Abstract
Purpose
To characterize optical coherence tomography angiography (OCT-A) vessel density of primary open angle glaucoma (POAG) patients with unilateral visual field (VF) loss.
Design
Cross-sectional study
Participants
Thirty-three POAG patients with VF defect in one eye (mean VF MD −3.9 ± 3.1 decibels [dB]) and normal VF in the other eye (mean VF MD −0.2 ± 0.9 dB), and 33 healthy eyes.
Methods
All subjects underwent OCT-A imaging, spectral domain (SD)-OCT imaging, and VF testing. OCT-A retinal vascular measurements were summarized as whole image vessel density (wiVD), circumpapillary vessel density (cpVD), and parafoveal vessel density (pfVD). Inter-eye differences in vascular measures, as well as SD-OCT retinal nerve fiber layer (RNFL), macular ganglion cell complex (mGCC) thickness, and rim area measurements in glaucoma and healthy eyes were compared. Areas under the receiver operating characteristic curves (AUROC) were used to evaluate diagnostic accuracy for differentiating between unaffected eyes of POAG patients and healthy eyes.
Main Outcome Measures
Difference in OCT-A vessel density and SD-OCT structural parameters between unaffected eyes of POAG patients with the fellow affected eyes and healthy controls
Results
Mean wiVD in unaffected eyes of POAG patients (52.0%) was higher than in their fellow affected eyes (48.8%) but lower than in healthy eyes (55.9%; P<0.001). Mean cpRNFL thickness, mGCC thickness, and rim area measurement in unaffected eyes of POAG patients (87.5 μm, 87.7 μm and 1.0 mm2) were also higher than those measurements in their fellow eyes (76.5 μm, 79.5 μm and 0.8 mm2; P<0.001) and lower than in healthy eyes (98.0 μm, 94.5 μm, and 1.4 mm2; P<0.001). AUROCs for differentiating unaffected eyes of POAG patients from healthy eyes were highest for wiVD (0.84), followed by mGCC (0.78) cpRNFL (0.77), and pfVD (0.69).
Conclusions
OCT-A measures detect changes in retinal microvasculature before visual field damage is detectable in POAG patients and these changes may reflect damage to tissues relevant to the pathophysiology of glaucoma. Longitudinal studies are needed to determine whether OCT-A measures can improve the detection and/or prediction of the onset and progression of glaucoma.
INTRODUCTION
Despite being a bilateral disease, glaucomatous neuropathy often presents asymmetrically with asymmetric visual field (VF) loss.1, 2 Several studies have documented the presence of subclinical glaucomatous changes in various structural regions such as neuroretinal rim, retinal nerve fiber layer (RNFL), macular ganglion cell complex (mGCC), lamina cribrosa and prelaminar tissue in fellow eyes of glaucoma patients with unilateral VF loss.3–12 Structural abnormalities of the optic nerve head and RNFL are known to often precede the development of VF damage detected by standard automated perimetry (SAP).13, 14 Moreover, perimetrically unaffected eyes of glaucoma patients have been shown to be at higher risk for developing VF abnormalities.15–18 There only has been limited evidence on the retrobulbar hemodynamics,19, 20 and vascular structure of the choroid21–23 in glaucoma eyes with unilateral VF damage.
Although the pathogenesis of primary open angle glaucoma (POAG) remains unclear,24 a potential pathogenic role for ocular blood flow and the microvascular networks of the retina has long been recognized.25–27 The recent development of optical coherence tomography angiography (OCT-A) allows visualization of retinal microvasculature with a high level of precision. Further, OCT-A provides reproducible quantitative measurement of the vascular networks in various retinal regions.28 Earlier studies using OCT-A in glaucoma have demonstrated that vessel density measurements in the optic disc, peripapillary retina, macula, and choroidal structures are associated with the severity of glaucomatous visual field damage.29–34 Most recently it has been shown that OCT-A is capable of detecting microvascular attenuation of the peripapillary and macular regions even in perimetrically intact hemiretinae of eyes with single-hemifield VF defects.35
The aim of the present study was to compare the microvasculature of healthy eyes with affected and unaffected eyes of POAG patients with unilateral VF damage.
METHODS
POAG patients and healthy subjects from the Diagnostic Innovations in Glaucoma Study (DIGS) (clinicaltrials.gov identifier, NCT00221897) were included. All study methods adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act and were approved by the institutional review boards at the University of California, San Diego. Informed consent was obtained from all participants.
Study Participants
This was a cross-sectional observational study including 33 POAG patients with unilateral VF loss and 33 healthy controls enrolled from the DIGS subjects who completed optic disc and macular OCT-A imaging (Avanti AngioVue; Optovue, Inc., Fremont, CA, USA), and optic nerve head and macular imaging using spectral domain optical coherence tomography (SD-OCT; Avanti; Optovue, Inc., Fremont, CA, USA). Details of the DIGS protocol and eligibility criteria have been previously described.36
All participants underwent ophthalmological examination, including assessment of best corrected visual acuity (BCVA), slit lamp biomicroscopy, intraocular pressure (IOP) measurement with Goldmann applanation tonometry, gonioscopy, central corneal thickness (CCT) measured with ultrasound pachymetry (DGH Technology, Inc., Exton, PA), dilated fundus examination, simultaneous stereophotography of the optic disc, VF testing by SAP (Humphrey Field Analyzer; 24-2 Swedish interactive threshold algorithm; Carl-Zeiss Meditec), SD-OCT, and OCT-A imaging. Perimetry and all imaging tests were conducted within a 6-month period.
Systemic measurements included systolic and diastolic blood pressure (BP) measured at the height of the heart with an Omron Automatic blood pressure instrument (model BP791IT; Omron Healthcare, Inc., Lake Forest, IL). Mean arterial pressure was derived as: 1/3 systolic BP + 2/3 diastolic BP. Mean ocular perfusion pressure (MOPP) was calculated as the difference between 2/3 of mean arterial pressure and IOP.
Inclusion criteria common to all subjects were: (1) Age ≥18, (2) Open angle on gonioscopy, (3) BCVA of 20/40 or better. Exclusion criteria were: (1) history of intraocular surgery (except uncomplicated cataract or glaucoma surgery), coexisting retinal pathology, non-glaucomatous optic neuropathy, uveitis, or ocular trauma, (2) diagnosis of Parkinson’s disease, Alzheimer’s disease, dementia, or history of stroke, (3) diabetic or hypertensive retinopathy, (4) unreliable visual fields, (5) poor-quality OCT-A or SD-OCT scans. Participants with systemic hypertension and/or diabetes mellitus were included unless they met exclusion criterion 3.
