Abstract
Objective
Lactate is an intermediate of glucose metabolism that has been implicated in the pathogenesis of insulin resistance. This study evaluated the relationship between glucose kinetics and plasma lactate concentration ([LAC]) before and after manipulating insulin sensitivity by progressive weight loss.
Methods
Forty people with obesity (BMI=37.9±4.3 kg/m2) were randomized to weight maintenance (n=14) or weight loss (n=19). Subjects were studied before and after 6 months of weight maintenance and before and after 5%, 11% and 16% weight loss. A hyperinsulinemic-euglycemic clamp procedure in conjunction with [6,6-2H2]glucose tracer infusion was used to assess glucose kinetics.
Results
At baseline, fasting [LAC] correlated positively with endogenous glucose production rate (r=0.532, p=0.001) and negatively with insulin sensitivity, assessed as the insulin-stimulated glucose disposal (r=−0.361, p=0.04). Progressive (5% through 16%) weight loss caused a progressive decrease in fasting [LAC], and the decrease in fasting [LAC] after 5% weight loss was correlated with the decrease in endogenous glucose production (r=0.654, p=0.002) and the increase in insulin sensitivity (r=−0.595, p=0.007).
Conclusion
This study demonstrates the inter-relationships among weight loss, hepatic and muscle glucose kinetics, insulin sensitivity, and [LAC], and suggests that [LAC] can serve as an additional biomarker of glucose-related insulin resistance.
Keywords: insulin sensitivity, glucose kinetics, obesity
INTRODUCTION
The use of plasma glucose as fuel involves its transport into cells where it is metabolized to pyruvate in the cytosol before entry into the mitochondria for complete oxidation in the tricarboxylic acid cycle. Pyruvate that does not enter the mitochondria is converted to lactic acid, which rapidly dissociates to lactate and hydrogen. Therefore, lactate is a product of incomplete glucose metabolism. During resting conditions, plasma lactate concentration ([LAC]) increases when flux through glycolysis exceeds the rate of mitochondrial oxidation. Accordingly, an increase in [LAC] could be an indicator of impaired glucose metabolism. In fact, fasting [LAC] is higher in people with obesity (1, 2) and type 2 diabetes (3, 4, 5) than in healthy lean people. An increase in [LAC] can also influence glucose metabolism by providing a gluconeogenic precursor to the liver and by disrupting muscle insulin signaling and insulin-mediated muscle glucose uptake (6, 7, 8, 9). Therefore, an increase in [LAC] can be both a biomarker and a cause of impaired glucose metabolism.
Weight loss improves multi-organ insulin sensitivity and insulin-mediated glucose metabolism in people with obesity (10). The therapeutic effect of weight loss on glucose metabolism suggests weight loss should decrease fasting [LAC], which could contribute to the beneficial effect of weight loss on metabolic function. However, the effect of weight loss on lactate metabolism is not clear because of conflicting results among studies. Weight loss has been shown to decrease fasting [LAC] in people with obesity and metabolic syndrome (2), but not in metabolically healthy people with obesity or people with obesity and type 2 diabetes (11, 12). The reason(s) for the inconsistency among studies is not clear, but could be related to differences in sample size and health status of the participants.
The purpose of this study was to evaluate the relationship between glucose kinetics and [LAC], and determine whether weight loss-induced changes in insulin sensitivity and endogenous glucose production are associated with changes in lactate metabolism. To this end, we evaluated [LAC] and glucose kinetics during basal conditions and during glucose and insulin infusion before and after progressive (5%, 11% and 16%) weight loss in people with obesity.
METHODS
Subjects
Forty men and women (BMI=37.9 ± 4.3 kg/m2, 44 ± 12 years old) were enrolled in this study. The assessment of lactate metabolism was made while subjects participated in a study that involved evaluating insulin sensitivity during progressive weight loss, conducted from January 2011 until October 2015 (10). No subject had diabetes or other serious illnesses, were taking medications known to interfere with insulin action or lactate metabolism, or consumed excessive alcohol (>21 drinks/week for men and >14 drinks/week for women). Written informed consent was obtained from all subjects before their participation in this study, which was approved by the Institutional Review Board of Washington University School of Medicine in St. Louis, MO.
