Abstract
Interleukin (IL)-15 overexpression in eosinophilic gastrointestinal disorders is reported, but IL-15’s role in promoting eosinophilia gastroenteritis is largely unknown. Therefore, we generated enterocyte-overexpressed IL-15 transgenic mice using Fabpi-promoter. The Fabpi-IL-15 (iIL-15) transgenic mice show induced IL-15 levels in the jejunum with a marked increase in jejunum eosinophils. However, no induction of eosinophilia in the blood or any other gastrointestinal segment was observed. Eosinophilia in the jejunum villus was substantially higher in iIL-15 mice compared to wild type mice. In addition, goblet cell hyperplasia was also observed in the jejunum of iIL-15 mice. Furthermore, a significant correlation between induced IL-15 transcript and the IL-18 transcripts was observed. Therefore, to further understand the role of IL-18 in IL-15 mice associated gastrointestinal disorders; we generated iIL-15/IL-18Rα−/− mice. Using these mice, we found that IL-18 has an important role in promoting IL-15-induced eosinophilia. Since intestinal IL-15 overexpression is reported in food intolerance; we examined OVA intolerance in iIL-15 mice. The OVA-sensitized and challenged iIL-15 mice experienced weight loss, diarrhea, and eosinophilia in the jejunum. Taken together, our findings demonstrate that intestinal IL-15 overexpression induces IL-18-dependent eosinophilia and immunoglobulin in the intestine that promotes food allergic responses.
Keywords: Eosinophils, Goblet cells, Fabpi-promoter, intestine, interleukin-15
ETOC BLURB
Study demonstrate that intestinal IL-15 overexpression induces classified induction of IL-18 dependent eosinophils and induced imunoglobulin levels in the intestine that promotes food allergic responses.
INTRODUCTION
IL-15 is an important pleiotropic cytokine that is involved in the regulation of Th1 and Th2 immune responses1 and is expressed by a large number of cell types including intestinal epithelial cells, monocytes, macrophages, and dendritic cells (DC).2 Previously, it has been reported that enterocytes produce and respond to IL-153 and IL-15 potently stimulates intraepithelial lymphocytes.4 These reports attract our attention to the role of IL-15 in eosinophilic gastroenteritis. IL-15 overexpression was reported in both the lamina propria and intestinal epithelium of patients with active celiac disease and the patients treated with a gluten-free diet.5 IL-15 overexpression was detected mainly in the enterocyte cell surface, rather than found secreted in the patients with celiac disease, suggesting a role in regulating intraepithelial lymphocytes through the cell-to-cell contact. Earlier, we reported induced IL-15 in eosinophilic esophagitis (EoE) patients and showed that IL-15Rα gene-deficient mice were protected from the induction of EoE.6 IL-15 is a growth and survival factor for iNKT cells and mechanistically we showed that iNKT cells upon activation by IL-18 releases eosinophil active cytokines that promote eosinophils accumulation in the esophagus.7, 8 Notably, we and others reported that the overexpression of IL-18 promotes lung and esophageal eosinophilia8, 9 and the levels of IL-18 correlate with the tissue eosinophilia in human EoE.7 Therefore, we tested the hypothesis that synergy of IL-15 and IL-18 may have an important role in eosinophil-associated inflammatory gastrointestinal diseases (EGID). Accordingly, we examine the role of IL-18 in intestinal IL-15 overexpressed mice in promoting intestinal eosinophilia and associated disorders. The intestinal IL-15 overexpressed (iIL-15) mice were generated using the intestine-specific rat fatty acid binding protein (Fabpi) promoter10 and IL-18Rα-deficient intestinal IL-15 overexpressed mice by cross-breeding iIL-15 and endogenous IL-18Rα deficient mice. Fabpi promoter has been extensively used to generate small intestine enterocytes specific gene overexpressed mice.10 Taken together, intestinal epithelium induced IL-15 overexpressed mice showed IL-18-dependent induced intestinal eosinophilia, Goblet cells in the small intestine that promote food allergen associated allergic responses.
RESULTS
Analysis of Fabpi-IL-15 transgenic mice
The phenotype of intestinal specific Fabpi-IL-15 transgenic mice generated, using the construct shown in Figure 1A, was assessed by real-time PCR as described in the method section.11, 12 The expression level of IL-15 was measured in the jejunum of three different mouse lines generated in our facility along with wild type mice. All three mice had increased IL-15 expression in the intestine than did wild type. The mouse line 1.3 showed the highest expression with ~ 15-fold increase in relative IL-15 mRNA expression (Figure 1B) and ~ 10-fold increase in protein levels (Figure 1C) compared to wild type mice in the jejunum of Fabpi-IL-15 transgenic (iIL-15) mice. Additionally, iIL-15 mice showed induced IFN-γ, but not TNF-α protein in the jejunum compared to wild type mice (Figure 1D, E).
Figure 1. Generation of intestine specific promoter (Fabpi) driven IL-15 transgenic mice.
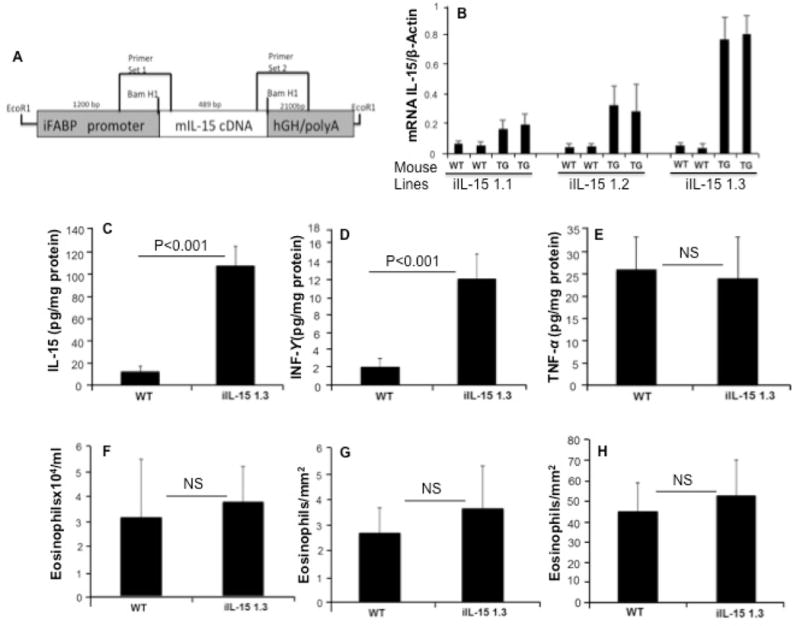
The cDNA fragment encoding the open reading frame of murine IL-15 was cloned into the BamHI site present in a 3.5-kb EcoRI fragment containing 1200bp of rat Fabpi promoter linked to 2100bp of the hGH gene. The position of the PCR primers used for the detection of the Fabpi-IL-15 was indicated (A). The intestinal IL-15 mRNA expression of three lines of Fabpi-IL-15 transgenic mice was generated and gene product was quantified by performing real time PCR analysis. (B). The iIL-15 line # 3 showed >10-fold increase of IL-15 protein levels compared to WT mice (C). iIL-15 mice showed induced IFN-γ (D), not TNF-α (E) protein in the jejunum compared to wild type mice. Eosinophil levels in the peripheral blood (F) esophagus (G) and colon (H) have been shown in wild type and iIL-15 transgenic mice. The results are representative of three separate experiments and presented as the mean ± S.D; Experiment “n” = 2, 4 mice/group), NS-not significant.
