Abstract
Objective
Dietary methionine restriction (MR) improves biomarkers of metabolic health, in part through coordinated increases in energy intake and energy expenditure (EE). Some metabolic benefits of dietary MR are secondary to its effects on energy balance so this study’s purpose was to examine how age at initiation of MR influences its effects on energy balance and body composition.
Methods
Energy balance was examined in rats provided Control or MR diets for 9 months after weaning or in rats between 6 and 12 months of age.
Results
Rats provided the Control diet for 9 months after weaning increased their body weight (BW) and fat mass by 5- and 8-fold, respectively, while BW and fat accumulation were reduced to 50% of Controls in the MR group. In adult rats fed the respective diets between 6 and 12 months of age, dietary MR increased energy intake by ~23% but the 15% increase in EE was sufficient to prevent increases in BW or fat mass.
Conclusions
Dietary MR produces comparable increases in EE in young, growing animals and in mature animals, but young animals continue to deposit new tissue because of the proportionately larger effect of MR on energy intake relative to maintenance requirements.
Introduction
Dietary methionine restriction (MR) produces an integrated series of metabolic and physiological responses that develop quickly after introducing the diet and improve many biomarkers of metabolic health (1–8). Two prominent physiological responses are increases in energy intake and expenditure (2), with the larger effect on energy expenditure (EE) slowing ongoing fat deposition by increasing the proportion of total energy intake required for maintenance of existing tissue. When the decrease in net energy available to support growth is integrated over time, it significantly limits normal age-associated growth and expansion of adipose tissue. The MR diet also increases in vivo insulin sensitivity through a combination of direct and indirect effects of the diet on liver, adipose tissue, and muscle (6). In addition, improvements in overall insulin sensitivity accrue from diet-induced reductions in adiposity. However, the extent to which increased EE and reductions in adiposity are required for diet-induced improvements in insulin sensitivity have not been clearly established.
Most studies of MR assess the responses of young mice or rats during the post-weaning phase of growth, but from a translational perspective, the more relevant strategy would be application of MR in adults with metabolic dysfunction. An additional concern with studying MR in a post-weaning model is that metabolic changes could be secondary to developmental effects associated with slowed growth. Several studies of MR have examined the metabolic phenotypes of rats after long term consumption of the MR diet (e.g., ~2 years) (1, 2). Although improvements in biomarkers of metabolic health were documented in both studies, concerns remain that these benefits are secondary to developmental effects associated with postweaning introduction of the diet. Therefore, an important objective of the present work was to obtain a side-by-side comparison of the effects of dietary MR on energy balance in the standard post-weaning MR model and also in an adult context where dietary MR was initiated after attainment of ~80% of mature size. Using these two experimental models, we report here that dietary MR held BW and body composition constant in adult rats between 6 and 12 months of age despite increasing weight adjusted food consumption by 20%. Dietary MR also increased energy intake and EE in young growing animals, but in this case, energy balance remained sufficiently positive to support a slower rate of growth and deposition of new tissue over the following 9 months.
Materials and Methods
Animals and Diets
All experiments were reviewed and approved by the Pennington IACUC using guidelines established by the National Research Council, the Animal Welfare Act, and the PHS Policy on humane care and use of animals. Two experiments were conducted using male F344 rats obtained from Harlan (Indianapolis, IN) at 5 weeks of age (Experiment 1) or 5 months of age (Experiment 2). In Experiment 1, rats were fed Purina rodent diet (#5001) until 35 days of age, then singly housed in shoebox cages with corncob bedding and fed Control diet for 7 days. Then rats were randomly assigned to one of two dietary treatment groups. Using the experimental feeding paradigm described previously (2), Control rats were provided purified diet containing 0.86% methionine and no cysteine while rats in the methionine-restricted group (MR) were provided the same diet with methionine restricted to 0.17% and no cysteine. The diets were formulated as extruded pellets and provided ad libitum(2). The energy content of both diets (Dyets Inc., Bethlehem, PA) was 15.96 kJ/g, with 18.9% of energy from fat (corn oil), 64.9% from carbohydrate, and 14.8% from a custom mixture of L-amino acids. The amino acid content of the diet on a weight basis was 14.1%. Details of diet composition are provided in Table 1. Temperature was maintained at 22–23° C and lights were on 12 h day from 7 AM-7 PM. In Experiment 2, five-months-old rats were fed Control diet for 1 month prior to assignment to Control or MR diet groups.
