Abstract
Background
Vagus Nerve Stimulation (VNS) is an FDA-approved method delivering electrical impulses for treatment of depression and epilepsy in adults. The vagus nerve innervates the majority of visceral organs and cervix, but potential impacts of VNS on the progress of pregnancy and the fetus are not well studied.
Methods
We tested the hypothesis that VNS in pregnant dams does not induce inflammatory changes in the cardiorespiratory control regions of the pups’ brainstem, potentially impacting the morbidity and mortality of offspring. Pregnant dams were implanted with stimulators providing intermittent low or high frequency electrical stimulation of the sub-diaphragmatic esophageal segment of the vagus nerve for 6–7 days until delivery. After birth, we collected pup brainstems that included cardio-respiratory control regions and counted the cells labeled for pro-inflammatory cytokines (IL-1β, IL-6, TNFα) and HMGB1.
Results
Neither pup viability nor number of cells labeled for pro-inflammatory cytokines in nTS nor XII was impaired by VNS. We provide evidence suggesting that chronic VNS of pregnant mothers does not impede the progress or outcome of pregnancy.
Conclusions
VNS does not cause preterm birth, affect well-being of progeny, or impact central inflammatory processes that are critical for normal cardiovascular and respiratory function in newborns.
INTRODUCTION
Vagus nerve stimulation (VNS) has wide-ranging clinical applications including FDA-approved treatments for epilepsy and depression in adults. Over 75,000 patients have been implanted with VNS devices (1). VNS is currently being investigated as a clinical treatment for sepsis and rheumatoid arthritis because it suppresses peripheral inflammation and may be important in modulating neuroinflammation (2,3). The effects of VNS have been correlated with serum TNF concentrations as a way to assess the efficacy of VNS to treat endotoxemia (4). The anti-inflammatory effect also seems to depend upon reduction of inflammation via descending cholinergic efferent output (4,5). While VNS has therapeutic value in adult patients, its impact on pregnancy, birth, and on fetal development and well-being remain largely unknown. Due to the inevitability of pregnancy in women undergoing VNS treatment, the impact of this therapy on pregnancy and fetal well-being is an important consideration—particularly with the increasing number of vagus nerve stimulators implanted over the past decade with expanding FDA approval (1). Case reports of VNS therapy in pregnant women found no adverse effects on pregnancy or the postpartum neonate (6,7), though these studies did not include physiological endpoints or extend to the use of animal models.
The vagus nerve interconnects the medulla, the heart and lungs, the stomach and other viscera, including the colon and female reproductive tract (8), thus any change in vagal activity would be expected to have an impact on these systems. Approximately 80% of vagus fibers carry afferent information to the CNS while 20% of the vagus is efferent information to the periphery (9). Though the uterus is virtually denervated during pregnancy, cervix remodeling involves parasympathetic innervation from the vagus and pelvic nerves to the cervix (10), in part via bNOS and CGRP fibers (11). These sensory neuropeptides mediate inflammatory responses in other tissues (11). We have previously shown that vagus nerve transection reduces the presence of macrophages in the cervix but also resulted in distension of the bladder and stomach in our previous work—suggesting that descending vagal input is key to normal cervical function (12) as well as autonomic tone to the viscera. Since vagus nerve transection in pregnant rats has such a significant impact, investigating the effect of VNS on the progression of pregnancy and inflammatory processes in the fetus is warranted and understanding the role of vagus nerve stimulation on pregnancy and pup outcome are the major motivators for this work.
VNS may be an effective treatment during pregnancy for infections that result from a compromised cervix immune barrier, as may occur with chorioamnionitis or premature rupture of fetal membranes (13–16), which exposes the developing fetus to pro-inflammatory cytokines and is associated with neonatal morbidity and brain injury. These cytokines are part of the developmental signals that regulate prenatal neural connectivity (17) in specific brain regions and there is limited evidence to indicate a reduced size of brainstem nuclei following systemic treatment with endotoxin, a prenatal inflammatory agent (18). Inflammation in these regions causes impaired breathing responses to hypoxia in neonatal rat pups (19,20). Thus, in this study we tested the hypothesis that maternal VNS would interfere with parturition and alter the level of pro-inflammatory cytokines in cardio-respiratory regions of the brainstem. We tested this hypothesis by using immunohistochemistry to label for pro-inflammatory cytokines and the transcription factor high mobility group box 1 (HMGB1). Our objective was to determine if chronic VNS impacts morbidity and mortality in infants born to mothers with implanted vagal stimulators.