Intestinal IL-15 (iIL-15) overexpression does not affect blood, esophagus, and colon eosinophilia in mice
Next, we evaluated the hypothesis that overexpression of IL-15 in intestinal enterocytes would have an effect on the blood eosinophils in the transgenic mice. Our analysis showed that 8-weeks-old Fabpi-IL-15 transgenic (iIL-15) mice have comparable eosinophilia in the blood (Figure 1F) or tissues like esophagus (Figure 1G) and colon (Figure 1H) to age and sex matched wild type mice. Taken together, these results demonstrate that ectopic expression of IL-15 in intestinal enterocytes has no effect on peripheral eosinophilia or other gastrointestinal segments, except the jejunum.
iIL-15 overexpression induces jejunum eosinophilia in mice
It was relevant to determine whether Fabpi promoter driven overexpression of IL-15 would induce the accumulation of eosinophils specifically in the jejunum. Notably, Fabpi transgene expression has been restricted only to the jejunum.10, 13 The jejunum of iIL-15 transgenic mice shows induced tissue eosinophilia compared with wild type mice. Few eosinophils were detected in wild type mice and these eosinophils were mainly restricted to the crypt region with very few detected in the villus (Figure 2 A–B), whereas, eosinophil accumulation in the jejunum occurred in the lamina propria, submucosa, and villus including in the epithelial layer of iIL-15 transgenic mice (Figure 2 C–D). Interestingly, the iIL-15 transgenic mice showed highly elongated villus and induced eosinophilia compared to wild type mice (Figure 2 A–D). The quantitative analysis indicated total eosinophil levels were increased ~ 4-fold in the jejunum of iIL-15 transgenic mice compared to wild type mice; however, the eosinophil numbers in the villus only area was increased >10-fold (Figure 2 E).
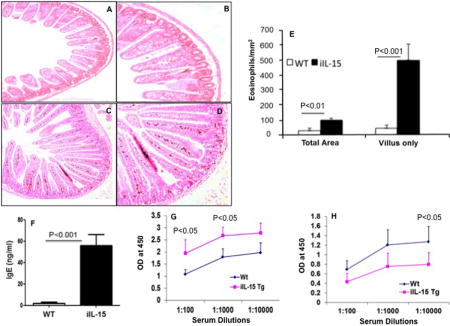
Figure 2. Baseline eosinophils in wild type and iIL-15 transgenic mice.
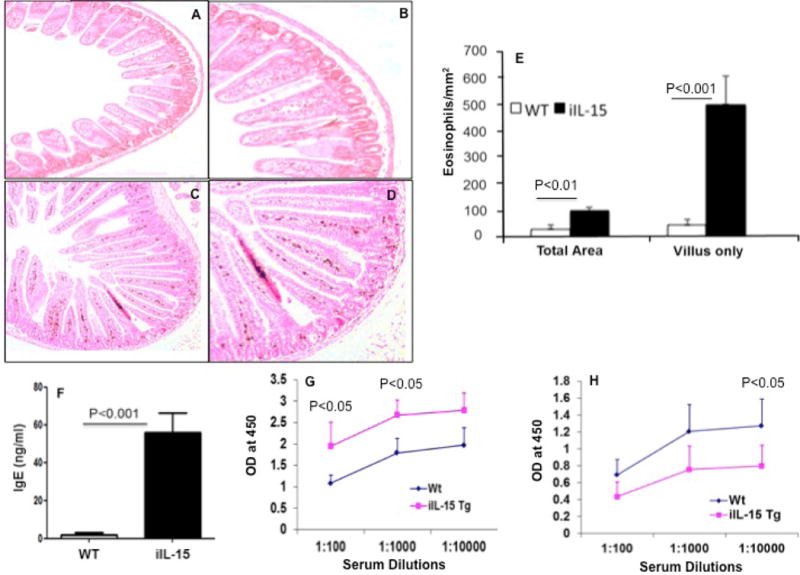
A representative photomicrograph of anti-MBP stained jejunum section from wild type mice (A, B) and Fabpi-IL-15 transgenic mice (C, D) detected eosinophils. The morphometric analysis shows eosinophil levels in WT and IL-15 transgenic (iIL-15) mice (E). Levels of immunoglobulin of IgE (F) IgG1 (G) and IgG2a (H) levels were shown in the iIL-15 mice compared to WT mice. Eosinophil levels were expressed as the mean eosinophils/mm2 ± S.D; Experiment “n”= 3, 3-4 mice/group).
Analysis of Immunoglobulin (IgE, IgG1, and IgG2a) levels in iIL-15 transgenic mice
In a number of allergic diseases, immunoglobulin (Ig) levels were elevated along with the IL-15;14 but the direct relationship of induced tissue IL-15 to the induction of blood immunoglobulin (Ig) levels is not well understood. Therefore, we examined IgE, IgG1, and IgG2a levels in the blood of wild type and iIL-15 mice by commercially available ELISA kit (BD Biosciences). Interestingly, 8 weeks old iIL-15 mice showed induced levels of IgE, IgG1 (Figure 2, F–G), but reduced levels of IgG2a in the blood compared to the age and sex matched wild type mice (Figure 2H). These data provide the evidence that IL-15 contributes to the regulation of immunoglobulin levels in mice.
Overexpression of IL-15 correlates with the levels of eosinophils
Next, we examined whether intestinal IL-15 gene expression correlates with induced eosinophil accumulation in the jejunum. Accordingly, a statistical correlation study was performed on the transcript expression of IL-15 to the jejunum eosinophilia. First, the β-Actin normalized IL-15 mRNA levels and the number of eosinophils in approximately 20-30 villus/mice were examined in 10 weeks old iIL-15 mice compared to wild type mice (Figure 3 A, B). Second, the IL-15 mRNA expression in the jejunum and eosinophils number in the villus of 6, 8, and 10 weeks old iIL-15 transgenic mice were analyzed for determining the statistical correlation. A highly significant correlation (r2=0.529, p<0.001) in the levels of eosinophil and IL-15 mRNA was observed (Figure 3C).
