Abstract
Gsα, the alpha stimulatory subunit of heterotrimeric G proteins that activates downstream signaling through the adenylyl cyclase and cAMP/PKA pathway, plays an important role in bone development and remodeling. The role of Gsα in mesenchymal stem cell (MSC) differentiation to osteoblasts has been demonstrated in several mouse models of Gsα inactivation. Previously, using mice with heterozygous germline deletion of Gsα (Gnas+/p−), we identified a novel additional role for Gsα in bone remodeling, and showed the importance of Gnas in maintaining bone quality by regulating osteoclast differentiation and function. In this study, we show that postnatal deletion of Gsα (CreERT2;Gnasfl/fl) leads to reduction in trabecular bone quality parameters and increased trabecular osteoclast numbers. Furthermore, mice with deletion of Gsα specifically in cells of the macrophage/osteoclast lineage (LysM-Cre;Gnasfl/fl) showed reduced trabecular bone quality and increased trabecular osteoclasts, but to a reduced extent compared to the CreERT2;Gnasfl/fl global knockout. This demonstrates that while Gsα has a cell autonomous role in osteclasts in regulating bone quality, Gsα expression in other cell types additionally contribute. In both of these mouse models, cortical bone was more subtly affected than trabecular bone. Our results support that Gsα is required postnatally to maintain trabecular bone quality and that Gsα function to maintain trabecular bone is regulated in part through a specific activity in osteoclasts.
Keywords: GNAS, Gsα, LysM, CreERT2, osteoclast, bone remodeling, trabecular bone
Introduction
Skeletal bone formation and maintenance occur through the processes of bone modeling and remodeling which are directed by coordinated activities of osteoblasts, osteoclasts, and osteocytes. Rates of bone formation by osteoblasts and bone resorption by osteoclasts are balanced and regulated through a network of signaling pathways to maintain bone quality [1–3]. GNAS, which encodes the α-subunit of stimulatory G-protein (Gsα) of adenylyl cyclase and activates cAMP signaling, plays important roles in skeletal development and maintenance. Previously, mouse models with global, as well as osteoblast- and osteocyte-specific, deletion of Gnas revealed important functions of the Gsα pathway in various stages of skeletal bone formation including mesenchymal stem cell commitment, osteoblast differentiation, and mineralization [4–8].
In additional to osteoblasts and osteogenesis, cAMP/PKA signaling has also been shown to regulate osteoclastogenesis, and other pathways such as Wnt/β-catenin and calmodulin-dependent kinase have been shown to act as upstream regulators of this process[9,10]. Using a mouse model with heterozygous germline inactivation of Gsα, we demonstrated that Gsα maintains bone quality by regulating osteoclast differentiation and bone resorption activity [11]. However, given that the mouse model used globally reduced Gsα expression, we could not distinguish the contributions of a cell-autonomous role of Gsα in osteoclasts versus additional cell types.
In this study, we investigated the effects of osteoclast-specific reduction of Gsα on bone maintenance through two in vivo approaches. Since osteoclasts do not begin to remodel bone and regulate skeletal bone quality until post-natal life, we induced postnatal global deletion of Gnas (CreERT2;Gnasfl/fl) to bypass effects of decreased Gsα during embryonic skeletal development. We additionally induced Gsα deletion specifically in cells of the macrophage/osteoclast lineage. In both models, we examined the impact of Gnas deletion on skeletal bone quality and osteoclasts. Our data suggest that postnatal Gsα deletion during bone remodeling stages adversely impacts trabecular bone quality and concomitantly increases in vivo osteoclast number. Similar effects were detected with specific macrophage/osteoclast lineage Gnas deletion indicating that Gsα influences trabecular bone quality at least in part by Gsα function in osteoclasts.
Methods
Animals
All animal experiments were performed in accordance with the relevant regulations and guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC), University of Pennsylvania.