MATERIALS AND METHODS
Adult Long Evans rats (Harlan, Inc, Indianapolis, IN) were individually housed in a temperature- and humidity-controlled vivarium with a 12-h light/dark cycle with food and water ad libitum. Animal care and usage followed National Institutes of Health guidelines and all experimental procedures were approved by the LLU Institutional Animal Care and Use Committee (IACUC).
Dam Surgery and VNS
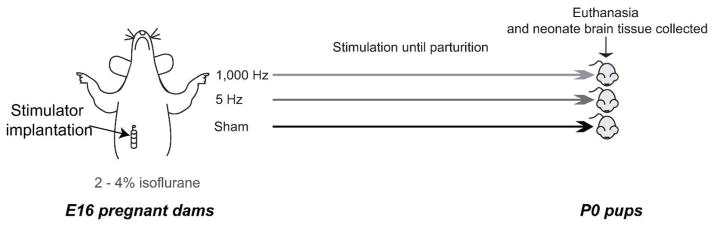
Two days after acclimatization to the vivarium, we performed surgery on pregnant dams at day 16 post-breeding, under 2 – 4% isoflurane-oxygen gas anesthesia (Figure 1, overview of study design and timeline). A midline incision was made below the rib cage to expose the sub-diaphragmatic vagus nerve, which was then separated from the connective sheath and vasculature to obtain an accessible length of approximately one centimeter. The vagus nerve was isolated at the sub-diaphgramatic portion of the esophagus following previously described procedures (12). After gentle, blunt dissection, a glass hook and fine forceps were used to separate the posterior vagal trunk and isolate a length of nerve sufficient to allow electrodes to encircle the nerve. VNS stimulators were fabricated by Harald Stauss Scientific and activated via a Hall Effect switch. We used the RNS model stimulator (http://haraldstauss.com/HaraldStaussScientific/default.html) to maximize current density over the course of the stimulation period. The electrode-wrapped vagus nerve was covered with a thin film of a silicone-based sealant (Kwik-sil, WPI, Sarasota, FL) to isolate and insulate the stimulation area. The electrode wires were connected to a battery-powered, implantable stimulator unit that was inserted into the abdominal cavity. Following surgery, the abdomen wall was sutured and the skin secured with clips. Pregnant dams were randomly assigned to groups in which the implanted VNS device was set to deliver 5 Hz or 1000 Hz at 1mA amplitude with 500 μs pulse width or no current (Sham). Our stimulation parameters were based upon parameters commonly used in clinical practice and pre-clinical studies assessing the effect of vagal nerve stimulation on selective fiber blockade (9,21).
Figure 1. Vagus Nerve Stimulation Protocol.
Vagus nerve stimulation protocol and timeline for experiments. In each treatment group: 1,000 Hz, 5 Hz, and Sham, we implanted stimulators with helical electrode contacts encircling the sub-diaphragmatic, esophageal branch of the vagus nerve at embryonic day 16 (E16) in pregnant dams. Pregnancies proceeded to birth and the pups were euthanized and brainstems removed within 12 hours after birth.
Stimulation duration was 30 min (on-time) at the given frequency for that animal and was followed by an off-time of 5.5 h repeated 4× per day. The programmed frequencies were confirmed each day using a digital AM/FM radio. Weight and food consumption were monitored daily to assess well-being of the dams and verify the stimulation regimen. Thus, the pups assessed for this project were exposed to either 5 Hz or 1000 Hz over five to six days post-VNS stimulator implantation since dams were allowed to continue in the study until parturition (typically gestational day 21 to 22 in rats).