Study protocol
Subjects were randomly assigned to weight maintenance (n=14 [5 withdrew after being informed of their randomization and 1 dropped out], 11 women and 3 men) or diet-induced weight loss (n=19 [1 dropped out], 16 women and 3 men) using a computerized randomization list provided by the statistician of the study (Figure 1). The characteristics of each group have been previously reported (10). Subjects in the weight loss group attended weekly individual behavior education sessions and dietary counseling sessions, and were prescribed a low-calorie diet (50-55% of energy as carbohydrate, 30% of energy as fat, and 15-20% of energy as protein) of self-prepared foods to achieve 5% weight loss. After 5% weight loss was achieved, solid and liquid meal replacements were provided as needed to achieve the 10% and 15% weight loss targets. All subjects in the weight loss group were studied before and after 5% loss; 9 subjects continued to lose weight and were studied again after ~11% and ~16% weight loss. After subjects achieved each weight loss target, a weight maintenance diet was prescribed to maintain a stable body weight (<2% change) for at least 3 weeks before repeat testing was performed to avoid the potential effect of energy imbalance on our outcome measures. Subjects in the weight loss group were studied before and after a median (quartiles) of 3.5 (2.9, 4.6), 6.8 (6.0, 8.6) and 10.4 (9.6, 10.4) months for 5%, 11% and 16% weight loss, respectively. Subjects randomized to weight maintenance were studied at baseline and after 6 months.
Figure 1. CONSORT diagram of the study.
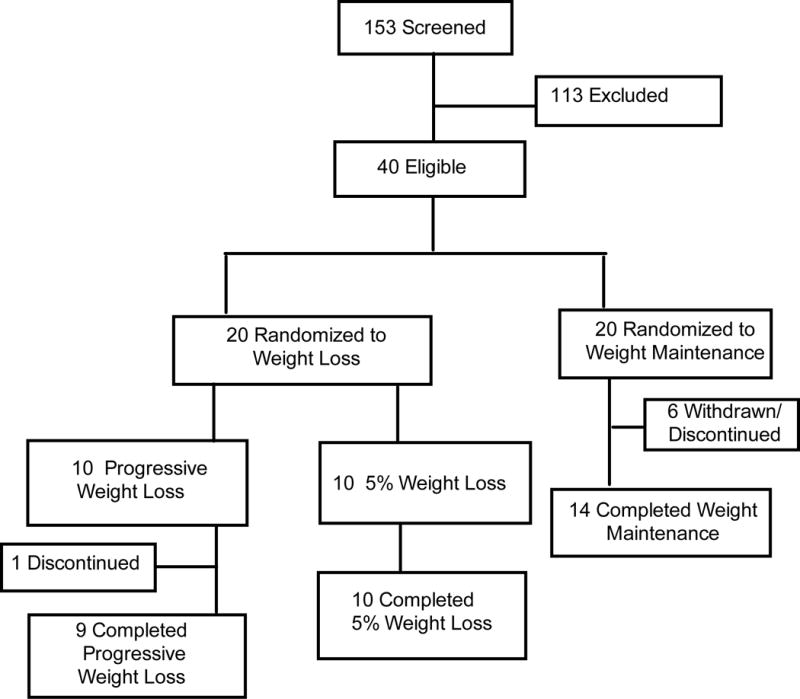
Flow of participants through screening procedures, baseline testing, randomization into weight loss and weight maintenance, and post-intervention testing. Adopted from ref. (10).
Body fat mass and fat-free mass were determined by using dual-energy X-ray absorptiometry (13). A hyperinsulinemic-euglycemic clamp procedure, in conjunction with [6,6-2H2]glucose tracer infusion, was used to assess glucose kinetics during basal and insulin-stimulated conditions (10). The rate of insulin infusion (50 mU/m2 body surface area/min) was designed to achieve typical postprandial plasma insulin concentrations (14). Blood samples were obtained from an indwelling radial arterial catheter.
