Abstract
Background
Interleukin-6 is a gp130 utilizing cytokine that is consistently associated with allergic diseases like asthma and urticaria in humans where mast cells are known to play a critical role. However, the role of IL-6 in allergic disease in not known. IL-6 was reported to enhance degranulation of in vitro-derived mast cells, but the effect of IL-6 on mediator release from human in situ-matured tissue-isolated mast cells had not been reported.
Methods
Human mature mast cells were isolated and purified from normal skin tissue from different donors. The expression of surface-expressed IL-6 receptors was demonstrated by flow cytometry. The effect of IL-6 on FcεRI-induced degranulation, PGD2 biosynthesis, and cytokine production was determined with β-hexosaminidase release assay, Western blotting, quantitative real-time PCR, and ELISA. The small molecule inhibitor of STAT-3, C188-9, was used to demonstrate STAT3 dependency.
Results
IL-6 significantly potentiated FcεRI-induced PGD2 biosynthesis, but had no effect on degranulation. IL-6 also induced VEGF gene expression and protein secretion, and enhanced FcεRI-induced IL-8 production. Mechanistically, IL-6 enhanced FcεRI-induced COX-2 expression, PGD2 biosynthesis, and VEGF production in a STAT3 dependent manner.
Conclusion
Here, we demonstrate that IL-6 is a potentiator of FcεRI-induced PGD2 biosynthesis, and can induce or enhance production of pro-angiogenesis factors VEGF and IL-8 from human in situ-matured skin mast cells.
Keywords: Mast cells, IL-6, Prostaglandin D2, COX-2, STAT3, VEGF
1. Introduction
Mature mast cells are tissue resident cells that are classically defined as the cell type responsible for allergic reactions, which are associated with crosslinking of the high affinity receptor for immunoglobulin E, FcεRI, by allergen [1]. The causative agents of allergies are pre-formed mediators like histamine and serine neutral proteases that are stored in cytoplasmic granules and released immediately after FcεRI crosslinking, and prostaglandins and leukotrienes that are rapidly biosynthesized from arachidonic acid. Mast cells also produce cytokines and chemokines that recruit other cell types and contribute to allergic inflammation. In addition to their role in allergy, mast cells are also involved in host defense against parasites and insect or reptile venom [2]. Mast cells have also been implicated in traditionally non-allergic diseases like diabetes [3,4] and various cancers [5,6].
Interleukin-6 (IL-6) is a pleiotropic cytokine involved in inflammation [7,8]. In humans, IL-6 levels are correlated with severity or pathogenesis of allergic diseases including asthma [9–12], urticaria [13,14], and mastocytosis [15,16] in which mast cells play a critical role. The exact role of IL-6 in allergic disease is not known, but IL-6 has been shown to modulate the development, survival, proliferation, adhesion, and chemokinesis of in vitro-derived mast cells [17–20]. In fact, IL-6 is routinely added to cultures of human cord blood CD34+ progenitors together with stem cell factor (SCF) to promote mast cell development in vitro [17,18,21,22]. Recently, it was shown that prolonged exposure to IL-6 could enhance FcεRI-induced degranulation of cord blood-derived mast cells (CBMCs) [23]. Thus, IL-6 appears to contribute to allergic disease by regulating mast cell development and reactivity.
The IL-6 receptor (IL-6R) is comprised of the shared signaling subunit gp130 and the IL-6 binding subunit IL-6Rα [7,24,25]. Gp130 (130 kDa) is a signal transducing receptor subunit shared by members of the IL-6 family of cytokines that includes IL-6, IL-27, leukemia inhibitory factor, oncostatin M, ciliary neurotrophic factor, cardiotrophin 1, cardiotrophin-like cytokine, IL-11, and IL-35. IL-6Rα exists as transmembrane (80 kDa) and soluble (sIL-6Rα, 50 – 55 kDa) forms due to alternative splicing or proteolytic cleavage [26]. IL-6 can signal through membrane-bound IL-6Rα/gp130 complexes (classical signaling), or, alternatively, IL-6 can bind sIL-6Rα to form a soluble IL-6/sIL-6Rα complex that then binds to surface-expressed gp130 (trans-signaling). In this way, IL-6 can induce signaling in cells that do not express membrane-bound IL-6Rα on their surface. The expression of membrane-bound IL-6Rα and gp130 on in vitro-derived mast cells from human cord blood has been shown [17,23], but expression on human tissue-isolated mast cells had not been determined.
IL-6-induced gp130 signals JAK kinases [27] leading to phosphorylation and activation of Signal Transducer and Activator of Transcription 3 (STAT3), which has been implicated in mast cell degranulation and anaphylaxis [28–30]. STAT-3 induces transcription of Suppressor of Cytokine Signaling 3 (SOCS3), which serves to inhibit IL-6R signals [31] and regulate Th2-mediated allergic disease [32]. Although STAT3 has been implicated as positive regulator of degranulation, the effect of IL-6R classical signaling on mediator release from human in situ-matured mast cells had not been reported.
In this study, we determined that human skin mast cells constitutively express membrane-bound IL-6Rα and gp130 that can form functional receptors for IL-6, and investigate the effect of IL-6R classical signaling on FcεRI-induced effector function. Overall, the findings indicate that IL-6 contributes to allergic inflammation in humans by potentiating FcεRI-induced PGD2 biosynthesis from mast cells, and suggests a role for IL-6 in mast cell-mediated angiogenesis.
2. Materials and Methods
2.1 Isolation, purification, and culture of human skin mast cells
Human skin mast cells were isolated and purified from fresh surgical specimens of human skin tissue that were obtained from the Cooperative Human Tissue Network of the National Cancer Institute, as approved by the human studies Internal Review Board at University of South Carolina. The tissue was mechanically disrupted with surgical scissors and then digested 3 × 1 h at 37°C with collagenase type II (Worthington Biochemical, Lakewood, NJ), hyaluronidase from bovine testes, and DNase I (Sigma-Aldrich, St. Louis, MO) in HBSS wash buffer (1X HBSS, 0.04% NaHCO3, 1% fetal bovine serum, 1% HEPES, 0.1% CaCl2) containing amphotericin B and Antibiotic/Antimycotic solution. After each digestion period, the samples were filtered through 40 μm nylon cell strainers. The filtered cells were collected by centrifugation, washed and re-suspended with wash buffer. After the final digestion, the collected cells were separated on Percoll by density centrifugation. The cells at the interface of buffer and Percoll layers were collected, washed and re-suspended at 5×105 cells/ml in serum-free X-VIVO 15™ media (Lonza, Walkersville, MD) containing recombinant human stem cell factor (SCF, 100 ng/ml) (PeproTech, Rocky Hill, NJ). The cells were transferred onto 24-well plates and maintained under standard culture conditions (37°C, 5% CO2) with weekly media changes. Purity was assessed by metachromatic staining with acidic toluidine blue, and by immunofluorescence staining for FcεRI expression with PE-labeled anti-human FcεRI antibody (clone AER-37 (CRA)) and mouse IgG2bk isotype control (BioLegend, San Diego, CA). Greater than 95% purity was achieved by 6 weeks of culture, and the mast cells were used thereafter.
2.2 IgE sensitization and FcεRI crosslinking
Mast cells (106 cells/ml) were incubated in X-VIVO 15™ media containing SCF (100 ng/ml) and 1 μg/ml chimeric human anti-NP IgE (human Fc + mouse Fab) (clone JW8/1)(AbD Serotec, Raleigh, NC) overnight at 37°C, 5% CO2. After washing to remove unbound IgE, the mast cells were re-suspended at 106 cells/ml in X-VIVO 15™ media or Tyrode’s buffer (135 mM NaCl, 1 mM MgCl2, 20 mM Hepes, 5 mM KCl, 1.8 mM CaCl2, 5.6 mM glucose; pH 7.4, 0.05% bovine serum albumin), and activated with the hapten 4-hydroxy-3-nitrophenylacetyl conjugated to bovine serum albumin at a 16:1 molar ratio (NP-BSA; Biosearch Technologies, Novato, CA) at the indicated concentration at 37°C for the indicated amount of time.
