Abstract
Objective
Trauma is a potent exposure that can have implications for health. However, little research has considered whether trauma exposure is related to endothelial function, a key process in the pathophysiology of cardiovascular disease (CVD). We tested whether exposure to traumatic experiences was related to poorer endothelial function among midlife women, independent of CVD risk factors, demographic factors, psychosocial factors, or a history of childhood abuse.
Methods
272 nonsmoking peri- and postmenopausal women aged 40–60 without clinical CVD completed the Brief Trauma Questionnaire, the Child Trauma Questionnaire, physical measures, a blood draw, and a brachial ultrasound for assessment of brachial artery flow mediated dilation (FMD). Relations between trauma and FMD were tested in linear regression models controlling for baseline vessel diameter; demographics, depression/anxiety, CVD risk factors, health behaviors, and additionally, a history of childhood abuse.
Results
Over 60% of the sample had at least one traumatic exposure, and 18% had three or more exposures. A greater number of traumatic exposures was associated with lower FMD, indicating poorer endothelial function in multivariable models [beta, b(standard error, SE-1.05 (.40), p=.01]. Relations between trauma exposure and FMD were particularly pronounced for three or more trauma exposures [b(SE)=−1.90(.71), p=.008, relative to no exposures, multivariable].
Conclusions
A greater number of traumatic exposures was associated with poorer endothelial function. Relations were not explained by demographics, CVD risk factors, mood/anxiety, or a by history of childhood abuse. Women with greater exposure to trauma over life may be at elevated CVD risk.
Keywords: trauma, cardiovascular diseases, endothelial dysfunction, women, flow mediated dilation
Introduction
Cardiovascular diseases (CVD) are the leading cause of death in women.1 Psychosocial factors are increasingly appreciated to be important to the development of CVD.2, 3 One potent psychosocial exposure is trauma. Exposure to trauma is particularly prevalent among women. For example, the National Institute of Justice’s National Violence Against Women Survey indicated a lifetime incidence of assault of 51.1% among US women,4 and the Centers for Disease Control and Prevention report that 43.9% of women will experience sexual violence during their lifetime.5
While trauma is a well-established risk factor for mental health problems, emerging data indicate its potential importance to the development of chronic disease. Some data indicates that a history of trauma exposure may be associated with increased risk for CVD.6–11 However, there are several limitations to this work. Most existing work considers self-reported CVD outcomes, findings which are limited by potential biases in CVD event detection and reporting.12, 13 Use of subclinical CVD measures among women who do not yet have clinical CVD can address this limitation. In fact, use of subclinical CVD indices is important when studying midlife women, among whom rates of clinical CVD are low.14, 15 One measure of the vasculature indexes the vascular endothelium, the single cell layer lining the vessel critical to multiple aspects of vascular health and function. Endothelial injury and dysfunction is an initiating event in atherosclerosis,16 and measures of endothelial function are prospectively associated with later CVD.17 Finally, as opposed to inquiring about trauma among individuals with CVD, investigation of the trauma-CVD risk association in individuals who have not yet experienced a CVD event is important, as the CVD event itself can represent a traumatic exposure.6, 18, 19
Among nonsmoking midlife women free of clinical CVD, we tested whether women with more traumatic exposures over life would have poorer endothelial function at midlife. We hypothesized that associations between trauma exposure and endothelial dysfunction would persist controlling for critical factors, including standard and novel CVD risk factors, depressed mood, and anxiety. Given documented relations of a history of childhood abuse with CVD risk20, 21 and relations of a history of childhood abuse with risk for later adult trauma exposure,22 we also consider the role of a history of childhood abuse in associations between trauma exposure and endothelial function at midlife.