Figure 3. Jejunum eosinophilia correlates with the IL-15 tissue expression.
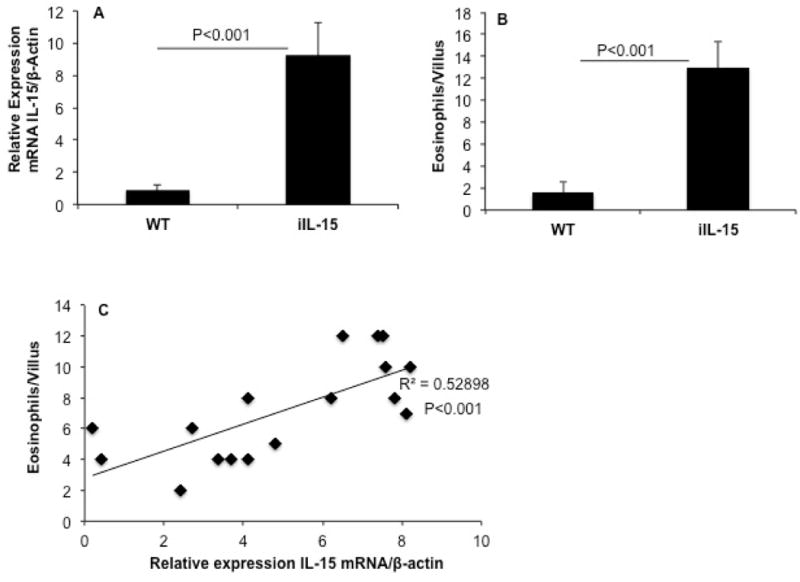
The relative expression of IL-15 mRNA and tissue number of eosinophils in the villus of jejunum of wild type and iIL-15 mice (A) and anti-MBP immunostained analysis eosinophils/villus are shown (B). Statistical correlation of eosinophil /villus with the IL-15 mRNA expression has been shown (C). The data was expressed as mean ± SD. (r2 = 0.52), P <0.001; Experiment “n”= 3, 4 mice/group.
Goblet Cell hyperplasia detected in iIL-15 transgenic mice
Goblet cells are present throughout the GI tract and they are the main source of mucins production and provide innate defense.15 To determine whether IL-15 overexpression induces Goblet cell hyperplasia, we assessed Goblet cells by performing PAS staining in the jejunum tissue sections of wild type and iIL-15 mice. Interestingly, an increase in a number of Goblet cells was observed in the jejunum of iIL-15 mice compared to wild type mice (Figure 4A, B). The increased extracellular mucus staining in the tissue sections was also observed in IL-15 overexpressed mice compared to the wild type mice. The quantitative analysis indicated that iIL-15 transgenic mice had a > 2.5-fold increase in the levels of jejunum Goblet cells compared to wild type mice (p<0.01, Figure 4 C).
Figure 4. Induced Goblet cells hyperplasia in the jejunum of iIL-15 mice.
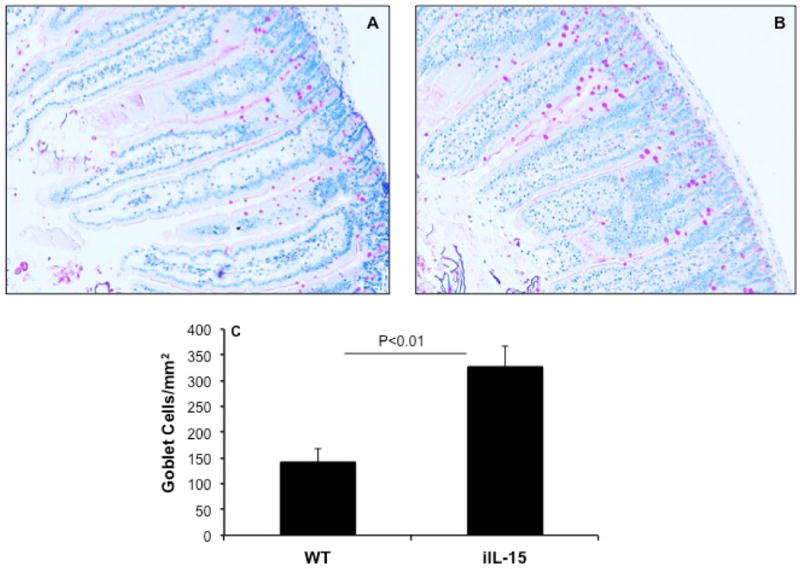
PAS stained jejunum tissue section of WT (A) and iIL-15 (B) mice detected Goblet cells (Photomicrograph presented are ×400 of original magnification). Morphometric quantitation of Goblet cells in iIL-15 mice compared to wild type mice (C). The data was expressed as mean ± SD; Experiment “n”= 3, 4 mice/group.
IL-15 and IL-18 interaction are critical in promoting jejunum eosinophilia and Goblet cell hyperplasia in iIL-15 mice
IL-15 and IL-18 are potent immunostimulatory cytokines that have the ability to induce numerous inflammatory diseases. In addition, both these cytokines are reported to be present in higher levels in a number of gastrointestinal diseases including EoE.6 Therefore, we were interested in examining the cooperative role of IL-15 and IL-18 in promoting intestinal eosinophilia. First, we examined the IL-18 protein levels in the jejunum of iIL-15 transgenic mice and found significantly increased levels of IL-18 in the jejunum overexpressing IL-15 (p<0.001, Figure 5A). Next, we determined the association between IL-18 and IL-15 gene-expression in the intestine of iIL-15 transgenic mice. Our real time PCR analysis indicated a significant correlation (r2=0.5064, p<0.001) between IL-15 and IL-18 mRNA expression in the different age groups (6, 8 and 10 weeks) of iIL-15 transgenic mice (Figure 5B). Third, to determine whether IL-15 induced eosinophilia and Goblet cell hyperplasia are mediated by IL-18, we generated IL-18Rα deficient iIL-15 transgenic mice. The IL-18Rα deficient mice were cross-bred with the iIL-15 transgenic mice as described earlier12 and offspring identified that contained IL-18Rα deficiency in iIL-15 transgenic mice. Anti-MBP immunostaining analysis detected significantly reduced jejunum eosinophilia in iIL-18Rα-deficient-iIL-15 transgenic mice compared to the iIL-15 mice (p<0.01, Figure 5C). Further, the PAS staining detected highly reduced jejunum Goblet cells in iIL-18Rα-deficient-iIL-15 transgenic mice compared to the iIL-15 transgenic mice (p<0.05, Figure 5D). These data indicate that IL-18 mediates IL-15 induced intestinal eosinophilia and Goblet cell hyperplasia in iIL-15 transgenic mice.