2.3 PGD2 and degranulation assays
Degranulation was measured by β-Hexosaminidase release assay. After sensitization with anti-NP IgE, the mast cells were washed and resuspended at 106 cells/ml in Tyrode’s buffer, pre-treated as describe in figure legends, and activated with NP-BSA for 30 min. After the activation period, mast cells and buffer were separated by centrifugation (2000 rpm × 5 min), and the pelleted cells were lysed with an equal volume of 1% Triton X-100. β-Hexosaminidase activity in supernatant and cell lysate was determined by the release of p-nitrophenol from substrate p-nitrophenyl N-acetyl-β-D-glucosaminide (pNAG; Sigma-Aldrich, St. Louis, MO) as described [33,34]. In a 96-well plate, 5 μl of supernatant or lysate were mixed with 45 μl of 4mM p-Nitrophenyl N-acetyl-β-D-Glucosaminide (pNAG) in citric acid buffer (pH 4.5) and incubated for 1 h at 37°C. The reaction was stopped with 150 μl of 0.2 M glycine, pH 10.7. Absorbance values at 405 nm were acquired with a BioTek Synergy HT microplate reader (BioTek, Winooski, VT). Percent β-hexosaminidase release was calculated from the absorbance values according to the formula: % β-hexosaminidase release = ((supernatant)/(supernatant + lysate)) × 100. PGD2 in the supernatants was measured by commercial assay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol.
2.4 Quantitative Real Time PCR
Gene expression was determined by quantitative real time PCR. RNA was isolated with the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized from total RNA with iScript cDNA Synthesis kit, and PCR was performed using iQ SYBR® Green Supermix (Bio-Rad, Hercules, CA). The kits were used according to the manufacturer’s instructions. The PCR reaction mix was composed of 2 μl of cDNA, 1 μl each of sense and antisense primers (10 μM each) and 12.5 μl of iQ SYBR® Green Supermix in a final volume of 25 μl. A hot-start PCR protocol (95°C × 5 min, (95°C × 30 sec, 55°C × 30 sec, 72°C × 30 sec) × 35 cycles, 95°C × 1 min, 55°C × 1 min) was performed on a CFX Connect Real Time PCR Detection System (Bio-Rad, Hercules, CA). Fold change in expression was determined by the 2ΔΔCt method with β2 microglobulin (B2M) as the reference gene. The oligonucleotide primers used were (5′-3′; forward and reverse): VEGF (CCATGCAGATTATGCGGATCAAA and CACCAACGTACACGCTCCAG), SOCS3 (GCTCCAAGAGCGAGTACCAG and CTGTCGCGGATCAGAAAGGT), gp130 (TCT GGGAGTGCTGTTCTGCTT and TGTGCCTTGGAGGAGTGTGA), IL-6Rα (CTCATCTTTCTACAGACTACG and GACCATCCATGTTGTGAATG), COX-1 (TCTTGCTGTTCCTGCTCCTG and GTTGGAGCGCACTGTGAGTA), COX-2 (ACTGCTCAACACCGGAATTT and CAAGGGAGTCGGGCAATCAT), and B2M (TGGGTTTCATCCATCCGACA and CTGCTTACATGTCTCGATCCC).
2.5 Western blotting
106 mast cells were lysed with 0.1 ml Tris-Glycine SDS Sample Buffer (Life Technologies, Carlsbad, CA) containing 1% β-mercaptoethanol and 1 mM Na3VO4. Protein equivalents of 5×105 cells/lane were separated by SDS-PAGE, and then transferred onto nitrocellulose membranes. After transfer, the membranes were blocked for 1 h at room temperature with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE). Two-color staining for total and phosphorylated STAT3 was performed with the primary antibodies Stat3 (79D7) rabbit mAb and phospho-STAT3 (Tyr705)(3E2) mouse mAb or phospho-STAT3 (Ser727)(6E4) mouse mAb (Cell Signaling Technology, Danvers, MA), and secondary antibodies goat anti-rabbit IRDye 680RD and goat anti-mouse 800CW (LI-COR Biosciences, Lincoln, NE). The blots were incubated overnight at 4°C with primary antibodies, and for 1 h at room temperature with secondary antibodies. The blots were scanned on an Odyssey® CLx Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
2.6 Flow Cytometry
Human skin mast cells (106) were washed with FACS buffer (1% BSA, 0.04% NaN3 in PBS), and Fc receptors were blocked with Human TruStain FcX™ (5 μl/106 cells) (BioLegend, San Diego, CA) and 1% normal human serum for 15 min on ice to prevent non-specific binding. The mast cells were then stained with PE-labeled anti-human CD130 (gp130) (clone 2E1B02), CD126 (IL-6Rα) (clone UV4), FcεRIα (clone AER-37 (CRA-1)), or respective isotype control, mouse IgG2a,k, IgG1,k, IgG2b,k (BioLegend, San Diego, CA) for 1 h on ice. After washing twice, the mast cells were re-suspended in 200 μl FACS buffer and run on a Beckman Coulter Cytomics FC500. Data was analyzed with FlowJo software (FlowJo LLC, Ashland, OR).
2.7 Cytokine Measurements
Mast cells (106 cells/ml) were sensitized with anti-NP IgE, pre-treated or not with IL-6 as indicated, and activated with NP-BSA for 24 h at 37°C in X-VIVO 15™ media containing SCF (100 ng/ml) and soybean trypsin inhibitor (100 μg/ml) (SBTI; Sigma-Aldrich, St. Louis, MO). After the activation period, mast cells and media were separated by centrifugation (2000 rpm × 5 min), and secreted cytokines in supernatant were measured by enzyme-linked immunosorbent assay (ELISA). VEGF was measured using commercial kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocol. TNF and IL-8 were measured using capture (purified) and detection (biotinylated) rat antibodies, and serially diluted recombinant cytokine standards for standard curves (BD Biosciences, San Jose, CA) in a 384-well format as described [35]. The plates were developed with the substrate peroxidase 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS; Sigma-Aldrich, St. Louis, MO). Absorbance values at 405 nm were obtained with a BioTek Synergy HT microplate reader (BioTek, Winooski, VT), and cytokine concentrations determined using Gen5 Data Analysis Software.
2.8 Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 6.0c for Mac OS X, GraphPad Software, La Jolla California USA, www.graphpad.com
3. Results
3.1 Human skin mast cells constitutively express membrane-bound gp130 and IL-6Rα
The IL-6 receptor is comprised of gp130 and IL-6Rα subunits. Gp130 is a membrane bound protein whereas IL-6Rα exists as membrane-bound and secreted forms. Both gp130 and IL-6Rα have been shown expressed on in vitro-derived mast cells [17,23], but their expression on human in situ-matured mast cells had not been reported. Here, we demonstrate by flow cytometry that human skin mast cells constitutively express gp130 (Figure 1A) and membrane-bound IL-6Rα (Figure 1C) on their surface. Net Mean Fluorescence Intensity (MFI) of gp130 staining was 778 ± 89 compared to 204 ± 9 for IgG isotype control (n=3, p=0.003) (Figure 1B), and of IL-6Rα staining was 937±37 compared to 722.5 ± 7.5 for IgG isotype control (n=3, p=0.03) (Figure 1D). Because mouse bone marrow-derived mast cells (BMMCs) reportedly require IL-10 for gp130 expression [36], we determined the effect of IL-10 on gp130 expression on human skin mast cells. Interestingly, treatment with IL-10 (10 ng/ml) for 24 h resulted in a significant increase in gp130 mRNA expression (3.5 ± 0.7 fold change, n=3, p=0.02) (Figure 1E), but did not enhance the surface expression of gp130 (Figure 1F). Thus, primary in situ-matured mast cells from human skin constitutively express gp130 and membrane bound IL-6Rα.
