ(12 df) = 21.181, p=.048). All stages showed fixed point attractor dynamics: fatigue became less stable over time; stress generally became more stable over time. Coupling relationships of stress on fatigue show evidence for shifts in regulatory relationships with one another across the MT.
Conclusions
Results are suggestive of general dysregulation via disruptions to coupling relationships of stress and fatigue across the MT. Findings support a holistic approach to understanding symptoms and supporting women during the MT.
Keywords: Menopausal transition, stress, fatigue, dynamics, attractors
Introduction
Women undergo physiological, psychological, and behavioral changes during the menopausal transition (MT) that impact quality of life and health outcomes.1–3 Post-menopausal women score lower on quality of life metrics than pre-menopausal women, a gap partially explained by post-menopausal women’s 10-fold higher risk of suffering vasomotor disorders, 3-fold higher risk for psychosocial impairment, 5-fold higher risk for physical disorders, and 3-fold higher risk for sexual disorders1.
Of symptoms experienced by women over the course of the menopausal transition (MT), fatigue is clinically significant because it may be disabling4 and is one of the most common and distressing symptoms associated with menopause. In a cross-sectional study of 300 women, 85.3% of post-menopausal women and 46.5% of peri-menopausal women reported symptoms of physical and mental exhaustion compared to just 19.7% of the pre-menopausal women.5 There may be financial consequences for midlife women experiencing fatigue. For example, a small qualitative study found that women teaching kindergarten were more likely to retire due to fatigue as they aged, thus having fiscal consequences for their retirement and for schools losing experienced teachers.6
Perceived stress is another frequently reported and bothersome symptom experienced by women during the MT. The literature links perceived stress and MT-related symptoms.7–9 For example, a cross-sectional study examining stressful life events and symptom severity found that post-menopausal women seeking treatment had more severe symptoms than a healthy comparison group.9 Perceived stress may be higher during late transition stage than late reproductive stage10 with suggested links to a history of sexual abuse and depressed mood, while improvement in perceived stress was associated with reduced role burden, improved perception of social support, and income adequacy.11 Studies of cancer patients have shown that fatigue, a common symptom of the MT and early post-menopause, is a major source of frustration and psychological stress, and that ameliorating fatigue reduces psychological stress.12
The co-occurrence of stress and fatigue experienced by women has been studied with respect to the workplace13,14 with work-related stress associated with fatigue, even when controlling for depression and demographic variables.14 However, stress and fatigue have not yet been longitudinally examined with respect to midlife women and the MT. Furthermore, the dynamics of stress and fatigue have not been studied, nor studied with respect to women’s health specifically. Given the incidence of midlife women experiencing the potentially fatiguing and stressful phenomenon of “the sandwich generation” (simultaneously caring for children and parents and often working outside the home, as well), it is an important gap in our understanding of midlife women’s health.15 While it is feasible that perceived stress could cause symptoms experienced during the menopausal transition to worsen, it is equally plausible that worsening of these symptoms could be a source of perceived stress.
The interactive dynamics of stress and fatigue within the context of the MT are particularly interesting as their mutual co-regulatory functions may vary across different stages of the transition. Just as stress is the body’s way of mounting a response to meet the demands of changes or challenges, fatigue is a natural by-product of prolonged exposure to stress. Further, just as stress can drive fatigue, fatigue can down-regulate or up-regulate stress depending on whether fatigue spurs restorative health behaviors (i.e. increased sleep) or interferes with healthy coping (i.e. insomnia) in an individual. Thus, we thought it important to explore the dynamic and likely bidirectional coupling (mutual influencing) of stress and fatigue across the MT.
Stress and Fatigue: A Nonlinear Dynamical Systems Approach
The MT may be considered to be a key example of a developmental transition that challenges resilience over time. Nonlinear dynamical systems theory provides the best approach to studying menopause from this perspective.16 A primary problem with research regarding the MT to date is that symptoms tend to be attributed to the MT rather than to other aspects, both past and present, of women’s lives.17 The present study aims to take into account the underlying systemic structures and physiologic and psychological processes prior to and across transition, rather than looking for the simpler causal influences of isolated variables. More specifically, rather than looking at high or low values on variable x or y for influence on “symptoms” experienced during the MT, the present study will examine changes within and among two key variables, aiming to understand systemic regulatory processes more directly and also more holistically. It is our view that this broader, structural and systemic approach may be necessary to more fully understand the complex and systemic mechanisms underlying biopsychosocial resilience as individuals move through ubiquitous biopsychosocial life transitions such as menopause. Specifically, if stress and fatigue shift in their relationship to one another, then one may lose one’s ability to flexibly bounce back following higher loads of stress or fatigue. Therefore, evidence of shifts in the relationships among variables like stress and fatigue across key life transitions such as the MT may improve one’s understanding of the underlying mechanisms of resilience in complex biopsychosocial systems.
Two central biopsychosocial regulatory processes that drive menopausal symptoms are stress and fatigue. Stress and fatigue each fluctuate over time (a) in their levels (i.e., high versus low), (b) in their flexibility (e.g. chronicity vs. lability), and (c) in their mutual influence on one another (e.g., coupling strength). Additionally, stress and fatigue may remain at a high or low level, termed an attractor. An attractor is how a system will naturally gravitate and remain, unless perturbed. Changes in each of these dynamical features may have functional significance. Apart from mean levels of stress, for example, the ability for stress to rise in response to a challenge and then return to a lower stable set point may be used to define one’s stress resilience.16,18,19 Similarly, mean levels of fatigue say little about one’s resilience compared to one’s ability to become revitalized after sufficient rest.
Certainly, understanding a key handful of factors that are associated with mean levels of stress, fatigue, and other health parameters may provide some useful information about one’s health and functioning. However, to understand resilience during the MT, the most important question is: How well do post-menopausal women maintain their ability to recover from stress and fatigue? McEwen and Wingfield20 defines the underlying mechanisms of resilience in general terms as “maintaining stability through change”, which begs the question: What are the structural factors that underlie one’s ability to remain flexible during the menopausal transition? Therefore, it may prove especially useful to apply models and methods capable of quantifying the relative stability of change dynamics to better understand resilience in complex biopsychosocial systems as women move through key transitions such as the MT. In this context resilience refers to the capacity to adapt to perturbations, including the ability to rebound or bounce back to a higher level of functioning, recover to the same level of functioning or experience some degree of improvement without returning to a prior level of functioning.21
A closely related question to how stability is maintained through change pertains to the maintenance of self-regulatory relationships among key parameters like stress and fatigue over time. How are these key parameters connected to one another? And how might changes in their dynamical coupling impact resilience during the MT and early post-menopause? On a practical level, the potential for stress and fatigue to serve circular regulatory functions is fairly clear. High levels of stress may adaptively drive up levels of fatigue, resulting in the corrective response of increased sleep and rest. In a complementary fashion, prolonged fatigue may increase stress, ideally resulting in some adaptive correction through problem-solving, social support, or shifts in one’s mindset.