Processing of brainstems, immunohistochemistry, and analyses
Within 12 hours of delivery, dams were asphyxiated with CO2. Pups were deeply anesthetized with isofluorane and their brainstems removed, rinsed with chilled saline, and stored in 4% paraformaldehyde for 24h before transfer to 30% sucrose for 48h to provide cryoprotection. Brainstems were frozen in Tissue-Tek OCT and sectioned at 20μm on a Leica CM 3050S cryostat. Sections of brainstem were stained using immunohistochemistry with selective antibodies for IL-6 (1:100 sc-1265-r, Santa Cruz), TNFα (1:100 ab6671, Abcam), IL-1β (1:100 ab9722, Abcam) and HMGB-1 (1:1000 ab18256, Abcam) as previously described (22). The number of cells expressing IL-6, TNFα, IL-1β, and HMGB1 protein were counted using a Zeiss Axio Imager A1 and unbiased stereology (Stereologer2000, Stereology Resource Center). All cell counts were normalized to volume assessed to account for variability in tissue morphology across sections. Photographs were acquired within Stereologer and post-processing was done using GIMP (http://gimp.org) and Adobe Illustrator CS6. We analyzed all data using one-way analysis of variance (ANOVA) with the Duncan post-hoc test (SPSS, v22, IBM) with a p<0.05 considered significant.
RESULTS
VNS effects on pregnancy, parturition, and pup viability
Neither surgery nor VNS interfered with either the progression of pregnancy or the parturition process. Pregnancy proceeded to term and litters had delivered in Sham (n=4) and VNS (5 Hz n=10, 1000 Hz n=11) groups by day 21–22 post-breeding (see Figure 1 for timing of VNS surgery and exposure). Pups were viable—breathing, with vigorous movement and vocalization when held. The pups were pink, and had milk present in their stomachs when harvest of tissue began, consistent with the metrics used to assess neonatal well-being using an Apgar scale (23). This is consistent with data seen from VnX pups from previous work (12). Table 1 summarizes the gestational age at delivery, number of live pups per litter, number of pups resorbed in utero, and postpartum pup mortality for each treatment group (5 Hz, 1000 Hz, and Sham).
Table 1.
VNS effects on pregnancy outcome and demographic features. Gestational age at delivery, number of live pups, number of pups resorbed, and postpartum pup mortality are reported here.
| 5 Hz | 1000 Hz | Sham | |
|---|---|---|---|
| Gestational Age at Delivery | E22 | E21 | E22 |
| Liveborn pups | 10 | 11 | 4 |
| Number of pups resorbed in utero | 0 | 1 | 0 |
| Postpartum pup mortality | 0 | 1 | 0 |
Brainstem cell counts
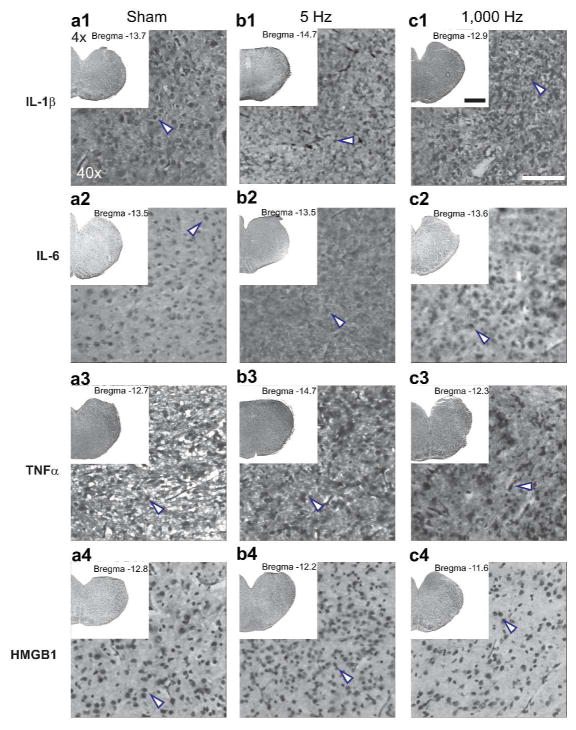
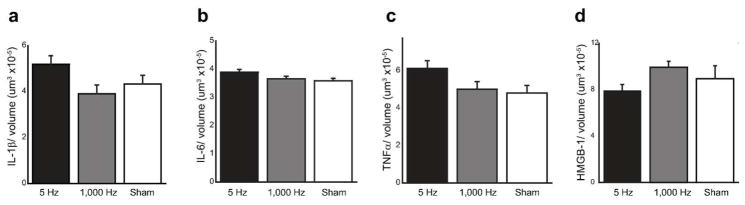
We used unbiased stereology to count the number of specific stained cells in pups from each group in the nTS from −12.0 to −14.7 mm (relative to Bregma) which encompasses the critical cardiorespiratory control regions in the brainstem. In all treatment groups, we counted > 500 cells across the nTS. Compared to Sham controls, the number of IL-1β (Figure 2, panels a1 to c1), IL-6 (Figure 2, panels a2 to c2), TNFα (Figure 2, panels a3 to c3), and HMGB1 (Figure 2, panels a4 to c4) labeled were not significantly different in pups from dams that received the 5 Hz or 1000 Hz VNS. Table 2 shows the summary counts (mean ± standard deviation) and p value for each marker. Figure 3 shows summary histograms with mean cell counts for Il-1β (panel a), IL-6 (panel b), TNFα (panel c) and HMGB1 (panel d) (± standard deviation) for all treatments and stains.