Glaucomatous VF damage was defined as a glaucoma hemifield test (GHT) outside normal limits and a pattern standard deviation (PSD) outside 95% normal limits confirmed on at least two consecutive, reliable (fixation losses and false-negatives ≤33% and ≤15% false-positives) tests with consistent glaucomatous damage (focal or diffusely narrowed neuroretinal rim, focal or diffuse retinal nerve fiber layer loss on optic disc stereophotographs graded by masked experts). Diagnosis of POAG with unilateral VF loss was defined as having one eye diagnosed with repeatable glaucomatous VF damage, with the contralateral eye showing no VF defects. Contralateral eyes of glaucoma patients were required to have consistently normal and reliable VF results from at least > 2 SAP tests. In addition, they required having no test points with a probability level less than 2% or no clusters of ≥ 3 adjacent points with a probability of less than 5% on the pattern deviation (PD) probability plots. Appearance of the optic disc was not considered in the determination of eligibility for patients within the POAG group.
Healthy eyes were required to have IOP < 21 mmHg with no history of elevated IOPs, normal-appearing optic disc, intact neuroretinal rim and RNFL, and a minimum of two reliable normal VF tests. One eye from each healthy subject was selected randomly for inclusion in the analysis.
SD-OCT Imaging
All subjects underwent optic nerve head (ONH) and macular imaging using the Avanti SD-OCT system with a 70-kHz axial line rate, 840-nm central wavelength, 22-μm focal spot diameter, and 5-μm axial resolution in tissue. Circumpapillary RNFL (cpRNFL) thicknesses measurements were obtained using the ONH map protocol, and the macular ganglion cell complex (mGCC) thickness was obtained using the mGCC scanning protocol. The ONH map protocol calculates cpRNFL thicknesses in a 10-pixel-wide band along a 3.45-mm-diameter circle centered on the ONH. The mGCC scanning protocol is a 7×7 mm2 raster scan composed of one horizontal B scan with 933 A-scans, and 15 vertical B scans with 933 A-scans per B-scan. The mGCC thickness was measured from the internal limiting membrane (ILM) to the inner plexiform layer (IPL) boundary. Only good-quality ONH and macular scans, defined by scans with a signal strength index (SSI) of more than 37, and without segmentation failure or artifacts such as missing or blank areas were included in the analysis.
OCT-A Image Acquisition and Processing
OCT-A imaging was performed using the Angiovue on the same day as SD-OCT imaging and by the same operator. The Angiovue provides noninvasive characterization of the retinal vasculature by using the motion contrast technique, and an efficient OCT angiography algorithm, the split-spectrum amplitude-decorrelation angiography (SSADA). Details have been described elsewhere.28 Briefly, SSADA algorithm detects motion of the red blood cells by measuring variations in the reflectance amplitude between the consecutive B-scans at the same location. The software (version 2016.1.0.35) generates high-resolution three-dimensional visualization of the perfused retinal vasculature at the capillary level. Vascular information is characterized as a vessel density map (Figure 1–2), and quantitatively as vessel density (%). Vessel density is automatically calculated as the proportion of measured area occupied by flowing blood vessels, which were defined by pixels with decorrelation values above the SSADA threshold level.
Figure 1.
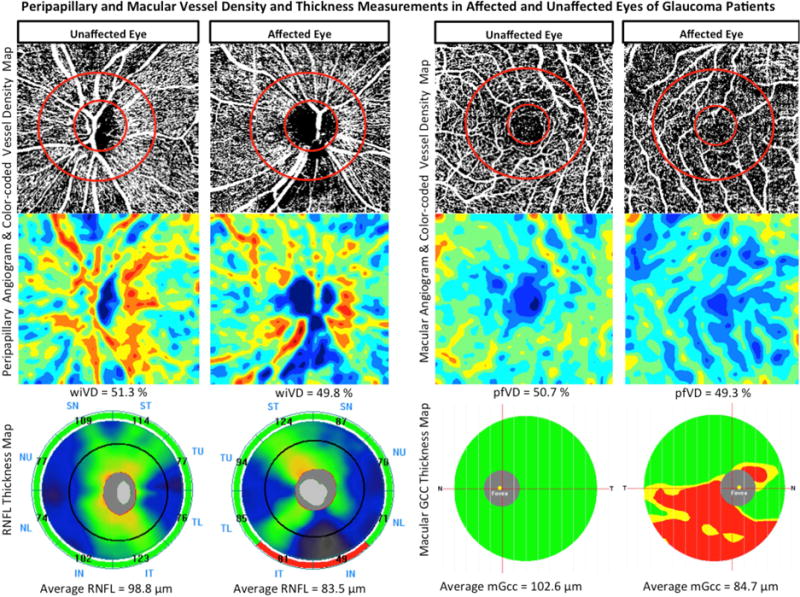
Top row: Vessel density map of the peripapillary retinal nerve fiber layer (left) and macular superficial layer (right) showing sparser microvascular networks in both regions of the affected eye compared with the unaffected eye of a glaucoma patient. Middle row: Area vessel density color-coded map of the peripapillary (left) and macular (right) regions in which the orange color indicates a vessel density of greater than 50% perfused vessels, dark blue indicates no perfused vessels detected, and intermediate vessel density values vary from yellow to light blue. Bottom row: Retinal nerve fiber layer (RNFL) thickness map (left) and Macular ganglion cell complex (mGCC) thickness map (right) demonstrating thinner RNFL and mGCC in the affected eye of glaucoma patient.
Figure 2.
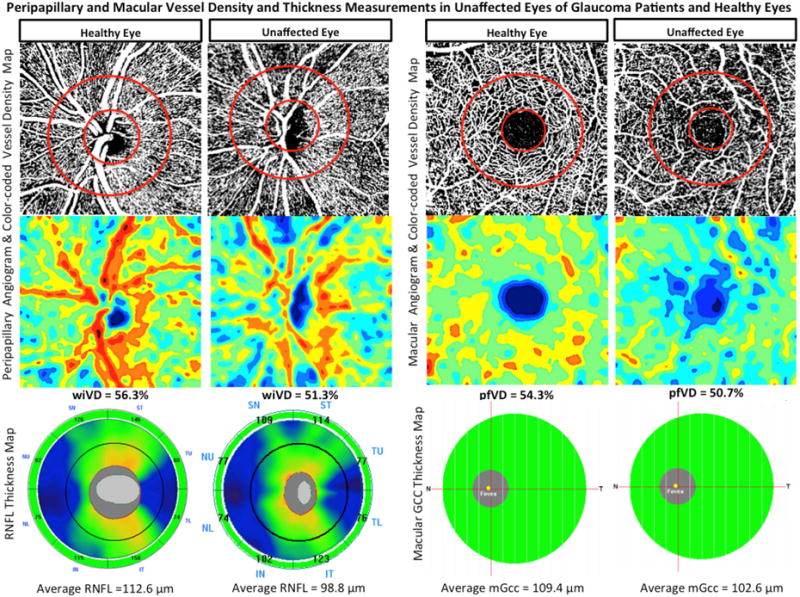
Top row: Vessel density map of the peripapillary retinal nerve fiber layer (left) and macular superficial layer (right) showing denser microvascular networks in both regions of an age-matched healthy eye compared to perimetrically-unaffected eye of a glaucoma patient. Bottom row: Area vessel density color-coded map of the peripapillary (left) and macular (right) regions in which the orange color indicates a vessel density of greater than 50% perfused vessels, dark blue indicates no perfused vessels detected, and intermediate vessel density values vary from yellow to light blue.