A recent line of investigations has tested the idea that a network approach (i.e., symptoms causing one another within a complex network structure) may be more valid in the sense of more accurately modeling than the traditional “disease” approach (i.e., a latent condition exists which causes individual symptoms) in psychopathology.22–24 The evidence gathered thus far has suggested that the structure of symptom networks (i.e., generally looser symptom connectivity) is predictive of resilience from psychopathology. For example, the density of network ties among symptoms predicts severity and relapse in depressive disorders.24
Just as the “symptoms” of conditions like depression may actually arise from a dysregulated network structure (e.g., fatigue causing insomnia, which causes anhedonia and so on), a similar network approach may explain the loss of resilience that can occur during the menopausal transition. Shifts in coupling strength, or coordination among nodes across the transition, would be a key marker for such a shift in resiliency.16,25
The present study aims to use an approach to science that is able to capture such phenomena through the use of modeling approaches that are inherently nonlinear, dynamical and systemic.
The Present Study: Topological change and Coupling across Menopause
Our method for capturing the dynamics of stress and fatigue involves studying the changes in each over time. Dynamical systems models can be thought of as an expansion on growth models whereby the observed trajectories are theorized to be a combination of patterns through time, error, and the reactions to perturbations. We begin to capture these three components by using models that focus on the relationships amongst derivatives. That is, a growth model is articulating change in terms of current value, change in the current value per unit of time (velocity), and error. A systems model need merely examine how that current value predicts the velocity combining the possibility for nonlinear trajectories over time and inherent depictions of the stability of the pattern under an assumption that the system is constantly being perturbed.26
In this particular case, we generated discrete changes over time in stress and fatigue. We then predicted these changes as a function of current stress and fatigue. Such a model allows a linear dynamic in that stress and fatigue can combine to depict attractive behavior while also allowing for the inclusion of coupling, which we believe to be particularly relevant to resiliency. In this case, coupling comes out as the extent to which values in one variable are able to uniquely predict changes in the other – changes in stress and fatigue predicting one another over time.
We then further this logic into a nonlinear dynamic circumstance through two ways, consistent with theory: (1) We allow the model parameters to be different at different stages of the MT and (2) We test for interactions between levels of stress and fatigue to allow stress and fatigue to have differential prediction as a function of one another. The first nonlinear method will examine possible changes to stability and stress-fatigue coupling at each stage of the MT. Do the dynamics change from late reproductive stage to early post-menopause? The second, phase dependent, method allows for a finer look at coupling across different levels of stress and fatigue. For example, it could be that coupling between fatigue and stress only occur when stress is particularly high, allowing fatigue to serve a specific regulatory function selectively, only on the highest levels of stress. The purposes of this study were to (1) investigate relationships among stress and fatigue during the menopausal transition and (2) employ innovative analyses of coupling dynamics to investigate system resilience or rigidity.
Methods
Participants
Participants for this study were drawn from the XX Midlife Women’s Health Study (XMWHS) which is a longitudinal study of women experiencing the menopausal transition (the parent study has been described in detail elsewhere).27 Women entered the study between 1990 and 1992. Those eligible for the parent study included women ages 35–55 with at least one ovary who had had a period within the previous 12 months, were not pregnant or lactating, and were able to speak and read English. This age range was chosen because the menopausal transition typically begins at an average age of 47 years, with the final menstrual period occurring when a woman is around 51 years old. This age range allows researchers to follow women over the transition from late reproductive stage to early post-menopause.26 After an initial in-person interview (n=508) with a registered nurse trained to interview, participants began providing data in the form of an annual health report and monthly (or quarterly) health diary and menstrual calendar (n=390).
Eligible participants for this study (n=56) were those who completed 60 or more annual health reports and monthly health diaries. Women were staged using the STRAW+10 criteria28 according to late reproductive (LR) stage, early menopausal transition (ET) stage, late menopausal transition (LT) stage, or within 5 years of the final menstrual period, the early post-menopausal (EPM) stage. An individual woman may have contributed data over more than one stage as we followed them over several years. Women not eligible for this study included those who had a prior hysterectomy, or received chemotherapy or radiation therapy. The total number of observations across the sample was 4,224. Of this total, 356 observations lacked menopausal stage information, so there was a subtotal of 3,868 observations with menopausal stage information (i.e., LR, ET, LT, EPM). Participants completed on average 69.07 monthly observations (SD=22.61). Table 1 presents the descriptive statistics of observations by menopausal stage. Women had observations on average for 3.44 menopausal stages (SD=.76).
Table 1.
Descriptive statistics of fatigue/stress raw score observations
| Menopausal stage | ||||
|---|---|---|---|---|
| Late Reproductive | Early Transition | Late Transition | Early Post-Menopause | |
| Number of women with data from each stage1 | 46 | 52 | 49 | 42 |
| Mean observations (SD) within women | 32.11 (23.93) | 19.98 (16.59) | 12.73 (10.54) | 17.33 (10.63) |
| Total observations per stage | 1,477 | 1,039 | 624 | 728 |
Note. Number is only of fatigue observations. Descriptive statistics for stress observations are identical to fatigue.
Numbers of women with data from each stage are not mutually exclusive.
Change scores were made from the “raw score” stress and fatigue variables. Because change scores are the difference scores between consecutive months of observations, this lead to there being fewer change score observations than raw score observations, since not all observations were made consecutively. That is, sometimes the timing between observations exceeded 1 monthly unit, and so prevented the creation of a corresponding change score for that raw score observation. Thus, of the 3868 raw score observations with menopausal stage information, a smaller total of 2316 change score observations were derived from raw scores with consecutive monthly responding. The average number of change scores per participant was 41.36 (SD=15.06). Table 2 presents the descriptive statistics of the stress and fatigue change scores.
Table 2.
Descriptive statistics of fatigue/stress change score observations in model
| Menopausal stage | ||||
|---|---|---|---|---|
| Late Reproductive | Early Transition | Late Transition | Early Post-Menopause | |
| Number of women with data from each stage1 | 45 | 42 | 27 | 14 |
| Mean observations (SD) within women | 25.69 (20.21) | 17.12 (14.84) | 11.00 (11.35) | 10.29 (16.01) |
| Total observations per stage | 1,156 | 719 | 297 | 144 |
Note. Number is only of fatigue change score observations. Descriptive statistics for stress change score observations are identical to fatigue change score.
Numbers of women with data from each stage are not mutually exclusive.
Stages of reproductive aging using the STRAW+10 Criteria
Stages of reproductive aging using the STRAW+10 Criteria were used to classify women’s menstrual cycle patterns for those not taking any type of estrogen or progesterone. Menstrual calendar data were used to determine whether women were in the LR, ET, LT, or EPM stage. Classification is based on staging criteria originally developed by Mitchell, Woods, and Mariella29, and validated by the ReSTAGE collaboration.30 Stages include LR (typified by subtle changes in menstrual cycle length), ET (increased variability in menstrual cycle length with persistent difference of 7+ days in length of consecutive cycles), LT (occurrence of amenorrhea of 60 days or longer and menstrual cycles are characterized by increased variability in cycle length, extreme fluctuations in hormonal levels, and increased prevalence of anovulation) and EPM (first 6 years since the final menstrual period). Symptoms are often the most frequent, severe and/or bothersome in the LT and EPM stages.31–34
Measures
The health diary included a symptom checklist that included questions about perceived stress and fatigue. Diary data were obtained on days 5, 6, and 7 of the menstrual cycle in pre-menopausal women (post-menopausal women provided diaries on a monthly schedule).