Figure 1. Human skin mast cells constitutively express membrane-bound IL-6Rα and gp130.
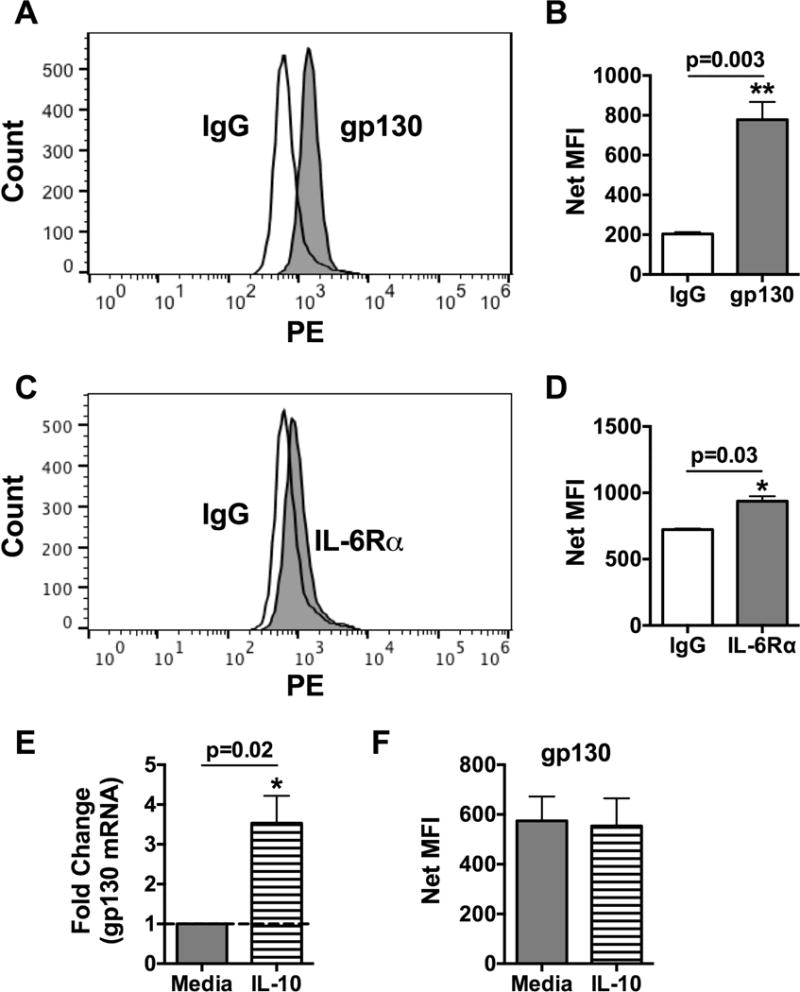
Human skin mast cells at rest were stained with PE-labeled anti-gp130 or anti-IL-6Rα and analyzed by flow cytometry (A, C). Significance was determined by analysis of Mean Fluorescence Intensity (MFI) (B, C). The effect of IL-10 on gp130 expression was determined by culturing human skin mast cells with IL-10 (10 ng/ml) for 24 h, and analyzing gp130 gene expression by quantitative real-time PCR (E) and surface expression by flow cytometry (F). Bars represent mean ± S.E.M. of values from 3 separate experiments with mast cells from different donors. Significance was determined with Student’s t-test. *, p<0.05 and **, p<0.01.
3.2 Human skin mast cells express functional membrane bound receptors for IL-6 capable of transmitting potent IL-6-induced signals via classical signaling
The constitutive expression of gp130 and membrane bound IL-6Rα on human skin mast cells suggested that these cells could be directly stimulated by IL-6 via classical signaling. To determine if gp130 and IL-6Rα subunits formed functional signaling complexes, we analyzed the phosphorylation status of Signal Transducer and Activator of Transcription 3 (STAT3), a known target of gp130 signals that is implicated in mast cell degranulation and anaphylaxis reactions [28–30], in human skin mast cells stimulated with IL-6. As demonstrated with Western blot, stimulation of human skin mast cells for 15 minutes with 1, 10 or 100 ng/ml of recombinant IL-6 induced a dose-dependent increase in STAT3 phosphorylation (Figure 2A, left side). For comparison, we stimulated skin mast cells in parallel with IL-27, which also utilizes gp130 and has been implicated in the regulation of mast cells and human allergic disease [37–40]. As expected, IL-27 induced STAT3 phosphorylation (Figure 2A, right side). However, STAT3 phosphorylation induced with IL-6 was significantly and dramatically greater than that induced by IL-27 (Figure 2B); thus, demonstrating the potency of IL-6 induced gp130 signals. Next, we analyzed the expression of Suppressor of Cytokine Signaling 3 (SOCS3), a downstream target of STAT3, in human skin mast cells stimulated with IL-6 or IL-27. In time-course experiments, SOCS3 mRNA expression was rapidly induced, peaking within 1h of treatment with 100 ng/ml of IL-6 (Figure 2C) or IL-27 (Figure 2D). Reflective of the difference in intensity of STAT3 phosphorylation induced by IL-6 or IL-27, the IL-6-induced increase in SOCS3 mRNA was dramatically greater than that induced with IL-27 at 1 h post-treatment (362 ± 128 versus 9 ± 0.3). By 3 h post-treatment, the SOCS3 mRNA level in IL-27-treated mast cells had returned to baseline levels, and in IL-6-treated mast cells was considerably reduced with continued temporal regression. Thus, human skin mast cells express functional membrane-bound IL-6 receptors capable of transmitting highly potent gp130 signals via direct stimulation with IL-6 (classical signaling).
Figure 2. IL-6 induces STAT3 phosphorylation and SOCS3 expression via classical signaling in human skin mast cells.
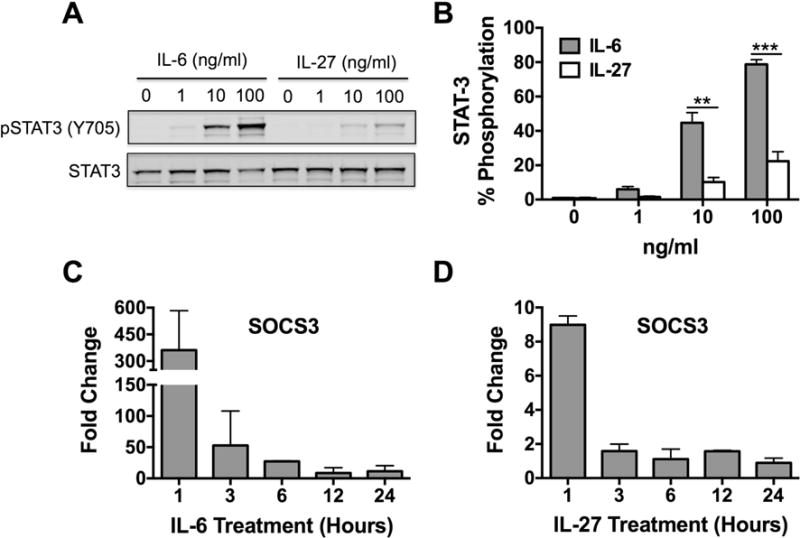
To determine the functionality of membrane-bound gp130 and IL-6Rα, STAT3 phosphorylation and SOCS3 expression was analyzed in IL-6-treated human skin mast cells. For comparison, parallel studies were performed with IL-27, which also utilizes gp130. STAT3 phosphorylation was determined by SDS-PAGE and Western blotting of whole cell lysates prepared from skin mast cells treated with IL-6 or IL-27 at 1, 10, or 100 ng/ml for 15 min (A). STAT3 phosphorylation was quantified with Western blot band intensities (B). SOCS3 expression was determined by quantitative real-time PCR of total RNA from skin mast cells treated with IL-6 (100 ng/ml) (C) or IL-27 (D) for 1, 3, 6, 12, or 24 h. Fold change was calculated using the 2ΔΔCt method with B2M as the reference gene. >2-fold change was considered significant. Bars represent mean ± S.E.M. of values from 3 separate experiments with mast cells from different donors. Significance was determined with Student’s t-test. **, p<0.01 and ***, p<0.001. Representative blot is shown.