Figure 5. IL-18 has a role in promoting jejunum eosinophilia and Goblet cell hyperplasia in iIL-15 mice.
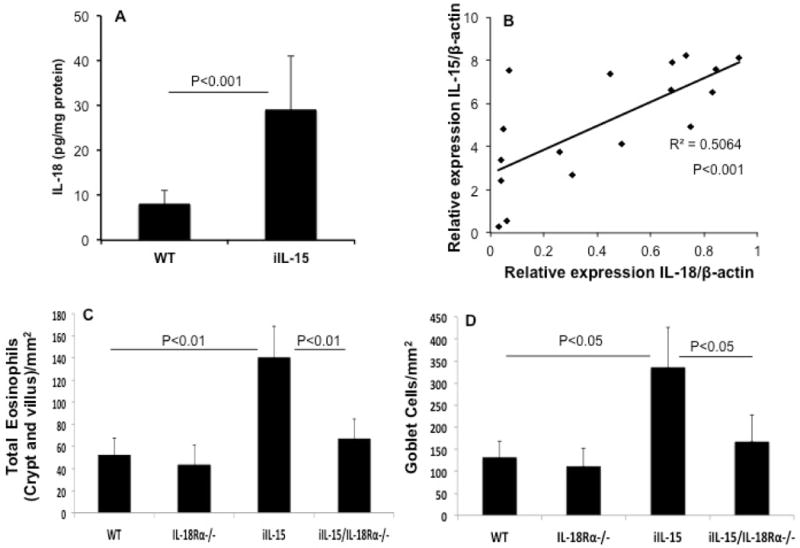
The protein level of IL-18 is induced in iIL-15 mice compared to the WT mice (A). A strong correlation in the levels of IL-15 and IL-18 mRNA is observed in the jejunum of iIL-15 mice (B). Morphometric analysis detected significant induction of jejunum eosinophilia in iIL-15 mice compared to WT, IL-18Rα-deficient mice and IL-18Rα-deficient-iIL-15 transgenic mice (C). Goblet cell hyperplasia in the jejunum of iIL-5 mice compared to the IL-18Rα-deficient-iIL-15 transgenic mice (D). The data was expressed as mean ± SD; Experiment “n”= 3, 4 mice/group.
IL-18 has a role in IL-15-induced eosinophil active Th2 cytokines in iIL-15 mice
Further, to establish the role of IL-18 in IL-15-induced intestinal eosinophilia, we examined eosinophil active cytokines like IL-4, iL-5, IL-13, IL-33 and TSLP in wild type mice, IL-18Rα-deficient mice, iIL-15 mice and IL-18Rα-deficient iIL-15 transgenic mice. The iIL-15 transgenic mice showed significantly induced mRNA levels of IL-4, IL-5 and IL-13 compared to wild type, IL-18Rα gene-deficient mice and IL-18Rα-deficient iIL-15 transgenic mice in the jejunum region (Supplementary figure 1 A-C). However, a comparable level of IL-33 and TSLP mRNA was observed in between wild type mice, IL-18Rα gene-deficient mice, iIL-15 mice and IL-18Rα-deficient iIL-15 transgenic mice (Supplementary figure 1 D, E). A similar significantly elevated protein level of IL-4, IL-5, and IL-13 was also observed in the jejunum region of intestine iIL-15 transgenic mice compared to wild type, IL-18Rα gene-deficient mice and IL-18Rα-deficient iIL-15 transgenic mice (Supplementary figure 1 F-H).
IL-15 intestinal overexpressed mice show intolerance to the food allergen
IL-15 has been reported induced in a number of inflammatory gastrointestinal diseases including EoE6 and IL-15 blockade resulted in the reversal of autoimmune intestinal damage.16 Therefore, we next determined the role of IL-15 overexpression in food tolerance by challenging the mice with OVA following the protocol described in the method section. We observed that each iIL-15 transgenic mouse started losing body weight after the 1st OVA-challenge that continued through the last OVA challenge; whereas, an increase in weight was observed in OVA-challenged wild type mice and might be associated with higher water intake in the first week of allergen challenge. No significant change in weight gain or loss was observed in sensitized mice challenged with saline (Figure 6 A). The gain or loss of the mouse body weight was divided by each mouse’s original body weight and percent gain or loss was calculated using standard method to calculate their percentage change in body weight. Diarrhea in iIL-15 transgenic mice was recorded in 20-30% of mice (2 out of 6 mice) after the 5th challenge and 100% (6 out of 6 mice) of mice showed diarrhea after the 6th challenge. Diarrhea recovery was observed in 60% of mice (4 out of 6 mice) after the 7th challenge, and following the 8th and 9th OVA-challenge, 70-80% (5 out of 6 mice) of the mice again showed the occurrence of diarrhea. In contrast, none of the sensitized and OVA challenged wild type mice showed the symptoms of diarrhea (Figure 6 B). We also examined the mast cells and eosinophil numbers in the jejunum of OVA and Alum-sensitized and saline or OVA challenged wild type and iIL-15 transgenic mice. The sensitized wild type and iIL-15 transgenic mice showed a comparable number of mast cells in the jejunum following saline- or OVA-challenge (Figure 6 C). However, an increased jejunum eosinophilia and Goblet cells hyperplasia was observed in OVA-challenged iIL-15 transgenic mice compared to OVA-challenged wild type mice (Figure 6 D,E). Further, to understand the mechanism operational in jejunum eosinophilia, Goblet cells hyperplasia and food intolerance in iIL-15 transgenic mice, we examined eosinophil active cytokines and mucin gene expression levels in saline and OVA -allenged wild type and iIL-15 transgenic mice. The iIL-15 transgenic mice showed significantly induced mRNA levels in the jejunum region of IL-4, IL-5 and IL-13, IL-18, TNFα and MUC5A in iIL-15 mice compared to wild type, which further stimulated following OVA-challenged iIL-15 mice compared to OVA-challenged wild type mice (Figure 6 F–K). These data indicate that intestinal IL-15 overexpression promotes food intolerance that may be associated with the induction of Th2 cytokines, mucin genes and IL-18 in particular.
Figure 6. OVA intolerance was observed in iIL-15 transgenic mice.