Fatigue
Fatigue was assessed with a single question from the annual health report and monthly diaries (“Think back over the last 24 hours and rate overall how severe was each symptom listed below – fatigue”) and was rated on a scale of 0 to 4 (0 not present, 1 minimal, 2 mild, 3 moderate, 4 extreme). Test retest reliability was .39.
Perceived stress
Perceived stress was assessed with one question from the annual health report and monthly diaries: “How stressful was today?” Participants answered using a scale ranging from 1 to 6 where 1 indicated none, and 6 indicated extremely. Test retest reliability was .37.
Overview of Data Analytic Strategy
Fatigue and stress were converted into discrete differences between the reported value one step into the future minus the current value. These differences were positive when stress/fatigue is increasing over time and negative when decreasing. Consecutive time-points that were greater than 30 days apart were treated as missing data to maintain some degree of temporal consistency in the changes. We then treated the changes in fatigue and changes in stress as simultaneous outcomes through a multivariate multilevel model in Mplus.35
We utilized a multilevel structural equation model, an analytic technique designed to account for data dependency. In this case, we accounted for two different dependencies. The first involved multiple measures of fatigue and stress from each individual (captured through the two level model). The second involved having two different dependent variables, changes in stress and fatigue respectively (captured by modeling stress and fatigue simultaneously with the inclusion of error covariances between them). This method is analogous to conducting regressions within the dependencies, saving out coefficients and then summarizing and predicting these coefficients. However, structural equation modeling is a maximum likelihood estimation procedure and therefore instead generates results as if these steps were carried out, rather than actually taking each step. As a set, we can depict the basic model as a pair of nested equations, one for change in fatigue as the dependent variable and one for the change in stress. For a given individual (i) at a given point in time(t) the basic equations were:
| (1) |
| (2) |
The betas (β) represent average effects across individuals. The errors terms (e) were captured as separate error variances for each of the dependent variables with a covariance between them to account for any remaining dependency.
This linear dynamic equation is capable of capturing three different kinds of effects: (1) do individuals’ stress and fatigue function homeostatically, returning to some combined level of stress and fatigue? (2) How stable is homeostasis for stress and fatigue: if a person moves away from their homeostatic level, how quickly do they return? And (3), do stress and fatigue relate to one another in terms of if one is moved away from their homeostatic level, do we see patterns consistent with the other being further stabilized or disrupted? The homeostatic nature of stress and fatigue (1) can be assessed through beta 1 and beta 5 (the effects of how a variable predicts its own change). When both are negative, it indicates that when diverging from the homeostatic value for stress and fatigue people invariably return to that value. The stability or resiliency of stress and fatigue (2) are captured by the steepness of the negative slopes. A steeper slope indicates that a person returns to the homeostatic level following a perturbation more quickly.
The third question about the relationship between stress and fatigue (3) are captured by beta 2 and beta 4 (the crossover or coupling effects). For example, if a person alters their diet (a perturbation), this might temporarily induce an increase in stress, Beta 2 implies that this would carry over into changes in fatigue which could then carry back through to stress via beta 4.
In this paper, we also utilize prediction plots of where we would expect a person’s fatigue and stress to go next as a visual way to interpret these effects. This involves creating a vector plot where combinations of values in stress and fatigue are factored forward to ask where the equations would predict the values to be after some period of time. The homeostatic point (or set point) is the point in which all the arrows converge. The length of arrows captures the stability as longer arrows bring one back to the homeostatic point faster. Swirling depictions capture coupling. For example, if only the coupling effects existed, and momentum effects (beta1 and beta 5) were zero, the model would then depict a cycle (i.e., an orbit around a fixed point) where upon the emergent pattern is a repeating cycle for both fatigue and stress over time. Graphically, this would create a circle on these prediction plots.
Adding in main effects of other variables essentially depict the change in location of the set point. However, building nonlinear models (in this case through the inclusion of interactions) alters the form of the relationships and can depict changes in the properties of dynamic patterns (i.e. the stability and coupling relationships can also change).26 For example, a variable that interacts with their own effects (beta 1 and beta 5) can strengthen, weaken and even extinguish the homeostatic nature of stress and fatigue. Variables that interact with the coupling effects (beta 2 and beta 4) can alter the coupling relationships. As resilience may be a case of multiple forms of altering the underlying topology simultaneously (e.g. changing the coupling, the strength, and location of the dynamic properties) we included all plausible linear interactions. Specifically, we included interactions within both equations between stress and fatigue. The utilized models were therefore:
| (3) |
| (4) |
To account for different attractor dynamics during each stage, we then treated the model as a stacked or multiple group analysis in Mplus. This essentially allows for different estimates of every coefficient for each stage and also allows the possibility to compare stages through equality constraints of sets of parameters. We also chose to person-center fatigue and stress bringing the focus onto intrinsic dynamics where the models characterize deviations from one’s own average. We excluded random effects with the exception of the residual variances by using the ‘complex’ estimation method to avoid oversaturation of the model. The resultant model utilized FIML with the Yuan and Bentler adjustment for non-normality.36 Significance is reported at alpha=.05, two-tailed.
Results
Sample
Descriptive Statistics for this subsample
The women who were eligible for inclusion had a mean age of 35 years (SD=4.71) years at the beginning of the study, a mean of 16 years of education (SD=2.57), and a median family income of $22,660 (SD $6,900). Most of the eligible participants were currently employed (93%), 73% were married or partnered, 19% were divorced or widowed, and 7% had never been partnered or married. Eligible women described their ethnicity at the start of the study as 2% African American, 7% Asian American, 91% White and no one self-described as Hispanic or other. Refer to Table 3 for a comparison of women eligible for this study and those determined ineligible. As demonstrated in Table 3, all women considered for this study are of similar age, education and income to those excluded. Women included in analyses were more likely to be white than those who were not eligible for these analyses. The groups were similar with respect to employment and marital status.
TABLE 3.
Sample Characteristics of Eligible and Ineligible Women for Inclusion
| Eligible women (n=56) |
Ineligible women (n= 451) |
||
|---|---|---|---|
| Characteristics | Mean (SD) | Mean (SD) | Pa |
| Age, y | 35(4.71) | 42(4.68) | .45 |
| Years of education | 16(2.57) | 16(2.82) | .80 |
| Family income, $ | 22,660(6,900) | 17,950(7,650) | .82 |
| Characteristics | n(%) | n(%) | Pb |
| Currently employed | .03 | ||
| Yes | 52(92.9) | 388(86) | |
| No | 4(7.1) | 63(14) | |
| Race/ethnicity | .05 | ||
| African American | 1(1.8) | 57(12.6) | |
| Asian/Pacific Islander | 4(7.1) | 39(8.6) | |
| White | 51(91.1) | 339(75.2) | |
| Other (Hispanic, mixed) | 0 | 16(3.5) | |
| Marital status | .86 | ||
| Married/partnered | 41(73) | 306(67.8) | |
| Divorced/widowed/not partnered | 11(19.17) | 114(23.5) | |
| Never married/partnered | 4(7.1) | 31(6.9) |
Independent t test.