Methods
Study Sample
We recruited 304 late perimenopausal (2–12 months amenorrhea) and postmenopausal (≥12 months amenorrhea)23 nonsmoking women aged 40–60 from the surrounding community (Pittsburgh, Pennsylvania, USA). As women were recruited into a study on hot flashes, by design, half of the women reported daily hot flashes, and half reported no hot flashes in the past three months (for further details on study design and sample characteristics, see 24). Exclusion criteria included hysterectomy and/or bilateral oophorectomy; history of heart disease, stroke, arrhythmia, gynecological cancer, pheochromocytoma, pancreatic tumor, kidney failure, seizures, Parkinson’s disease, Raynaud’s Phenomenon; current pregnancy; or having used the following medications in the past 3 months: oral/transdermal estrogen or progesterone, selective estrogen receptor modulators, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, gabapentin, insulin, beta blockers, calcium channel blockers, alpha-2 adrenergic agonists, or other antiarrhythmic agents. Women who were undergoing chemotherapy, hemodialysis, or peritoneal dialysis were also excluded.
Of the 304 women, 32 women were excluded due to missing flow mediated dilation (FMD) data because of poor scan quality or movement during the exam. Excluded women did not differ on any study variables than included women. 272 women were included in primary models.
Design and Procedures
Women were recruited from the community via advertisements, mailings, and online message boards. Participants underwent physical measurements, ambulatory monitoring, a blood draw, and a carotid artery ultrasound. Procedures were approved by the University of Pittsburgh Institutional Review Board. Participants provided written, informed consent.
Measures
Trauma and abuse exposure
Trauma exposure in adulthood was assessed via items from the Brief Trauma Questionnaire developed for the Nurses’ Health Study II25 which was adapted from the Brief Trauma Interview.26, 27 This brief self-report questionnaire is designed to assess a history of traumatic events, and the version administered in the present study included nine items querying about the following specific events rated as yes or no having ever occurred: serious accidents, natural disasters, life threatening illness, being beaten or mugged, unwanted sexual contact, death of a child, sexual harassment, threat of injury or violence, or witnessing a severe injury or death. As reported by Koenen and colleagues25 interrater reliability for the presence of Criterion A1 trauma exposure according to the DSM-IV was high [average kappa =.70 (range .74–1.00) for all events except illness (.60)]. Exposures were considered separately as well as summed, with the sum categorized as none, 1–2, or 3 or more exposures.
Child abuse history was assessed via a separate questionnaire, the 28-item Child Trauma Questionnaire (CTQ), a validated measure of child abuse and neglect experienced at or before age 18.28 It included questions about emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect. The CTQ has strong test-retest reliability (0.79–0.86), internal consistency (α=.75–.94), and convergent validity.28, 29 The CTQ was considered as a total continuous scale score.
Brachial ultrasound
After an overnight fast, FMD was measured after 10 minutes of supine rest by high resolution B-mode ultrasound imaging of the right brachial artery, 2–10 cm proximal to the antecubital crease, by trained sonographers using a standardized protocol. Images were obtained at rest (baseline) and after 5 minutes of forearm blood flow occlusion (post-deflation) with a pneumatic tourniquet set to 50 mm Hg above the participant’s systolic blood pressure. For baseline diameters, digitized images were recorded for 20 seconds. Immediately after deflation, images were recorded continuously for three minutes. The arterial diameter was measured as the distance between the anterior and posterior arterial wall media-adventitia interfaces on images captured on the R wave using edge-detection software. All images for this study were read by a single trained reader using the Brachial Analysis System (MIA, University of Iowa) software.30 The reading software allows continuous tracking of the brachial artery diameter across these images so that the peak change in diameter can be accurately determined. FMD was calculated as the maximum percentage of change in arterial diameter, relative to baseline. This methodology at this laboratory has shown reproducibility (ICC’s=0.70–0.72).31