Sample analyses and calculation of glucose kinetics
[LAC] was measured on a Beckman DxC600 autoanalyzer, using reagents also from Beckman (Brea, CA) (15). Plasma glucose tracer-to-tracee ratio (TTR) was determined by using gas chromatography-mass spectroscopy (16). Glucose rate of appearance (Ra) in plasma during basal conditions provides an index of hepatic glucose production rate, and was calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR during the last 30 min of the basal period. During the clamp procedure, glucose rate of disappearance (Rd) from plasma provides an index of insulin-stimulated muscle glucose uptake, and was calculated as the sum of endogenous glucose Ra and the rate of infused (exogenous) glucose. Insulin sensitivity was determined as the relative increase in glucose Rd during insulin infusion.
Statistical Analysis
A two-way repeated measures ANOVA with group (weight loss versus maintenance) and time (pre-versus post-intervention) as factors was used to evaluate the effects of 5% weight loss on [LAC]; and significant interactions were followed by Tukey’s post hoc procedure. A one-way repeated measures ANOVA was used to assess the effect of progressive weight loss on [LAC]. Effects of time were followed by simple contrasts to assess differences from baseline and trend analysis to assess the linear, quadratic, and cubic components of the overall time-related change. Pearson’s correlation was used to evaluate the relationship between [LAC] and glucose kinetics. Results are shown as means ± SD. A P-value of 0.05 or less was considered statistically significant. The sample size was based on the primary outcome of the original study (change in insulin sensitivity during weight loss) as reported previously (10). Statistical analyses were performed by using SPSS (version 24, IBM, Armonk, NY).
RESULTS
Inter-relationships among [LAC], glucose production rate, and insulin sensitivity
At baseline, there was a three-fold range in [LAC] from 0.6 mmol/L to 1.9 mmol/L. However, fasting [LAC] correlated positively with glucose Ra (Figure 2A) and negatively with skeletal muscle insulin sensitivity, assessed as the relative increase in glucose Rd during insulin infusion (Figure 2B). Insulin infusion had a variable effect on [LAC], which ranged from a 76% decrease to a 100% increase; the insulin-induced change in [LAC] was positively correlated with the insulin-stimulated increase in glucose Rd (Figure 2C). The relationship between fasting [LAC] and glucose Rd during clamp or the absolute increase in glucose Rd during insulin infusion was not statistically significant (r=−217, p= 0.225 and r=−266, p= 0.134, respectively).
Figure 2. Interrelationship between plasma lactate concentration ([LAC]) and glucose kinetics.
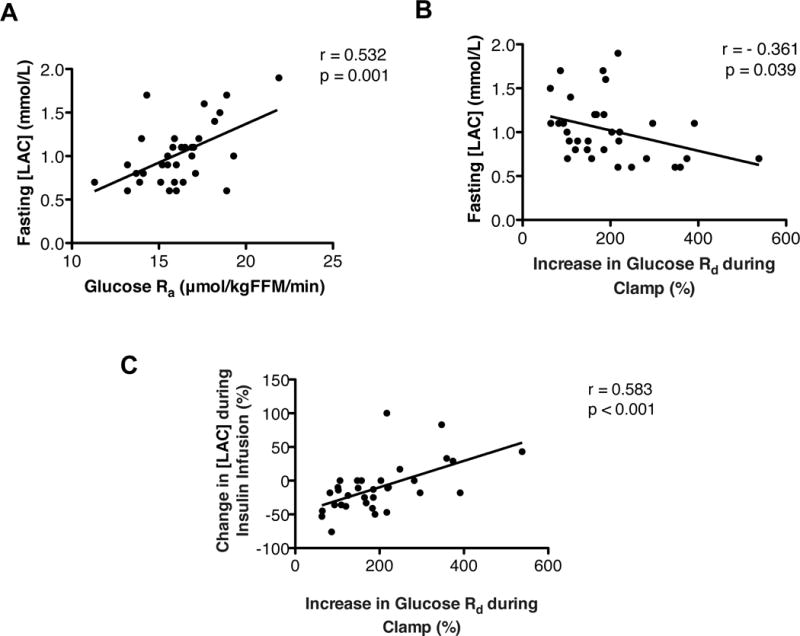
(A) Relationship between fasting [LAC] and the glucose rate of appearance (Ra) into plasma. (B) Relationship between fasting [LAC] and the relative increase in glucose rate of disappearance (Rd) during a hyperinsulinemic-euglycemic clamp procedure. (C) Relationship between the relative change in [LAC] and the relative increase in glucose Rd during a hyperinsulinemic-euglycemic clamp procedure.