In this report, vessel density in the peripapillary RNFL was analyzed in 4.5 × 4.5 mm OCT-A scans centered on the ONH and parafoveal vessel density was analyzed in 3 × 3 mm OCT-A scans centered on the fovea. Vessel density within the RNFL was measured from the ILM to the RNFL posterior boundary. Whole image vessel density (wiVD) was measured over the entire scan field and circumpapillary vessel density (cpVD) was calculated within a 750-μm-wide elliptical annulus extending from the optic disc boundary. Macular vessel density was measured within the inner retinal layers extending from the ILM to IPL, which is consistent of superficial vascular plexus. Parafoveal vessel density (pfVD) was calculated in a 1.5 mm-wide parafoveal, circular annulus with an outer diameter of 3 mm and an inner diameter of 1 mm centered on the fovea.
Image quality was reviewed for all ONH and macular scans according to the Imaging Data Evaluation and Analysis (IDEA) Reading Center standard protocol. Trained graders (AY, PICM, MHS) reviewed scans and excluded poor quality images, which were defined as images with SSI of less than 48, poor clarity images, residual motion artifacts noted as irregular vessel pattern or disc boundary on the enface angiogram, local weak signal, or RNFL segmentation errors.
Statistical Analysis
Descriptive statistics were presented as the mean and standard deviation (SD). The independent two-sample student t-test was used for comparison of continuous variables between glaucoma and healthy eyes; a two-tailed paired t-test analysis was used to compare measurements between perimetrically affected and unaffected eyes of glaucoma patients. The Chi-square test was employed to compare categorical variables between groups.
Area under the receiver operating characteristic (AUROC) curves were used to determine the diagnostic accuracy of vessel density and structural measurements to differentiate between unaffected eyes of glaucoma patients and healthy eyes. A bootstrap resampling procedure (n = 1000 resamples) was used to obtain the confidence intervals for AUROCs.
Pairwise comparisons of AUROCs were performed using the method suggested by Pepe et al. to determine the statistical significance of differences between the AUROCs.37
Statistical analyses were performed using Stata software version 14 (StataCorp, College Station, TX, USA). A P value of less than 0.05 was considered to be statistically significant.
RESULTS
The study population consisted of 33 POAG patients (mean age, 70.2 ± 11.4 years) with VF defects in one eye (mean VF MD −3.9 ± 3.1 decibels [dB]), and normal VF test results in the other eye (mean VF MD, −0.2 ± 0.9 dB), and 33 healthy subjects (67.9 ± 8.6 years). The clinical and ocular characteristics of the study population are described in table 1. There were no significant differences between glaucoma patients and healthy subjects in any baseline clinical characteristics, including age, gender, race, self-reported history of diabetes mellitus and systemic hypertension, systemic antihypertensive and diabetes medications, and systemic blood pressure measurements (all P>0.05).
Table 1.
Demographics and Ocular Characteristics of Study Population.
| Healthy Subjects (n=33) |
Glaucoma Patients | P-valueA | P-valueB | P-valueC | ||
|---|---|---|---|---|---|---|
|
Perimetrically Unaffected Eyes (n=33) |
Perimetrically Affected Eyes (n=33) |
|||||
| Age (years) | 68.9 (63.9–70.0) | 70.2 (66.1–74.2) | 0.198† | |||
| Gender (Male/Female) | 9/24 | 15/18 | 0.125† | |||
| Ethnicity (AD/ED) | 12/21 | 6/27 | 0.097† | |||
| Systolic BP(mm Hg) | 129.5 (122.9–136.1) | 129.5 (122.8–136.2) | 0.990† | |||
| Diastolic BP(mm Hg) | 83.5 (79.2–87.9) | 79.2 (75.3–83.1) | 0.136† | |||
| MAP(mm Hg) | 98.9 (97.2–101.1)) | 96.0 (95.2–99.8) | 0.347† | |||
| Self-Reported Diabetes n (%) | 15.2 | 6.1 | 0.427† | |||
| Self-Reported Hypertension n (%) | 39.4 | 60.6 | 0.139† | |||
| Diabetes medication, n (%) | 12.1 | 6.1 | 0.672† | |||
| Antihypertensive medication, n (%) | 39.4 | 57.6 | 0.218† | |||
| Topical glaucoma medication, n (%) | 0.0 | 60.6 | 93.9 | <0.001† | <0.001‡ | <0.001† |
| IOP (mm Hg) | 14.5 (13.5–15.5) | 15.0 (13.5–16.5) | 14.2 (13.1–16.3) | 0.581† | 0.378‡ | 0.632† |
| MOPP (mmHg) | 51.4 (48.3–52.1) | 49.0 (47.5–51.4) | 49.7 (46.4–51.6) | 0.463† | 0.542‡ | 0.287† |
| CCT (μm) | 545.0 (530.0–560.1) | 535.8 (520.2–551.3) | 538.9 (521.3–553.5) | 0.388† | 0.116‡ | 0.346† |
| Spherical equivalent (D) | −0.67 (−0.6–0.5) | −0.84 (−1.2∓0.01) | −0.99 (−1.1∓0.02) | 0.285† | 0.138‡ | 0.462† |
| Disc Area (mm2) | 2.0 (1.9–2.1) | 2.0 (1.9–2.1) | 1.9 (1.9–2.0) | 0.961† | 0.614‡ | 0.732† |
| SAP Mean Deviation (dB) | 0.2 (−0.2–0.7) | −0.2 (−0.5–0.2) | −3.9 (−5–2.8) | 0.148† | <0.001‡ | <0.001† |
| SAP Pattern Standard Deviation (dB) | 1.6 (1.5–1.7) | 1.7 (1.6–1.9) | 5.6(4.4–6.9) | 0.113† | <0.001‡ | <0.001† |
Abbreviations: AD: African descent; ED: European descent; BP: Blood pressure; MAP: Mean arterial blood pressure; IOP: Intraocular pressure; MOPP: Mean ocular perfusion pressure; CCT: Central corneal thickness; D: Diopters; SAP: Standard automated perimetry.
Continuous variables are shown as mean (95% confidence interval).
Statistical significance tested by independent sample t-test†, and Paired t-test‡ between mean values of:
Perimetrically unaffected eyes of glaucoma patients with healthy eyes
Pairwise comparisons between both eyes of glaucoma patients
Perimetrically affected eyes of glaucoma patients with healthy eyes
As expected, affected glaucoma eyes had worse MD, and PSD, smaller rim area, and thinner RNFL and mGCC compared to healthy eyes (all P<0.001). The vessel density measurements were also significantly lower in affected glaucoma eyes compared to healthy eyes in both peripapillary and macular regions (both P<0.001) (Table 2).