CreERT2 mice (Jackson Laboratories, stock no. 008463) [12] were crossed to Gnasfl/fl mice [4–8] to generate CreERT2;Gnasfl/+ mice which were subsequently crossed with Gnasfl/fl mice to obtain CreERT2;Gnasfl/fl mice. Cre-negative mice from the same litters were used as controls. At 4 weeks of age, mice were injected intraperitoneally with tamoxifen dissolved in corn oil (1 mg/100 μl) on three successive days at 50 μg/g body weight. Three mice were harvested at 4 weeks and at 6 weeks post-injection. LysM-Cre mice (Jackson Laboratories, stock no. 004781) [13] were crossed with Gnasfl/fl mice to generate LysM-Cre;Gnasfl/+ mice which were subsequently crossed with Gnasfl/fl mice to obtain LysM-Cre;Gnasfl/fl mice and Cre-negative (Gnasfl/fl) control mice. Mice were analyzed at 3–4 weeks of age by histology and at 12 weeks by micro-computed tomography. Both male and female mice were used in these studies. All mouse lines were maintained on a C57BL/6 background (crosses used mice from Jackson Laboratories, stock no. 000664).
Micro-computed tomography (μCT)
Femurs from CreERT2;Gnasfl/fl and Cre-negative littermates at 6 weeks after tamoxifen injections or from 12 week old LysM-Cre;Gnasfl/fl and Cre-negative littermates were processed for μCT scanning and bone volume quantification using previously described methods [11].
Histology
One femur per mouse was harvested for μCT; each contralateral limb was processed for histology. TRAP staining was performed using the leukocyte acid phosphatase kit (Sigma 387A) as described previously [11]. Briefly, paraffin embedded slides were de-paraffinized, hydrated through a graded series of ethanol to deionized water and then stained following the recommended procedure. After staining for TRAP for 1 hour, slides were rinsed thoroughly with deionized water, counterstained with methyl green, and allowed to air dry. Femurs from CreERT2;Gnasfl/fl and Cre negative mice harvested at 4 weeks after tamoxifen injections and femurs from 3–4 week old LysM-Cre;Gnasfl/fl and Cre negative littermates were stained for TRAP and multi-nucleated osteoclasts (≥3 nuclei) were counted along trabeculae in the distal femur proximal to the growth plate.
Real-time PCR
Real-time PCR to measure Gsα expression was performed as described previously. Briefly, soft tissue was removed from femur and tibiae, ends were cut, and bone marrow was flushed out. The mid-diaphyseal region was frozen in liquid nitrogen then crushed in a tissue lyser (TissueLyser LT, Qiagen). RNA was extracted with Trizol, and cDNA was prepared using High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific). Gsα primer sequences were published previously [11]. Data were normalized to Gapdh.
Statistical Analyses
Analyses of CreERT2;Gnasfl/fl and Cre-negative controls: In order to accommodate for the small litter size and the higher variation between the multiple litters, Student’s t-test was performed to compare μCT data after setting the Cre-negative littermate controls to 1. Unpaired two-sided Student’s t-test was performed using absolute osteoclast numbers per mm. For real time qRT-PCR data, Cre-negative littermate controls were set to 1.
Analyses of LysM-Cre;Gnasfl/fl and Cre-negative controls: Similar to above, unpaired two-sided Student’s t-test was performed to compare μCT, real time qRT-PCR data, and osteoclast numbers after setting the littermate controls to 1.