3.3 IL-6 has no effect on FcεRI-induced degranulation of human skin mast cells
IL-6 was recently shown to enhance the degranulation of in vitro-derived CBMCs [23]. Therefore, given the association between severity of allergic disease and IL-6 in humans, we sought to determine the effect of IL-6 on FcεRI-induced degranulation of human in situ-matured mast cells. Human skin mast cells were sensitized with chimeric human anti-NP IgE, pre-treated with 1, 10 or 100 ng/ml of recombinant human IL-6 for 1 h, and then challenged with NP-BSA (100 ng/ml) for 30 min. Degranulation was determined by β-hexosaminidase assay. As demonstrated, IL-6 had no effect on FcεRI-induced degranulation of human skin mast cells (Figure 3A). In the converse set of experiments, IgE-sensitized human skin mast cells were pre-treated or not with IL-6 at a high concentration (100 ng/ml), and then challenged with 0.1, 1, 10, or 100 ng/ml of NP-BSA. As shown, IL-6 failed to enhance degranulation induced with a dose range of NP-BSA representing sub-maximal to maximal levels of FcεRI crosslinking (Figure 3B). Noteworthy, IL-6 failed to enhance degranulation of human skin mast cells even at a concentration (100 ng/ml) that strongly induced the phosphorylation STAT3 (Figure 2A), which has itself been implicated in mast cell degranulation [28–30]. Since human MCTC mast cells are known to spontaneously secrete IL-6 (Figure 3C) [41], and prolonged exposure to IL-6 was reported to enhance degranulation of in vitro-derived cord blood-derived mast cells (CBMCs) [23], we sought to determine if the potential or capacity of skin mast cells for degranulation was somehow affected by endogenous IL-6. To address this, we cultured human skin mast cells with neutralizing anti-IL-6 antibody (1 μg/ml) or isotype control for up to 3 days, and measured FcεRI-induced degranulation at daily intervals. As shown, β-hexosaminidase release from mast cells cultured with neutralizing anti-IL-6 antibody was nearly identical to that from mast cells cultured with non-specific IgG control or media alone at each time-point (Figure 3D). Thus, gp130 signaling initiated by exogenous IL-6 does not enhance or inhibit FcεRI-induced degranulation of human skin mast cells. Moreover, long term exposure to endogenously produced IL-6 does not appear to influence the capacity or potential of human skin mast cells for degranulation.
Figure 3. IL-6 does not induce or enhance FcεRI-induced degranulation of human skin mast cells.
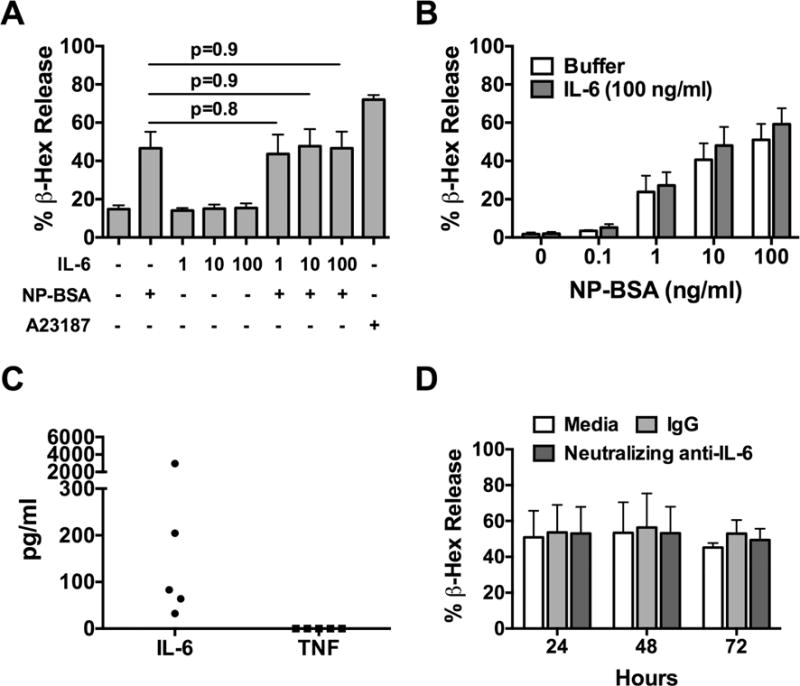
Human skin mast cells were sensitized with anti-NP IgE, and challenged with NP-BSA for 30 min. Degranulation was determined with β-hexosamindase release assay. (A) Dose-dependent effect of IL-6 on FcεRI-induced degranulation induced with NP-BSA (100 ng/ml). (B) Effect of IL-6 (100 ng/ml) on degranulation induced with NP-BSA at 0.1, 1, 10, or 100 ng/ml. (C) Spontaneous release of IL-6. The amount of IL-6 and TNF in 7-day culture media of mast cells was quantified with ELISA. Each data point represents mast cells from a single donor (n=5). (D) Effect of endogenously produced IL-6 on degranulation. Mast cells were cultured with neutralizing anti-IL-6 mAb or isotype control (1 μg/ml) for 24, 48, or 72 h, and degranulation was determined. Bars represent mean ± S.E.M. of values from at least 3 different experiments with mast cells from different donors. Analysis was determined by Student’s t-test.
3.4 IL-6 potentiates FcεRI-induced PGD2 biosynthesis
Mast cells are major producers of PGD2 [42], a critical component of allergic airways inflammation [43]. Since IL-6 had no effect on mast cell degranulation, we sought to determine the effect on FcεRI-induced PGD2 biosynthesis. To do so, IgE-sensitized human skin mast cells were pre-treated with 1, 10, or 100 ng/ml of IL-6 for 1 h, and then challenged with NP-BSA (100 ng/ml) for 30 min. PGD2 in cell-free media was quantified by enzyme immunoassay. As shown, IL-6 significantly and dose-dependently enhanced the amount of PGD2 secreted from FcεRI-activated human skin mast cells (Figure 4A). In the converse set of experiments, IgE-sensitized human skin mast cells were pre-treated or not with IL-6 (100 ng/ml) and then challenged with 0.1, 1, 10, or 100 ng/ml of NP-BSA. As shown, mast cells treated with IL-6 produced more PGD2 than control mast cells with significant differences detected with NP-BSA at 10 ng/ml or 100 ng/ml (Figure 4B). Thus, IL-6 potentiates FcεRI-induced PGD2 biosynthesis from human skin mast cells.
Figure 4. IL-6 potentiates FcεRI-induced PGD2 biosynthesis from human skin mast cells.
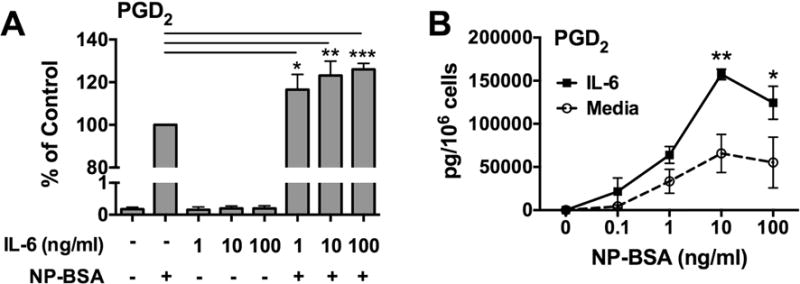
To determine the effect of IL-6 on PGD2 production, human skin mast cells were sensitized with anti-NP IgE, pre-treated with IL-6 for 1 h, and then challenged with NP-BSA for 30 min. IgE-sensitized human skin mast cells were pre-treated with IL-6 at 1, 10, or 100 ng/ml, and then challenged with NP-BSA (A), or pre-treated with IL-6 (100 ng/ml) and challenged with NP-BSA at 1, 10, or 100 ng/ml (B). Secreted PGD2 in cell-free supernatants was measured by enzyme immunoassay. Bars represent mean ± S.E.M. of values from at least 3 separate experiments with mast cells from different donors. Significance was determined with one-way ANOVA. *, p<0.05; **, p<0.01.
