Abstract
Aims
Large-scale clinical trials and translational studies have demonstrated that weight loss achieved through diet and physical activity reduced the development of diabetes in overweight individuals with prediabetes. These interventions also reduced the occurrence of metabolic syndrome and risk factors linked to other chronic conditions including obesity-driven cancers and cardiovascular disease. The Healthy Living Partnerships to Prevent Diabetes (HELP PD) was a clinical trial in which participants were randomized to receive a community-based lifestyle intervention translated from the Diabetes Prevention Program (DPP) or an enhanced usual care condition. The objective of this study is to compare the 12 and 24-month prevalence of metabolic syndrome in the two treatment arms of HELP PD.
Materials and Methods
The intervention involved a group-based, behavioral weight-loss program led by community health workers monitored by personnel from a local diabetes education program. The enhanced usual care condition included dietary counseling and written materials.
Results
HELP PD included 301 overweight or obese participants (BMI 25–39.9 kg/m2) with elevated fasting glucose levels (95–125 mg/dl). At 12 and 24 months of follow-up there were significant improvements in individual components of the metabolic syndrome: fasting blood glucose, waist circumference, HDL, triglycerides and blood pressure and the occurrence of the metabolic syndrome in the intervention group compared to the usual care group.
Conclusions
This study demonstrates that a community diabetes prevention program in participants with prediabetes results in metabolic benefits and a reduction in the occurrence of the metabolic syndrome in the intervention group compared to the enhanced usual care group.
Keywords: metabolic syndrome, lifestyle intervention, diabetes prevention, obesity
INTRODUCTION
The metabolic syndrome is a constellation of clinical features1 which in combination identify individuals who are at increased risk for developing diabetes, some cancers, and cardiovascular disease including stroke.2–4 Diseases related to metabolic syndrome represent the leading causes of death in the United States, making interventions to improve the components of metabolic syndrome especially needed. Programs specifically designed to promote weight loss have been successful in improving these parameters but have been criticized as being too expensive for dissemination to the general population.
The Diabetes Prevention Program (DPP) and the Finnish Diabetes Prevention Study (FDPS) were successful in altering the metabolic profiles of participants and such benefits were found to be long lasting. The DPP5,6 reported that the prevalence of metabolic syndrome at 3 years among those who had metabolic syndrome at baseline was significantly lower in the lifestyle intervention group compared to the placebo group.7 Similarly in the FDPS,8 with a mean follow-up of 3.9 years, the prevalence of metabolic syndrome decreased significantly in the intervention group compared to the control group.9 The Healthy Partnerships to Prevent Diabetes (HELP PD) trial was a single center randomized controlled trial (RCT) targeting overweight individuals with elevated fasting glucose levels (95–125 mg/dL)10 and was successful in reducing fasting glucose in the intervention arm compared to the enhanced usual care arm at one and two years.11,12 The cost of the intervention program was approximately one-third that of DPP as HELP PD employed community health workers to facilitate the intervention groups.13 The intervention resulted in weight loss in most participants in the first 6 months comparable to DPP14 and the weight loss was maintained in a significant number of participants for a 24 month period.11,12 This paper summarizes the impact of the intervention on 12 and 24 month changes in the metabolic syndrome and its individual components in these individuals.
METHODS
The design and methods, recruitment details, and primary outcome measures of this study have previously been reported.10–16 Briefly, this single center RCT of overweight and obese participants (BMI 25–39.9 kg/m2) with elevated fasting blood glucose between 95 and 125 mg/dl were randomly assigned to either the group-based lifestyle weight loss (LWL) intervention or enhanced usual care (EUC).10 Exclusion criteria were diabetes, cardiovascular disease in the past 6 months, uncontrolled hypertension, medications affecting glucose metabolism, and major chronic illnesses that would affect participation and/or limit lifespan.15 Overall the goal of recruitment was to be representative of the community so that the intervention could be translated to the general population; 301 individuals were enrolled in the trial and data was collected during 2007–2011.15 There was no racial or gender bias in the selection of participants. Outcomes were assessed every 6 months and included fasting blood glucose, weight, waist circumference, triglycerides, blood pressure, LDL and HDL cholesterol.10 All participants provided written informed consent and the study was approved by the Wake Forest School of Medicine Institutional Review Board. The analyses described in this report were performed in 2015–2016.