Figure 2.
Immunohistochemistry staining for cytokines and HMGB1. From top to bottom, we show IL-1β (a1-c1), IL-6 (a2-c2), TNFα (a3-c3), and HMGB1 (a4-c4) immuno-staining for each treatment (Sham, 5 Hz, 1,000 Hz). Each panel shows an inset photomicrograph (4×) with the distance from Bregma and higher resolution photomicrograph at 40×. Arrows (blue outline) indicate stained cells. Scalebar = 1mm (4×) and 100 μm (40×).
Table 2.
Cell counts per volume across stimulation groups. Each inflammatory marker and the mean number of cells expressing that marker are reported here ± standard deviation.
| 5 Hz | 1000 Hz | Sham | P value | |
|---|---|---|---|---|
| IL-1β | 5.19 × 10−5 ± 1.69 × 10−5 | 3.91 × 10−5 ± 7.18 × 10−6 | 4.34 × 10−5 ± 1.78 × 10−5 | 0.154 |
| IL-6 | 3.89 × 10−5 ± 1.06 × 10−5 | 3.66 × 10−5 ± 1.05 × 10−5 | 3.59 × 10−5 ± 1.47 × 10−5 | 0.684 |
| TNFα | 6.12 × 10−5 ± 2.97 × 10−5 | 5.01 × 10−5 ± 6.97 × 10−6 | 4.80 × 10−5 ± 1.32 × 10−5 | 0.277 |
| HMGB1 | 7.91 × 10−5 ± 1.25 × 10−5 | 9.92 × 10−5 ± 1.28 × 10−5 | 8.94 × 10−5 ± 2.73 × 10−5 | 0.099 |
Figure 3.
Summary histograms of unbiased stereology results for immunolabeling. All results are reported as the number of stained cells versus stimulation parameter. Markers shown are IL-1β (panel a), IL-6 (panel b), TNFα (panel c), and HMGB1 (panel d). Cell count numbers were divided by volume of area of interest to normalize for tissue size, then averaged (reported as average ± standard deviation). N=3 for all groups.
In addition to the canonical, early-onset pro-inflammatory cytokines, we stained for HMGB1, due to its putative anti-inflammatory role. We saw a non-significant increase in HMGB1 staining in the NTS in the 1,000 Hz stimulation group when compared to 5 Hz and Sham treatments, which is opposite of the trend seen with IL-6 and TNFα.
DISCUSSION
In this study, we found that maternal VNS does not significantly increase the expression of pro-inflammatory cytokines in the cardio-respiratory control regions of the medulla, does not interfere with the parturition process, does not cause preterm birth, nor negatively impacts viability of the pups born to VNS-treated dams. Because we saw no ill-effects of maternal VNS, our data suggests that there is no impairment of the physiological processes essential for normal birth and viability of neonates. These findings support and extend observations in case reports describing the effectiveness of VNS therapy during pregnancies in which VNS has been used to treat depression and refractory epilepsy. The authors’ of these reports found no impact on the timing of delivery of the mothers’ infants (6,24). An additional study of eight pregnancies in which the mothers received VNS for refractory epilepsy showed no adverse effects on either pregnancy or neonatal viability (7). Collectively, these findings support the suggestion that VNS may be included in therapeutic approaches to diseases during pregnancy.