3.5 IL-6 enhances FcεRI-induced COX-2 expression and PGD2 production by a STAT3-dependent mechanism
To begin to understand the mechanism, we analyzed the effect of IL-6 on expression of COX-1 and COX-2, key enzymes in the eicosanoid pathway leading to prostaglandin biosynthesis [44]. Human skin mast cells were sensitized with anti-NP IgE, pretreated with 100 ng/ml or a dose range of 1 – 100 ng/ml of IL-6 for 1 h, and then activated with NP-BSA (100 ng/ml) for 1 h. Changes in expression of COX-1 and COX-2 were determined by quantitative real time PCR. As expected since COX-2 is an inducible enzyme whereas COX-1 is constitutively expressed, the expression of COX-2, but not COX-1, was induced with FcεRI crosslinking (Figure 5A and C). In accordance with the ability to potentiate PGD2 biosynthesis (Figure 4), IL-6 significantly enhanced the FcεRI-induced expression of COX-2 (Figure 5A and B). IL-6 alone had no effect on the expression of either enzyme. Noteworthy, IL-6 had no effect on expression of hematopoietic prostaglandin D synthase (HPGDS), which catalyzes the conversion of prostaglandin H2 (PGH2) to PGD2 [45], or RasGRP4, which regulates HPGD2 expression in mast cells [46] (not shown).
Figure 5. IL-6 enhances FcεRI-induced COX-2 expression and PGD2 biosynthesis by a STAT3-dependent mechanism in human skin mast cells.
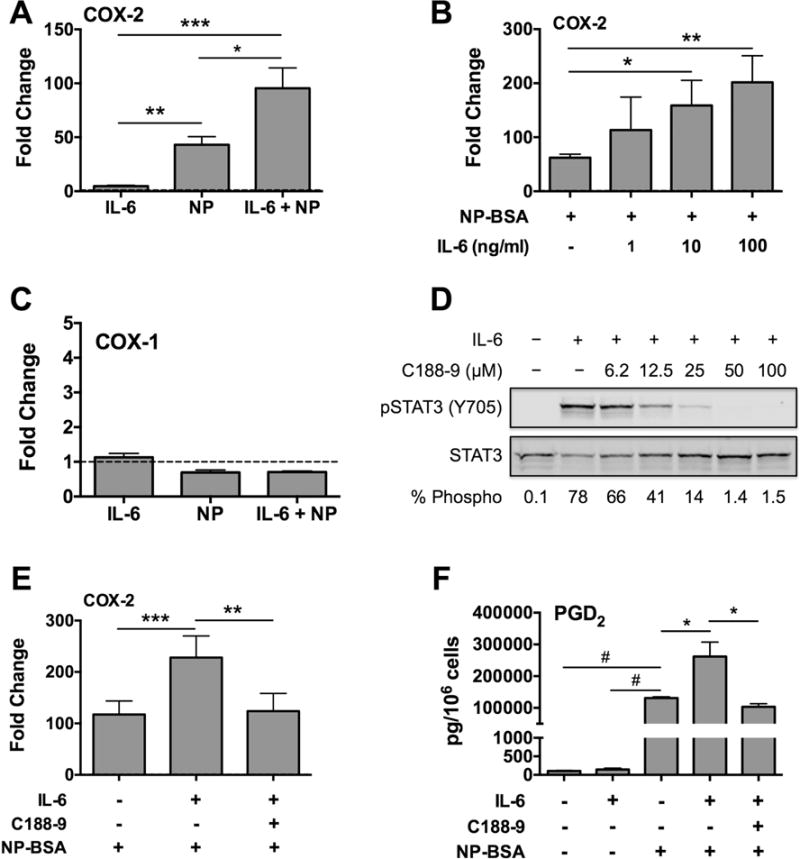
IgE-sensitized mast cells were pre-treated with IL-6 (100 ng/ml) for 1 h, and challenged with NP-BSA (100 ng/ml) for 1 h. The effect of IL-6 on FcεRI-induced expression of COX-2 (A, B) and COX-1 (C) was determined by quantitative real-time PCR. (D) Western blot demonstrating the efficacy of C188-9 to inhibit IL-6-induced STAT3 phosphorylation. (E) Quantitative real-time PCR analysis showing that C188-9 inhibits IL-6-enhanced COX-2 expression. (F) Inhibition of IL-6-induced potentiation of FcεRI-induced PGD2 biosynthesis with C188-9 (25 μM). Graph bars represent mean ± S.E.M. of values from 3 separate experiments with mast cells from different donors. Significance was determined with one-way ANOVA. *, p<0.05; **, p<0.01; ***, p<0.01; #, p<0.001.
To confirm that the IL-6-induced increase in COX-2 and PGD2 was due to STAT3 activation, we used the small molecule inhibitor of STAT-3 tyrosine phosphorylation C188-9 [47]. First, we determined the efficacy of C188-9 to inhibit IL-6-induced STAT3 phosphorylation at the activating tyrosine 705 in human skin mast cells. In dose curve experiments, human skin mast cells were pre-treated or not with C188-9 (6.25 to 100 μM) for 1 h, and then stimulated with IL-6 (100 ng/ml) for 15 min. STAT3 phosphorylation was determined by Western blot analysis of whole cell lysates. As shown, C188-9 dose-dependently inhibited the IL-6-induced phosphorylation of STAT3 at the Y705 (Figure 5D). Next, we sought to determine if C188-9 would inhibit the IL-6-induced enhancement of FcεRI-induced COX-2 expression. Human skin mast cells were sensitized with anti-NP IgE, pre-treated with C188-9 (25 μM) for 1 h, and then stimulated with IL-6 (100 ng/ml) for 1 h. The mast cells were then activated with NP-BSA (10 ng/ml) for an additional 1 h. As demonstrated by quantitative real-time PCR, C188-9 completely inhibited the IL-6-induced increase in COX-2 expression (Figure 5E). Similarly, C188-9 was shown to be highly effective at inhibiting the IL-6-induced biosynthesis of PGD2 (Figure 5F). Together, these data confirm that the enhancing effect of IL-6 on FcεRI-induced PGD2 biosynthesis and COX-2 expression was indeed due to STAT3 activation.
3.6 IL-6 induces VEGF production and enhances FcεRI-induced IL-8 secretion
The ability of mast cells to produce VEGF is abundantly documented [48]. Having shown that human skin mast cells express functional membrane-bound IL-6Rα and gp130, we sought to determine if classical signaling with IL-6 could induce or enhance FcεRI-induced VEGF production from these cells. To do so, human skin mast cells were sensitized with anti-NP IgE, pre-treated with 1, 10, or 100 ng/ml of IL-6 for 1 h, and then challenged with NP-BSA (100 ng/ml) for 24 h. Secreted VEGF, IL-8 and TNF in supernatants were measured with ELISA. As shown, IL-6 alone induced a significant increase in secretion of VEGF compared to untreated skin mast cells (Figure 6A). A similar increase in VEGF secretion was also observed from FcεRI-activated mast cells pre-treated with IL-6 indicating an additive rather than potentiating effect. To confirm that VEGF production was induced directly with IL-6, we treated non-sensitized human skin mast cells with IL-6 (100 ng/ml), and measured changes in VEGF mRNA expression at 1, 3, 6, 12, and 24 h by quantitative real-time PCR. As shown, VEGF mRNA expression was increased >3-fold at 6 hours post IL-6, and remained elevated at 12 and 24 h compared to untreated mast cells (Figure 6B). We further demonstrate that the IL-6-induced production of VEGF was inhibited with C188-9, the inhibitor of STAT3 phosphorylation at the activating tyrosine 705 (Figure 6C). Thus IL-6 alone can induce VEGF production by a STAT3-dependent mechanism in human in situ-matured mast cells. IL-6 alone did not induce IL-8 production, but significantly enhanced FcεRI-induced IL-8 secretion (Figure 6D). In contrast, IL-6 had no effect on production of TNF (Figure 6E).