Interventions
The LWL intervention was adapted from the DPP curriculum5 to be delivered by community health workers (CHWs) in group settings.10 The objective was to achieve weight loss through reductions in caloric intake of approximately 500 kcal/day and moderate intensity exercise of 180 minutes/week to achieve weight loss of .3 kg per week for the first 6 months (Phase 1) for 5%-7% total weight loss. Months 7–24 (Phase 2) were focused on weight maintenance but weight loss goals were encouraged as long as BMI did not fall below 20 kg/m2. Participants met in groups of 8–12 in community sites such as recreation centers, and sessions were led by the CHWs who were trained, monitored, and supervised by registered dietitian nutritionists (RDN) affiliated with the local diabetes education program.16 In addition, participants met in individual sessions with an RDN during months 1, 3 and 6. Group sessions were weekly during Phase 1 and then monthly in Phase 2. The CHWs also contacted the participants by phone once a month in Phase 2.
The EUC comparison arm was designed to offer more than what was usual care for participants to enhance continued participation in the trial. EUC consisted of two individual sessions focusing on healthy lifestyle with an RDN during the first three months of the trial and a monthly newsletter which addressed healthy lifestyle behaviors and community resources.
Outcome Measures
Metabolic Syndrome was determined using current ATP (Adult Treatment Panel III) / NCEP (National Cholesterol Education Program) guidelines1, 17–19 as the combination of any three of the following five conditions: waist circumference male ≥ 102cm (40in) female ≥ 88 cm (35in); fasting triglycerides ≥ 150 mg/dl or drug treatment for elevated triglycerides; HDL cholesterol male (<40mg/dl) and female (<50mg/dl) or drug treatment for low HDL cholesterol; blood pressure ≥ 130 systolic or ≥ 85 diastolic or drug treatment for elevated blood pressure and fasting plasma glucose (FPG) ≥ 100mg/dl or drug treatment for elevated blood glucose.17 All parameters were reported at baseline, 12 months and 24 months for both the LWL and EUC groups. Waist circumference was also assessed at 6 months while blood pressure and fasting plasma glucose were also assessed at 6 and 18 months. All blood tests were performed after an 8 hour fast and samples were processed by a central lab masked to the participants’ intervention assignment. Glucose was measured using a timed endpoint method supplied by Beckman Coulter for the Synchron LX analyzer. Waist circumference was measured using a Gulick II 150 cm anthropometric tape with the subject in a recumbent position without clothing touching the skin midpoint between the inferior margin of the last rib and the iliac crest.20
Statistical Analysis
Constrained longitudinal data analysis (repeated measures analysis of variance with the baseline treated as a response and baseline means constrained to be equal in the two groups)21 was used to assess the effect of the HELP intervention on the components of the metabolic syndrome (waist circumference, SBP, DBP, fasting blood glucose, triglycerides, and HDL) over time. Note that some outcomes (e.g., SBP, DBP, and glucose) were measured every six months while others (e.g., triglycerides, HDL) were measured every year. All available data were used in the analyses; results are reported at baseline, 12, and 24 months for all outcomes. An unstructured covariance matrix was used to model the within patient correlations over time. Linear contrasts were used to assess the intervention effect at 12 and 24 months. Chi-square tests were used to assess treatment differences in the proportion of individual metabolic components and the metabolic syndrome. Hochberg’s modified Bonferroni step-up multiple test procedure was used to adjust p-values for multiple contrasts for each outcome.22 Chi-square tests and t-tests were used to assess differences in participant characteristics between those who did and did not drop out of the study. SAS version 9.4 was used to perform the analyses.