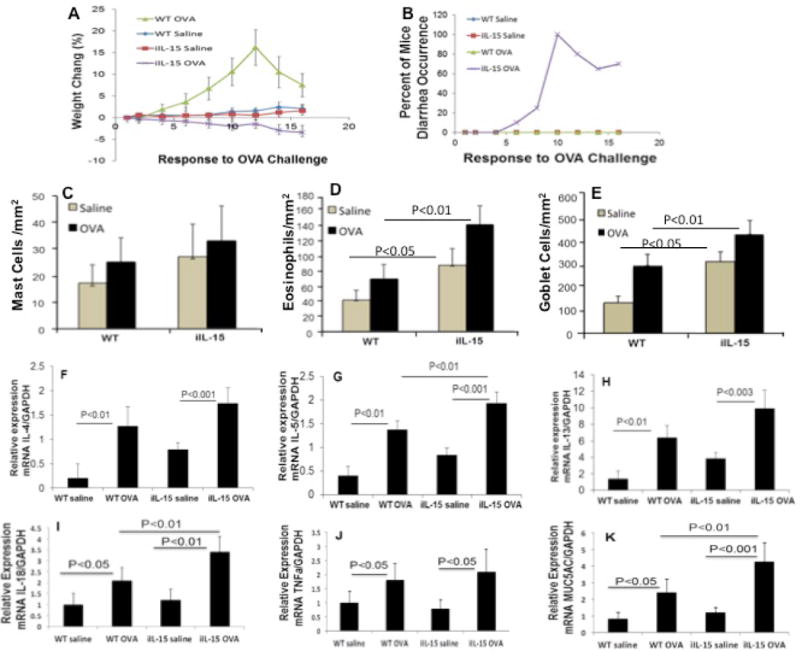
OVA/Alum sensitized and OVA challenged iIL-15 mice show dose dependent decrease of body weight (A) and occurrence of diarrhea (B) compared to the sensitized and challenged WT mice. No weight gain or loss was observed in sensitized and saline challenged WT and iIL-15 mice (A, B). Both WT and iIL-5 mice show comparable number of mast cells in saline and OVA challenged mice (C). OVA challenged mice detected induced jejunum eosinophilia compared to WT mice (D). The mRNA levels of IL-4, IL-5, IL-13, IL-18, TNFα and MUC5A in the jejunum region of saline or ova challenged WT and iIL-15 mice (F-K). The data was expressed as mean ± SD; Experiment “n”= 2, 3 mice/group.
DISCUSSION
Antigen presenting cells and epithelial cells are the major source of IL-153, 6, 17 and we present evidence that eosinophilia and epithelial cell hyperplasia occurs in the esophagus and intestine following the induction of experimental, human EoE and other EGID.18, 19 Th2-associated cytokines (IL-4 IL-5, and IL-13)20, 21 are implicated in a number of eosinophil-associated gastrointestinal disorders; but the significance and mechanism of induced IL-15 in EGID is completely overlooked. IL-15 is induced in a number of food and other allergen-induced gastrointestinal diseases including EoE and celiac disease.6, 22, 23 It is well reported that IL-15 is an iNKT cell growth and survival factor that is also implicated in inducing IgE and eosinophil active cytokines and chemokines.7, 8 Food-specific IgE levels have been detected in the majority of eosinophilic gastroenteritis patients. Notably, the correlation of IL-15 mRNA levels with esophageal eosinophils in patients with active EoE was earlier reported.6 Both macrophages and dendritic cells are the rich source of IL-15 and have a role in both innate and adaptive immunity. Under certain conditions, these cells produce a number of mediators that activate T cells to generate inflammatory cytokines.24, 25 We earlier reported increased IL-15 expressed macrophages in experimental EoE.6 In addition, IL-15-dependent induction of IL-18 has been shown in mice26 and we reported that IL-15 responsive iNKT cells in response to IL-18 release eosinophil associated Th2 cytokines that promote EoE pathogenesis and both have a correlation with tissue eosinophilia.7, 8 Therefore, in the current study, we dissected the consequences of overexpressing IL-15 in enterocytes using an enterocyte-specific promoter (Fabpi).10, 13 Experimental analyses of IL-15 intestine transgenic mice have established several fundamental principles. First, Fabpi promoter driven IL-15 overexpression in the intestine demonstrates a significant impact on regulating eosinophil accumulation only in the small intestine, resulting in induced jejunum eosinophilia compared to wild type mice. The esophagus and colon do not show any induced eosinophilia in Fabpi-IL-15 intestinal transgenic mice, unlike the small intestine. No induction of blood eosinophilia in iIL-15 mice indicates that intestinal expression of IL-15 may not directly influence the hematopoietic organs in expanding eosinophils in the peripheral blood. Further, these studies also demonstrated that expression of IL-15 in enterocytes induces a change in the regional distribution of eosinophils within the intestinal tract. In wild type mice, most eosinophils reside within the base of the crypt; few were detected in lamina propria of the intestine. In contrast, IL-15 transgenic mice have a marked accumulation of lamina propria eosinophils within the villi, consistent with the expression of the Fabpi transgene in villus-associated enterocytes.10, 13 Increased expression of IL-15 in enterocytes likely induces the selective migration of lamina propria eosinophils from the base to within the villus and even infiltrated the epithelium. The intraepithelial eosinophils were also observed in almost all eosinophilic associated gastrointestinal disorders (EGID) and are a characteristic feature of the disease state.27, 28 Additionally, we show that iIL-15 transgenic mice also induce mast cells. More importantly, we found that the IL-15 mRNA expression in the jejunum strongly correlated with induced jejunum eosinophilia. Additionally, Goblet cell hyperplasia and induced mucin staining in the jejunum of iIL-15 transgenic mice was observed, which was consistent with earlier reported studies that an association between IL-15 and induced Goblet cells has a role in innate defense.15, 29, 30 Earlier, we showed that IL-15 exposed T cells induce STAT5-regulated eosinophil active cytokines IL-4, IL-5, and IL-13,6, 31 and IL-13 induction was associated with the development of Goblet cell hyperplasia.32 Similarly, in multiple studies, peripheral or tissue eosinophilia has been shown to be dependent on IL-5;33–35 and recently, we showed that IL-18 activated iNKT cells also promote eosinophilia in EGID.7 Notably, IL-15 is a growth and survival factor for iNKT cells and iNKT activation has been shown to promote eosinophil active cytokines. Herein, we provide direct evidence that induced IL-15 has a role in promoting intestinal eosinophilia. Further, to confirm that IL-15 and IL-18 cooperation promotes intestinal eosinophilia, we first examined the IL-18 levels in the intestinal tissue of IL-15 transgenic mice. Highly induced IL-18 was observed in iIL-15 transgenic mice and statistical correlation data indicated a strong correlation between IL-15 and IL-18 gene expression in the intestine. Further, to establish the role of IL-18 in promoting jejunum eosinophilia in iIL-15 overexpressed mice, we generated IL-18Rα gene-deficient iIL-15 overexpressed mice. These mice showed a significant reduction of jejunum eosinophilia compared to iIL-15 mice. The data indicated that indeed IL-18 cooperates in promoting jejunum eosinophilia in iIL-15 mice.