Figure 6. IL-6 induces VEGF synthesis and enhances FcεRI-induced IL-8 production in human skin mast cells.
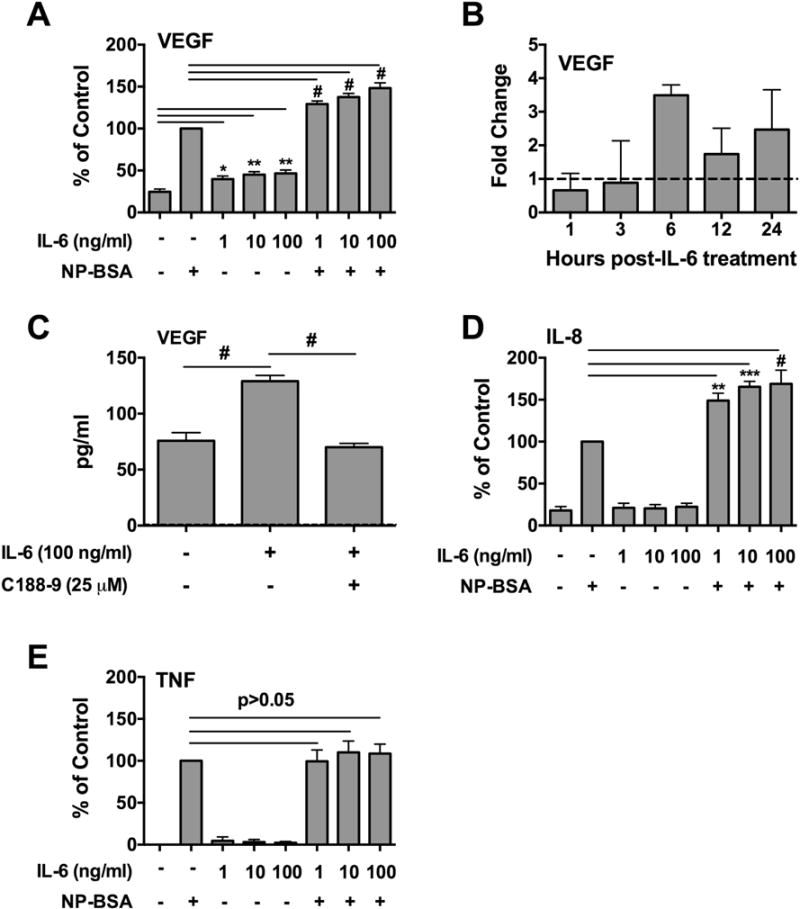
Human skin mast cells were sensitized with anti-NP IgE, pre-treated with IL-6 at 1, 10, or 100 ng/ml for 1 h, and then challenged with NP-BSA (100 ng/ml) for 24 h. Secreted VEGF (A, C), IL-8 (D) and TNF (E) in cell-free media were measured with ELISA. (B) IL-6-induced VEGF mRNA expression was analyzed by quantitative real-time PCR using total RNA isolated from mast cells treated with IL-6 (100 ng/ml) for 1, 3, 6, 12, and 24 h. >2-fold change was considered significant. (C) C188-9 inhibits IL-6-induced VEGF production. Bars represent mean ± S.E.M. of values from 3 separate experiments with mast cells from different donors. Significance was determined by ANOVA. *, p<0.05, **, p<0.01, ***, p<0.001, #, p<0.0001.
3.7 FcεRI and gp130 signals synergize to enhance STAT3 phosphorylation
STAT3 phosphorylation occurs at two main residues: tyrosine 705 and serine 727. Initial phosphorylation at Y705 activates STAT3 [49,50], whereas phosphorylation at S727 is required for maximal transcriptional activity of STAT3 [51]. To determine the effect of FcεRI signaling on IL-6-induced STAT3 phosphorylation, human skin mast cells were sensitized with anti-NP IgE, and treated with IL-6 (100 ng/ml), NP-BSA (100 ng/ml), or IL-6 + NP-BSA for 15 min. STAT3 phosphorylation at Y705 and S727 was determined by Western blot analysis of whole cell lysates (Figure 7). As expected, IL-6 alone strongly phosphorylated STAT3 at Y705, but, surprisingly, did not phosphorylate S727. Conversely, FcεRI signals alone did not phosphorylate STAT3 at Y705, but, interestingly, did phosphorylate S727 albeit at low but detectable levels. Importantly, phosphorylation of STAT3 at both Y705 and S727 was increased in human skin mast cells treated with IL-6 + NP-BSA. Thus, signals from FcεRI and gp130 synergize to enhance STAT3 phosphorylation at both Y705 and S727.
Figure 7. FcεRI and gp130 signals synergize to enhance STAT3 phosphorylation in human skin mast cells.
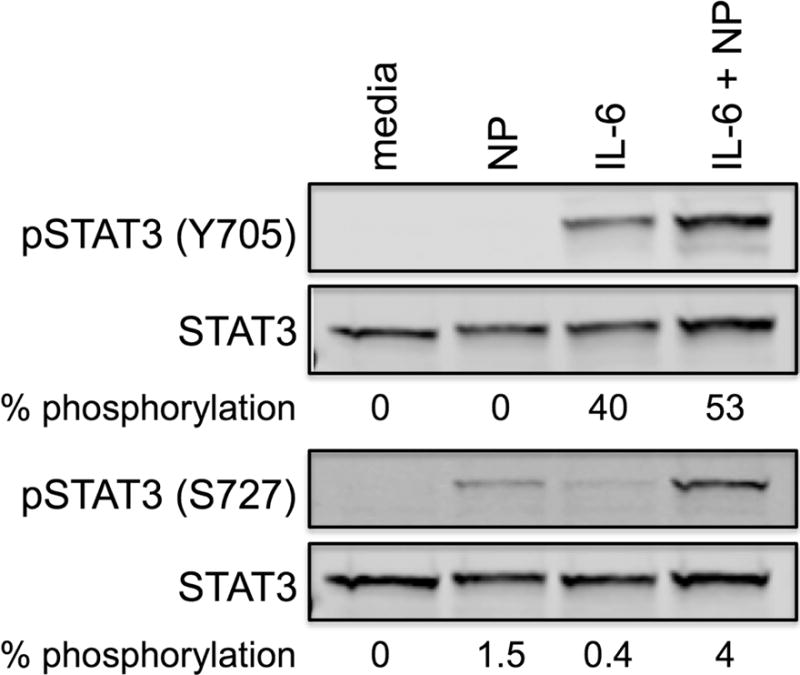
Western blot showing phosphorylation of STAT3 at tyrosine 705 and serine 727. Human skin mast cells were sensitized with anti-NP IgE, and then treated with IL-6 (100 ng/ml), NP-BSA (100 ng/ml), or IL-6 + NP for 15 min. STAT3 phosphorylation was determined by SDS-PAGE and Western blot analysis of whole cell lysates. Blot is representative of two separate experiments with mast cells from different donors.