RESULTS
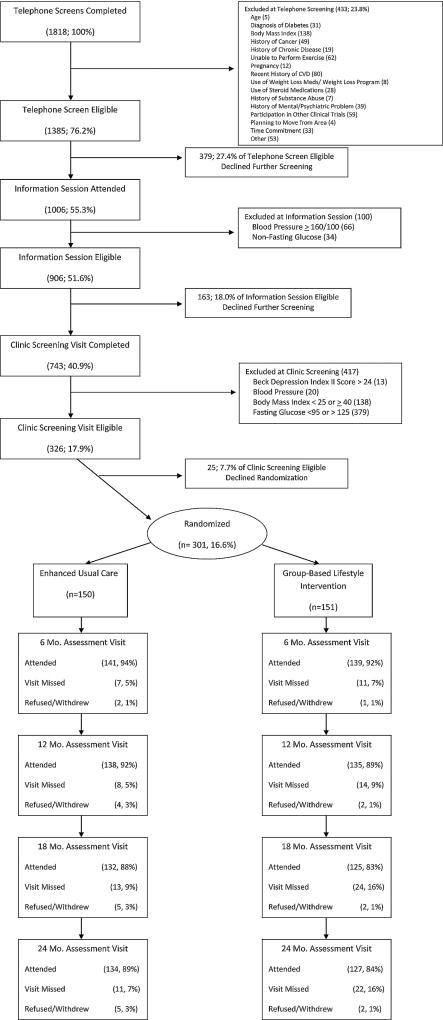
A total of 301 participants were randomized between 8/2007 and 4/2009, 151 to LWL and 150 to the EUC condition. Figure 1 illustrates the recruitment, screening, randomization, and retention process. Baseline characteristics for randomized participants are summarized in Table 1. Ages ranged from 34 to 81 with a median of 58 years; 57% were female and 27% were minority. Most participants were married (70%), employed full or part-time (66%), and lived with other people (81%). BMI ranged from 24.6 to 40.5 with a median of 32.8; by CDC criteria, 27% of participants were overweight and 73% were obese. Baseline characteristics were similar in the two groups.
Figure 1.
Screening, randomization, and follow-up in the HELP PD study
Table 1.
Baseline participant characteristics by arm
| Intervention (N=151) |
Control (N=150) |
Total (N=301) |
|
|---|---|---|---|
|
|
|||
| Characteristic | # ( %) | # ( %) | # ( %) |
| Sex | |||
| Male | 64 (42) | 64 (43) | 128 (43) |
| Female | 87 (58) | 86 (57) | 173 (57) |
| Race | |||
| White | 111 (74) | 109 (73) | 220 (73) |
| Non-white | 40 (26) | 41 (27) | 81 (27) |
| Age (years) – Median (Range) | 57 (36, 81) | 58 (37, 78) | 58 (34, 81) |
| < 50 | 42 (28) | 33 (22) | 75 (25) |
| 50 – 59 | 51 (34) | 51 (34) | 102 (34) |
| 60+ | 58 (38) | 66 (44) | 124 (41) |
| Marital Status | |||
| Never Married | 12 ( 8) | 5 ( 3) | 17 ( 6) |
| Married/Living together | 105 (70) | 107 (71) | 212 (70) |
| Divorced/Widowed/Separated | 34 (23) | 38 (25) | 72 (24) |
| Employment | |||
| Full or part-time | 98 (65) | 100 (67) | 198 (66) |
| Other | 53 (35) | 50 (33) | 103 (34) |
| Number in House | |||
| 1 | 26 (17) | 31 (21) | 57 (19) |
| 2 | 84 (56) | 77 (51) | 161 (53) |
| 3 | 20 (13) | 24 (16) | 44 (15) |
| 4+ | 21 (14) | 18 (12) | 39 (13) |
| Metabolic Condition | |||
| Obesity | 131 (87) | 123 (82) | 254 (84) |
| Blood Pressure | 109 (72) | 103 (69) | 212 (70) |
| Glucose | 102 (68) | 103 (69) | 205 (68) |
| Triglycerides | 50 (33) | 53 (35) | 103 (34) |
| HDL | 82 (54) | 73 (49) | 155 (51) |
| Number of Conditions | |||
| 0 | 2 ( 1) | 1 ( 1) | 3 ( 1) |
| 1 | 8 ( 5) | 16 (11) | 24 ( 8) |
| 2 | 33 (22) | 29 (19) | 62 (21) |
| 3 | 47 (31) | 50 (33) | 97 (32) |
| 4 | 46 (30) | 39 (26) | 85 (28) |
| 5 | 15 (10) | 15 (10) | 30 (10) |
| Medications | |||
| Any | 116 (77) | 125 (83) | 241 (80) |
| Hypertension | 78 (52) | 78 (52) | 156 (52) |
| Lipids | 53 (35) | 61 (41) | 114 (38) |
| Triglycerides | 7 ( 5) | 10 ( 7) | 17 ( 6) |
| BMI – Median (Range) | 32.9 (24.6, 39.9) | 32.4 (24.6, 40.5) | 32.8 (24.6, 40.5) |
| ≥ 30 | 112 (74) | 107 (71) | 219 (73) |
The estimates of the prevalence of the metabolic syndrome components are also shown in Table 1 and descriptive statistics for the metabolic components of metabolic syndrome at baseline are shown in Table 2. Overall, 70% of the participants met the criteria for the metabolic syndrome. Baseline levels of the individual metabolic components did not differ significantly between treatment groups. By ATP/NCEP criteria, 84% were obese based on waist circumference, 70% had elevated blood pressure, 68% had elevated glucose levels, 34% had elevated triglycerides, and 51% had low HDL levels. Roughly 50% of the participants in both treatment groups reported taking hypertension medications at baseline. In comparison, only 5% of LWL and 7% of EUC participants reported taking triglyceride medications.