Covariates
Height and weight were measured via a fixed stadiometer and a calibrated balance beam scale and BMI calculated (kg/m2). Seated blood pressure (BP) was measured via a Dinamap device after 10-min rest. Women reported a lifetime history of a range of psychiatric disorders, including PTSD. Medical and reproductive history was assessed by standard instruments. Menopause status was obtained from reported menstrual bleeding patterns.23 Parity was classified by the total reported number of live births. Use of medications [antidepressants, antihypertensives, lipid-lowering, glucose-lowering, beta agonists, anticonvulsants] was reported. Anxiety was assessed via the State-Trait Anxiety Inventory,32 depressive symptoms by the Center for Epidemiologic Studies Depression scale,33 perceived stress via the Cohen perceived stress scale,34 and sleep quality via the Pittsburgh Sleep Quality Index.35 Leisure-time physical activity was assessed via the International Physical Activity Questionnaire.36 Hot flashes were assessed by 24 hour ambulatory physiologic monitoring.24 Sleep time was assessed over three days via actigraphy (Actiwatch 2, Respironics, Inc., Murrysville, PA) in 1-min epochs and analyzed and scored (Philips Actiware v6.0.0 software) according to established methods.37 Total sleep time was calculated as: [(time in bed)-(sleep onset latency)-(minutes of wakefulness between sleep onset time and final wake time)]. Glucose, high-density lipoprotein cholesterol (HDL-C), and triglycerides were measured enzymatically (Vital Diagnostics, Lincoln, RI; intra- and inter assay coefficients of variation (CV), respectively, were: glucose: 1.9%, 2.4%; HDL: 1.8%, 2.6%; triglycerides: 1.8%, 3.7%). Total cholesterol was determined enzymatically (intra- and inter-assay CVs: 3.5%, 6.7%) and LDL-C was calculated using the Friedewald formula.38 Insulin was measured via radioimmunoassay (intra- and inter-assay CVs: 4.8%, 10.5%). Homeostatic model assessment (HOMA), reflecting insulin resistance, was calculated.39 C-reactive protein was assessed using a high sensitivity reagent set (Beckman Coulter, Brea, CA; intra- and inter-assay CVs: 5.5%, 3.0%). Interleukin-6 was assessed with an R&D Systems high sensitivity ELISA (Minneapolis, MN; intra- and inter-assay CVs: 9.1%, 10.2%).
Data analysis
Sum of trauma exposures, HOMA, triglycerides, C-reactive protein, and interleukin-6 values were natural log transformed and leisure time physical activity square root transformed for analysis. Differences between participants by included/excluded status were tested using linear regression, Wilcoxon rank sum, and chi-square tests. Associations between trauma exposure and FMD were tested in linear regression models. Covariates were factors associated with FMD at p<.20: baseline lumen diameter, age, race/ethnicity, leisure time physical activity, state anxiety, parity, beta agonist medications. BMI and SBP were forced into models. To test the role of childhood abuse in relations between lifetime trauma exposure and FMD, a separate set of models included CTQ scores as an additional covariate in multivariable models testing relations between Brief Trauma Questionnaire scores in relation to FMD. We also tested interactions between CTQ scores and Brief Trauma Questionnaire scores in relation to FMD in multivariable models. In secondary models, a reported history of PTSD (six women) was considered as an additional covariate or as an exclusion. Additional covariates were education, financial strain, depressive symptoms, perceived stress, antidepressants, general self-rated health, lipids, HOMA, BP-lowering, lipid-lowering, and diabetes medications, hot flashes, CRP, and IL-6. Residual analysis and diagnostic plots were conducted to verify model assumptions. Analyses were performed with SAS v9.2 (SAS Institute, Cary, NC). Models were 2-sided at α=0.05.
Results
Participants were on average 54 years old, postmenopausal, overweight, and White (Table 1). The mean (SD) FMD was 7.39% (4.04). Over 60% (N=164) of the sample had at least one traumatic exposure. Eighteen percent of the women had three or more traumatic exposures (Table 2). The most common exposure was unwanted sexual contact, reported by 22% of the sample.
Table 1.