χ2 test.
All fit indices suggested that the model adequately depicts the relationships (χ2(9 df) = 7.638, p=.57, correction factor=4.9244; RMSEA 90%CI = .000 <=.000 <=.032; CFI = 1.00). The level-1 and level-2 sample sizes for LR were n1=46 and n2=1477, for ET were n1=52 and n2=1039, for LT n1=49 and n2=624, and for EPM were n1=42 and n2=728. These sample sizes correspond to the raw scores because Mplus uses full information maximum likelihood to estimate the model using all the sample data, including the raw scores with missing change scores. The R2 values for each outcome in each group accounted for between 37–48% of changes in fatigue and 40–45% of changes in stress in the data. To test for the equivalency of the four reproductive aging stages (LR, ET, LT, and EPM), we equated all own and coupling parameters across groups. This induced a significant loss in model fit (χ2Δ(12 df) = 21.181, p=.048) suggesting that the four stages, in fact, differed in their dynamics. To illustrate the higher order emergent dynamics for each stage, we estimated trajectories from the equations using the Runge-Kutta 4th order algorithm and overlaid them onto a Fatigue by Stress space akin to a velocity flow field.37 Trajectory starting points were one standard deviation above, at the mean, and below the mean for fatigue and stress respectively for each group. All four stage-specific patterns were homeostatic (i.e., attractors; stress and fatigue returning to set-points), but they differed in the location of their set-points, the strength of the attraction, and the coupling relationships between stress and fatigue (see Figure 1). Note that the labels in Figure 1 are: Late Reproductive (1), Early Transition (2), Late Transition (3) and Early Post-Menopause (4).
Fig. 1.
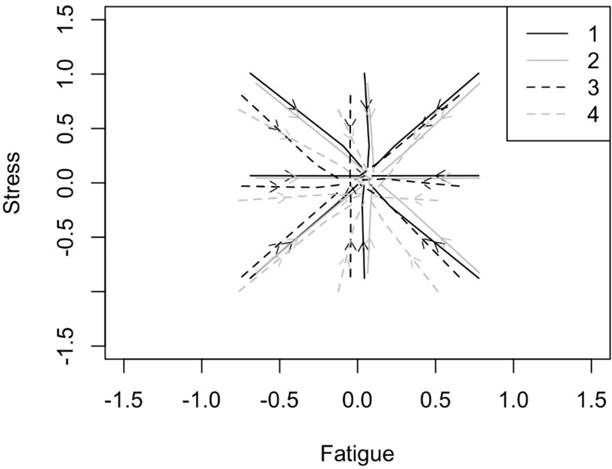
Velocity flow field diagram depicting trajectories of stress and fatigue at each of the four stages of menopause (1 = LR, 2 = ET; 3 = LT; 4 = EPM).
Given that the data were person-centered (change is depicted in standard-deviation units), the overall location of attraction is expected to closely approximate zero. As such, slight shifts from zero at each stage may indeed represent meaningful shifts within the intrinsic stability of each parameter (i.e., stress and fatigue) across the menopausal stages. Within the model under this centering logic, changes in the intercepts from the equations directly represent the set points. Figure 2 illustrates these intercepts in relation to one another and in relation to means. Overall, the intercepts did not differ from one another (determined by comparing the model to one where the intercepts were equated, χ2Δ(6 df) = 9.385, p=.153). However, some individual intercepts were different from zero while others were not. For both the LR and ET stages, the set point for fatigue is significantly higher than individual’s mean fatigue (p=.017 and p=.001, respectively). This was not true for the later stages (p=.537 for LT and p=.208 for EPM), nor were any of the stress set points significantly different from the individual’s means.
Fig. 2.
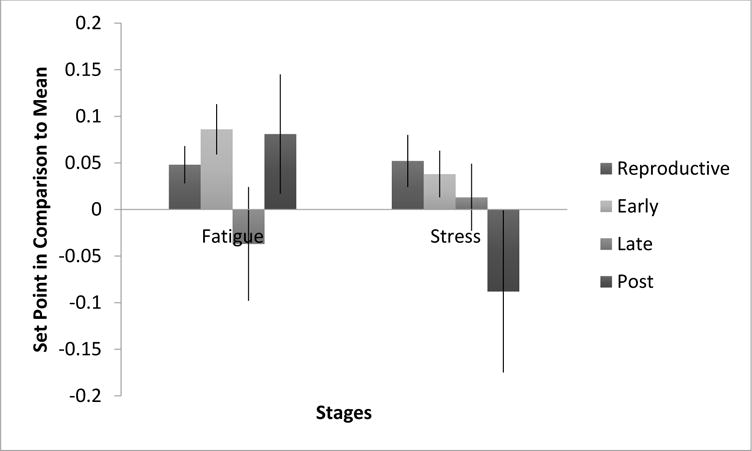
Set point differences and standard error bars. Reproductive stage N1 = 1477, N2 = 46; Early stage N1 = 1039, N2 = 52; Late stage N1 =624, N2 = 49; Post stage N1 = 728, N2 = 42.
Note that the labels in Figures 2–5 are: Late Reproductive (Reproductive), Early Transition (Early), Late Transition (Late) and Early Post-Menopause (Post).
Fig. 5.
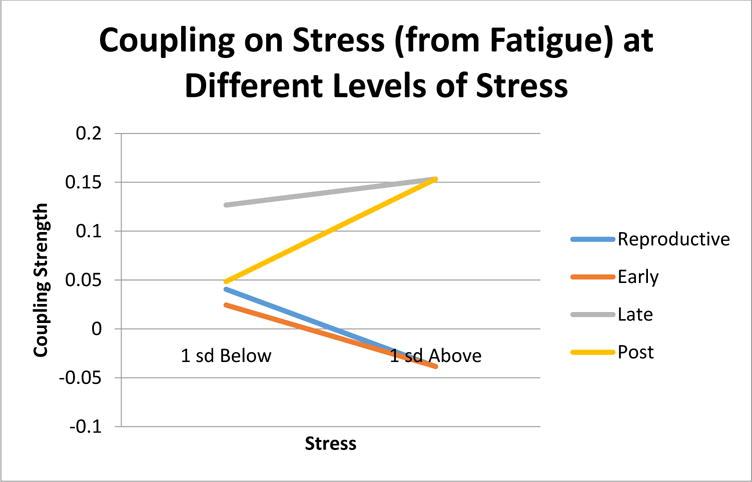
Coupling of fatigue on stress at different levels
Effects of how each variable predicts its own changes represents a form of Lyapunov exponent characterizing the strength of attraction towards the set point, but also the influence of perturbations within the system.26 The inclusion of the interactions to account for phase dependent coupling allow these effects to differ at levels of one variable in regards to the other (e.g. the attraction of fatigue can differ at levels of stress and vice versa). However, our centering procedure modifies the interpretation of the main effects to the average within person effects. The more negative the coefficient, inherently the more stable the attractor in that dimension. That is, when perturbed, the more negative slope indicates less effect as a function of the same size of perturbation and faster return to the set point. In all cases, the coefficient was significantly different from zero (all p values less than .001).