Table 2.
Baseline levels of components of the metabolic syndrome
| Intervention (N=151)
|
Control (N=150)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Median | Min | Max | Mean | SD | Median | Min | Max |
| Waist | ||||||||||
| Males | 108.1 | 9.0 | 106.0 | 91.6 | 131.3 | 108.2 | 10.3 | 108.5 | 87.4 | 132.7 |
| Females | 102.6 | 8.9 | 103.2 | 80.6 | 127.3 | 101.5 | 10.1 | 102.3 | 79.9 | 123.6 |
| SBP | 126.7 | 13.8 | 126.0 | 93.0 | 164.0 | 127.5 | 14.3 | 126.5 | 101.0 | 169.0 |
| DBP | 73.5 | 9.4 | 74.0 | 49.0 | 106.0 | 72.9 | 9.3 | 73.0 | 51.0 | 106.0 |
| Glucose | 105.4 | 12.5 | 103.0 | 73.0 | 189.0 | 105.7 | 10.0 | 105.5 | 86.0 | 133.0 |
| Trig | 139.2 | 89.8 | 116.0 | 33.0 | 711.0 | 132.1 | 80.3 | 121.5 | 40.0 | 694.0 |
| HDL | ||||||||||
| Males | 41.1 | 10.1 | 39.0 | 24.0 | 83.0 | 39.6 | 9.0 | 38.5 | 19.0 | 61.0 |
| Females | 50.0 | 12.4 | 49.0 | 28.0 | 93.0 | 51.7 | 11.4 | 50.0 | 33.0 | 89.0 |
Overall, 87% of the participants completed the two-year study period; 84% of the LWL participants and 89% of the EUC participants. Those who dropped out were similar to those who completed the study in gender, race, age, marital status, employment, household number, and all of the metabolic parameters. Obese participants were more likely to drop out (16%) than those who were overweight (6%), p = .025, but that was true for both arms (18% of LWL participants and 14% of EUC participants dropped out).
Changes in the Components of Metabolic Syndrome over Time
Least squares estimates for the metabolic components, obtained from the constrained longitudinal models including time and treatment group, are shown in Table 3. It is seen that waist circumference decreased significantly over the first year in the LWL group and increased over the subsequent year while it remained relatively stable across time in the EUC group. Compared to the EUC group, waist circumference was significantly lower in the LWL group at all post-randomization times. Systolic blood pressure decreased significantly in both arms, but the two arms did not differ significantly at one or two years. Diastolic blood pressure (DBP) decreased similarly in the two groups over the first year; however, at two years DBP was significantly lower for the LWL group. Fasting blood glucose improved significantly for the LWL group during the first year and increased slightly thereafter while it improved slightly during the first year in the EUC group before increasing beyond baseline levels during the second year. Compared to the EUC group, fasting blood glucose was significantly lower in the LWL group at one and two years. Triglyceride levels decreased significantly over the first year in the LWL group and remained significantly lower than baseline at two years; they decreased non-significantly in the EUC group. Triglyceride levels were significantly lower in the LWL group at one year but not at two years. HDL remained relatively unchanged in the LWL group and decreased significantly over time in the EUC group. HDL levels were significantly higher in the LWL group at both one and two years.
Table 3.