Sample characteristics
| Trauma Exposure | ||
|---|---|---|
|
| ||
| Yes (N=164) | No (N=108) | |
| Age, M (SD) | 54.13 (3.94) | 54.00 (3.93) |
| Race, N (%) | ||
| White | 119 (72.56) | 79 (73.15) |
| African American, Hispanic, Other | 45 (27.44) | 29 (26.85) |
| BMI, M (SD) | 28.57 (6.30) | 29.74 (7.52) |
| SBP, mmHg, M (SD) | 120.6 (15.25) | 117.8 (12.83) |
| DBP, mmHg, M (SD) | 70.31 (9.32) | 69.38 (8.62) |
| HDL, M (SD) | 62.64 (15.54) | 62.86 (13.48) |
| LDL, M (SD) | 131.5 (34.27) | 130.4 (33.19) |
| Triglycerides, M (SD) | 113.52 (66.62) | 103.21 (44.13) |
| HOMA, M (SD) | 2.67 (1.61) | 2.68 (1.70) |
| Parity, Median (IQR) | 2.0 (1,3) | 2.0 (1, 3) |
| Self-rated health, N (%) | ||
| Excellent | 40 (24.39) | 31 (38.70) |
| Very good | 70 (42.68) | 52 (48.15) |
| Good/fair/poor | 54 (32.93) | 25 (23.15) |
| Education, N (%) | ||
| High school, vocational school | 63 (38.41) | 51 (47.22) |
| College education or higher | 101 (61.59) | 57 (52.78) |
| Financial strain (yes), N (%) | 51 (31.10) | 32 (29.63) |
| Physical activity, leisure time, IPAQ, M (SD) | 786.76 (1113.16) | 982.03 (1135.28) |
| State anxiety (Speilberger), M (SD) | 32.18 (10.04) | 32.24 (9.66) |
| Depressive symptoms (CESD), M (SD) | 8.24 (8.37) | 7.26 (7.89) |
| Perceived stress, M (SD) | 4.59 (2.95) | 4.42 (2.80) |
| Childhood abuse/neglect (CTQ score), M (SD) | 39.86 (14.78) | 32.76 (9.92) |
| Hot flashes, physiologic, number / 24 hrs, M (SD) | 9 (9) | 8 (8) |
| CRP, M (SD) | 2.89 (3.48) | 3.05 (3.86) |
| IL6, M (SD) | 1.99 (1.83) | 2.04 (2.82) |
| Medications, N (%) | ||
| BP-lowering | 23 (14.02) | 22 (20.37) |
| Lipid-loweringa | 15 (9.15) | 21 (19.44) |
| Diabetes | 6 (3.66) | 4 (3.70) |
| Beta-agonist | 7 (4.27) | 7 (9.48) |
| Anticonvulsants | 3 (1.83) | 0 (0.00) |
| Antidepressants | 3 (1.83) | 3 (2.78) |
Differs between trauma exposure group at p<.05
Depressive symptoms, triglycerides, HOMA, IL6, CRP log transformed, physical activity square root transformed for comparison
Table 2.
Prevalence of exposure to traumatic events
| N (%) | |
|---|---|
| Have you ever… | |
| Been in a serious car accident or a serious accident? | 49 (18.01) |
| Been in a major natural or human made disaster? | 26 (9.56) |
| Had a very serious or life-threatening illness? | 17 (6.25) |
| Been attacked, beaten or mugged by anyone? | 53 (19.49) |
| Been made or pressured into having some type of unwanted sexual contact? | 60 (22.06) |
| Experienced the death of one of your own children? | 18 (6.62) |
| Experienced sexual harassment at work that was either physical or verbal? | 53 (19.49) |
| Been in any other situation in which you were seriously injured? | 37 (13.6) |
| Witnessed a situation in which someone was seriously injured or killed? | 58 (21.32) |
| Sum exposures | |
| 0 | 108 (39.71) |
| 1–2 | 114 (41.91) |
| 3+ | 50 (18.38) |
Women with a greater number of traumatic exposures, particularly three or more, had lower FMD, a marker of poorer endothelial function (Table 3; Figure 1). The single exposure most strongly associated with lower FMD was having a history of a major accident. Associations were not explained by covariates associated with FMD. We also considered a range of additional covariates, including educational attainment, additional CVD risk factors, and additional cardiovascular medications, and relations persisted.
Table 3.