4. Discussion
Here, we show that human skin mast cells constitutively express membrane bound IL-6Rα and gp130 on their surface capable of forming a functional IL-6R, and demonstrate for the first time that IL-6-induced classical signals act to potentiate FcεRI-induced PGD2 biosynthesis. Supporting this observation, we found that IL-6 treatment enhanced the FcεRI-induced expression of COX-2, which is directly involved in PGD2 biosynthesis. Moreover, the IL-6-induced enhancement in COX-2 expression was prevented with C188-9, a small molecule inhibitor of STAT3 phosphorylation. To our knowledge, this is the first demonstration that IL-6R classical signals can potentiate IgE-dependent PGD2 production from human tissue-resident mast cells. These novel findings suggest that IL-6, whose levels are consistently correlated with severity of allergic disease, contribute to allergic inflammation by enhancing the production of inflammatory PGD2. For example, given that mast cells are significant producers of PGD2, a major contributor to allergic asthma [43], our findings suggest that IL-6 contributes to asthma severity in humans by acting on mast cells via IL-6R classical signals to increase PGD2 production.
In contrast to a recent study showing that IL-6 could enhance degranulation of in vitro-derived mast cells [23], we found no evidence to support an enhancing effect of IL-6 on degranulation of human in situ-matured skin mast cells. This is particularly interesting because STAT3, which was strongly phosphorylated by IL-6 in human skin mast cells, has been implicated in mast cell degranulation [28,29]. It is possible that STAT3 is not as critical for degranulation as believed. In fact, it was reported that degranulation of human mast cells is dependent on STAT3, whereas degranulation of mouse mast cells is independent of STAT3 [30]. If STAT3 were critical for degranulation, it is expected that both human and mouse would be equally dependent on STAT3 for degranulation. The reason for the different response of human in vitro-derived CBMCs and in vivo-matured skin mast cells to IL-6 is not clear, but could be due to differences in maturation stage and/or developmental environment. Of note, IL-6 is routinely added to CBMC cultures to promote mast cell development in vitro, but is not required to maintain human skin mast cells ex-vivo. In addition, IL-6 could have different effects on human mast cells from different tissue. For example, IL-6 was shown to protect human lung MCT mast cells from IL-4-induced apoptosis, but had no protective effect on MCTC mast cells from lung or skin [41]. Therefore, it is of interest to determine if IL-6 enhances degranulation of human lung MCT mast cells similar to that reported for in vitro-derived CBMCs. Thus, IL-6 could differentially impact human mast cells at different developmental stages. Further investigation is required to firmly establish STAT3 as a regulator of mast cell degranulation.
Mast cells have been shown to localize around tumors [5], and are implicated in the development of various cancers [5,6]. However, their exact role in classically non-allergic diseases is not known. Moreover, it is not entirely clear how mast cells become activated to participate in tumor development or progression. However, it is well documented that mast cells produce pro-angiogenesis VEGF and express VEGF receptors [48]. VEGF production from human mast cells can be induced not only by FcεRI crosslinking, but with prostaglandin E2 (PGE2) [52], adenosine [53], and IL-9 [54]. Our novel finding that IL-6 alone can induce VEGF production and enhances FcεRI-induced IL-8 from human skin mast cells adds to the growing list of substances that can trigger the production of VEGF and other angiogenesis-related factors from mast cells. The fact that skin mast cells spontaneously secrete IL-6 (Figure 3C) [41], suggests a possible autocrine mechanism in which mast cell-secreted IL-6 induces VEGF production, and also recruits other mast cells to the site of inflammation where additional pro-angiogenesis factors can be produced. Thus, these findings suggest that IL-6 could be a link that connects mast cells to traditionally non-allergic diseases like cancer.
The ability of IL-6 to induce (i.e. VEGF) or enhance (i.e. IL-8) some cytokines but not others (i.e. TNF) is interesting. Although the data presented here cannot explain why different cytokines respond differently to IL-6 ± FcεRI stimulation, we speculate that the differential response is due to expression of different response elements within the promoter regions of the cytokine genes. Further studies will be necessary to determine why some cytokines but not others can be induced with IL-6.
The novel findings from this study are that IL-6 potentiates FcεRI-induced PGD2 biosynthesis, and induces VEGF production, from human in situ-matured skin mast cells. These findings indicate that IL-6 contributes to allergic disease in humans by increasing the amount of inflammatory PGD2 produced from tissue-resident mast cells, and suggest a role for IL-6 in mast cell-mediated angiogenesis.
General Significance.
These findings from this study indicate that IL-6 contributes to human allergic disease by enhancing the production of inflammatory PGD2 from tissue-resident mast cells. Moreover, the data suggest a novel role for IL-6 in mast cell-mediated angiogenesis.
Highlights.
Human skin mast cells constitutively express membrane-bound IL-6Rα and gp130.
IL-6 potentiates FcεRI-induced PGD2 biosynthesis in human skin mast cells.
IL-6 enhances FcεRI-induced COX-2 expression and PGD2 biosynthesis by a STAT3 dependent mechanism.
IL-6 induces de novo production of VEGF in human skin mast cells by a STAT3 dependent mechanism.
IL-6 enhances FcεRI-induced IL-8 production from human skin mast cells.
Acknowledgments
This study was supported by Research Development Fund Grant from USC School of Medicine (GG), Magellan Scholar Grant from Office of Undergraduate Research (JD), American Association of Immunologists Careers in Immunology Fellowship (GG and CM), and National Institutes of Health grant 1P20GM103641 (GG).
Abbreviations
- IgE
Immunoglobulin E
- PGD2
Prostaglandin D2
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- IL-6
interleukin-6
- VEGF
vascular endothelial growth factor
- CBMCs
cord blood-derived mast cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukai K, Tsai M, Starkl P, Marichal T, Galli SJ. IgE and mast cells in host defense against parasites and venoms. Semin Immunopathol. 2016;38:581–603. doi: 10.1007/s00281-016-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlos D, Yaochite JNU, Rocha FA, Toso VD, Malmegrim KCR, Ramos SG, et al. Mast cells control insulitis and increase Treg cells to confer protection against STZ-induced type 1 diabetes in mice. Eur J Immunol. 2015;45:2873–2885. doi: 10.1002/eji.201545498. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1:269–279. doi: 10.1158/2326-6066.CIR-13-0119. [DOI] [PubMed] [Google Scholar]
- 6.Ribatti D. Mast cells as therapeutic target in cancer. Eur J Pharmacol. 2016;778:152–157. doi: 10.1016/j.ejphar.2015.02.056. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 9.Morjaria JB, Babu KS, Vijayanand P, Chauhan AJ, Davies DE, Holgate ST. Sputum IL-6 concentrations in severe asthma and its relationship with FEV1. Thorax. 2011;66:537. doi: 10.1136/thx.2010.136523. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick AM, Higgins M, Holguin F, Brown LAS, Teague WG. National Institutes of Health/National Heart, Lung, and Blood Institute’s Severe Asthma Research Program, The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125:851–857.e18. doi: 10.1016/j.jaci.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama A, Kohno N, Sakai K, Kondo K, Hirasawa Y, Hiwada K. Circulating levels of soluble interleukin-6 receptor in patients with bronchial asthma. Am J Respir Crit Care Med. 1997;156:1688–1691. doi: 10.1164/ajrccm.156.5.9610070. [DOI] [PubMed] [Google Scholar]