To test the equivalency of these effects across reproductive aging stages, we conducted a chi-square difference test equating the like coefficients across stages. The constraints significantly worsened model fit (χ2Δ(6 df) = 21.491, p=.001) suggesting that the stabilities of the stress and fatigue dimensions of the attractors vary by stage. Chi-square difference tests revealed the fatigue dimension of attractor significantly weakens from LR (β=−.965) to ET (β=−.858) (χ2Δ(1 df) = 4.389, p=.036), and weakens again from ET to LT (β=−.788) (χ2Δ(1 df) = 5.012, p=.025). The fatigue attractor strength did not significantly change from LT to EPM (χ2Δ(1 df) = 2.235, p=.135). As for the stress dimension of the attractor, it strengthened from ET (β=−.818) to LT (β=−.965) (χ2Δ(1 df) = 6.834, p=.009, and nearly significantly weakened, but did not meet criteria, from LT to EPM (β=−.842) (χ2Δ(1 df) = 3.640, p=.056. In sum, fatigue showed a pattern of decreasing stability from LR to EPM and stress showed a pattern of increasing stability.
Coupling is represented by the crossover prediction of stress predicting changes in fatigue and fatigue predicting changes in stress (the curvature observed in the trajectories of Figure 1). Since we envisioned the possibility that coupling could vary across different levels of stress and fatigue, we specifically interpret the interactions as if the variable predicting its own changes moderated the coupling relationships. Coupling is best interpreted both in terms of its deviation from zero and in the interpretation of the sign. The deviation from zero indicates the occurrence of coupling, that the current level of stress or fatigue perturbs the level of the other variable carrying over the influence of one outcome to the other. The sign indicates the form of this perturbation - positive values indicate that when one is higher, it pushes the other variable to be higher. Negative values indicate that when one is higher it pushes the other to be lower.
Figure 4 illustrates the relationships of stress on changes in fatigue. For the LR stage, there was significant coupling under all circumstances (βmain effect=.062, p=.029; βinteraction=.009, p=.792) indicating that higher stress corresponded to a higher level of fatigue no matter the level of fatigue. During ET there was no evidence of coupling (βmain effect=.021, p=.494; βinteraction=−.035, p=.314). This was also true for LT (βmain effect=−.005, p=.948; βinteraction=−.082, p=.182) For EPM, coupling on fatigue from stress was phase-dependent upon the level of fatigue (βmain effect=.106, p=.172; βinteraction=−.098, p=.030). Specifically, stress was coupled when fatigue was low.
Fig. 4.
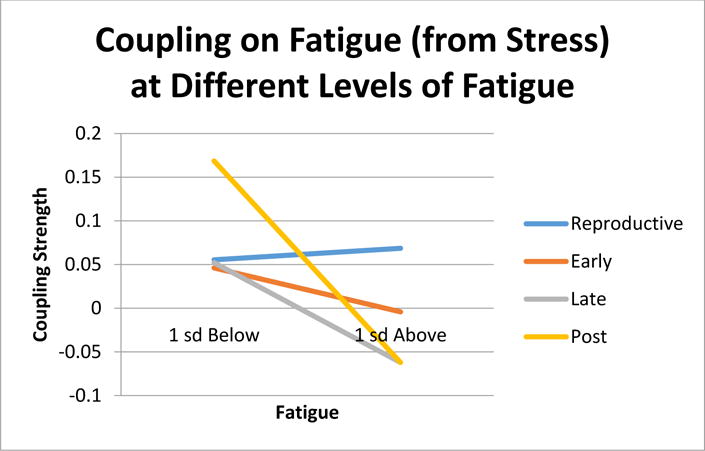
Coupling effects of stress on fatigue at high and low levels of fatigue.
The coupling of fatigue on changes in stress had quite a different pattern, illustrated in Figure 5. Fatigue showed no coupling during ET (βmain effect=.001, p=.978; βinteraction=−.042, p=.166). During LT, coupling was constantly positive (βmain effect=.14, p=.035; βinteraction=.016, p=.822). And as before, we observed phase dependent coupling only in the EPM stage (βmain effect=−.076, p=.284; βinteraction=−.149, p=.036). Here positive coupling between fatigue and changes in stress occurred under higher stress, allowing changes in fatigue to correspond to more changes in stress. In sum, coupling only occurred during LR, LT and EPM. During LR changes in stress predicted changes in fatigue. During LT, changes in fatigue predicted changes in stress. During EPM, both coupling directions appeared, but phase dependently such that only one of the coupling directions appeared at a given point in time.
Discussion
The present study aimed to directly measure the regulatory dynamics of stress and fatigue across the stages of the MT and early post-menopause. Because there is no prior research directly analyzing stability and coupling of women’s experiences across the full menopausal transition, our hypotheses were somewhat open-ended and exploratory: (a) a decrease in attractor strength in key parameters during the transition in response to biopsychosocial stress – signaling a loss of coherence in the dynamics of stress and of fatigue; and (b) shifts in the regulatory coupling relationships between stress and fatigue from late reproductive stage to early post-menopause. In general, the evidence did support each of these hypotheses.
Overall, the dynamical menses-by-menses time-series model provided an adequate fit to changes in each parameter over time, with R2 values across MT stages ranging 37–48% for fatigue, and 40–45% for stress. Furthermore, there was a significant loss in model fit (χ2Δ(12 df) = 21.181, p=.048) when including menopausal stages, suggesting that the four stages, in fact, differed in their dynamics.
All stages showed fixed point attractor dynamics for stress and fatigue, and there was some mixed evidence for changes to attractor location for fatigue between ET and LT (see Figure 1). Overall, the intercepts did not differ from one another (determined by comparing the model to one where the intercepts were equated, χ2Δ(6 df) = 9.385, p=.153). However, some individual intercepts were different from zero (the individual’s mean) while others were not. For both the LR and ET stages, the set point for fatigue is significantly higher than individual’s mean fatigue (p=.017 and p=.001, respectively). This was not true for the later stages (p=.537 for LT and p=.208 for EPM). One logical interpretation of this result is that when a woman becomes more fatigued than usual, there is a tendency for fatigue to hang around longer – what goes up, doesn’t tend to come down as quickly. None of the stress set points differed significantly from the individual’s means (see Figure 2).