The importance of these findings is underscored by the choice of physiologically relevant VNS characteristics. We used low frequency and current stimulation parameters that mimicked those seen in clinical use (25). Additionally, we used a higher stimulus frequency (1000 Hz) that blocks a significant proportion of the afferent traffic carried via the vagus (21,26). Neither VNS stimulus regimes resulted in significant changes in IL-1β, IL-6, TNFα, or HMGB1 across our treatment groups. We focused upon the early onset cytokines and HMGB1 since they represent the “first responders” of inflammatory regulation. HMGB1 is a chromatin-associated protein that plays a key role in transcription and regulation of gene expression. It has also been implicated in the anti-inflammatory response observed in other studies of VNS (27,28). We also constrained our focus to the brainstem regions critical for cardio-respiratory control and implicated in neonatal morbidity and mortality (29,30). In the brain, interleukin-6 (IL-6) is typically produced in response to the early upregulation of pro-inflammatory cytokines like IL-1β via the NFκB pathway (31,32). In addition to IL-6, TNFα and IL-1β are produced and released as part of the early inflammatory response (33). IL-1β is a key pro-inflammatory cytokine that can positively feedback to the NFκB (31) signaling cascade and exacerbate the production of pro-inflammatory cytokines to amplify the inflammatory response (34). TNFα triggers local inflammatory responses and serves as a co-factor for modulation of presynaptic release in the CNS (35) and the succeeding cascade of both pro- and anti-inflammatory cytokines and chemokines. The trophic role of TNFα in modulation of brainstem autonomic circuits is not yet known but it has been shown to be necessary for appropriate synaptic formation (35). Work has previously been done in murine models looking at obesity-induced inflammatory changes in the expression of IL-6 in the hypothalamus (17), and this is an area of research that warrants further research.
VNS has been implicated in the modulation of inflammatory tone and been suggested as a therapy to alleviate the impact of chronic inflammation. VNS upregulates the expression of TNFα (5) systemically in non-pregnant adult murine models, and this has been shown to activate other cytokines, such as IL-1β, and HMGB1 (4) which is implicated in both pro- and anti-inflammatory responses to VNS as part of HPA axis activation (36,37). IL-1β is the proto-typical early-onset pro-inflammatory cytokine and LPS injection induces local expression of IL-1β in respiratory control centers of the brainstem (19,20). Because these cytokines are upregulated very quickly and early in the inflammatory response, their expression in response to inflammation may provide an early indication of the efficacy of VNS in either promoting or blocking their expression and subsequent changes in inflammatory tone.
The work we report here suggests that VNS lacks significant negative impact on the production and release of cytokines and does not impair the timing of parturition or the viability of the newborn. An obvious limitation of our study is that we constrained our stimulus parameters to two sets (relatively low and high) and we did not employ animal models of inflammation or seizure to determine the efficacy of VNS as an intervention. Further studies will need to be performed to determine if there are stimulus parameters for VNS that are inherently pro-inflammatory and anti-inflammatory.
Limitations of the study
Our work is innovative in assessing the role of VNS in a pregnant rat model but there are inherent limitations to our study. Because we used implantable stimulators with limited battery, we were limited in the current density available to block using kilohertz frequency alternating current. Other investigators have used anesthetized animal models to perform kilohertz frequency stimulation and show complete neural block of peripheral nerves at higher frequencies than we used in our experiments (21,26,38–40). We used a maximum of 1000 Hz for our stimuli after consulting with the vendor of our implantable stimulators to assure continuous stimulation over the entire experimental time period. Additionally, we did not assess anti-inflammatory markers, however, HMGB1 is implicated in both anti- and pro-inflammatory roles (37,41). Future work will incorporate a more extensive panel of pro- and anti-inflammatory markers and a wider range of stimulus parameters to assess the impact that VNS has on immune state in both pregnant mothers and their offspring.
Further neurodevelopmental evaluation of neonates exposed to vagal nerve stimulation would provide a broader understanding of longer-term consequences of maternal VNS therapy. Assessing systemic c-reactive protein (CRP) and pro-calcitonin as well as pro- and anti-inflammatory cytokines in babies born to these mothers would provide further information about the immune response evoked by VNS. Ultimately, the goal is to understand the effects of VNS on neuroinflammatory processes that contribute to the physiology of pregnancy, parturition, and fetal neurodevelopment. It may be possible to tune VNS to arrest or prophylactically prevent chorioamnionitis or other sources of inflammation in the mother and improve maternal and fetal outcome.
Acknowledgments
Financial Support: Supported in part by NIH HD954931. Imaging was performed in the Advanced Imaging and Microscopy Core with support from NSF Grant MRI-DBI 0923559 and the Loma Linda University School of Medicine Pediatrics Research Fund.