Relation between violence exposure and FMD
| FMD (%) B(SE) | |||
|---|---|---|---|
|
| |||
| Model 1 | Model 2 | Model 3 | |
| Serious accident | −1.65 (.59)c | −1.61 (.62)b | −1.78 (.60)c |
| Disaster | −1.27 (.78) | −1.25 (.81) | −1.17 (.79) |
| Serious illness | .41 (.95) | .31 (.98) | .36 (.95) |
| Attacked | −.58 (.57) | −.75 (.60) | −.74 (.60) |
| Sexual assault | −.48 (.55) | −.44 (.57) | −.69 (.59) |
| Death of child | −1.22 (.91) | −1.23 (.94) | −1.58 (.95)a |
| Sexual harassment | −.66 (.57) | −.76 (.60) | −.95 (.60) |
| Serious injury | .41 (.66) | .42 (.68) | .22 (.70) |
| Witness violence | −.24 (.55) | −.27 (.57) | −.48 (.58) |
| Any exposure | −.84 (.47)a | −.88 (.49)a | −1.03 (.49)b |
| Sum exposurese | −.73 (.37)b | −.75 (.39)b | −1.05 (.40)c |
| Number of exposuresd | |||
| 1–2 | −.64 (.51) | −.67 (.53) | −.75 (.51) |
| 3+ | −1.30 (.64)b | −1.37 (.68)b | −1.90 (.71)c |
p<.10;
p<.05;
p<.01
Relative to no exposures
log transformed for analysis
Each exposure considered in separate model
Model 1: Baseline diameter, age, race, BMI, SBP, beta agonist medications, anticonvulsant medications, parity, leisure time physical activity, state anxiety
Model 2: Model 1 covariates + education, diabetes, HOMA, lipids (LDL, HDL, triglycerides), additional medications (antihypertensive, lipid-lowering, anti-diabetic, antidepressant)
Model 3: Model 1 covariates + child trauma history (child trauma questionnaire)
Figure 1.
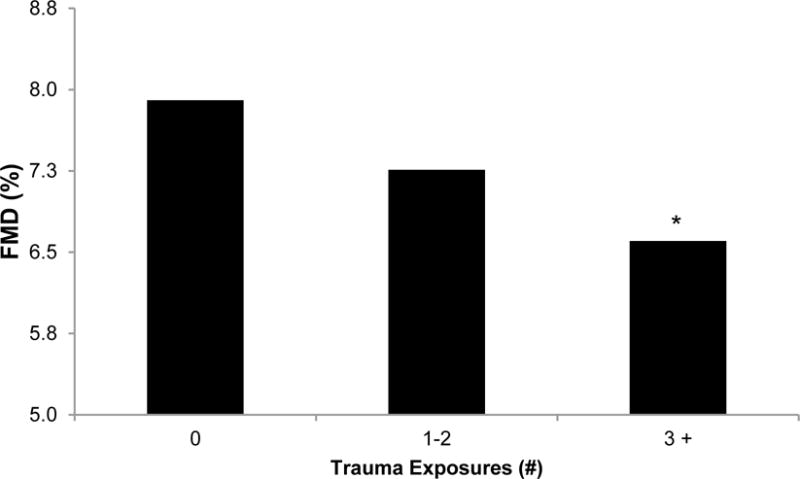
Adjusted mean FMD by number of traumatic exposures
Means adjusted for baseline diameter, age, race, BMI, SBP, beta agonist medications, anticonvulsant medications, parity, leisure time physical activity, state anxiety
*p<.05 relative to no exposure
We considered the role of childhood abuse in these relations. As expected, the correlation between a history of trauma exposure (Brief Trauma Questionnaire score) and childhood abuse (CTQ score) was moderate and statistically significant (ρ=.26, p<.0001). When including childhood abuse history in multivariable models of lifetime trauma exposure in relation FMD, associations between lifetime trauma and FMD persisted (Table 3). There was no evidence of a synergy between child abuse and lifetime trauma exposure, as interactions between trauma exposure and childhood abuse in relation to FMD were not significant (p’s>.20).
We conducted several additional analyses. We considered additional covariates of depressive symptoms, perceived stress, hot flashes, self-rated health, financial strain, alcohol use, sleep, and inflammatory markers (CRP, IL6) and findings were unchanged (data not shown). Six women reported a history of PTSD. To consider whether findings were driven by these women with PTSD, we first included PTSD as an additional covariate, and next alternatively excluded women with PTSD, and findings remained (data not shown). We considered any differences in relations between trauma exposure and FMD by race/ethnicity; interactions were not significant (interaction p’s>.10). Given prior evidence of modification of relations of trauma exposure and cardiovascular outcomes by sleep time,40 we additionally explored whether sleep time modified associations between trauma exposure and FMD. We found indication of interaction between accident history and actigraphy-assessed sleep time (interaction p=.03), with relations between accident history and lower FMD particularly pronounced among women sleeping <6 hours per night (<6 hours: b=−2.87, SE=1.17, p=.02; ≥6 hours: b=−1.36, SE=.72, p=.06).