Table 2.
Structural and Vascular Measurements in Healthy Perimetrically Affected and Perimetrically Unaffected Eyes.
| Healthy Eyes (n=33) |
Glaucoma Patients | Healthy vs. unaffected eyes P-valueA |
Affected vs. unaffected eyes P-valueB |
Healthy vs. affected eyes P-valueC |
||
|---|---|---|---|---|---|---|
|
Perimetrically Unaffected Eyes (n=33) |
Perimetrically Affected Eyes (n=33) |
|||||
| Whole image vessel density (%) | 55.9 (3.2) | 52.0 (3.1) | 48.8 (4.0) | <0.001* | <0.001* | <0.001* |
| Circumpapillary vessel density (%) | 62.7 (3.6) | 61.4 (3.4) | 58.0 (4.8) | 0.116 | <0.001* | <0.001* |
| Parafoveal vessel density (%) | 54.5 (2.8) | 51.6 (4.1) | 51.1 (4.3) | 0.005 | 0.537 | <0.001* |
| Average RNFL thickness (μm) | 98.0 (9.0) | 87.5 (10.2) | 76.5 (10.6) | <0.001* | <0.001* | <0.001* |
| Rim area (mm2) | 1.4 (0.3) | 1.0 (0.3) | 0.8 (0.3) | <0.001* | <0.001* | <0.001* |
| Average mGCC thickness (μm) | 94.5 (6.2) | 87.7 (6.5) | 79.5 (6.5) | 0.001* | <0.001* | <0.001* |
Values are shown in mean ± Standard deviation. RNFL: Retinal nerve fiber layer; mGCC: macular ganglion cell complex.
The comparison was performed by using independent samples t-test.
The comparison was performed by using paired t-test.
The comparison was performed by using independent samples t-test.
Uncorrected P values are presented. P values less than 0.003 (.05/18) after adjustment for multiple comparisons based on Bonferroni correction are considered statistically significant and noted with an asterisk (*).
The intra-individual analyses showed that there were no differences in the mean CCT, spherical equivalent (SE) refractive error, IOP, and MOPP between both eyes of glaucoma patients (paired t-test, all P>0.05). However, affected eyes of glaucoma patients had worse mean VF MD (−3.9 ± 3.1 dB), and PSD (5.6 ± 3.6 dB) compared to unaffected eyes (−0.2 ± 0.9 dB, and 1.7 ± 0.4 dB; P<0.001). Affected eyes also had thinner rim area (0.8 ± 0.3 mm2), cpRNFL (76.5 ± 10.6 m), and mGCC (79.5 ± 6.5 μm) compared to their fellow unaffected eyes (1.0 ± 0.3 mm2, 87.5 ± 10.2 μm, 87.7 ± 6.5 μm; P<0.001). Moreover, wiVD (48.8 ± 4.0 %) and cpVD (58.0 ± 4.8 %) measurements in the affected eyes were significantly lower than those in unaffected eyes (52.0 ± 3.1 %, 61.4 ± 3.4 %; P<0.001), whereas the differences in pfVD measurements in affected (51.1 ± 4.3 %) and unaffected eyes (51.6 ± 4.1 %) did not reach statistical significance (P=0.537, Figure 3).
Figure 3.
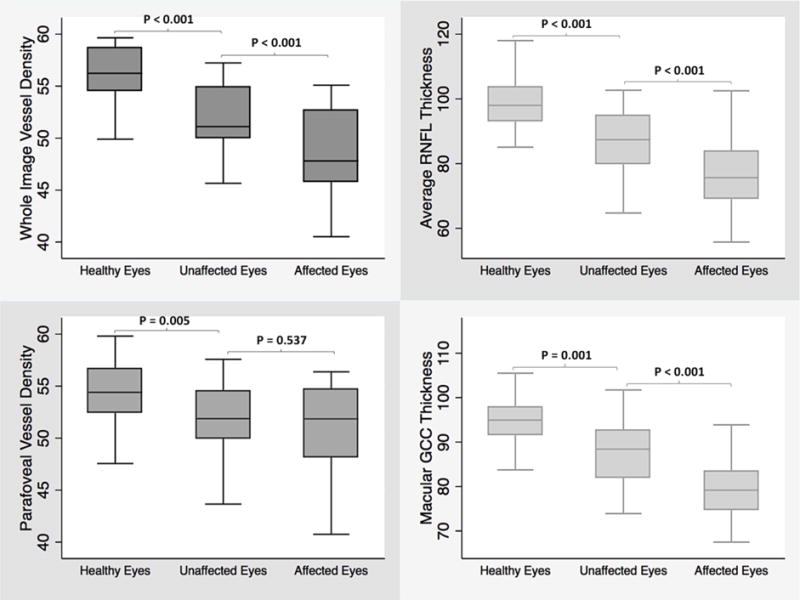
Boxplots illustrating the distribution of whole image vessel density (top left), average retinal nerve fiber layer thickness (top right), parafoveal vessel density (bottom left), and macular ganglion cell complex thickness (bottom right) measurements in healthy, unaffected and affected eyes of glaucoma patients with unilateral visual field loss. The medians are represented by horizontal line in the gray box. Error bars denote interquartile range
Mean CCT, SE, IOP, and MOPP in unaffected eyes were not statistically different from those in healthy eyes (P>0.05). The mean VF MD and PSD of the unaffected and healthy eyes were also statistically similar (independent samples t-test, P = 0.15 and P= 0.11, respectively). However, unaffected eyes of glaucoma patients had on average lower rim area (1.0 ± 0.3 mm2), cpRNFL (87.5 ± 10.2 μm), and mGCC (87.7 ± 6.5 μm) thickness measurements compared to healthy eyes (1.4 ± 0.3 mm2, 98.0 ± 9.0 μm, 94.5 ± 6.2 μm; all P<0.001). Unaffected eyes of POAG patients also showed sparser vascular networks in both peripapillary and macular regions compared to healthy eyes (Figure 2). Specifically, in the macular region, pfVD (51.6 ± 4.1 %) was significantly lower in unaffected eyes of glaucomatous patients than in healthy eyes (54.5 ± 2.8 %; P=0.005). In the peripapillary region however, although wiVD measurement in unaffected eyes (52.0 ± 3.1 %) was significantly lower than healthy eyes (55.9 ± 3.2 %; P<0.001), the difference in cpVD measurement did not reach statistical significance (61.4 ± 3.4 % vs. 62.7 ± 3.6%; P=0.116).
The AUROC for differentiating between unaffected eyes of POAG patients and healthy eyes was highest for wiVD (0.84 ± 0.05), followed by mGCC thickness (0.78 ± 0.06), average RNFL thickness (0.77 ± 0.06), and pfVD (0.69 ± 0.07) (Figure 4). However, in the pairwise comparison of AUROCs, the differences did not reach statistical significance (P>0.05).