Effects of weight loss on lactate and glucose metabolism
Compared with subjects randomized to weight maintenance, 5% weight loss caused a decrease in fasting [LAC] (Figure 3A) and an increase in the relative change in [LAC] induced during insulin infusion (Figure 3B). The relative decrease in fasting [LAC] after 5% weight loss correlated with both the relative decrease in basal glucose Ra (Figure 3C) and the relative increase in insulin sensitivity (assessed as a relative increase in glucose Rd during a hyperinsulinemic-euglycemic clamp procedure) (Figure 3D). Progressive 5% to 16% weight loss caused a progressive decline in fasting [LAC] (Figure 4A) and a progressive increase in the relative change in [LAC] induced by insulin infusion (Figure 4B), in conjunction with a progressive increase in muscle insulin sensitivity reported previously (10).
Figure 3. Effect of moderate (5%) weight loss on plasma lactate concentration ([LAC]) during postabsorptive conditions and a hyperinsulinemic-euglycemic clamp procedure.
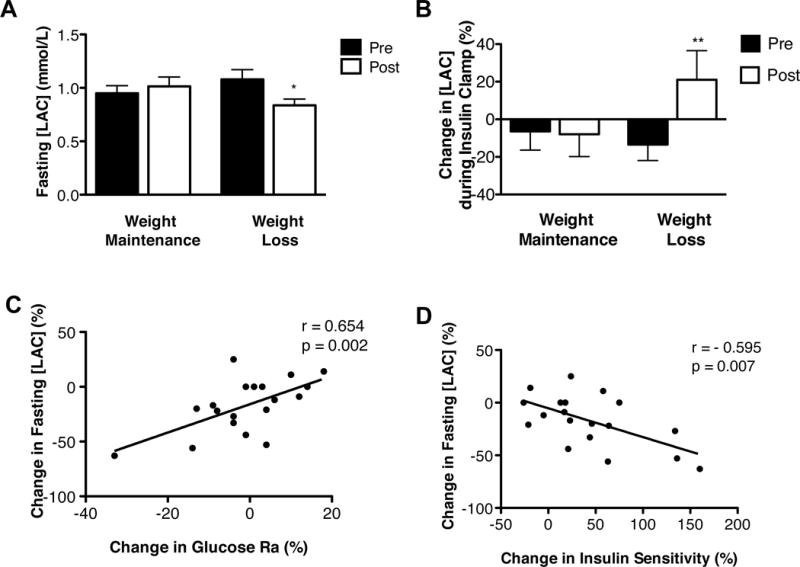
(A) Fasting [LAC] before and after weight maintenance and 5% weight loss. *Value significantly different from corresponding weight maintenance value, interaction p=0.009 evaluated by using two-way repeated measures ANOVA. (B) Change in [LAC] induced by a hyperinsulinemic-euglycemic clamp procedure before and after weight maintenance and 5% weight loss. **Value different from corresponding weight maintenance value, interaction p=0.06 evaluated by using one-way repeated measures ANOVA. (C) Relationship between the relative change in fasting [LAC]pl with the relative change in glucose rate of appearance (Ra). (D) Relationship between the relative change in fasting [LAC] with the relative change in insulin sensitivity [assessed as the percent increase in glucose rate of disappearance during a hyperinsulinemic-euglycemic clamp procedure].