Results
Postnatal homozygous deletion of Gnas/Gsα adversely alters trabecular bone
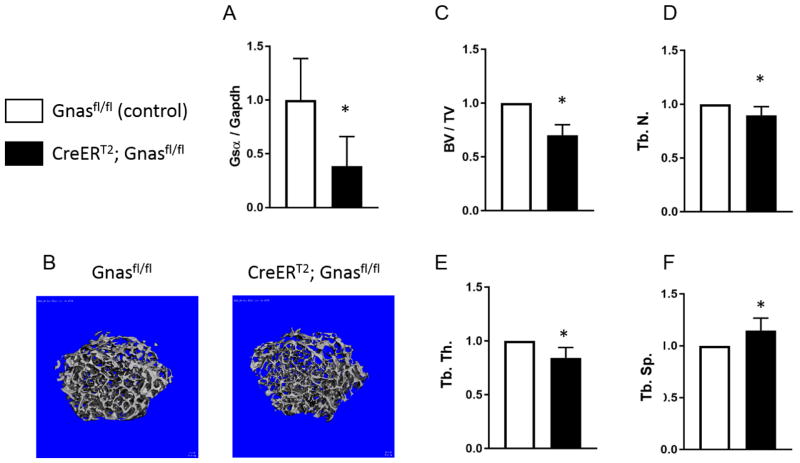
Since osteoclasts do not begin to remodel bone and regulate skeletal bone quality until post-natal life, we induced global deletion of Gnas (CreERT2;Gnasfl/fl) post-natally in order to examine effects of Gnas/Gsα depletion independently of embryonic skeletal development. At 4 weeks of age, CreERT2;Gnasfl/fl mice and control (Cre-negative) mice were treated with tamoxifen to induce Gnas deletion. RNA from mid-diaphyseal cortical bone harvested 6 weeks after the injection showed a significant decrease in Gsα mRNA expression by RT-PCR in CreERT2;Gnasfl/fl mice compared to control (Cre-negative) bone (Figure 1A). To investigate the postnatal role of Gsα on bone remodeling, we examined the femurs at 6 weeks after tamoxifen injections by μCT (Figure 1B). Trabecular bone volume (20% reduction) and bone volume fraction (30% reduction) were significantly reduced in CreERT2;Gnasfl/fl mice compared to controls (Figure 1C). Furthermore, trabecular architecture showed dramatic changes between the genotypes, with CreERT2;Gnasfl/fl mice demonstrating significantly reduced trabecular number (10% lower) and trabecular thickness (15% lower) and increased trabecular spacing (15% higher) compared to littermate controls (Figure 1D–F). Of note, total volume at the distal femur was 15% higher in the mutants compared to controls suggesting that bone is increased overall (Supplementary Figure 1A) in the homozygous mutant mice.
Figure 1. Postnatal homozygous deletion of Gnas/Gsα negatively affects trabecular bone quality.
(A) Reduced Gsα mRNA expression in bone samples from CreERT2;Gnasfl/fl mice compared to control (Cre-negative) Gnasfl/fl samples was confirmed by RT-PCR (n = 5 per genotype). (B) Representative μCT scans of control and CreERT2;Gnasfl/fl mice at 6 weeks after tamoxifen injections (n = 5 per genotype) showing cross-section of trabecular bone from distal femurs. (C–F) Measured trabecular bone parameters show reduced bone volume fraction (BV/TV), trabecular number (Tb.N/mm), and trabecular thickness (Tb.Th) and increased trabecular spacing (Tb.Sp, mm) in CreERT2;Gnasfl/fl. *p < 0.05.
In comparison to the impact on trabecular bone, effects of Gnas inactivation on cortical bone at the mid-diaphysis of femur were more subtle. While total volume measured at the mid-diaphysis was significantly increased (12%) in mutants (Supplementary Figure 1B), bone volume fraction (BV/TV) and cortical thickness were marginally reduced in the CreERT2;Gnasfl/fl mice when compared with age-matched littermate controls (Supplementary Figure 1C, D), suggesting an overall loss of cortical bone in mutants.
CreERT2;Gnasfl/fl mice have increased osteoclast numbers
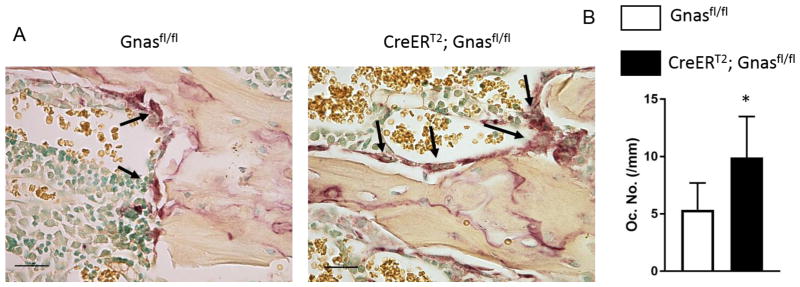
Previously, we determined that mice with germline heterozygous inactivation of Gnas/Gsα had increased endocortical osteoclast numbers that were associated with decreased cortical bone quality [11]. In order to determine whether bone quality and osteoclast numbers are altered by Gnas inactivation only during post-natal bone remodeling, femurs from CreERT2;Gnasfl/fl and littermate controls at 4 weeks after tamoxifen treatment were subjected to TRAP staining for osteoclast activity and the number of trabecular osteoclasts were counted. The numbers of multi-nucleated trabecular osteoclasts were significantly increased (Figures 2A, B) in CreERT2;Gnasfl/fl mice, supporting that postnatal homozygous deletion of Gsα affects bone maintenance and trabecular bone quality by increasing the numbers of bone-resorbing osteoclasts.