Figure 4.
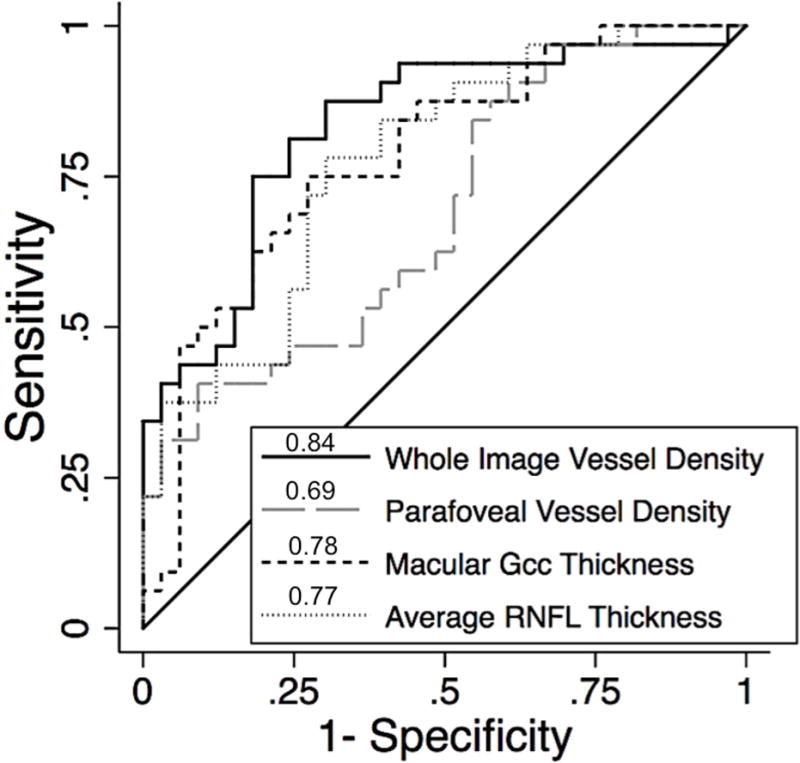
Area under the receiver operator characteristic curves (AUROCs) for differentiating between unaffected eyes of glaucoma patients with unilateral visual field loss and healthy eyes; Whole image vessel density (0.84), macular ganglion cell complex (0.78), average RNFL thickness (0.77), and parafoveal vessel density (0.69).
As structural measurements, such as RNFL, optic nerve head rim and cup area have been shown to be associated with disc size, we also evaluated the association of optic disc area on vessel density measurements. We found no statistically significant correlations between disc area measured by SD-OCT with both wiVD and cpVD measurements in healthy eyes (R2=0.008, P=0.521, and R2=0.005, P=0.723, respectively).
DISCUSSION
In the current study, we showed that vessel densities in both peripapillary and macular regions were significantly lower in both eyes of POAG patients with unilateral VF loss than healthy eyes of similar age (P<0.05). In addition, peripapillary vessel density in the affected eyes was lower than in their fellow unaffected eyes, whereas the difference in macular vessel density was similar in both eyes of the same patient (P>0.05).
In glaucoma, the sensitivity of OCT-A peripapillary vascular measurements for diagnosing glaucoma was shown to be high.30, 31, 38 Results of those studies serve as initial validation of OCT-A vessel density measurements for detection of changes relevant to glaucomatous damage. Findings of the present study provide further insight into the diagnostic performance of OCT-A vascular measurements in a clinically relevant scenario.
Structural changes in eyes of glaucoma patients with unilateral VF damage have been studied extensively.7–16 Our results of thinner rim, cpRNFL, and macular structure are consistent with studies showing detectable structural damage in perimetrically unaffected eyes of unilateral glaucoma patients. However, results of studies evaluating ocular vasculature in asymmetric or unilateral glaucoma are inconsistent, perhaps due to technical differences in imaging modalities and various aspects of ocular blood flow being studied. Drance et al. in 1968 performed ophthalmodynamography in unilateral POAG eyes and found lower diastolic perfusion pressure in eyes with VF loss compared to unaffected eyes and healthy controls.39 O’Brien and colleagues employed Doppler imaging in carotid arteries of glaucoma patients with asymmetric VF damage, and found higher arterial resistance in the internal carotid arteries on the side of greater VF loss.40 Fontana et al. showed that pulsatile orbital blood flow (POFB) was significantly lower in eyes of normal tension glaucoma (NTG) patients with and without VF loss compared to healthy eyes.41 Costa and associates used color Doppler imaging (CDI) in glaucoma patients with asymmetric glaucomatous damage, and found no difference between retrobulbar blood flow velocities of more damaged and less damaged eyes. However, they demonstrated that retrobulbar flow velocity in the less damaged eyes was lower than that of healthy controls.42 Consistent with the results of the present study, Nicolela et al.19 and Plange et al.20 showed that both eyes of glaucoma patients with asymmetric VF damage had significantly lower blood velocities and higher arterial resistive indices in retrobulbar vessels compared to healthy eyes. They also reported that decreased blood velocities were more pronounced in more affected eyes compared to the less affected eyes.
More recently, studies investigating choroidal structure using SD-OCT failed to find a difference in peripapillary and macular choroidal thickness between eyes of glaucoma patients with unilateral or asymmetric VF loss.21–23 Li et al. showed that peripapillary choroidal thickness measurements were similar between both eyes of POAG patients with unilateral VF loss and healthy eyes.21 Suh et al. also found no significant inter-eye difference in peripapillary choroidal thickness in patients with unilateral NTG.22 Mwanza et al. compared the macular choroidal thickness measurements between asymmetric POAG eyes with advanced glaucoma in one eye and no or mild glaucoma in the fellow eye. They reported no significant change in macular choroidal thickness of eyes with advanced POAG compared to that of fellow eyes with no glaucoma or with mild glaucoma.23 Each of these three studies suggested a lack of association between glaucoma and structural features of the choroid. Although SD-OCT provides better visualization of the posterior retinal structures with more reproducible measurement results, it only can reflect the static structure, not the hemodynamics of choroidal circulation. Unlike choroidal thickness measurements by SD-OCT, vascular measurements obtained by OCT-A reflect dynamic changes in the ocular vasculature.