Furthermore, since IL-15 levels were increased in food intolerance in a number of gastrointestinal diseases including celiac disease, we investigated the role of induced IL-15 in food intolerance. Our data indicate that iIL-15 mice are intolerant to OVA. The OVA-sensitization and intragastric OVA-challenge reduces body weight and triggers diarrhea in iIL-15 transgenic mice; whereas the same OVA-sensitized and saline challenged wild type mice were protected from losing body weight or induction of diarrhea. A similar weight gain in wild type OVA-sensitized and challenged mice were also reported earlier and are consistent with our observation.36 Interestingly, the iIL-15 mice following OVA-sensitization and challenge do not induce mast cells but induce eosinophilia. Additionally, we observed that IL-15 overexpression correlates with IL-18 in the jejunum of iIL-15 mice. Thus, iIL-15 transgenic mice food intolerance is not only associated with induced jejunum eosinophilia and Goblet cell hyperplasia but some other mechanism may also be operational in promoting food intolerance in iIL-15 mice.
Notably, mast cells induction and association in OVA-induced diarrhea in wild type mice was earlier reported; however, the OVA -hallenged iIL-15 do not show induced mast cells. No induction of mast cells was observed and it may be due to the different protocol regime of OVA-sensitization and challenge that has been used in previous studies.37, 38 Notably, IL-15 induced eosinophils and its role in gastrointestinal innate and adaptive immunity was observed. However, in contrast to the intestine, a different response of IL-15 also was observed and recently reported for lung airway obstruction in experimental asthma.31 The IL-15 induced protective lung airway responses are independent of tissue eosinophilia. The lung and intestine have very different environments, flora and mechanism, gastrointestinal segments from the stomach to colon have baseline eosinophilia;11 compared to no baseline eosinophils in the lungs at the healthy state. Taken together, the current study indicated that IL-15 overexpression, associated eosinophilia, and Goblet cell hyperplasia is mechanistically associated with the synergistic effect of IL-18 and IL-15 that needs further attention to understand eosinophil associated gastrointestinal disorders and food intolerance.
METHODS
Generation and characterization of Fabpi-IL-15 transgenic mice
Enterocytes have been demonstrated to be a chief source of a number of chemokines and cytokines in inflammatory lesions in patients with diverse gastrointestinal inflammatory disorders.39, 40 We were interested in examining the consequences of overexpressing IL-15 by intestinal enterocytes. The 1.2-kb 5′-flanking region of the rat Fabpi gene contains all of the necessary elements to promote specific expression of transgenes in enterocytes of the small intestine.10, 13 Therefore, oligonucleotides containing BamHI sites were ligated to both ends of a 489-bp fragment containing the entire coding region of murine IL-15 cDNA amplified by PCR incorporating an improved Kozak consensus sequence and BamHI restriction sites on both ends. Both cDNAs were ligated into the BamHI site of the PBSIF1178-hGHpgkNeo plasmid,10 which consists of a ~3.8-kb EcoRI fragment containing nucleotides −1178 to +28 of rat Fabpi promoter linked to nucleotides +3 to +2150 of the human growth hormone (hGH) gene (except for its 5′ regulatory sequences) as shown in Figure 1A. The transgene plasmid was propagated in Escherichia coli DH5α cells, and the transgene fragment was liberated by EcoRI endonuclease digestion and purified using the QIAEX DNA extraction kit (Qiagen Inc., Chatsworth, CA). After extensive dialysis, the linearized fragment was microinjected into the pronucleus of fertilized eggs from FVB/N mice (Transgenic Core Facility, Children’s Hospital Medical Center, Cincinnati, OH). Further, these FABPi-IL-15 transgenic mice were converted into Balb/c background by crossing with Balb/c mice for 10 generations and used in the studies. Specific pathogen-free wild type (Balb/c) mice and IL-18R-α gene- deficient mice (8-10 weeks old) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). The Tulane Institutional Animal Care and Use Committee (IACUC) approved the animal protocols that were employed in accordance with National Institute of Health (NIH) guidelines.
Identification and genotyping of Fabpi-iL-15 (iIL-15) transgenic mice
Transgenic mice were identified and further genotyped by two PCR reactions using an upstream mIL-15 primer with Fabpi forward primer, product size 244bp and downstream mIL-15 primers with hGH reverse primer, product size 323 bp as per the design is shown (Figure 1A). The primers used for the genotyping of mice are listed in Supplementary table 1A.
Generation of IL-18Rα-gene-deficient/Fabpi-IL-15 transgenic Mice
Mating IL-18 Rα gene-deficient mice with Fabpi-IL-15 mice generated IL-18Rα gene-deficient/IL-15 overexpressing mice as earlier described by us.12, 41
OVA sensitization and challenge protocol
Wild type and iIL-15 transgenic mice were sensitized with intraperitoneal injection of 200 mg OVA with 1 mg Alum on day 0 and day 7. The sensitized iIL-15 mice were challenged intragastric on day 14 with OVA (100 mg) 9 times on alternate days. During the protocol regime, individual mouse weight loss and diarrhea were recorded.
Eosinophil tissue analysis
All segments of the gastrointestinal tract including the esophagus, jejunum and colon of wild type and Fabpi-IL-15 transgenic mice (or any other generated gene-deficient-transgenic mice) were fixed with 4% paraformaldehyde/PBS, processed using standard histological techniques, and immunostained with antiserum against mouse major basic protein (MBP), as previously described.11, 12 Briefly, 5μm sections were blocked with normal goat serum and stained with a rat anti-murine MBP antiserum (gift of J. and N. Lee, Mayo Clinic, Scottsdale, AZ). The slides were washed and incubated with biotinylated goat anti-rat antibody and avidin-peroxidase complex (Vectastain ABC Peroxidase Elite kit; Vector Laboratories, Burlingame, CA). The slides were then developed by nickel diaminobenzidine, enhanced nickel cobalt chloride to form a black precipitate, and counterstained with nuclear fast red.
Quantification of eosinophils
Eosinophils were quantified by counting the anti-MBP positive cells in the epithelial mucosa and lamina propria of the esophagus. Eosinophil numbers and area of each esophageal tissue section were measured and calculated with the assistance of digital morphometric analysis (Luminera Corporation, Infinity Analyze 6.1.0) and expressed as eosinophils/mm2. Further, the same software was used to measure epithelial cell layer thickness. The intestinal sections were taken from the same position in each set of mice, and at least 4–5 random sections/mouse were analyzed. Using digital image capture, tissue regions associated with the entire lamina propria or with the lamina propria associated with villi only were quantified for the total MBP positive cell numbers relative to the total tissue area. Calculated eosinophil levels are expressed as eosinophils/mm2 as earlier reported.28, 42
Goblet cells and mucus production analysis
Jejunum samples were fixed with 4% paraformaldehyde in phosphate buffer (pH 7.4), embedded in paraffin, cut into 5-μm sections, and fixed to positively charged slides. Periodic acid Schiff reaction staining (Poly Scientific R&D Corp., Bay Shore, NY) was then performed on the tissue sections, according to the manufacturer’s recommendations. Results were expressed as the PAS+ mucus-producing cells/mm2.