Least Squares Means (SEs) for metabolic components at each time*
| Outcome | Time | Intervention (N=151) | Control (N=150) | p-value** |
|---|---|---|---|---|
| Waist | 0 | 104.7 (0.58) | 104.7 (0.58) | --- |
| 12 | 99.2 (0.67) | 104.0 (0.67) | <.001 | |
| 24 | 101.3 (0.71) | 104.5 (0.70) | <.001 | |
| SBP | 0 | 127.1 (0.81) | 127.1 (0.81) | --- |
| 12 | 123.1 (1.08) | 123.9 (1.07) | .534 | |
| 24 | 124.8 (1.23) | 126.1 (1.20) | .437 | |
| DBP | 0 | 73.2 (0.54) | 73.2 (0.54) | --- |
| 12 | 70.5 (0.70) | 71.6 (0.69) | .189 | |
| 24 | 71.6 (0.75) | 73.7 (0.73) | .048 | |
| Glucose | 0 | 105.5 (0.65) | 105.5 (0.65) | --- |
| 12 | 101.2 (0.88) | 104.1 (0.87) | .015 | |
| 24 | 103.3 (0.99) | 107.7 (0.97) | .003 | |
| Triglycerides | 0 | 135.7 (4.9) | 135.7 (4.9) | --- |
| 12 | 107.2 (4.9) | 128.7 (4.9) | <.001 | |
| 24 | 112.3 (5.8) | 124.9 (5.7) | .083 | |
| HDL | 0 | 46.4 (0.70) | 46.4 (0.70) | --- |
| 12 | 46.1 (0.86) | 42.4 (0.86) | <.001 | |
| 24 | 46.8 (0.98) | 43.7 (0.96) | .004 |
Restricting baseline means to be equal
Adjusted for multiple comparisons within an outcome using the Hochberg step-up procedure
The Metabolic Syndrome
The number and percentage of participants who met the ATP/NCEP criteria for the metabolic syndrome are shown in Table 4 by time and treatment group. These results fairly well mimic the results of the continuous components presented above. While not always statistically significant, the proportions of participants who met the criteria for each metabolic condition as well as the overall criteria for the metabolic syndrome were greater in the EUC group compared to the LWL group. At one year, 14% fewer LWL participants met the waist circumference criteria (p=.011), 15% fewer had elevated fasting blood glucose (p=.012), 14% fewer had elevated triglycerides (p=.020), and 12% fewer had low HDL levels (p=.068). Overall, at one year, 53% of the LWL participants had the metabolic syndrome compared to 69% of the EUC participants (p=.006). Results were similar at two years. At that time, 13% fewer LWL participants had waist circumference criteria (p=.011), 16% fewer had elevated fasting blood glucose (p=.008), 5% fewer had elevated triglycerides (p=.392), and 10% fewer had low HDL levels (p=.078). Overall, at two years, 61% of the LWL participants had the metabolic syndrome compared to 77% of the EUC participants (p=.006).
Table 4.
Individual components of metabolic syndrome and overall metabolic syndrome status by time and arm
| Condition | Time | Intervention | Control | p-value* |
|---|---|---|---|---|
|
|
||||
| n / N ( %) | n / N ( %) | |||
| Waist Circumference | 0 | 131/151 (87) | 123/150 (82) | 256 |
| 12 | 89/135 (66) | 110/138 (80) | .011 | |
| 24 | 87/127 (69) | 110/134 (82) | .011 | |
| Blood Pressure | 0 | 109/151 (72) | 103/150 (69) | .504 |
| 12 | 83/135 (61) | 91/138 (66) | .443 | |
| 24 | 86/127 (68) | 100/133 (75) | .364 | |
| Fasting Blood Glucose | 0 | 102/151 (68) | 103/150 (69) | .835 |
| 12 | 74/135 (55) | 96/138 (70) | .012 | |
| 24 | 79/127 (62) | 105/134 (78) | .008 | |
| Triglycerides | 0 | 50/151 (33) | 53/150 (35) | .685 |
| 12 | 28/135 (21) | 48/138 (35) | .020 | |
| 24 | 31/127 (24) | 39/134 (29) | .392 | |
| HDL | 0 | 82/151 (54) | 73/150 (49) | .328 |
| 12 | 71/135 (53) | 90/138 (65) | .068 | |
| 24 | 63/127 (50) | 81/134 (60) | .078 | |
| Metabolic Syndrome | 0 | 108/151 (72) | 104/150 (69) | .677 |
| 12 | 71/135 (53) | 95/138 (69) | .006 | |
| 24 | 77/127 (61) | 103/134 (77) | .006 | |
| Number of | 0 | 3.1 (1.1) | 3.0 (1.2) | .431 |
| Metabolic Conditions | 12 | 2.6 (1.2) | 3.2 (1.2) | <.001 |
| Mean (SD) | 24 | 2.7 (1.3) | 3.2 (1.2) | <.001 |
Post-randomization p-values adjusted for multiple comparisons within an outcome using the Hochberg step-up procedure
Of the 135 LWL participants with baseline and one-year data, 6 participants who did not have the metabolic syndrome at baseline were classified as developing it at one year (worsened) while 29 with the metabolic syndrome at baseline did not have it at one year (improved). For the 138 EUC participants with both measurements, 12 worsened and 16 improved. Of the 127 LWL participants with baseline and two-year data, 14 worsened and 25 improved. For the 134 EUC participants with both measurements, 20 worsened and 11 improved.