Figure 2. Mice with postnatal homozygous deletion of Gnas/Gsα show increased trabecular osteoclast numbers.
(A) TRAP staining of femurs at 4 weeks after tamoxifen injection shows multi-nucleated osteoclasts along trabecular bone. (B) Quantification of trabecular osteoclast numbers (Oc.N/mm) at 4 weeks after tamoxifen injection in control and CreERT2;Gnasfl/fl mice (n= 4 per genotype). *p < 0.05
Deletion of Gnas/Gsα specifically in macrophage/osteoclast lineage cells negatively affects trabecular bone quality but less severely than in CreERT2;Gnasfl/fl mice
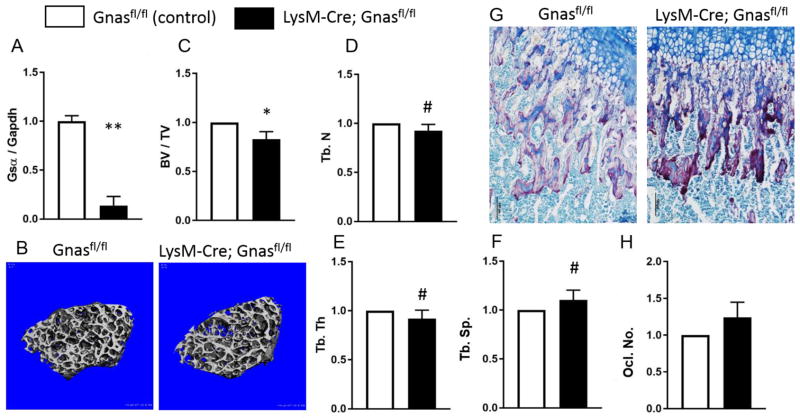
Increased trabecular osteoclasts in CreERT2;Gnasfl/fl mice, along with our previous data from mice with germline heterozygous Gnas/Gsα deletion showing increased osteoclast numbers in vivo and enhanced osteoclast differentiation in vitro, led us to investigate whether Gnas/Gsα has a cell-autonomous role in osteoclasts to regulate bone quality. We generated mice with homozygous deletion of Gsα in cells of the macrophage/osteoclast lineage using the LysM-Cre mouse model. Macrophages (pre-osteoclasts) isolated from LysM-Cre;Gnasfl/fl mice showed a 80% decrease in Gsα mRNA expression by RT-PCR compared to Cre-negative cells, confirming the deletion of Gsα in the mutants (Figure 3A). MicroCT scans of femurs (Figure 3B) showed a statistically significant decrease in trabecular BV/TV (18%) in 12-week-old LysM-Cre;Gnasfl/fl mice compared to controls (Figure 3C). In addition, trabecular microarchitecture parameters, trabecular number, and trabecular thickness were marginally decreased (8%, p ≤ 0.1) while trabecular spacing was marginally increased (10%, p < 0.1) compared to littermate controls (Figure 3D–F). These results were, however, less pronounced than the effects in CreERT2;Gnasfl/fl mice.
Figure 3. Macrophage-specific Gnas/Gsα deletion reduced trabecular bone quality less severely than global deletion of Gnas/Gsα.