Conclusions
In this sample of nonsmoking midlife women free of clinical CVD, women with a history of trauma exposure, particularly three or more traumatic exposures, had lower FMD, indicating poorer endothelial function. Associations between trauma exposure and endothelial function were not explained by multiple potentially confounding or explanatory factors, including psychosocial or demographic factors, mood, anxiety, CVD risk factors, or even a history of childhood abuse or neglect. These data support the importance of considering trauma exposure as an important psychosocial factor in midlife women’s cardiovascular health.
Prior work has linked trauma exposure to reported cardiovascular outcomes.9, 41 The present finding is novel in its use of vascular ultrasound measures of endothelial function among individuals free of CVD. Use of vascular ultrasound provides a subclinical disease marker and can indicate potential early vascular dysfunction before clinical disease is present. Considering subclinical indicators is useful for indexing aspects of cardiovascular health among midlife women, who typically show clinical CVD only decades later.1, 14 Further, using subclinical measures among an asymptomatic population avoids potential biases associated with self-reported CVD (e.g., contact with the medical system, health literacy),12, 13 and provides the opportunity for intervention and prevention before frank disease is present. Finally, investigating subclinical CVD before clinical CVD is evident avoids the potentially interpretative difficulty of the clinical CVD event itself (e.g., heart attack, stroke) serving as a trauma.19 In sum, the present data underscore the importance of particular prevention efforts aimed at women with a history of multiple traumatic exposures, and this work may also point to a possible mechanism, endothelial dysfunction, linking trauma exposure to later clinical CVD.
Over 60% of the women in this study reported exposure to one or more traumatic experiences, and 18% of the women endorsed three or more traumatic exposures. These figures are consistent with other work.9 Women with a greater number of exposures had the lowest FMD, pointing to the importance of multiple lifetime exposures.41 The most common exposure was a history of sexual assault, reported by 22% of the women, yet the exposure most strongly related to endothelial function was having been in an accident (18% of the sample). We considered an explanatory factor of ongoing physical health issues in these relations, yet associations persisted with adjustment for a range of physical health measures, including self-rated health, health status, and markers of inflammation. It is notable that women with a history of major illness in the present study did not have lower FMD than their counterparts without such a history. In sum, the present work indicates that a greater number of traumatic exposures was related to lower FMD.
A notable aspect of the current work was our consideration of both lifetime trauma exposure and child abuse/neglect. Our prior work has found a history of child abuse and neglect associated with greater adult carotid atherosclerosis.20 However, controlling for childhood trauma exposure, assessed via a validated child trauma inventory, did not reduce associations between trauma and FMD. Further, we found no evidence of an interactive or additive effect of childhood and lifetime trauma in relation to FMD. Findings support an independent association between adult exposures from childhood exposures in relation to CVD risk.
We considered a range of possible confounders and pathways that might account for associations between trauma exposure and endothelial function. Controlling for multiple traditional and novel CVD risk factors, comorbidities, and medications did not explain associations. Controlling for depression, anxiety, and other negative affective symptoms did not reduce associations between trauma exposure and endothelial function, suggesting that the trauma exposure itself was important irrespective of an ongoing affective response to it. Excluding women endorsing a history of PTSD did not alter findings, supporting findings consistent with other work indicating relations between trauma exposures to health outcomes independent of PTSD.9, 41 Finally, neither hot flashes or sleep characteristics explained associations between trauma exposure and FMD. However, there was some evidence that relations between accident history and lower FMD was particularly prominent among women with shorter sleep, a finding broadly consistent with our prior work.40 Although many pathways were considered here, pathways deserving further investigation in future work include the hypothalamic pituitary adrenal axis,42 the nitric oxide pathway,43 and epigenetic alterations,44, 45 mechanisms potentially impacted by traumatic experiences and associated with CVD risk.