In terms of attractor strength, the homeostatic pull of stress and fatigue, the results suggest that fatigue became less stable (less pull) from LR to LT and continuing into EPM; however, stress generally became more stable (stronger pull from LR to LT; see Figure 3). We had predicted decreased stability for both variables, and so this hypothesis was only partially supported (for fatigue only). The opposite shift was observed for stress as was predicted – increasing stability across the menopausal transition. Of course, there is no clear way to interpret whether increasing or decreasing stability is healthy or not without considering levels. For example, if stress is more stable and also higher, this would be bad, whereas if stress is generally low, stability is good. At this point, all we can say is that stability appears to change across the MT, with fatigue becoming less stable and stress becoming more stable.
Fig. 3.
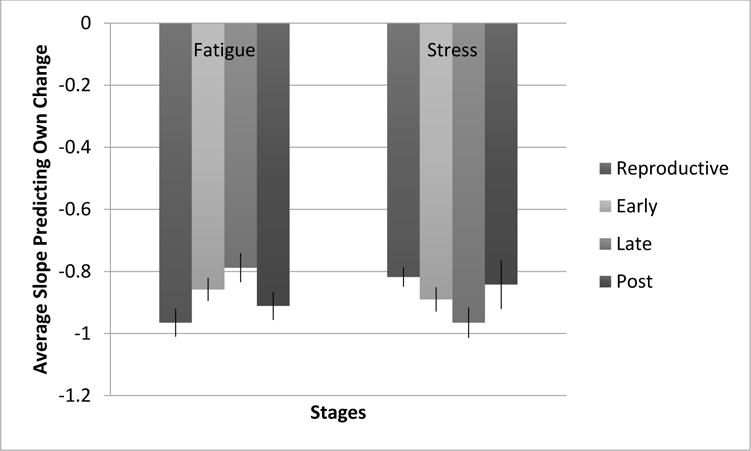
Attractor strength across menopausal stages with standard error bars. More negative slopes indicate greater attractive strength.
The results for coupling have more clear-cut implications for health and resilience (though clearly this study is only a first step), with both stress and fatigue showing evidence for shifting their regulatory relationships with one another across the MT. For the impact of stress on fatigue: In LR, increasing stress was associated with increasing fatigue (across all levels of stress). This is likely a smooth healthy regulatory function early on, whereby up-tics in one’s stress during a first menses pull for a proportional uptick in fatigue during the next menses, no matter the degree of stress. Interestingly, during the middle stages of transition (i.e., from ET to LT) the coupling relationship disappears – which suggests a disconnection or breaking down of this adaptive regulatory function. After the MT, fatigue is once again driven by stress; however, the smooth proportionality now appears warped. No longer smoothly connected across all levels of stress, fatigue is driven by stress only at the lower fatigue levels (see Figure 4). This may indicate some loss of resilience since the regulatory function of fatigue is no longer operating when one is at higher levels of fatigue.
Regarding the opposite relationship, fatigue driving stress, it appears that at the early years (LR and ET) there is no coupling relationship: being tired doesn’t make one stressed. This seems to make sense in terms of healthy functioning because there is no obvious reason why fatigue should be stressful. However, during LT, fatigue at any level of stress tends to make one even more stressed. Various explanations may be offered for this phenomenon, for example that fatigue becomes more threatening once one has reached LT because of negative experiences with being fatigued during the transition. Whatever the explanation, it would appear ideal for one’s resilience from fatigue or for stress regulation. Further, the warping effect appears again at EPM but with the opposite pattern as stress driving fatigue. Fatigue is apparently only stressful for post-menopausal women when they are at higher levels of stress (see Figure 5). Again, this result may indicate that the MT is a time when women learn to react negatively to their own fatigue levels during the menopausal transition, with a lingering sensitivity during EPM when their stress levels are high.
Putting these two sets of coupling results together, it appears that these women tended to go into the MT with a smooth healthy regulatory function of increasing stress leading to increasing fatigue, and fatigue not leading to stress at all. During the MT, stress loses its driving relationship to fatigue, while fatigue forms a driving relationship with stress. Finally, at EPM the connections in both directions are left significantly warped, with stress driving up fatigue only when stress is low, and fatigue driving up stress only when stress is high.
Altogether, these results are suggestive of some general dysregulation via disruptions to coupling relationships across the MT, and likely serving to decrease one’s ability to bounce back from either stress or fatigue (i.e., resilience). Nevertheless, these interpretations are also somewhat speculative and have been derived post-hoc. Numerous other interpretations may be offered, and so replication with a more specific set of hypotheses will be necessary before any firm conclusions may be drawn.
Despite their preliminary nature, the present results are cutting edge in providing support for a more holistic approach to understanding and supporting women’s transition through menopause. First, health care providers may wish to sensitively consider their assumptions about the MT in order to provide a more accurate and helpful story about what the MT actually is.
There is evidence that positive health behaviors are related to fewer symptoms in this population,41 but how should such messages be tailored? Rather than viewing the MT as a time of symptoms (based perhaps on a faulty disease metaphor), the MT may more accurately be described as one of several critical biopsychosocial life transitions. Nevertheless, most women should be taught to expect some degree of self-regulatory shifts to the dynamics of key hormonally-driven experiences, such as stress, fatigue, and changes in mood. Patients may benefit from being taught to understand self-regulation in terms of network dynamics; beyond temporary increases or decreases in stress or fatigue, they may also find that it is more difficult to manage their stress as they did before because it no longer triggers fatigue. Or, they may find that when they get tired, it is accompanied by stress for no good purpose. The present study only examined these two parameters and found signs of dysregulation across the MT. Numerous other processes may shift similarly, and different patients may have unique sets of increase or decrease in coupling processes across the biopsychosocial spectrum. Through increased awareness of these particular shifts, as well as the use of intentional strategies to compensate for loss of automatic regulatory functions (e.g., purposefully resting when stressed) women may be empowered to understand how the transition process works, and how to take better holistic care of themselves as they move through the transition.
The present study had a number of limitations that should be considered prior to any applications and any subsequent follow up investigations. First, the sampling interval was menses-by-menses. As such, it started at around a one-month lag, and became more irregular across the phases of the MT. For those concerned with regular sampling intervals in time-series analysis, this could be viewed as problematic because regularity in sampling is usually ideal for time-series designs. Similarly, one could argue that the data were under-sampled, inasmuch as stress and fatigue shift more quickly than a month-to-month pace.
On the other hand, biological time is arguably different than chronological time, and it would be a hard stretch to try to form inferences about the impact of menopause on stress and fatigue if the sampling had been done outside of the menstrual period. In an ideal scenario, the data would have been collected on a daily rate, and then within and between menstrual periods could have been investigated. With more than a minimum of 60 months of data collection, however, it would have been quite difficult to obtain daily samples, especially given the lack of cell phone and other mobile data collection devices at the time this study was launched.
A second, related, limitation of the present study is the regulatory interpretations made between stress and fatigue when the lag interval is one menstrual period (several weeks to a month long). One could argue that it would be more appropriate to consider the regulatory functions between stress and fatigue as occurring within a single cycle. Indeed, hourly measurements could hypothetically be collected across 3–4 days within a cycle to test similar hypotheses at a more micro-temporal level. However, the shifts to the monthly dynamics, in both stability and regulatory influence may also be seen as rather impressive precisely because the lag is so long. If the stability of both stress and fatigue, as well as their driver-driven dynamics, are showing permanent shifts at a monthly lag, it is highly unlikely that they are not shifting at a more micro (hourly or daily) scale as well.