DISCUSSION
The HELP PD trial successfully demonstrated that a weight loss program utilizing community health workers (CHWs) in community settings was able to significantly reduce fasting glucose in the intervention (LWL) arm compared to the control group (EUC). Just as important are the effects on other metabolic risk factors comprising the metabolic syndrome, a highly prevalent condition in these overweight study subjects. It is increasingly being recognized that the constellation of metabolic conditions should be addressed and treated for the prevention of not only cardiovascular disease but diabetes and obesity-driven cancers including postmenopausal breast cancer and prostate cancer as well.2–4
Calculator tools for the 10-year risk assessment for having a heart attack use the following parameters: age, gender, total cholesterol, HDL cholesterol, smoker and systolic blood pressure but fail to include obesity as measured by BMI and waist circumference. Increased waist circumference was in the metabolic syndrome range in more than 80% of the study subjects at baseline and dropped to 66% in the LWL group at 12 months and 69% at 24 months. The EUC group had no significant reduction in waist circumference and remained in the metabolic syndrome range at 12 months (80%) and 24 months (82%). This program of weight loss through caloric restriction and modest exercise benefits multiple metabolic parameters and the subsequent metabolic syndrome. In particular, changes in waist circumference as a measure of adiposity, fasting glucose, fasting triglycerides, diastolic blood pressure and HDL levels were positively impacted by this program. These benefits were attained at one year and sustained over a two-year period.
Because trained CHWs were able to carry out the main delivery and documentation of the intervention, translation into other community settings appears to both affordable and sustainable. Cost is much more modest with CHWs implementing the protocol with support from staff affiliated with a diabetes care center or similar facility. Recent efforts to scale up diabetes prevention interventions on a national level could have the added benefit of reaching populations at risk for other costly chronic diseases.23 The prospect of duplicating this program in other community settings would allow the intervention to reach many more individuals at high risk of developing diabetes, cancer, and heart disease.
Limitations
There are limitations that require mentioning. The HELP PD study was a single-center trial that was implemented in a mid-sized city in North Carolina, USA. Participants were highly educated and Latinos were underrepresented due to our inability to offer the LWL intervention in Spanish. These limitations make it more difficult to generalize to other populations and the HELP PD intervention will need to be assessed in other settings.
CONCLUSIONS
Overall, this study demonstrates that a community-based diabetes prevention program in participants with prediabetes also resulted in metabolic benefits and a reduction in the occurrence of the metabolic syndrome in the intervention group compared to the enhanced usual care group.