Several limitations deserve mention. We assessed a range of potential exposures using an adapted measure implemented in other major cohort studies (NHS II). However, not all possible exposures were assessed, and an abbreviated version of this measure was administered (excluding items pertaining to nursing practice, for example). Thus, some misclassification of exposure is possible. Further, women were recalling these exposures rather than assessing them prospectively; thereby these reports may be subject to potential for influences of memory. Further, women reported a history of PTSD, but not all PTSD symptoms were assessed, and PTSD history was not established via diagnostic interview. Although a wide range of possible confounders were assessed and considered carefully, residual confounding is a consideration in observational studies, and this study cannot establish directionality or causality. Further, brachial ultrasound is a validated measure of endothelial function highly correlated with coronary endothelial function,46 it is not a direct measure, which would require more invasive procedures not amenable to a sample of this size. Finally, the sample included women and had somewhat limited racial ethnic diversity; men and more racially/ethnically diverse samples should be considered in future work.
This study had several strengths. This study included a well-characterized sample of non-smoking midlife women free of clinical CVD. Vascular endothelial function was assessed via a widely-used and validated marker of vascular endothelial function. Multiple possible confounders and mechanisms were measured and considered here. Exposures during both adulthood as well as childhood were assessed and considered.
In summary, women with a history of three or more lifetime traumatic exposures had poorer endothelial function than women without a trauma history. The most prevalent exposure was sexual assault, yet the most potent single exposure for endothelial function was a history of having been in an accident. Relations were not explained by psychological factors such as anxiety or depressive symptoms, standard CVD risk factors, general health status, or a childhood history of abuse or neglect. These data underscore the potential value of considering trauma exposure as an important cardiovascular health issue among women.
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health, National Heart Lung and Blood Institute (R01HL105647, K24123565 to Thurston) and the University of Pittsburgh Clinical and Translational Science Institute (NIH Grant UL1TR000005). The National Institutes of Health funded this work and approved the initial study design, but was not involved in the conduct of the study; collection, management, analysis, or interpretation of the data; nor the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest
Thurston: MAS Innovation (consulting), Guidepoint (consulting). von Känel: Vifor AG Switzerland (honorarium); no other authors have disclosures to declare.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: current research and future directions. Psychosom Med. 2010;72(9):842–54. doi: 10.1097/PSY.0b013e3181f6934f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45(5):637–51. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Tjaden P, Thoennes N. Full Report of the Prevalence, Incidence, and Consequences of Violence Against Women: Findings From the National Violence Against Women Survey. Rockville, MD: National Criminal Justice Reference Service; 2000. [Google Scholar]
- 5.Breiding M, Smith S, Basile K, Walters M, Chen J, Merrick M. Prevalence and Characteristics of Sexual Violence, Stalking, and Intimate Partner Violence Victimization — National Intimate Partner and Sexual Violence Survey, United States, 2011. MMWR. 2014;63(SS08):1–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):548–56. doi: 10.1016/j.pcad.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J. 2013;166(5):806–14. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol. 2009;28(1):125–30. doi: 10.1037/0278-6133.28.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumner JA, Kubzansky LD, Elkind MS, et al. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132(4):251–9. doi: 10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol. 2013;62(11):970–8. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenen KC, Sumner JA, Gilsanz P, et al. Post-traumatic stress disorder and cardiometabolic disease: improving causal inference to inform practice. Psychol Med. 2017;47(2):209–25. doi: 10.1017/S0033291716002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mnatzaganian G, Braitberg G, Hiller JE, Kuhn L, Chapman R. Sex differences in in-hospital mortality following a first acute myocardial infarction: symptomatology, delayed presentation, and hospital setting. BMC Cardiovasc Disord. 2016;16(1):109. doi: 10.1186/s12872-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes JS, Arispe IE, Moy E. Heart disease and prevention: race and age differences in heart disease prevention, treatment, and mortality. Med Care. 2005;43(3 Suppl):I33–41. [PubMed] [Google Scholar]