Blood eosinophil and serum IgE, IgG2a and IgG1 analysis
Peripheral blood samples were collected in heparinized tubes (Becton Dickinson, Franklin Lakes, NJ) from the inguinal artery of anesthetized mice. Eosinophil levels were directly determined with Discombe’s stain.12, 28 Total serum IgE, IgG2a, IgG1 levels were measured by ELISA (BD Biosciences, San Diego, CA) analysis using the manufacturer’s protocol.
Quantitative PCR
Total RNA was isolated from the intestinal samples using TRIZOL (Invitrogen, Indianapolis, IN). The RNA samples (500 ng) were subjected to reverse transcription using iScript reverse transcriptase (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. IL-4, IL-5 IL-13, IL-15, IL-18 TNFα, TSLP, and lMUC5AC levels were quantified using gene-specific primers by real-time PCR (IQ5, Bio-Rad) as reported earlier.41, 43, 44 Results were then normalized to mouse β-actin or GAPDH amplified from the same cDNA mix and expressed as relative gene expression as described earlier.6, 7, 12 Specific transcripts from cDNA were amplified using the gene-specific primers are listed in table 1B.
ELISA analysis
Cytokine levels in intestinal homogenates were measured using ELISA kits for IL-4, IL-5, IL-13, IL-15, IL-18 (eBiosciences, San Diego, CA), INF-ϒ and TNF-α (R & D Systems, Minneapolis, MN). The detection limits were 4 pg/ml for IL-4, IL-5, IL-13, IL-15, IL-18 and 15 pg/ml for IFN-γ and TNF-α.
Statistical analysis
Data are expressed as the mean ± standard deviation (S.D.). Statistical significance comparing different sets of mice was determined by Student’s t-test.
Supplementary Material
Supplementary Figure 1. Relative transcripts analysis levels in the intestine of mice. The relative mRNA levels of IL-4, IL-5, IL-13, IL-33 and TSLP were analyzed in wild type, iIL-15, IL-18Rα-gene deficient mice and IL-18Rα-gene deficient-iIL-15 transgenic mice (A-E). The protein levels of IL-4, IL-5 and IL-13 were measured in wild type, iIL-15, IL-18Rα-gene deficient mice and IL-18Rα-gene deficient/iIL-15 transgenic mice (F-H). Data was expressed as mean ± SD, n=5 mice/group (experiment “n=2; 5 mice/group), NS-Not significant.
Supplementary Table 1. Gene specific primer sequences used for PCR reactions are presented in Table 1A and Table 1B.
Acknowledgments
This work was supported in part by the grant NIH R01 AI080581 (AM). The authors also thank Drs James and Nancy Lee (Mayo Clinic) for anti-MBP and the transgene core facility of Cincinnati Children’s Hospital, Cincinnati, OH for generating iIL-15 transgenic mice and Dr Marc Rothenberg, CCHMC for providing previously used Fabpi promoter.12
Footnotes
DR ANIL MISHRA (Orcid ID: 0000-0003-4266-4684)
CONFLICT OF INTEREST
This author discloses the following: Anil Mishra has served as a consultant for Axcan Pharma, Aptalis, Elite Biosciences, Calypso Biotech SA, and Enumeral Biomedical. The remaining authors disclose no conflicts of interest.
References
- 1.Lodolce JP, Burkett PR, Koka RM, et al. Regulation of lymphoid homeostasis by interleukin-15. Cytokine & growth factor reviews. 2002;13:429–39. doi: 10.1016/s1359-6101(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 2.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 3.Reinecker HC, MacDermott RP, Mirau S, et al. Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology. 1996;111:1706–1713. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- 4.Ebert EC. Interleukin 15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterology. 1998;115:1439–1445. doi: 10.1016/s0016-5085(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 5.Mention JJ, Ben B, Ahmed M, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–745. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X, Wang M, Mavi P, et al. Interleukin-15 expression is increased in human eosinophilic esophagitis and mediates pathogenesis in mice. Gastroenterology. 2010;139:182–93 e7. doi: 10.1053/j.gastro.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niranjan R, Rajavelu P, Ventateshaiah SU, et al. Involvement of interleukin-18 in the pathogenesis of human eosinophilic esophagitis. Clinical immunology. 2015;157:103–13. doi: 10.1016/j.clim.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutt P, Shukla JS, Ventateshaiah SU, et al. Allergen-induced interleukin-18 promotes experimental eosinophilic oesophagitis in mice. Immunology and cell biology. 2015;93:849–57. doi: 10.1038/icb.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang MJ, Choi JM, Kim BH, et al. IL-18 induces emphysema and airway and vascular remodeling via IFN-gamma, IL-17A, and IL-13. American journal of respiratory and critical care medicine. 2012;185:1205–1217. doi: 10.1164/rccm.201108-1545OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweetser DA, Hauft SM, Hoppe PC, et al. Transgenic mice containing intestinal fatty acid-binding protein-human growth hormone fusion genes exhibit correct regional and cell-specific expression of the reporter gene in their small intestine. Proc Natl Acad Sci U S A. 1988;85:9611–9615. doi: 10.1073/pnas.85.24.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A, Hogan SP, Lee JJ, et al. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719–1727. doi: 10.1172/JCI6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra A, Hogan SP, Brandt EB, et al. Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking. J Biol Chem. 2002;277:4406–4412. doi: 10.1074/jbc.M110424200. [DOI] [PubMed] [Google Scholar]
- 13.Sweetser DA, Birkenmeier EH, Klisak IJ, et al. The human and rodent intestinal fatty acid binding protein genes. A comparative analysis of their structure, expression, and linkage relationships. J Biol Chem. 1987;262:16060–16071. [PubMed] [Google Scholar]
- 14.Ruckert R, Herz U, Paus R, et al. IL-15-IgG2b fusion protein accelerates and enhances a Th2 but not a Th1 immune response in vivo, while IL-2-IgG2b fusion protein inhibits both. Eur J Immunol. 1998;28:3312–3320. doi: 10.1002/(SICI)1521-4141(199810)28:10<3312::AID-IMMU3312>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Kim JJ, Khan WI. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens. 2013;2:55–70. doi: 10.3390/pathogens2010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DePaolo RW, Abadie V, Tang F, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosman D, Kumaki S, Ahdieh M, et al. Interleukin 15 and its receptor. Ciba Foundation symposium. 1995;195:221–229. doi: 10.1002/9780470514849.ch15. [DOI] [PubMed] [Google Scholar]