Footnotes
Disclosure: Funding for this project was provided through a Pediatrics Research Initiative grant from the LLU department of Pediatrics.
References
- 1.Chakravarthy K, Chaudhry H, Williams K, Christo PJ. Review of the Uses of Vagal Nerve Stimulation in Chronic Pain Management. Curr Pain Headache Rep. 2015;19:54. doi: 10.1007/s11916-015-0528-6. [DOI] [PubMed] [Google Scholar]
- 2.Meneses G, Bautista M, Florentino A, et al. Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J Inflamm Lond Engl. 2016;13:33. doi: 10.1186/s12950-016-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mihaylova S, Killian A, Mayer K, Pullamsetti SS, Schermuly R, Rosengarten B. Effects of anti-inflammatory vagus nerve stimulation on the cerebral microcirculation in endotoxinemic rats. J Neuroinflammation. 2012;9:183. doi: 10.1186/1742-2094-9-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 5.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 6.Husain MM, Stegman D, Trevino K. Pregnancy and delivery while receiving vagus nerve stimulation for the treatment of major depression: a case report. Ann Gen Psychiatry. 2005;4:16. doi: 10.1186/1744-859X-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Menachem E. Vagus nerve stimulation for treatment of seizures? Arch Neurol. 1998;55:231–2. doi: 10.1001/archneur.55.2.231. [DOI] [PubMed] [Google Scholar]
- 8.Collins JJ, Lin CE, Berthoud HR, Papka RE. Vagal afferents from the uterus and cervix provide direct connections to the brainstem. Cell Tissue Res. 1999;295:43–54. doi: 10.1007/s004410051211. [DOI] [PubMed] [Google Scholar]
- 9.Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Yellon SM. Contributions to the dynamics of cervix remodeling prior to term and preterm birth. Biol Reprod. 2017;96:13–23. doi: 10.1095/biolreprod.116.142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirby LS, Kirby MA, Warren JW, Tran LT, Yellon SM. Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig. 2005;12:578–85. doi: 10.1016/j.jsgi.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Clyde LA, Lechuga TJ, Ebner CA, Burns AE, Kirby MA, Yellon SM. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biol Reprod. 2011;84:587–94. doi: 10.1095/biolreprod.110.086207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallapur SG, Nitsos I, Moss TJM, et al. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med. 2009;179:955–61. doi: 10.1164/rccm.200811-1728OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol. 2014;41:83–103. doi: 10.1016/j.clp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Perlman JM. White matter injury in the preterm infant: an important determination of abnormal neurodevelopment outcome. Early Hum Dev. 1998;53:99–120. doi: 10.1016/s0378-3782(98)00037-1. [DOI] [PubMed] [Google Scholar]
- 16.Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol N Y N 1989. 2012;67:287–94. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- 17.Sanders TR, Kim DW, Glendining KA, Jasoni CL. Maternal obesity and IL-6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology. 2014;155:2566–77. doi: 10.1210/en.2013-1968. [DOI] [PubMed] [Google Scholar]
- 18.Duncan JR, Cock ML, Scheerlinck J-PY, et al. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res. 2002;52:941–9. doi: 10.1203/00006450-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Balan KV, Kc P, Mayer CA, Wilson CG, Belkadi A, Martin RJ. Intrapulmonary lipopolysaccharide exposure upregulates cytokine expression in the neonatal brainstem. Acta Paediatr Oslo Nor 1992. 2012;101:466–71. doi: 10.1111/j.1651-2227.2011.02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafri A, Belkadi A, Zaidi SIA, Getsy P, Wilson CG, Martin RJ. Lung inflammation induces IL-1β expression in hypoglossal neurons in rat brainstem. Respir Physiol Neurobiol. 2013;188:21–8. doi: 10.1016/j.resp.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel YA, Butera RJ. Differential fiber-specific block of nerve conduction in mammalian peripheral nerves using kilohertz electrical stimulation. J Neurophysiol. 2015;113:3923–9. doi: 10.1152/jn.00529.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RL, Murray ST, Camacho DK, Wilson CG. Vagal nerve stimulation attenuates IL-6 and TNFα expression in respiratory regions of the developing rat brainstem. Respir Physiol Neurobiol. 2016;229:1–4. doi: 10.1016/j.resp.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Antonelli MC, editor. Softcover reprint of the original. 1. Springer; 2016. Perinatal Programming of Neurodevelopment. 2015 edition. Place of publication not identified. [Google Scholar]