- 14.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the american heart association. Circulation. 2011;123(11):1243–62. doi: 10.1161/CIR.0b013e31820faaf8. [Practice Guideline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. quiz 89-90. [DOI] [PubMed] [Google Scholar]
- 16.Celermajer DS. Endothelial dysfunction: Does it matter? Is it reversible? J Am Coll Cardiol. 1997;30(2):325–33. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 17.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 18.Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PLoS One. 2012;7(6):e38915. doi: 10.1371/journal.pone.0038915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edmondson D, Richardson S, Fausett JK, Falzon L, Howard VJ, Kronish IM. Prevalence of PTSD in survivors of stroke and transient ischemic attack: a meta-analytic review. PLoS One. 2013;8(6):e66435. doi: 10.1371/journal.pone.0066435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurston RC, Chang Y, Derby CA, et al. Abuse and subclinical cardiovascular disease among midlife women: findings from the Study of Women’s Health Across the Nation. Stroke. 2014;45(8):2246–51. doi: 10.1161/STROKEAHA.114.005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich-Edwards JW, Mason S, Rexrode K, et al. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. 2012;126(8):920–7. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitfield CL, Anda RF, Dube SR, Felitti VJ. Violent childhood experiences and the risk of intimate partner violence in adults - Assessment in a large health maintenance organization. J Interpers Violence. 2003;18(2):166–85. [Google Scholar]
- 23.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–68. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurston RC, Chang Y, Barinas-Mitchell E, et al. Menopausal hot flashes and carotid intima media thickness among midlife women. Stroke. 2016;47(12):2910–5. doi: 10.1161/STROKEAHA.116.014674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koenen KC, De Vivo I, Rich-Edwards J, Smoller JW, Wright RJ, Purcell SM. Protocol for investigating genetic determinants of posttraumatic stress disorder in women from the Nurses’ Health Study II. BMC Psychiatry. 2009;9:29. doi: 10.1186/1471-244X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnurr P, Spiro A, Vielhauer M, Findler M, Hamblen J. Trauma in the lives of older men: findings from the Normative Aging Study. J Clin Geropsychol. 2002;8:175–87. [Google Scholar]
- 27.Schnurr P, Lunney C, Sengupta A, Spiro A., III A longitudinal study of retirement in older male veterans. J Consult Clin Psychol. 2005;73(3):561–6. doi: 10.1037/0022-006X.73.3.561. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 29.Walker EA, Gelfand A, Katon WJ, et al. Adult health status of women with histories of childhood abuse and neglect. Am J Med. 1999;107(4):332–9. doi: 10.1016/s0002-9343(99)00235-1. [DOI] [PubMed] [Google Scholar]
- 30.Sonka M, Liang W, Lauer R. Flow-mediated dilation in brachial arteries: computer analysis of ultrasound image sequences. CVD Prevention. 1998;1:147–55. [Google Scholar]
- 31.Harris KF, Matthews KA, Sutton-Tyrrell K, Kuller LH. Associations between psychological traits and endothelial function in postmenopausal women. Psychosom Med. 2003;65(3):402–9. doi: 10.1097/01.psy.0000035720.08842.9f. [DOI] [PubMed] [Google Scholar]
- 32.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 33.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 35.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 36.Craig CL, Marshall A, Sjostrom M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 37.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 38.Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 39.Matthews D, Hosker J, Rudenski A, Naylor B, Teacher D, Turner R. Homeostasis model assessment: insulin resistance and B cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 40.Thurston RC, Chang Y, Barinas-Mitchell E, et al. Child abuse and neglect and subclinical cardiovascular disease among midlife women. Psychosom Med. 2017;79(4):441–9. doi: 10.1097/PSY.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sledjeski EM, Speisman B, Dierker LC. Does number of lifetime traumas explain the relationship between PTSD and chronic medical conditions? Answers from the National Comorbidity Survey-Replication (NCS-R) J Behav Med. 2008;31(4):341–9. doi: 10.1007/s10865-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68(5):657–61. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- 43.Bersani FS, Wolkowitz OM, Lindqvist D, et al. Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav Immun. 2016;52:153–60. doi: 10.1016/j.bbi.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Lam LL, Emberly E, Fraser HB, et al. Factors underlying variable DNA methylation in a human community cohort. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17253–60. doi: 10.1073/pnas.1121249109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houtepen LC, Vinkers CH, Carrillo-Roa T, et al. Genome-wide DNA methylation levels and altered cortisol stress reactivity following childhood trauma in humans. Nat Commun. 2016;7:10967. doi: 10.1038/ncomms10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]