- 18.Teitelbaum JE, Fox VL, Twarog FJ, et al. Eosinophilic esophagitis in children: Immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122:1216–1225. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 19.Foster B, Foroughi S, Yin Y, et al. Effect of anti-IgE therapy on food allergen specific T cell responses in eosinophil associated gastrointestinal disorders. Clinical and molecular allergy. 2011;9:1–7. doi: 10.1186/1476-7961-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaffe JS, James SP, Mullins GE, et al. Evidence for an abnormal profile of interleukin-4 (IL-4), IL-5, and gamma-interferon (gamma-IFN) in peripheral blood T cells from patients with allergic eosinophilic gastroenteritis. Journal of clinical immunology. 1994;14:299–309. doi: 10.1007/BF01540983. [DOI] [PubMed] [Google Scholar]
- 21.Beyer K, Castro R, Birnbaum A, et al. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. The Journal of allergy and clinical immunology. 2002;109:707–713. doi: 10.1067/mai.2002.122503. [DOI] [PubMed] [Google Scholar]
- 22.Weinbrand-Goichberg J, Segal I, Ovadia A, et al. Eosinophilic esophagitis: an immune-mediated esophageal disease. Immunologic research. 2013;56:249–260. doi: 10.1007/s12026-013-8394-y. [DOI] [PubMed] [Google Scholar]
- 23.Abadie V, Jabri B. IL-15: a central regulator of celiac disease immunopathology. Immunological reviews. 2014;260:221–234. doi: 10.1111/imr.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas M, Schachterle W, Oberle K, et al. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirman I, Vainer B, Nielsen OH. Interleukin-15 and its role in chronic inflammatory diseases. Inflammation research: official journal of the European Histamine Research Society. 1998;47:285–289. doi: 10.1007/s000110050331. [DOI] [PubMed] [Google Scholar]
- 26.Sattler A, Dang-Heine C, Reinke P, et al. IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur J Immunol. 2015;45:2286–2298. doi: 10.1002/eji.201445313. [DOI] [PubMed] [Google Scholar]
- 27.Rayapudi M, Rajavelu P, Zhu X, et al. Invariant natural killer T-cell neutralization is a possible novel therapy for human eosinophilic esophagitis. Clinical & translational immunology. 2014;3:1–10. doi: 10.1038/cti.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra A, Hogan SP, Brandt EB, et al. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107:83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasnain SZ, Gallagher AL, Grencis RK, et al. A new role for mucins in immunity: insights from gastrointestinal nematode infection. The international journal of biochemistry & cell biology. 2013;45:364–374. doi: 10.1016/j.biocel.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Jarry A, Dorso L, Gratio V, et al. PAR-2 activation increases human intestinal mucin secretion through EGFR transactivation. Biochemical and biophysical research communications. 2007;364:689–694. doi: 10.1016/j.bbrc.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 31.Venkateshaiah SU, Zhu X, Rajavelu P, et al. Regulatory effects of Interleukin (IL)-15 on allergen-induced airway obstruction. The Journal of allergy and clinical immunology. 2017 doi: 10.1016/j.jaci.2017.05.025. https://doi.org/10.1016/j.jaci.2017.05.025. [DOI] [PMC free article] [PubMed]
- 32.Herbert DR, Yang JQ, Hogan SP, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. The Journal of experimental medicine. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broide D, Sriramarao P. Eosinophil trafficking to sites of allergic inflammation. Immunological reviews. 2001;179:163–172. doi: 10.1034/j.1600-065x.2001.790116.x. [DOI] [PubMed] [Google Scholar]
- 34.Coeffier E, Joseph D, Vargaftig BB. Role of interleukin-5 in enhanced migration of eosinophils from airways of immunized guinea-pigs. Br J Pharmacol. 1994;113:749–756. doi: 10.1111/j.1476-5381.1994.tb17057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–294. doi: 10.1016/j.coi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Saldanha JC, Gargiulo DL, Silva SS, et al. A model of chronic IgE-mediated food allergy in ovalbumin-sensitized mice. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2004;37:809–816. doi: 10.1590/s0100-879x2004000600005. [DOI] [PubMed] [Google Scholar]
- 37.Brandt EB, Strait RT, Wang Q, et al. Oral antigen-induced intestinal anaphylaxis requires IgE-dependent mast cell degranulation. The Journal of allergy and clinical immunology. 2003;111:S339–342. [Google Scholar]
- 38.Brandt EB, Strait RT, Hershko D, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacDermott RP. Chemokines in the inflammatory bowel diseases. Journal of clinical immunology. 1999;19:266–272. doi: 10.1023/a:1020583306627. [DOI] [PubMed] [Google Scholar]
- 40.MacDermott RP, Sanderson IR, Reinecker HC. The central role of chemokines (chemotactic cytokines) in the immunopathogenesis of ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 1998;4:54–67. doi: 10.1097/00054725-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Rothenberg ME, MacLean JA, Pearlman E, et al. Targeted disruption of the chemokine eotaxin partially reduces antigen induced tissue eosinophilia. Journal of Experimental Medicine. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verma AK, Manhoar M, Upparahalli Venkateshaiah S, et al. Role of vasoactive intestinal peptide in promoting the pathogenesis of eosinophilic esophagitis (EoE) Cellular and Molecular Gastroenterology and Hepatology. 2017 doi: 10.1016/j.jcmgh.2017.09.006. doi.org/10.1016/j.jcmgh.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manohar M, Verma AK, Upparahalli Venkateshaiah S, et al. Role of eosinophils in the initiation and progression of pancreatitis pathogenesis. American journal of physiology Gastrointestinal and liver physiology. 2017;2017 doi: 10.1152/ajpgi.00210.2017. ajpgi 00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra A, Wang M, Pemmaraju VR, et al. Esophageal Remodeling Develops as a Consequence of Tissue Specific IL-5-Induced Eosinophilia. Gastroenterology. 2008;134:204–214. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Relative transcripts analysis levels in the intestine of mice. The relative mRNA levels of IL-4, IL-5, IL-13, IL-33 and TSLP were analyzed in wild type, iIL-15, IL-18Rα-gene deficient mice and IL-18Rα-gene deficient-iIL-15 transgenic mice (A-E). The protein levels of IL-4, IL-5 and IL-13 were measured in wild type, iIL-15, IL-18Rα-gene deficient mice and IL-18Rα-gene deficient/iIL-15 transgenic mice (F-H). Data was expressed as mean ± SD, n=5 mice/group (experiment “n=2; 5 mice/group), NS-Not significant.
Supplementary Table 1. Gene specific primer sequences used for PCR reactions are presented in Table 1A and Table 1B.