With the advent of OCT-A, there has been a renewed interest in investigating the role of the microvasculature of the optic nerve head, peripapillary region and macula and, particularly, the associations of their vessel densities with functional measurements in glaucoma.29–31, 33, 38, 43 Findings of the present study support these previous ones. However, each of these earlier studies have compared glaucoma eyes with healthy eyes from different individuals. In the current study, this association was evaluated with both an intra-individual as well as inter-individual comparison approach. The intra-individual approach is advantageous in that it avoids the effect of potential confounding factors on vascular measurements such as age, gender, ethnicity, systemic blood pressure, systemic conditions and medications.44 Moreover, using the patient’s own eye as an internal control minimizes inaccuracies derived from inter-individual anatomic variabilities.45 Therefore, results of a lower peripapillary vessel density in the affected eyes provide additional evidence to confirm the association between vascular loss in the peripapillary area and functional damage relevant to the pathophysiology of glaucoma.29–31, 33, 38, 43
Moreover, in the present study we observed that lower vessel density measurements could be detected even in eyes without detectable VF damage, and wiVD was the parameter with the highest diagnostic accuracy (AUROC=0.84) in differentiating between perimetrically unaffected eyes of POAG patients from healthy eyes. These results are consistent with reports of lower retrobulbar hemodynamic parameters in more affected eyes of POAG patients with unilateral VF loss.19, 20 They suggest that hemodynamic insufficiencies along with structural damage can precede detectable functional loss in unaffected eyes of POAG patients with unilateral VF loss. It still is unclear how these insufficiencies can affect the optic nerve, but the radial peripapillary capillaries (RPCs) are likely to be involved.
Retina ganglion cells (RGCs) and their axons are among the most highly metabolically active structures in humans, and are strongly dependent on their regional vascular supply. RPCs form a distinct network of capillary beds within the RNFL and are intimately tied to the RNFL axons, perhaps to meet the large energy requirements of the RGCs.46 Previous studies reported strong correlations between RPC morphology and RNFL thickness, and suggested that neurovascular co-patterning and functional crosstalk mechanisms could be linked to retinal homeostasis. In addition, due to their distinct parallel formation and paucity of anastomoses, RPCs are thought to be vulnerable to various pathological challenges such as pressure and ischemia.46–48 With the cross-sectional design of the current study, the temporal relationship between vascular attenuation and structural damage in both eyes of glaucoma patients compared to each other and to healthy eyes cannot be addressed.
An interesting finding of the present study was that although wiVD and pfVD in the unaffected eyes were significantly lower than healthy eyes, the differences in cpVD measurements among these groups were not significant (P > 0.05). In a previous study31, we also found that the differences in cpVD measurement of glaucoma suspects and healthy eyes did not reach statistical significance. In the same study, however, cpVD was significantly lower in POAG eyes compared with healthy eyes. In another study,35 it was shown that cpVD measurement in the perimetrically intact hemiretinae of glaucoma patients with single-hemifield VF defect was lower than age-matched healthy hemiretinae. These results suggest that the vascular attenuation in the RPC layer is widespread in perimetric glaucoma, and vascular dropout could be detected in both the peripheral and more central regions around the optic disc. In contrast, in glaucoma suspects and perimetrically unaffected eyes, vascular attenuation could only be found in the more peripheral regions of the peripapillary area.
An unexpected finding of the current study was that the macular vascular measurement was similar in both eyes of POAG patients despite their differences in the VF test results, thinner structural measurements and decreased peripapillary vessel density in the affected eyes. This could be explained in part by the smaller magnitude of difference in the mean vessel density measurements across groups in the macular region compared with the peripapillary region, which was also observed in previous studies.38, 43
One may argue that adjustment for multiple testing should be applied for comparing vessel density and thickness measurements between both eyes of glaucoma patient with unilateral VF loss and healthy eyes. Therefore, P values were adjusted for multiple comparisons based on Bonferroni correction. In this study, unaffected eyes of glaucoma patients had significantly lower wiVD, RNFL, rim, mGCC than healthy eyes (Table 2), even after applying Bonferroni correction with a cutoff P value of 0.003, which is known to be a conservative method of controlling multiple comparisons. Comparison between both eyes of glaucoma patients also showed that affected eyes had significantly lower wiVD, cpVD, RNFL, rim, mGCC than their unaffected fellow eyes even after Bonferroni correction.
The finding that pfVD has lower diagnostic accuracy in differentiating between unaffected eyes of glaucoma patients from healthy eyes is in accordance with previous reports. Rao et al.38 showed that even in differentiating glaucoma eyes from healthy eyes, macular vessel density has moderate diagnostic performance that is poorer than measurements in the peripapillary region. This poorer diagnostic accuracy may be attributed to the fact that the RGCs in the macular region are concentrated in the 4.5 mm foveal center that may remain intact in the early stages of the disease.49 Therefore, there is an overlap in the vascular function and vessel density distribution between healthy and glaucoma eyes. The glaucoma group in Rao et al’s report38 also predominantly consisted of individuals with pre-perimetric and early glaucoma, which may account for the relatively low diagnostic accuracy of macular vessel density in distinguishing between healthy and glaucoma eyes in their study.
This study has several limitations. First, defining glaucomatous VF damage by using the PD plot, which compares measurements to a normative database, does not preclude possible diffuse functional damage, which may occur in early glaucoma.50 As a result, it should be noted that perimetrically unaffected eyes reported in this study, and also some previous reports,5, 7, 8, 21 do not necessarily imply an absence of VF damage. Second, because of the cross-sectional design of the present study, we could only identify detectable microvascular attenuation, and were not able to establish any cause-and-effect relationships between peripapillary and macular vessel density with glaucomatous RGC and axonal damage.
Evidence of vascular changes in the fellow eyes of glaucoma patients with unilateral VF damage could provide support for a means of detecting and tracking glaucomatous progression, as well as detecting a site of early damage. Previous prospective studies in glaucoma suspects have suggested that baseline structural measurements are strong predictors of conversion into glaucoma,51–53 and the rate of RNFL loss was shown to be more than twice as fast in eyes developing glaucomatous VF damage compared with eyes that did not develop VF damage.54 However, longitudinal data regarding the prognostic value of attenuated baseline vasculature is limited. Schumann et al. demonstrated that inter-eye difference in progression of glaucomatous VF damage correlates with the difference in retrobulbar hemodynamic parameters being independent of the extent of the differences in glaucomatous optic disc damage and IOP.55 To the best of our knowledge, there are no prospective reports on the microvascular network of unilateral or asymmetric glaucoma. Therefore, longitudinal follow-up of these eyes would address the importance of lower vessel density values found in this study and another report of glaucoma suspects.31
Finally, due to the cross-sectional, observational design of the study we were unable to determine the potential confounding effect of ocular and systemic antihypertensive medications and systemic conditions on vessel density measurements across groups. Therefore, despite using an intra-individual approach, which avoids the effect of potential confounding factors on vessel density measurements, and despite finding no differences in BP, IOP or MOPP measurements, systemic conditions and the use of systemic medications across groups we cannot dismiss the possible confounding effect of these factors. Hence, exploring the potential impact of systemic and ocular conditions and the use of medications on vessel density measurements require longitudinal studies.
In conclusion, OCT-A detects vessel density dropout in fellow eyes of POAG patients with unilateral VF loss. The lower vessel density found in OAG eyes implies that quantitative OCT-A measurements suggests damage to tissues relevant to the pathophysiology of glaucoma. The finding of lower vessel density in perimetrically unaffected fellow eyes suggests that OCT-A can detect microvascular changes in eyes at high risk of developing glaucoma before detectable VF damage. Longitudinal studies are needed to determine whether OCT-A measures can improve the detection and/or prediction of the onset and progression of glaucomatous damage.