Figure 4. Effect of progressive weight loss on plasma lactate concentration ([LAC]).
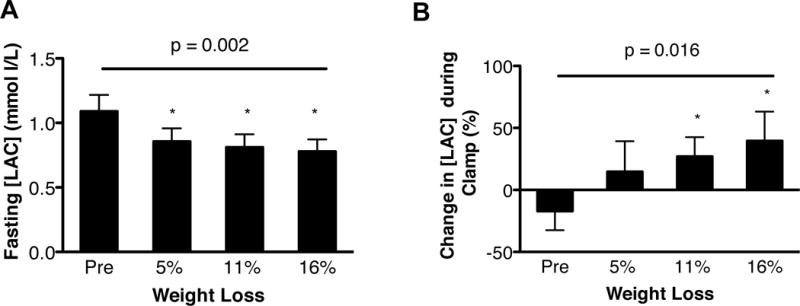
(A) Fasting [LAC] before and after progressive weight loss (evaluated by using one-way repeated measures ANOVA, p=0.002; linear effect of time, p=0.006). (B) Change in [LAC] during a hyperinsulinemic-euglycemic clamp procedure before and after progressive weight loss (evaluated by using one-way repeated measures ANOVA, p=0.016; linear effect of time, p=0.006). *Value significantly different from Pre value, p <0.05.
DISCUSSION
In this study, we evaluated the relationship between [LAC] and glucose kinetics and the effect of weight loss on lactate metabolism in people with obesity. We found fasting [LAC] was positively correlated with the rate of endogenous glucose production, but negatively correlated with insulin sensitivity, assessed as the relative increase in glucose Rd during the clamp procedure. To further investigate the relationship between [LAC] and insulin action, we assessed [LAC] in a subset of participants after weight loss-induced manipulation of insulin sensitivity. Progressive weight loss (5% through 16%) caused a progressive decrease in fasting [LAC], and the change in fasting [LAC] after 5% weight loss was directly correlated with a relative decrease in basal glucose Ra and relative increase in insulin-sensitivity. These data support the inter-relationship between hepatic and skeletal muscle glucose metabolism and [LAC], and suggest fasting [LAC] is a biomarker of glucose-related insulin resistance.
Our finding that fasting [LAC] is associated with insulin resistance is consistent with data from previous studies that have shown fasting [LAC] is higher in people with obesity and those with type 2 diabetes than in people who are lean and healthy (4, 5, 17, 18, 19). The design of our study is not able to determine the precise mechanism(s) responsible for the observed correlation between fasting [LAC] and insulin sensitivity and the progressive decline in fasting [LAC] with progressive weight loss. There is no storage depot for lactate, so circulating lactate represents a balance between production and plasma clearance. During postabsorptive, resting conditions, skeletal muscle (20) and adipose tissue (1, 21) are major sources of whole-body lactate production, whereas the splanchnic bed (liver and gut) is likely an important source of postprandial lactate production (22). Circulating lactate can be excreted by the kidneys or taken up by specific tissues and converted to glucose (primarily by liver and kidneys) (9, 23), or oxidized for fuel (primarily by heart and skeletal muscle) (24, 25). Presumably, during basal conditions, the weight loss-induced increase in insulin sensitivity and decrease in endogenous glucose production caused a decrease in the delivery of glucose to skeletal muscle and adipose tissue and increased the proportion of muscle and adipose tissue glucose uptake that was completely oxidized or converted to glycogen, thereby decreasing whole-body lactate production and fasting [LAC].