Acknowledgments
We would like to acknowledge members of the HELP PD research staff for their recruitment, retention, and data collection efforts. We are also particularly grateful to our community health workers who delivered the intervention and staff from the Joslin Diabetes Center at Wake Forest Baptist Medical Center, including Joyce M. Sydell, MEd, RD, LDN and Donna Kernodle, MPH, RD, CDE. This project is supported by Award # R18DK069901 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health. NIDDK had no role in the design of HELP PD; the collection, analysis and interpretation of study data; the preparation of this manuscript; and the decision to submit it for publication. C.P. researched data and wrote the manuscript. D.C. analyzed the data and wrote the manuscript. C.B., J.K., and M.V. researched data and reviewed/edited the manuscript. M.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The contents of this paper have not been previously published elsewhere. No financial disclosures were reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: NCT00631345; Clinicaltrials.gov
Financial Disclosure: No financial disclosures were reported by the authors of this paper.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009 Oct 20;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Lohmann AE, Goodwin PJ, Chlebowski RT, Pan K, Stambolic V, Dowling RJ. Association of Obesity-Related Metabolic Disruptions With Cancer Risk and Outcome. J Clin Oncol. 2016;34(35):4249–4255. doi: 10.1200/JCO.2016.69.6187. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher EJ, LeRoith D. Insulin, insulin resistance, obesity, and cancer. Curr Diab Rep. 2010;10(2):93–100. doi: 10.1007/s11892-010-0101-y. [DOI] [PubMed] [Google Scholar]
- 4.Kabat GC, Kim M, Chlebowski RT, et al. A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2046–2053. doi: 10.1158/1055-9965.EPI-09-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002 Dec;25(12):2165–71. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N.Engl.J.Med. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005 Apr 19;142(8):611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N.Engl.J.Med. 2001 May 3;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 9.Ilanne-Parikka P, Eriksson JG, Lindstrom J, Peltonen M, Aunola S, Hamalainen H, Keinanen-Kiukaanniemi S, Laakso M, Valle TT, Lahtela J, et al. Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care. 2008 Apr;31(4):805–7. doi: 10.2337/dc07-1117. [DOI] [PubMed] [Google Scholar]
- 10.Katula JA, Vitolins MZ, Rosenberger EL, Blackwell C, Espeland MA, Lawlor MS, Rejeski WJ, Goff DC. Healthy Living Partnerships to Prevent Diabetes (HELP PD): design and methods. Contemp.Clin.Trials. 2010 Jan;31(1):71–81. doi: 10.1016/j.cct.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katula JA, Vitolins MZ, Rosenberger EL, Blackwell CS, Morgan TM, Lawlor MS, Goff DC., Jr One-Year Results of a Community-Based Translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care. 2011 Jul;34(7):1451–7. doi: 10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katula JA, Vitolins MZ, Morgan TM, Lawlor MS, Blackwell CS, Isom SP, Pedley CF, Goff DC., Jr The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med. 2013 Apr;44(4 Suppl 4):S324–S332. doi: 10.1016/j.amepre.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawlor MS, Blackwell CS, Isom SP, Katula JA, Vitolins MZ, Morgan TM, Goff DC., Jr Cost of a group translation of the Diabetes Prevention Program: Healthy Living Partnerships to Prevent Diabetes. Am J Prev Med. 2013 Apr;44(4 Suppl 4):S381–S389. doi: 10.1016/j.amepre.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitolins MZ, Isom SP, Blackwell CS, et al. The healthy living partnerships to prevent diabetes and the diabetes prevention program: a comparison of year 1 and 2 intervention results. Transl Behav Med. 2016 doi: 10.1007/s13142-016-0447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackwell CS, Foster KA, Isom S, Katula JA, Vitolins MZ, Rosenberger EL, Goff DC., Jr Healthy Living Partnerships to Prevent Diabetes: recruitment and baseline characteristics. Contemp.Clin.Trials. 2011 Jan;32(1):40–9. doi: 10.1016/j.cct.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sydell J, Kernodle D, Blackwell CS, Vitolins MZ. Identifying, Training, and Monitoring Community Health Workers in A Community-Based Diabetes Prevention Study. JJ Community Medicine. 2015;1(1) [Google Scholar]
- 17.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002 Dec 1;156(11):1070–7. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw.Cardiol. 2005 Dec;4(4):198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Strazzullo P, Barbato A, Siani A, Cappuccio FP, Versiero M, Schiattarella P, Russo O, Avallone S, della VE, Farinaro E. Diagnostic criteria for metabolic syndrome: a comparative analysis in an unselected sample of adult male population. Metabolism. 2008 Mar;57(3):355–61. doi: 10.1016/j.metabol.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Williamson D, Kahn HS, Worthman C, Burnette J, Russell C. Precision of recumbent anthropometry. Am J Human Biology. 1993 May 27;5(2):159–67. doi: 10.1002/ajhb.1310050205. [DOI] [PubMed] [Google Scholar]
- 21.Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Statistics in Medicine. 2009 Sep;28(20):2509–2530. doi: 10.1002/sim.3639. [DOI] [PubMed] [Google Scholar]
- 22.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988 Dec;75(4):800–802. [Google Scholar]
- 23.Centers for Disease Control and Prevention. [Accessed November 21, 2016];National Diabetes Prevention Program. https://www.cdc.gov/diabetes/prevention/index.html.