Precise.
Vascular dropout in regions without detectable visual field damage in patients with POAG can be detected using OCT angiography.
Acknowledgments
Financial Disclosure(s): Adeleh Yarmohammadi: none; Linda M. Zangwill: Research support – Carl Zeiss Meditec, Heidelberg Engineering, National Eye Institute, Topcon, and Nidek; Patricia Isabel Manalastas: none; Nathan J. Fuller: none; Alberto Diniz-Filho: none; Luke Saunders: none; Min Hee Suh: none; Kyle Hasenstab: none, Robert N. Weinreb: Research support –Carl Zeiss Meditec, Genentech, Heidelberg Engineering, Konan, National Eye Institute, Optovue, Tomey and Topcon; Consultant – Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Eyenovia, Novartis, Sensimed and Topcon.
Supported in part by National Institutes of Health/National Eye Institute grants EY011008 (L.M.Z.), EY14267 (L.M.Z.), EY019869 (L.M.Z.), core grant P30EY022589; an unrestricted grant from Research to Prevent Blindness (New York, NY); grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck, and Santen.
Abbreviations and Acronyms
- -A
angiography
- CCT
central corneal thickness
- CI
confidence interval
- dB
decibels
- DIGS
Diagnostic Innovations in Glaucoma Study
- IOP
intraocular pressure
- MD
mean deviation
- μm
micrometers
- OCT
optical coherence tomography
- POAG
primary open-angle glaucoma
- PSD
pattern standard deviation
- RGC
retinal ganglion cell
- RNFL
retinal nerve fiber layer
- RPC
radial peripapillary capillaries
- SAP
standard automated perimetry
- SD
spectral domain
- SSADA
split-spectrum amplitude-decorrelation angiography.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poinoosawmy D, Fontana L, Wu JX, et al. Frequency of asymmetric visual field defects in normal-tension and high-tension glaucoma. Ophthalmology. 1998;105(6):988–91. doi: 10.1016/S0161-6420(98)96049-3. [DOI] [PubMed] [Google Scholar]
- 2.Lee AJ, Wang JJ, Rochtchina E, et al. Patterns of glaucomatous visual field defects in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol. 2003;31(4):331–5. doi: 10.1046/j.1442-9071.2003.00660.x. [DOI] [PubMed] [Google Scholar]
- 3.Caprioli J, Miller JM, Sears M. Quantitative evaluation of the optic nerve head in patients with unilateral visual field loss from primary open-angle glaucoma. Ophthalmology. 1987;94(11):1484–7. doi: 10.1016/s0161-6420(87)33264-6. [DOI] [PubMed] [Google Scholar]
- 4.Zeyen TG, Raymond M, Caprioli J. Disc and field damage in patients with unilateral visual field loss from primary open-angle glaucoma. Doc Ophthalmol. 1992;82(4):279–86. doi: 10.1007/BF00161015. [DOI] [PubMed] [Google Scholar]
- 5.Reus NJ, Lemij HG. Scanning laser polarimetry of the retinal nerve fiber layer in perimetrically unaffected eyes of glaucoma patients. Ophthalmology. 2004;111(12):2199–203. doi: 10.1016/j.ophtha.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Wollstein G, Garway-Heath DF, Poinoosawmy D, Hitchings RA. Glaucomatous optic disc changes in the contralateral eye of unilateral normal pressure glaucoma patients. Ophthalmology. 2000;107(12):2267–71. doi: 10.1016/s0161-6420(00)00352-3. [DOI] [PubMed] [Google Scholar]
- 7.Caprioli J, Nouri-Mahdavi K, Law SK, Badala F. Optic disc imaging in perimetrically normal eyes of glaucoma patients with unilateral field loss. Trans Am Ophthalmol Soc. 2006;104:202–11. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DM, Hwang US, Park KH, Kim SH. Retinal nerve fiber layer thickness in the fellow eyes of normal-tension glaucoma patients with unilateral visual field defect. Am J Ophthalmol. 2005;140(1):165–6. doi: 10.1016/j.ajo.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Da Pozzo S, Fanni D, Paoloni M, et al. Retinal nerve fibre layer of perimetrically unaffected eyes of glaucoma patients: an optical coherence tomography study. Clin Exp Ophthalmol. 2009;37(2):217–22. doi: 10.1111/j.1442-9071.2009.02004.x. [DOI] [PubMed] [Google Scholar]
- 10.Zangalli CS, Ahmed OM, Waisbourd M, et al. Segmental Analysis of Macular Layers in Patients With Unilateral Primary Open-Angle Glaucoma. J Glaucoma. 2016;25(4):e401–7. doi: 10.1097/IJG.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 11.Kwun Y, Han JC, Kee C. Comparison of lamina cribrosa thickness in normal tension glaucoma patients with unilateral visual field defect. Am J Ophthalmol. 2015;159(3):512–8 e1. doi: 10.1016/j.ajo.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Kim DW, Jeoung JW, Kim YW, et al. Prelamina and Lamina Cribrosa in Glaucoma Patients With Unilateral Visual Field Loss. Invest Ophthalmol Vis Sci. 2016;57(4):1662–70. doi: 10.1167/iovs.15-18453. [DOI] [PubMed] [Google Scholar]
- 13.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109(1):77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 14.Quigley HA, Katz J, Derick RJ, et al. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99(1):19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 15.Susanna R, Drance SM, Douglas GR. The visual prognosis of the fellow eye in uniocular chronic open-angle glaucoma. Br J Ophthalmol. 1978;62(5):327–9. doi: 10.1136/bjo.62.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana L, Armas R, Garway-Heath DF, et al. Clinical factors influencing the visual prognosis of the fellow eyes of normal tension glaucoma patients with unilateral field loss. Br J Ophthalmol. 1999;83(9):1002–5. doi: 10.1136/bjo.83.9.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen PP, Park RJ. Visual field progression in patients with initially unilateral visual field loss from chronic open-angle glaucoma. Ophthalmology. 2000;107(9):1688–92. doi: 10.1016/s0161-6420(00)00229-3. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros FA, Sample PA, Weinreb RN. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss. Am J Ophthalmol. 2004;137(5):863–71. doi: 10.1016/j.ajo.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Nicolela MT, Drance SM, Rankin SJ, et al. Color Doppler imaging in patients with asymmetric glaucoma and unilateral visual field loss. Am J Ophthalmol. 1996;121(5):502–10. doi: 10.1016/s0002-9394(14)75424-8. [DOI] [PubMed] [Google Scholar]
- 20.Plange N, Kaup M, Arend O, Remky A. Asymmetric visual field loss and retrobulbar haemodynamics in primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2006;244(8):978–83. doi: 10.1007/s00417-005-0227-9. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Bian A, Zhou Q, Mao J. Peripapillary choroidal thickness in both eyes of glaucoma patients with unilateral visual field loss. Am J Ophthalmol. 2013;156(6):1277–84 e1. doi: 10.1016/j.ajo.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Suh W, Cho HK, Kee C. Evaluation of peripapillary choroidal thickness in unilateral normal-tension glaucoma. Jpn J Ophthalmol. 2014;58(1):62–7. doi: 10.1007/s10384-013-0290-4. [DOI] [PubMed] [Google Scholar]