In contrast with the relationship between [LAC] and insulin sensitivity during postabsorptive conditions, [LAC] in response to either glucose ingestion or glucose infusion is lower in people who have insulin-resistant glucose metabolism than in those who are insulin sensitive (18, 26, 27, 28). The precise mechanism explaining this observation is not known, but it is likely that the impairment in tissue glucose uptake associated with insulin resistance limits the availability of glucose for metabolism to lactate (1, 5, 29, 30, 31), whereas the increase in tissue glucose uptake associated with insulin sensitivity increases glycolytic flux and lactate production. In our subjects, the relative change in [LAC] during the hyperinsulinemic-euglycemic clamp procedure progressively increased with progressive weight loss and the accompanying increase in insulin sensitivity, demonstrating that the impairment in postprandial lactate production associated with obesity is corrected by weight loss. However, we are not able to determine which tissues were responsible for the weight-loss induced increase in lactate production during the clamp procedure, which could involve any of the insulin-sensitive tissues that produce lactate, such as skeletal muscle, adipose tissue, liver, intestine, and kidney.
It is possible that the relationship we observed between [LAC] and insulin resistance represents an adverse effect of lactate on insulin action. Data from studies conducted in rodent models demonstrate that hyperlactemia affects cellular insulin signaling and impairs insulin-stimulated glucose uptake in skeletal muscle (6, 7, 8). However, these findings have not been confirmed in human subjects; experimental sodium lactate infusion, in conjunction with sodium hydroxide infusion to prevent lactic acidosis, did not have adverse effects on glucose disposal or insulin sensitivity in healthy lean people (32, 33). The reason for the discrepancy between studies in rodents and people is not clear, but it is possible the changes in plasma pH and [LAC] achieved during lactate infusion in people were not adequate to affect insulin action. Additional studies are needed to determine whether [LAC] is involved in the pathogenesis of insulin resistance in people with obesity.
This study demonstrates the inter-relationships among weight loss, hepatic and muscle glucose kinetics, insulin sensitivity, and [LAC] in people with obesity, and suggests fasting [LAC] can serve as an additional biomarker of glucose-related insulin resistance. Additional studies are needed to identify the precise mechanisms responsible for relationship between [LAC] and insulin action and to clarify the importance of circulating lactate in the pathogenesis of insulin resistance in people.
IMPORTANCE OF THE STUDY.
What is already known about this subject?
Lactate is an intermediate of glucose metabolism that has been implicated in the pathogenesis of insulin resistance and type 2 diabetes.
People with obesity and diabetes have higher fasting plasma lactate concentration ([LAC]) compared with people who are lean and healthy.
What does your study add?
This study demonstrates that fasting [LAC] is positively associated with the rate of endogenous glucose production, and negatively associated with insulin sensitivity, assessed as the relative increase in glucose disposal during a hyperinsulinemic-euglycemic clamp procedure.
Progressive (5% through 16%) weight loss caused a progressive decrease in fasting [LAC], whereas the relative decrease in fasting [LAC] in response to 5% weight loss was directly correlated with the relative decrease in endogenous glucose production and the relative increase in insulin-stimulated glucose disposal.
Fasting [LAC] is a potential biomarker of insulin resistance with respect to glucose metabolism.
Acknowledgments
The authors thank Freida Custodio and Jennifer Shew for technical assistance, the research coordinators of the Center for Human Nutrition and the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation.
Funding: This study was supported by National Institutes of Health grants DK 104995, DK 56341 (Nutrition Obesity Research Center), DK20579 (Diabetes Research Center), DK052574 (Digestive Disease Research Center), and RR024992 (Clinical and Translational Science Award), and grants from the Pershing Square Foundation and the Longer Life Foundation. MC is supported by a postdoctoral fellowship from the American Heart Association (17POST33060003).
Footnotes
Clinical Trial Registration: ClinicalTrials.gov, registration number: NCT01299519
Disclosure: The authors declare no conflict of interest.