(A) Gsα mRNA expression in bone marrow macrophages (osteoclast lineage cells) isolated from LysMCre;Gnasfl/fl mice is reduced relative to controls (Cre-negative; n= 4 per genotype). (B) Representative μCT scans of 12-week-old control and LysMCre;Gnasfl/fl mice showing cross-sections of trabecular bone from distal femurs. (C–F) Measured trabecular bone parameters show reduced bone volume fraction (BV/TV), trabecular number (Tb.N, and trabecular thickness (Tb.Th) and increased trabecular spacing (Tb.Sp) in LysMCre;Gnasfl/fl (n= 5 per genotype). **p< 0.01; *p < 0.05; #p≤0.1
TRAP staining for osteoclast activity appeared increased in CreERT2;Gnasfl/fl trabecular bone compared to control (Figure 3G). Although poor viability of mutant mice limited our ability to examine a larger sample size, quantification of multi-nucleated osteoclasts (from n=4 LysM-Cre;Gnasfl/fl and n=4 controls) identified a trend towards increased numbers in LysM-Cre;Gnasfl/fl mice (p < 0.2) compared to controls (Figure 3H). Reduced viability also limited our ability to examine LysM-Cre;Gnasfl/fl cells in osteoclast differentiation and pit formation assays, however data using cells heterozygous for Gnas knockout (n = 3 LysM-Cre;Gnasfl/+), showed enhanced differentiation, increased numbers of TRAP+ multi-nucleated cells, and higher pit formation/resorption area by mutant cells compared to littermate Cre-negative controls (n = 2) (also see Supplementary Figure 2 and Supplementary Information).
The more subtle effect on osteoclast functions by LysM-Cre;Gnasfl/fl or LysM-Cre;Gnasfl/+ mice compared with the statistically significant effects observed in CreERT2;Gnasfl/fl mice supports that while Gnas inactivation confers cell-autonomous osteoclast-specific effects on osteoclast function, Gnas additionally acts in other cell types that contribute to the regulation of osteoclast activity and function.
Discussion
The roles of GNAS/Gsα in skeletal development and maintenance have been demonstrated in MSCs, osteoblasts, and osteocytes through several mouse models [4–8]. Previously, we identified a role for Gsα in bone remodeling and maintenance of bone quality in regulating osteoclast activity and differentiation. In this follow up investigation, we further show that Gsα regulates bone quality at least in part through cell-specific functions in osteoclasts [11]. These investigations additionally reveal that Gsα is important not only during bone development, but also during postnatal bone remodeling.
Since osteoclasts do not begin to remodel bone and regulate skeletal bone quality until postnatal life [1–3,14], this investigation used a conditional Gnas knockout mouse model and induced deletion of Gnas/Gsα postnatally. When Gnas/Gsα was globally deleted through the CreERT2 recombination system, trabecular bone quality was significantly reduced along with significantly elevated osteoclast numbers. This delineates a role for Gsα in maintaining bone quality during postnatal remodeling independently of skeletal development.
Our earlier study [11] was the first to show that Gsα regulates skeletal development and maintenance through modulating osteoclast number and function. In vitro differentiation studies suggested that the role of Gsα in osteoclasts is cell-autonomous. However, the effects of deletion of Gsα in other cell types including osteoblasts on the overall skeletal phenotype and the potential impact of global Gsα deletion on osteoblast-osteoclast coupling precluded us from confirming a solely cell-autonomous role for Gsα in osteoclasts. In the current study, to overcome this limitation, we deleted Gsα in cells of the macrophage-lineage using LysM-Cre to inactivate Gnas. Mice with Cre driven by the LysM promoter has previously been used to study bone quality in several studies and has been shown to be reliable in gene deletion in macrophage/osteoclast-lineage cells [9,15,16].
Both conditional Gnas knockout mouse models used in this study demonstrated enhanced osteoclast function and activity upon Gnas inactivation. Postnatal global Gsα deletion during bone remodeling stages was shown to adversely impact trabecular bone quality and concomitantly increase in vivo osteoclast numbers. Specific macrophage/osteoclast lineage Gnas deletion also enhance osteoclast functions and negatively affected trabecular bone, however to a reduced degree, indicating that while Gnas/Gsα in osteoclasts influences trabecular bone quality, Gnas function in additional cell types also contribute.