A third limitation of the present study is that there was a substantial degree of missing data, with an average of 75 instances per individual representing 58% of measurements missing, and also the fact that this sample was generally very healthy throughout their transitions (average fatigue of 1.11 on a scale of 0 to 4 and stress of 2.37 on a scale of 1 to 6 across the sample). Further, growth models (which have some connections to systems models generally) have been argued to sometimes account for missingness in data that would otherwise be thought due to nonrandom missingness38. Again, however, these are not the sort of limitations that would increase the likelihood of a type-I error, but would instead place a damper on statistical power. Finding dynamical results with only 56 individuals with high levels of missing data may be seen as good support for the application of SEM and HLM approaches to nonlinear time-series investigations. Traditional time-series methods that depend on very long, complete, and clean data are not feasible in situations such as this.
A fourth limitation of the present study is that there may have been other cofounders involved in the dynamics of stress and fatigue during the MT. Specifically, it is possible that women may have had varying levels of coping, as well as varying stressors. Stressors may have included stress with family members, children, co-workers, financial stressors or health stresses. We could not account for any of these in our analyses.
Finally, as has already been emphasized, the current hypotheses were rather broad – essentially predicting shifts in both the stability of, and the driver-driven linkages between stress and fatigue across menopause. Following this first important step towards a more direct approach to understanding biopsychosocial dynamics and structural resilience processes, subsequent investigations should make more specific hypotheses about which shifts are likely to occur, and in which directions.
There is a wide and potentially groundbreaking set of follow-up investigations that may now be carried out using the methods developed for the present study, including follow-up studies using the present data set. For example, it may be interesting to derive individual parameters for change in stability and coupling strength (from the HLM analysis) to predict functional outcomes across this sample of women. One would predict that the greater the shifts in stability and coupling strengths, the greater would be the degree of post-menopausal problems experienced, such as anxiety, depression, fatigue, cortisol dysregulation and relational conflict. Other predictors could be fruitfully investigated apart from menopausal stage, such as attitudes toward menopause, key hormonal levels, or pre-menopausal transition functioning, each of which might hypothetically moderate changes to dynamical processes over time (e.g., attractor stability and coupling strength). Finally, other key variables are likely to form nodes within a broader network that includes stress and fatigue. A likely third variable, for example, would be hot flashes, which emerge specifically during the MT and which may help to explain to some extent the stability shifts and warped coupling between stress and fatigue that have been observed here. Specifically, higher levels of hot flashes in the nighttime may help to explain why fatigue becomes more stressful across the menopausal transition, as may the shift in one’s social roles that also tends to occur at around this time of life.
From the broader perspective, the present study may be viewed as supportive of the use of a nonlinear dynamical systems approach to understanding biopsychosocial resilience. Both science and practice should reconsider the very idea that something like menopause could have “symptoms,” which imply some latent “illness” that causes fatigue, stress, insomnia, hot-flashes, and interpersonal functioning and such in a linear and one-directional manner. Instead, practitioners and researchers alike should consider that these biological, psychological and social parameters are each potentially linked together within a self-regulatory network, where each may influence the other over time. Through this lens, features such as stuckness and linkage have more to do with health and resilience than solitary levels at any particular point in time. Indeed, such an approach is being fruitfully applied to reconsidering how psychiatric symptoms operate within a network model rather than the traditional latent disease process model.22,24
Such an approach may indeed be a more accurate and fruitful quantitative approach to both science, and also for patient care across each of the most common chronic conditions (e.g., overweight, diabetes, and heart disease). Distinct from acute care, where simple causes lead to clear illnesses, with simple treatments, the quantitative science of chronic conditions may benefit from a focus that is more holistic and consistent with the views of the most experienced and talented practitioners. Rather than focusing on ups and downs in simple signs and symptoms, quantitative research may move toward viewing the dynamics of person as a holistic network, viewing the movement within the net, and not just a snapshot of its nodes.
In sum, this study shows that the dynamic relationship between the body’s regulatory relationship between stress and fatigue changes dramatically over the course of the MT. We believe this insight will be important in helping to inform interventions for women. For example, if women are assisted in tracking important changes in the self-regulatory functions of stress and fatigue, they many then develop healthy compensatory habits, such as increasing the regularity of sleep schedules to assist in fatigue-related stress, or learning to better self-assess their stress levels and to take planned breaks even if they don’t feel immediately fatigued. A better understanding of the menopausal transition will also help lead to a better understanding of the differences among menopausal symptoms, mid-life stressors within a modern Western cultural context, and experiences that unfold through natural aging. On the broadest scope, understanding the dynamics of the menopausal transition may help us to better grasp the mechanisms that underlie human resilience in general.
Conclusion
We observed that fatigue became less stable and stress became more stable over time and that the two variables were less coupled during ET to LT, suggesting a breakdown of this adaptive function. The regulatory relationship between stress and fatigue changes over the course of the MT with distinctly different patterns by stage. These findings suggest changes in system-wide resilience during the MT. Suggestions are made to evaluate midlife women in a more holistic manner, to better understand this complex developmental transition.
Acknowledgments
Financial support: This work was supported in part by NIH National Library of Medicine (NLM) Training Program in Biomedical and Health Informatics at the University of Washington, Grant Nr. T15LM007442.
Footnotes
Conflicts & Disclosures: None
Contributor Information
Lisa Taylor-Swanson, University of Utah, College of Nursing.
Alexander E. Wong, University of Utah, Department of Psychology.
David Pincus, Chapman University, Department of Psychology.
Jonathan E. Butner, University of Utah, Department of Psychology.
Jennifer Hahn-Holbrook, Chapman University, Department of Psychology.
Mary Koithan, University of Arizona, College of Nursing.
Kathryn Wann, Chapman University, Department of Psychology.
Nancy Fugate Woods, University of Washington, School of Nursing.