References
- 1.Jansson PA, Larsson A, Smith U, Lonnroth P. Lactate release from the subcutaneous tissue in lean and obese men. J Clin Invest. 1994;93:240–246. doi: 10.1172/JCI116951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford SO, Ambrose MS, Hoogeveen RC, Brancati FL, Ballantyne CM, Young JH. Association of lactate with blood pressure before and after rapid weight loss. Am J Hypertens. 2008;21:1337–1342. doi: 10.1038/ajh.2008.282. [DOI] [PubMed] [Google Scholar]
- 3.Crawford SO, Hoogeveen RC, Brancati FL, Astor BC, Ballantyne CM, Schmidt MI, et al. Association of blood lactate with type 2 diabetes: the Atherosclerosis Risk in Communities Carotid MRI Study. Int J Epidemiol. 2010;39:1647–1655. doi: 10.1093/ije/dyq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 5.Avogaro A, Toffolo G, Miola M, Valerio A, Tiengo A, Cobelli C, et al. Intracellular lactate- and pyruvate-interconversion rates are increased in muscle tissue of non-insulin-dependent diabetic individuals. J Clin Invest. 1996;98:108–115. doi: 10.1172/JCI118754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi CS, Kim YB, Lee FN, Zabolotny JM, Kahn BB, Youn JH. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. Am J Physiol Endocrinol Metab. 2002;283:E233–240. doi: 10.1152/ajpendo.00557.2001. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi AM, Fabris R, Bassetto F, Serra R, Leturque A, Federspil G, et al. Hyperlactatemia reduces muscle glucose uptake and GLUT-4 mRNA while increasing (E1α)PDH gene expression in rat. American Journal of Physiology – Endocrinology And Metabolism. 1999;276:E922–E929. doi: 10.1152/ajpendo.1999.276.5.E922. [DOI] [PubMed] [Google Scholar]
- 8.Vettor R, Lombardi AM, Fabris R, Pagano C, Cusin I, Rohner-Jeanrenaud F, et al. Lactate infusion in anesthetized rats produces insulin resistance in heart and skeletal muscles. Metabolism – Clinical and Experimental. 46:684–690. doi: 10.1016/s0026-0495(97)90014-7. [DOI] [PubMed] [Google Scholar]
- 9.Jenssen T, Nurjhan N, Consoli A, Gerich JE. Failure of substrate-induced gluconeogenesis to increase overall glucose appearance in normal humans. Demonstration of hepatic autoregulation without a change in plasma glucose concentration. Journal of Clinical Investigation. 1990;86:489–497. doi: 10.1172/JCI114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zawadzki JK, Wolfe RR, Mott DM, Lillioja S, Howard BV, Bogardus C. Increased rate of Cori cycle in obese subjects with NIDDM and effect of weight reduction. Diabetes. 1988;37:154–159. doi: 10.2337/diab.37.2.154. [DOI] [PubMed] [Google Scholar]
- 12.van Aggel-Leijssen DP, Saris WH, Hul GB, van Baak MA. Short-term effects of weight loss with or without low-intensity exercise training on fat metabolism in obese men. Am J Clin Nutr. 2001;73:523–531. doi: 10.1093/ajcn/73.3.523. [DOI] [PubMed] [Google Scholar]
- 13.Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, et al. Metabolically normal obese people are protected from adverse effects following weight gain. The Journal of clinical investigation. 2015;125:787–795. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care. 2012;35:1316–1321. doi: 10.2337/dc11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Molecular Metabolism. 2016;5:538–551. doi: 10.1016/j.molmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, et al. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest. 2015;125:787–795. doi: 10.1172/JCI78425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Prato S, Bonadonna RC, Bonora E, Gulli G, Solini A, Shank M, et al. Characterization of cellular defects of insulin action in type 2 (non-insulin-dependent) diabetes mellitus. J Clin Invest. 1993;91:484–494. doi: 10.1172/JCI116226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovejoy J, Newby FD, Gebhart SS, DiGirolamo M. Insulin resistance in obesity is associated with elevated basal lactate levels and diminished lactate appearance following intravenous glucose and insulin. Metabolism. 1992;41:22–27. doi: 10.1016/0026-0495(92)90185-d. [DOI] [PubMed] [Google Scholar]