Studies of osteoblast differentiation and mineralization were not performed in this study in either of the two mouse models. In our previous study, we also showed that Gsα regulates osteoclast differentiation and function through enhancement of Nfatc1 levels via the PKA signaling pathway [11]. Future experiments on osteoblast differentiation and function, osteoblast-osteoclast coupling, and downstream signals regulating bone remodeling would uncover the various cellular and signaling mechanisms by which Gsα impacts bone maintenance.
Of particular note, our previous studies in mice with global germline heterozygous deletion of Gsα showed a more severe effect in cortical bone than trabecular bone [11]. In contrast, CreERT2;Gnasfl/fl mice showed a more severe adverse effect on trabecular bone quality and a subtle effect on cortical bone. This could be due to a varied number of reasons including differences in mouse ages and the time points examined, the levels of Gsα expression, and/or differing effects of these differences on osteoblasts, osteoclasts and other cell types. A significant impact on skeletal bone that depends on the levels of Gnas signaling through early development may also be a major influence on bone quality in cortical vs. trabecular bone. After initial skeletal development, trabecular bone undergoes more active remodeling than cortical bone [17]. Hence the effect of postnatal deletion of Gsα could plausibly have a larger impact on trabecular bone remodeling than cortical bone.
Surprisingly, despite decreased trabecular bone volume fraction and microarchitecture, CreERT2;Gnasfl/fl mice showed increased total volume at both the distal femur and mid-diaphysis suggesting an increase in overall size of the bone. With aging, there is increased osteoclast activity leading to loss of both trabecular and cortical bone (endosteal resorption at the mid-diaphysis). In order to compensate for the bone loss, the mid-diaphyseal region undergoes periosteal expansion via appositional growth which helps maintain the mechanical strength of long bones [1,2]. In the Gsα mutants, we observe a similar phenotype with increased osteoclasts and loss of bone. Although we did not measure bone strength by mechanical testing in the two mouse models in this current work, we did perform three-point bending tests in mice with heterozygous deletion of Gsα [11] and showed reduction in mechanical strength parameters (stiffness and peak load) in the Gsα mutants compared to littermate controls. Postnatal deletion of Gsα could cause a decrease in mechanical strength that would support the decreased trabecular and cortical bone that we observe by μCT. Hence, the increase in total bone volume at both the distal femur and mid-diaphysis could be an adaptive response to maintain integrity of the bone.
Overall, our data demonstrate that Gnas is an important regulator of postnatal skeletal bone remodeling. Our studies additionally support that Gnas/Gsα activity in osteoclasts plays important roles in trabecular bone quality and architecture, but also that cells in addition to osteoclasts contribute to postnatal maintenance of bone quality. The identities of the relevant interacting cells and the mechanisms through which Gnas/Gsα signaling in these cells are important considerations for future investigations.
Supplementary Material
Highlights.
Postnatal Gsα deletion impacts skeletal bone maintenance by reducing trabecular bone and increasing osteoclasts.
Gsα deletion in osteoclast progenitor cells decreases trabecular bone and increases osteoclasts.
Phenotype is less severe in mice with specific osteoclast Gsα deletion compared to postnatal global knockout.
Trabecular bone quality is regulated in part by Gsα activity in osteoclasts.