References
- 1.Blumel JE, Castelo-Branco C, Binfa L, et al. Quality of life after the menopause: a population study. Maturitas. 2000;34(1):17–23. doi: 10.1016/s0378-5122(99)00081-x. [DOI] [PubMed] [Google Scholar]
- 2.Tseng LA, El Khoudary SR, Young EA, et al. The association of menopause status with physical function: the Study of Women’s Health Across the Nation. Menopause. 2012;19(11):1186–1192. doi: 10.1097/gme.0b013e3182565740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009;62(2):153–159. doi: 10.1016/j.maturitas.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Sharpe M, Wilks D. Fatigue. BMJ. 2002;325(7362):480–483. doi: 10.1136/bmj.325.7362.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chedraui P, Aguirre W, Hidalgo L, Fayad L. Assessing menopausal symptoms among healthy middle aged women with the Menopause Rating Scale. Maturitas. 2007;57(3):271–278. doi: 10.1016/j.maturitas.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Cau-Bareille D. Factors influencing early retirement in a female-dominated profession: kindergarten teacher in France. Work. 2011;40(Suppl 1):S15–30. doi: 10.3233/WOR-2011-1265. [DOI] [PubMed] [Google Scholar]
- 7.Glazer G, Zeller R, Delumba L, et al. The Ohio Midlife Women’s Study. Health care for women international. 2002;23(6–7):612–630. doi: 10.1080/07399330290107377. [DOI] [PubMed] [Google Scholar]
- 8.Hunter MS. Depression and the menopause. BMJ. 1996;313(7067):1217–1218. doi: 10.1136/bmj.313.7067.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igarashi M, Saito H, Morioka Y, Oiji A, Nadaoka T, Kashiwakura M. Stress vulnerability and climacteric symptoms: life events, coping behavior, and severity of symptoms. Gynecol Obstet Invest. 2000;49(3):170–178. doi: 10.1159/000010241. [DOI] [PubMed] [Google Scholar]
- 10.Falconi AM, Gold EB, Janssen I. The longitudinal relation of stress during the menopausal transition to fibrinogen concentrations: results from the Study of Women’s Health Across the Nation. Menopause. 2016;23(5):518–527. doi: 10.1097/GME.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods NF, Mitchell ES, Percival DB, Smith-DiJulio K. Is the menopausal transition stressful? Observations of perceived stress from the Seattle Midlife Women’s Health Study. Menopause. 2009;16(1):90–97. doi: 10.1097/gme.0b013e31817ed261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson LE, Garland SN. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int J Behav Med. 2005;12(4):278–285. doi: 10.1207/s15327558ijbm1204_9. [DOI] [PubMed] [Google Scholar]
- 13.Ricci JA, Chee E, Lorandeau AL, Berger J. Fatigue in the U.S. workforce: prevalence and implications for lost productive work time. J Occup Environ Med. 2007;49(1):1–10. doi: 10.1097/01.jom.0000249782.60321.2a. [DOI] [PubMed] [Google Scholar]
- 14.Rose DM, Seidler A, Nübling M, et al. Associations of fatigue to work-related stress, mental and physical health in an employed community sample. BMC Psychiatry. 2017;17(1):167. doi: 10.1186/s12888-017-1237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillett JE, Crisp DA. Examining coping style and the relationship between stress and subjective well-being in Australia’s ‘sandwich generation’. Australas J Ageing. 2017;36(3):222–227. doi: 10.1111/ajag.12439. [DOI] [PubMed] [Google Scholar]
- 16.Pincus D, Metten A. Nonlinear dynamics in biopsychosocial resilience. Nonlinear Dynamics Psychol Life Sci. 2010;14(4):353–380. [PubMed] [Google Scholar]
- 17.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33(4):539–549. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of internal medicine. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- 19.Sterling P, Eyer J. Allostasis: A new paradigm to explain arosal pathology. New York, NY: Wiley; 1998. [Google Scholar]
- 20.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 21.Szanton SL, Gill JM. Facilitating resilience using a society-to-cells framework: a theory of nursing essentials applied to research and practice. ANS Adv Nurs Sci. 2010;33(4):329–343. doi: 10.1097/ANS.0b013e3181fb2ea2. [DOI] [PubMed] [Google Scholar]
- 22.Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Annu Rev Clin Psychol. 2013;9:91–121. doi: 10.1146/annurev-clinpsy-050212-185608. [DOI] [PubMed] [Google Scholar]
- 23.Borsboom D, Cramer AO, Schmittmann VD, Epskamp S, Waldorp LJ. The small world of psychopathology. PloS one. 2011;6(11):e27407. doi: 10.1371/journal.pone.0027407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wigman JT, van Os J, Borsboom D, et al. Exploring the underlying structure of mental disorders: cross-diagnostic differences and similarities from a network perspective using both a top-down and a bottom-up approach. Psychol Med. 2015;45(11):2375–2387. doi: 10.1017/S0033291715000331. [DOI] [PubMed] [Google Scholar]
- 25.Pincus SM. Greater signal regularity may indicate increased system isolation. Math Biosci. 1994;122(2):161–181. doi: 10.1016/0025-5564(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 26.Butner JE, Gagnon KT, Geuss MN, Lessard DA, Story TN. Utilizing topology to generate and test theories of change. Psychol Methods. 2015;20(1):1–25. doi: 10.1037/a0037802. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell ES, Woods NF. Cognitive symptoms during the menopausal transition and early postmenopause. Climacteric. 2011;14(2):252–261. doi: 10.3109/13697137.2010.516848. [DOI] [PubMed] [Google Scholar]
- 28.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. The Journal of clinical endocrinology and metabolism. 2012;97(4):1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell ES, Woods NF, Mariella A. Three stages of the menopausal transition from the Seattle Midlife Women’s Health Study: toward a more precise definition. Menopause. 2000;7(5):334–349. doi: 10.1097/00042192-200007050-00008. [DOI] [PubMed] [Google Scholar]
- 30.Harlow SD, Cain K, Crawford S, et al. Evaluation of four proposed bleeding criteria for the onset of late menopausal transition. The Journal of clinical endocrinology and metabolism. 2006;91(9):3432–3438. doi: 10.1210/jc.2005-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy OK, Iversen L, Aucott L, Hannaford PC. Factors associated with resilience or vulnerability to hot flushes and night sweats during the menopausal transition. Menopause. 2013;20(4):383–392. doi: 10.1097/gme.0b013e31827655cf. [DOI] [PubMed] [Google Scholar]
- 32.Duffy OK, Iversen L, Hannaford PC. The impact and management of symptoms experienced at midlife: a community-based study of women in northeast Scotland. BJOG: an international journal of obstetrics and gynaecology. 2012;119(5):554–564. doi: 10.1111/j.1471-0528.2012.03276.x. [DOI] [PubMed] [Google Scholar]
- 33.Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Taylor HJ, Mitchell ES. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: observations from the Seattle Midlife Women’s Health Study. J Womens Health (Larchmt) 2007;16(5):667–677. doi: 10.1089/jwh.2006.0138. [DOI] [PubMed] [Google Scholar]
- 34.Woods NF, Mariella A, Mitchell ES. Depressed mood symptoms during the menopausal transition: observations from the Seattle Midlife Women’s Health Study. Climacteric. 2006;9(3):195–203. doi: 10.1080/13697130600730663. [DOI] [PubMed] [Google Scholar]
- 35.Raudenbush S, Brennan R, Barnett R. A multivariate hierarchical model for studying psychological change within married couples. Journal of Family Psychology. 1995;9:161–174. [Google Scholar]
- 36.Yuan K, Bentler P. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociological Methodology. 2000;30:165–200. [Google Scholar]
- 37.Kutta M. Beitrag zur naherungsweisen integration totaler differentialgleichungen. Zeitschrift fur Mathematik und Physik. 1901;46:435–453. [Google Scholar]
- 38.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]