- 19.Williams RS, Heilbronn LK, Chen DL, Coster AC, Greenfield JR, Samocha-Bonet D. Dietary acid load, metabolic acidosis and insulin resistance – Lessons from cross-sectional and overfeeding studies in humans. Clin Nutr. 2016;35:1084–1090. doi: 10.1016/j.clnu.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Consoli A, Nurjhan N, Reilly JJ, Jr, Bier DM, Gerich JE. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990;86:2038–2045. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frayn KN, Humphreys SM. Metabolic characteristics of human subcutaneous abdominal adipose tissue after overnight fast. Am J Physiol Endocrinol Metab. 2012;302:E468–475. doi: 10.1152/ajpendo.00527.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis MA, Williams PE, Cherrington AD. Effect of glucagon on hepatic lactate metabolism in the conscious dog. Am J Physiol. 1985;248:E463–470. doi: 10.1152/ajpendo.1985.248.4.E463. [DOI] [PubMed] [Google Scholar]
- 23.Stumvoll M, Chintalapudi U, Perriello G, Welle S, Gutierrez O, Gerich J. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Invest. 1995;96:2528–2533. doi: 10.1172/JCI118314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman BC, Tsvetkova T, Lowes B, Wolfel EE. Myocardial FFA metabolism during rest and atrial pacing in humans. American Journal of Physiology – Endocrinology And Metabolism. 2009;296:E358–E366. doi: 10.1152/ajpendo.90747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs RA, Meinild AK, Nordsborg NB, Lundby C. Lactate oxidation in human skeletal muscle mitochondria. Am J Physiol Endocrinol Metab. 2013;304:E686–694. doi: 10.1152/ajpendo.00476.2012. [DOI] [PubMed] [Google Scholar]
- 26.Yki-Jarvinen H, Bogardus C, Foley JE. Regulation of plasma lactate concentration in resting human subjects. Metabolism. 1990;39:859–864. doi: 10.1016/0026-0495(90)90133-w. [DOI] [PubMed] [Google Scholar]
- 27.Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, et al. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 28.Zuniga-Guajardo S, Zinman B. The metabolic response to the euglycemic insulin clamp in type I diabetes and normal humans. Metabolism. 1985;34:926–930. doi: 10.1016/0026-0495(85)90140-4. [DOI] [PubMed] [Google Scholar]
- 29.Kelley DE, Mokan M, Mandarino LJ. Metabolic pathways of glucose in skeletal muscle of lean NIDDM patients. Diabetes care. 1993;16:1158–1166. doi: 10.2337/diacare.16.8.1158. [DOI] [PubMed] [Google Scholar]
- 30.Qvisth V, Hagstrom-Toft E, Moberg E, Sjoberg S, Bolinder J. Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am J Physiol Endocrinol Metab. 2007;292:E709–714. doi: 10.1152/ajpendo.00104.2006. [DOI] [PubMed] [Google Scholar]
- 31.Sjöstrand M, Holmäng A, Strindberg L, Lönnroth P. Estimations of muscle interstitial insulin, glucose, and lactate in type 2 diabetic subjects. American Journal of Physiology – Endocrinology And Metabolism. 2000;279:E1097–E1103. doi: 10.1152/ajpendo.2000.279.5.E1097. [DOI] [PubMed] [Google Scholar]
- 32.Ferrannini E, Natali A, Brandi LS, Bonadonna R, De Kreutzemberg SV, DelPrato S, et al. Metabolic and thermogenic effects of lactate infusion in humans. American Journal of Physiology – Endocrinology And Metabolism. 1993;265:E504–E512. doi: 10.1152/ajpendo.1993.265.3.E504. [DOI] [PubMed] [Google Scholar]
- 33.Paquot N, Schneiter P, Cayeux MC, Chiolero R, Temler E, Jequier E, et al. Effects of infused sodium lactate on glucose and energy metabolism in healthy humans. Diabete Metab. 1995;21:345–352. [PubMed] [Google Scholar]