- 23.Mwanza JC, Sayyad FE, Budenz DL. Choroidal thickness in unilateral advanced glaucoma. Invest Ophthalmol Vis Sci. 2012;53(10):6695–701. doi: 10.1167/iovs.12-10388. [DOI] [PubMed] [Google Scholar]
- 24.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 25.Kornzweig AL, Eliasoph I, Feldstein M. Selective atrophy of the radial peripapillary capillaries in chronic glaucoma. Arch Ophthalmol. 1968;80(6):696–702. doi: 10.1001/archopht.1968.00980050698002. [DOI] [PubMed] [Google Scholar]
- 26.Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38(Suppl):S3–6. doi: 10.1016/0039-6257(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 27.Weinreb RN, Harris A, editors. Ocular Blood Flow in Glaucoma. Amsterdam, The Netherlands: Kugler Publications; 2009. [Google Scholar]
- 28.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–25. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121(7):1322–32. doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Jia Y, Takusagawa HL, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol. 2015;133(9):1045–52. doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Invest Ophthalmol Vis Sci. 2016;57(9):OCT451–9. doi: 10.1167/iovs.15-18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suh MH, Zangwill LM, Manalastas PI, et al. Optical Coherence Tomography Angiography Vessel Density in Glaucomatous Eyes with Focal Lamina Cribrosa Defects. Ophthalmology. 2016;123(11):2309–17. doi: 10.1016/j.ophtha.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between Optical Coherence Tomography Angiography Vessel Density and Severity of Visual Field Loss in Glaucoma. Ophthalmology. 2016;123(12):2498–508. doi: 10.1016/j.ophtha.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh MH, Zangwill LM, Manalastas PI, et al. Deep Retinal Layer Microvasculature Dropout Detected by the Optical Coherence Tomography Angiography in Glaucoma. Ophthalmology. 2016;123(12):2509–18. doi: 10.1016/j.ophtha.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and Macular Vessel Density in Patients with Glaucoma and Single-Hemifield Visual Field Defect. Ophthalmology. 2017 doi: 10.1016/j.ophtha.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127(9):1136–45. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pepe M, Longton G, Janes H. Estimation and Comparison of Receiver Operating Characteristic Curves. Stata J. 2009;9(1):1. [PMC free article] [PubMed] [Google Scholar]
- 38.Rao HL, Pradhan ZS, Weinreb RN, et al. Regional Comparisons of Optical Coherence Tomography Angiography Vessel Density in Primary Open-Angle Glaucoma. Am J Ophthalmol. 2016;171:75–83. doi: 10.1016/j.ajo.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Drance SM, Wheeler C, Pattullo M. Uniocular open-angle glaucoma. Am J Ophthalmol. 1968;65(6):891–902. doi: 10.1016/0002-9394(68)92218-6. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien C, Saxton V, Crick RP, Meire H. Doppler carotid artery studies in asymmetric glaucoma. Eye (Lond) 1992;6(Pt 3):273–6. doi: 10.1038/eye.1992.51. [DOI] [PubMed] [Google Scholar]
- 41.Fontana L, Poinoosawmy D, Bunce CV, et al. Pulsatile ocular blood flow investigation in asymmetric normal tension glaucoma and normal subjects. Br J Ophthalmol. 1998;82(7):731–6. doi: 10.1136/bjo.82.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa VP, Sergott RC, Smith M, et al. Color Doppler imaging in glaucoma patients with asymmetric optic cups. J Glaucoma. 1994;3(Suppl 1):S91. doi: 10.1097/00061198-199400321-00012. [DOI] [PubMed] [Google Scholar]
- 43.Rao HL, Pradhan ZS, Weinreb RN, et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS One. 2017;12(3):e0173930. doi: 10.1371/journal.pone.0173930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao HL, Pradhan ZS, Weinreb RN, et al. Determinants of Peripapillary and Macular Vessel Densities Measured by Optical Coherence Tomography Angiography in Normal Eyes. J Glaucoma. 2017 doi: 10.1097/IJG.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 45.Asrani S, Rosdahl JA, Allingham RR. Novel software strategy for glaucoma diagnosis: asymmetry analysis of retinal thickness. Arch Ophthalmol. 2011;129(9):1205–11. doi: 10.1001/archophthalmol.2011.242. [DOI] [PubMed] [Google Scholar]
- 46.Yu PK, Balaratnasingam C, Xu J, et al. Label-Free Density Measurements of Radial Peripapillary Capillaries in the Human Retina. PLoS One. 2015;10(8):e0135151. doi: 10.1371/journal.pone.0135151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu PK, Cringle SJ, Yu DY. Correlation between the radial peripapillary capillaries and the retinal nerve fibre layer in the normal human retina. Exp Eye Res. 2014;129:83–92. doi: 10.1016/j.exer.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Henkind P. Radial peripapillary capillaries of the retina. I. Anatomy: human and comparative. Br J Ophthalmol. 1967;51(2):115–23. doi: 10.1136/bjo.51.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000;41(7):1774–82. [PubMed] [Google Scholar]
- 50.Henson DB, Artes PH, Chauhan BC. Diffuse loss of sensitivity in early glaucoma. Invest Ophthalmol Vis Sci. 1999;40(13):3147–51. [PubMed] [Google Scholar]
- 51.Zangwill LM, Weinreb RN, Beiser JA, et al. Baseline topographic optic disc measurements are associated with the development of primary open-angle glaucoma: the Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2005;123(9):1188–97. doi: 10.1001/archopht.123.9.1188. [DOI] [PubMed] [Google Scholar]
- 52.Lalezary M, Medeiros FA, Weinreb RN, et al. Baseline optical coherence tomography predicts the development of glaucomatous change in glaucoma suspects. Am J Ophthalmol. 2006;142(4):576–82. doi: 10.1016/j.ajo.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Mohammadi K, Bowd C, Weinreb RN, et al. Retinal nerve fiber layer thickness measurements with scanning laser polarimetry predict glaucomatous visual field loss. Am J Ophthalmol. 2004;138(4):592–601. doi: 10.1016/j.ajo.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 54.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121(7):1350–8. doi: 10.1016/j.ophtha.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schumann J, Orgul S, Gugleta K, et al. Interocular difference in progression of glaucoma correlates with interocular differences in retrobulbar circulation. Am J Ophthalmol. 2000;129(6):728–33. doi: 10.1016/s0002-9394(99)00481-x. [DOI] [PubMed] [Google Scholar]