Acknowledgments
This work was supported by Progressive Osseous Heteroplasia Association (POHA), the Center for Research in FOP and Related Disorders, the Ian Cali Endowment for Research in FOP and Related Disorders, the Cali-Weldon Professorship of FOP Research (EMS), the Penn Center for Musculoskeletal Diseases (NIH/NIAMS P30-AR06919), and US National Institute of Health grant R01-AR046831 (EMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 2010;11:219–227. doi: 10.1007/s11154-010-9153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeman E. Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr. 2009;19:219–233. doi: 10.1615/critreveukargeneexpr.v19.i3.40. [DOI] [PubMed] [Google Scholar]
- 3.Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3 doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JY, Aarnisalo P, Bastepe M, Sinha P, Fulzele K, Selig MK, Chen M, Poulton IJ, Purton LE, Sims NA, Weinstein LS, Kronenberg HM. Gsα enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121:3492–3504. doi: 10.1172/JCI46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha P, Aarnisalo P, Chubb R, Ono N, Fulzele K, Selig M, Saeed H, Chen M, Weinstein LS, Pajevic PD, Kronenberg HM, Wu JY. Loss of Gs(alpha) early in the osteoblast lineage favors adipogenic differentiation of mesenchymal progenitors and committed osteoblast precursors. J Bone Miner Res. 2014;29:2414–2426. doi: 10.1002/jbmr.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha P, Aarnisalo P, Chubb R, Poulton IJ, Guo J, Nachtrab G, Kimura T, Swami S, Saeed H, Chen M, Weinstein LS, Schipani E, Sims NA, Kronenberg HM, Wu JY. Loss of Gsα in the postnatal skeleton leads to low bone mass and a blunted response to anabolic parathyroid hormone therapy. J Biol Chem. 2016;291:1631–1642. doi: 10.1074/jbc.M115.679753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakamoto A, Chen M, Nakamura T, Xie T, Karsenty G, Weinstein LS. Deficiency of the G-protein α-subunit Gsα in osteoblasts leads to differential effects on trabecular and cortical bone. J Biol Chem. 2005;280:21369–21375. doi: 10.1074/jbc.M500346200. [DOI] [PubMed] [Google Scholar]
- 8.Fulzele K, Krause DS, Panaroni C, Saini V, Barry KJ, Liu XL, Lotinun S, Baron R, Bonewald L, Feng JQ, Chen M, Weinstein LS, Wu JY, Kronenberg HM, Scadden DT, Pajevic PD. Myelopoiesis is regulated by osteocytes through Gs alpha-dependent signaling. Blood. 2013;121:930–939. doi: 10.1182/blood-2012-06-437160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weivoda MM, Ruan M, Hachfeld CM, Pederson L, Howe A, Davey RA, Zajac JD, Kobayashi Y, Williams BO, Westendorf JJ, Khosla S, Oursler MJ. Wnt Signaling Inhibits Osteoclast Differentiation by Activating Canonical and Noncanonical cAMP/PKA Pathways. J Bone Miner Res. 2016;31:65–75. doi: 10.1002/jbmr.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon SH, Ryu JY, Lee Y, Lee ZH, Kim HH. Adenylate cyclase and calmodulin-dependent kinase have opposite effects on osteoclastogenesis by regulating the PKA-NFATc1 pathway. J Bone Miner Res. 2011;26:1217–1229. doi: 10.1002/jbmr.310. [DOI] [PubMed] [Google Scholar]
- 11.Ramaswamy G, Kim H, Zhang D, Lounev V, Wu JY, Choi Y, Kaplan FS, Pignolo RJ, Shore EM. Gs α Controls Cortical Bone Quality by Regulating Osteoclast Differentiation via cAMP / PKA and β-Catenin Pathways. Sci Rep. 2017;7:45140. doi: 10.1038/srep45140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 13.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/A:1008942828960. [DOI] [PubMed] [Google Scholar]
- 14.Ke HZ. In vivo characterization of skeletal phenotype of genetically modified mice. J Bone Miner Metab. 2005;23:84–89. doi: 10.1007/BF03026330. [DOI] [PubMed] [Google Scholar]
- 15.Soung DY, Kalinowski J, Baniwal SK, Jacome-Galarza CE, Frenkel B, Lorenzo J, Drissi H. Runx1-Mediated Regulation of Osteoclast Differentiation and Function. Mol Endocrinol. 2014;28:546–553. doi: 10.1210/me.2013-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ucer S, Iyer S, Bartell SM, Martin-Millan M, Han L, Kim H-N, Weinstein RS, Jilka RL, O’Brien CA, Almeida M, Manolagas SC. The Effects of Androgens on Murine Cortical BoneDoNot Require AR or ERa Signaling in Osteoblasts and Osteoclasts. J Bone Miner Res. 2015;30:1138–1149. doi: 10.1002/jbmr.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.