- 24.Houser MV, Hennessy MD, Howard BC. Vagal nerve stimulator use during pregnancy for treatment of refractory seizure disorder. Obstet Gynecol. 2010;115:417–9. doi: 10.1097/AOG.0b013e3181bd1a8b. [DOI] [PubMed] [Google Scholar]
- 25.Fang D, Shi S-Q, Shi L, et al. Direct electrical stimulation softens the cervix in pregnant and nonpregnant rats. Am J Obstet Gynecol. 2015;212:786.e1–786.e9. doi: 10.1016/j.ajog.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 26.Patel YA, Saxena T, Bellamkonda RV, Butera RJ. Kilohertz frequency nerve block enhances anti-inflammatory effects of vagus nerve stimulation. Sci Rep. 2017;7:39810. doi: 10.1038/srep39810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy G, Fishman JE, Xu D, et al. Vagal nerve stimulation modulates gut injury and lung permeability in trauma-hemorrhagic shock. J Trauma Acute Care Surg. 2012;73:338–342. doi: 10.1097/TA.0b013e31825debd3. discussion 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajbouj M, Merkl A, Schlaepfer TE, et al. Two-year outcome of vagus nerve stimulation in treatment-resistant depression. J Clin Psychopharmacol. 2010;30:273–81. doi: 10.1097/JCP.0b013e3181db8831. [DOI] [PubMed] [Google Scholar]
- 29.Martin RJ, Wilson CG. What to do about apnea of prematurity? J Appl Physiol. 2009;107:1015–6. doi: 10.1152/japplphysiol.00940.2009. [DOI] [PubMed] [Google Scholar]
- 30.Moorman JR, Lake DE, Ivanov PC. Early Detection of Sepsis--A Role for Network Physiology? Crit Care Med. 2016;44:e312–313. doi: 10.1097/CCM.0000000000001548. [DOI] [PubMed] [Google Scholar]
- 31.Doyle SL, O’Neill LAJ. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–13. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Reys LG, Ortiz-Pomales YT, Lopez N, et al. Uncovering the neuroenteric-pulmonary axis: vagal nerve stimulation prevents acute lung injury following hemorrhagic shock. Life Sci. 2013;92:783–92. doi: 10.1016/j.lfs.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Balan KV, Kc P, Hoxha Z, Mayer CA, Wilson CG, Martin RJ. Vagal afferents modulate cytokine-mediated respiratory control at the neonatal medulla oblongata. Respir Physiol Neurobiol. 2011;178:458–64. doi: 10.1016/j.resp.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drexler SK, Foxwell BM. The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. 2010;42:506–18. doi: 10.1016/j.biocel.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Santello M, Bezzi P, Volterra A. TNF$\alpha$ controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Kiss A, Tratsiakovich Y, Mahdi A, et al. Vagal nerve stimulation reduces infarct size via a mechanism involving the alpha-7 nicotinic acetylcholine receptor and downregulation of cardiac and vascular arginase. Acta Physiol Oxf Engl. 2017 doi: 10.1111/apha.12861. [DOI] [PubMed] [Google Scholar]
- 37.Andersson U, Antoine DJ, Tracey KJ. The functions of HMGB1 depend on molecular localization and post-translational modifications. J Intern Med. 2014;276:420–4. doi: 10.1111/joim.12309. [DOI] [PubMed] [Google Scholar]
- 38.Tanner JA. Reversible blocking of nerve conduction by alternating-current excitation. Nature. 1962;195:712–3. doi: 10.1038/195712b0. [DOI] [PubMed] [Google Scholar]
- 39.Kilgore KL, Bhadra N. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation J Int Neuromodulation Soc. 2014;17:242–254. doi: 10.1111/ner.12100. discussion 254–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowman BR, McNeal DR. Response of single alpha motoneurons to high-frequency pulse trains. Firing behavior and conduction block phenomenon. Appl Neurophysiol. 1986;49:121–38. doi: 10.1159/000100137. [DOI] [PubMed] [Google Scholar]
- 41.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med Camb Mass. 2008;14:476–84. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]