- 12.Neveu WA, Allard JL, Raymond DM, Bourassa LM, Burns SM, Bunn JY, et al. Elevation of IL-6 in the allergic asthmatic airway is independent of inflammation but associates with loss of central airway function. Respir Res. 2010;11:28. doi: 10.1186/1465-9921-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasperska-Zajac A, Sztylc J, Machura E, Jop G. Plasma IL-6 concentration correlates with clinical disease activity and serum C-reactive protein concentration in chronic urticaria patients. Clin Exp Allergy. 2011;41:1386–1391. doi: 10.1111/j.1365-2222.2011.03789.x. [DOI] [PubMed] [Google Scholar]
- 14.Fujii K, Konishi K, Kanno Y, Ohgou N. Acute urticaria with elevated circulating interleukin-6 is resistant to anti-histamine treatment. J Dermatol. 2001;28:248–250. doi: 10.1111/j.1346-8138.2001.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 15.Brockow K, Akin C, Huber M, Metcalfe DD. IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin Immunol. 2005;115:216–223. doi: 10.1016/j.clim.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Mayado A, Teodosio C, Garcia-Montero AC, Matito A, Rodriguez-Caballero A, Morgado JM, et al. Increased IL6 plasma levels in indolent systemic mastocytosis patients are associated with high risk of disease progression. Leukemia. 2016;30:124–130. doi: 10.1038/leu.2015.176. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita T, Sawai N, Hidaka E, Yamashita T, Koike K. Interleukin-6 directly modulates stem cell factor-dependent development of human mast cells derived from CD34(+) cord blood cells. Blood. 1999;94:496–508. [PubMed] [Google Scholar]
- 18.Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- 19.Schoeler D, Grützkau A, Henz BM, Küchler J, Krüger-Krasagakis S. Interleukin-6 enhances whereas tumor necrosis factor alpha and interferons inhibit integrin expression and adhesion of human mast cells to extracellular matrix proteins. J Invest Dermatol. 2003;120:795–801. doi: 10.1046/j.1523-1747.2003.12126.x. [DOI] [PubMed] [Google Scholar]
- 20.Misiak-Tłoczek A, Brzezińska-Błaszczyk E. IL-6, but not IL-4, stimulates chemokinesis and TNF stimulates chemotaxis of tissue mast cells: involvement of both mitogen-activated protein kinases and phosphatidylinositol 3-kinase signalling pathways. Apmis. 2009;117:558–567. doi: 10.1111/j.1600-0463.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 21.Kirshenbaum AS, Metcalfe DD. Growth of human mast cells from bone marrow and peripheral blood-derived CD34+ pluripotent progenitor cells. Methods Mol Biol. 2006;315:105–112. doi: 10.1385/1-59259-967-2:105. [DOI] [PubMed] [Google Scholar]
- 22.Lappalainen J, Lindstedt KA, Kovanen PT. A protocol for generating high numbers of mature and functional human mast cells from peripheral blood. Clin Exp Allergy. 2007;37:1404–1414. doi: 10.1111/j.1365-2222.2007.02778.x. [DOI] [PubMed] [Google Scholar]
- 23.Desai A, Jung MY, Olivera A, Gilfillan AM, Prussin C, Kirshenbaum AS, et al. IL-6 promotes an increase in human mast cell numbers and reactivity through suppression of suppressor of cytokine signaling 3. J Allergy Clin Immunol. 2016;137:1863–1871.e6. doi: 10.1016/j.jaci.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300:2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 26.Garbers C, Jänner N, Chalaris A, Moss ML, Floss DM, Meyer D, et al. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J Biol Chem. 2011;286:14804–14811. doi: 10.1074/jbc.M111.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. Embo J. 1995;14:1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erlich TH, Yagil Z, Kay G, Peretz A, Migalovich-Sheikhet H, Tshori S, et al. Mitochondrial STAT3 plays a major role in IgE-antigen-mediated mast cell exocytosis. J Allergy Clin Immunol. 2014;134:460–469. doi: 10.1016/j.jaci.2013.12.1075. [DOI] [PubMed] [Google Scholar]
- 29.Siegel AM, Stone KD, Cruse G, Lawrence MG, Olivera A, Jung MY, et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol. 2013;132:1388–1396. doi: 10.1016/j.jaci.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hox V, O’Connell MP, Lyons JJ, Sackstein P, Dimaggio T, Jones N, et al. Diminution of signal transducer and activator of transcription 3 signaling inhibits vascular permeability and anaphylaxis. J Allergy Clin Immunol. 2016;138:187–199. doi: 10.1016/j.jaci.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 32.Seki YI, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, et al. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9:1047–1054. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz LB, Austen KF, Wasserman SI. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J Immunol. 1979;123:1445–1450. [PubMed] [Google Scholar]
- 34.Schwartz LB, Lewis RA, Seldin D, Austen KF. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981;126:1290–1294. [PubMed] [Google Scholar]
- 35.Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol. 2005;175:2635–2642. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 36.Traum D, Timothee P, Silver J, Rose-John S, Ernst M, LaRosa DF. IL-10-induced gp130 expression in mouse mast cells permits IL-6 trans-signaling. Journal of Leukocyte Biology. 2012;91:427–435. doi: 10.1189/jlb.0411209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie M, Mustovich AT, Jiang Y, Trudeau JB, Ray A, Ray P, et al. IL-27 and type 2 immunity in asthmatic patients: association with severity, CXCL9, and signal transducer and activator of transcription signaling. J Allergy Clin Immunol. 2015;135:386–394. doi: 10.1016/j.jaci.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chae SC, Li CS, Kim KM, Yang JY, Zhang Q, Lee YC, et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52:355–361. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- 39.Wittmann M, Zeitvogel J, Wang D, Werfel T. IL-27 is expressed in chronic human eczematous skin lesions and stimulates human keratinocytes. J Allergy Clin Immunol. 2009;124:81–89. doi: 10.1016/j.jaci.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 41.Oskeritzian CA, Zhao W, Pozez AL, Cohen NM, Grimes M, Schwartz LB. Neutralizing endogenous IL-6 renders mast cells of the MCT type from lung, but not the MCTC type from skin and lung, susceptible to human recombinant IL-4-induced apoptosis. J Immunol. 2004;172:593–600. doi: 10.4049/jimmunol.172.1.593. [DOI] [PubMed] [Google Scholar]
- 42.Dichlberger A, Schlager S, Maaninka K, Schneider WJ, Kovanen PT. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. J Lipid Res. 2014;55:2471–2478. doi: 10.1194/jlr.M048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 44.Kawata R, Reddy ST, Wolner B, Herschman HR. Prostaglandin synthase 1 and prostaglandin synthase 2 both participate in activation-induced prostaglandin D2 production in mast cells. J Immunol. 1995;155:818–825. [PubMed] [Google Scholar]
- 45.Kanaoka Y, Urade Y. Hematopoietic prostaglandin D synthase, Prostaglandins Leukot. Essent Fatty Acids. 2003;69:163–167. doi: 10.1016/s0952-3278(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Yang Y, Stevens RL. RasGRP4 regulates the expression of prostaglandin D2 in human and rat mast cell lines. J Biol Chem. 2003;278:4725–4729. doi: 10.1074/jbc.C200635200. [DOI] [PubMed] [Google Scholar]
- 47.Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB, Tweardy DJ. Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood. 2011;117:5701–5709. doi: 10.1182/blood-2010-04-280123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marone G, Varricchi G, Loffredo S, Granata F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol. 2016;778:146–151. doi: 10.1016/j.ejphar.2015.03.088. [DOI] [PubMed] [Google Scholar]
- 49.Zhong Z, Wen Z, Darnell JE. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 50.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 51.Wen Z, Zhong Z, Darnell JE. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172:1227–1236. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 53.Detoraki A, Staiano RI, Granata F, Giannattasio G, Prevete N, de Paulis A, et al. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol. 2009;123:1142–9. 1149.e1–5. doi: 10.1016/j.jaci.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 54.Sismanopoulos N, Delivanis DA, Alysandratos KD, Angelidou A, Vasiadi M, Therianou A, et al. IL-9 induces VEGF secretion from human mast cells and IL-9/IL-9 receptor genes are overexpressed in atopic dermatitis. PLoS ONE. 2012;7:e33271. doi: 10.1371/journal.pone.0033271. [DOI] [PMC free article] [PubMed] [Google Scholar]