Table 1.
Composition of the Methionine-Restricted Dieta
| Ingredient | Concentration in Diet (%) | Ingredient | Concentration in Diet (%) |
|---|---|---|---|
|
| |||
| L-Arginine | 1.12 | L-Phenylalanine | 1.16 |
| L-Lysine | 1.80 | Glycine | 2.33 |
| L-Histidine | 0.33 | Dextrose | 20.00 |
| L-Leucine | 1.11 | Dyetrose | 5.00 |
| L-Isoleucine | 0.82 | Corn Starch | 43.25 |
| L-Valine | 0.82 | Cellulose Fiber | 5.00 |
| L-Threonine | 0.82 | Choline bitartrate | 0.20 |
| L-Tryptophan | 0.18 | Vitamin mix - AIN-76A | 1.00 |
| DL-Methionineb | 0.17 | Mineral mix - AIN-76 | 3.50 |
| Glutamic Acidc | 3.39 | Corn Oil | 8.00 |
Energy content of Control and Methionine-restricted diets is 15.96 kJ/g.
DL-methionine concentration of Control diet is 0.86%.
L-Glutamic acid concentration of Control diet is 2.70%.
Food consumption was measured by weighing food provided at the beginning of the feeding interval and unconsumed and wasted food 24 or 48 hours (h). The bedding was sifted through wire mesh to weigh unconsumed food. Body composition was determined by NMR spectroscopy (Bruker Minispec, Billerica, MA).
Experiment 1 – Juvenile Study
Two cohorts of 16 rats were provided the Control or MR diets for 3 months or 9 months beginning at 6 weeks of age. After 3 and 9 months on diet, 8 rats from each dietary group were transferred to indirect calorimeters for measurement of EE (Oxymax System, Columbus Instruments, Columbus, OH). Rats were acclimated in the chambers for 24 h prior to measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2) at 48-min intervals for 72 h. At the end of the 4-day period, the rats were removed from the calorimeter and euthanized. VO2 is expressed as liters (L) of O2 consumed per h, while Respiratory Exchange Ratio (RER) is the ratio of VCO2 produced to VO2 consumed. EE was calculated as (VO2 X (3.815 + (1.232 X RER)) X 4.019 kJ/h), expressed as kJ/h/rat as described by the manufacturer (Columbus Instruments, Columbus, OH). Group differences (e.g., Diet, Diet Duration, Time of Day, and Diet x Diet Duration x Time of Day interaction) in EE (kJ/h/rat) were compared using Analysis of Covariance (ANCOVA) (JMP Software, Version 12; SAS Institute Inc., Cary, NC), calculating least squares means that accounted for variation in EE attributable to differences in lean mass and fat mass among the rats. The significance of model effects and least squares means ± SEM for the three way interaction were compared using residual variance as the error term (9).
Experiment 2 - Adult Study
The effects of MR on energy balance in adult rats were examined in two cohorts of 16 rats beginning at 6 months of age. Rats were provided the Control or MR diets for 3 months or 6 months thereafter. BW, body composition, and food consumption were determined as before after 3 and 6 months in cohorts of 8 rats per group prior to measurement of EE as before. The effects of Diet, Diet Duration, and Time of Day on EE were assessed by ANCOVA as in Experiment 1.
Statistics
The energy balance response variables in Experiments 1 and 2 were analyzed using a 2-way ANOVA, with Diet and Diet Duration as main effects. The analysis tested for a Diet x Diet Duration interaction using residual variance as the error term. Response variables at each diet duration were compared using least squares means from the ANOVA with the Bonferroni correction. Protection against Type I errors was set at 5%.
Results
Experiment 1 – Juvenile Study
In Experiment 1, the initial BW of all rats was ~84 g and BW increased in Controls by 3.9-fold at 3 months and 5.1-fold at 9 months (Table 2). Mean fat mass in Controls increased by 5.4-fold and 8.2-fold at 3 and 9 months, respectively, while mean fat-free mass increased by 3.5-fold at 3 months and 4.2-fold at 9 months (Table 2). The accumulation of fat-free mass paralleled the accumulation of BW while the rate of fat accumulation over the same period exceeded the rate of accumulation of BW. MR slowed the increase in BW, fat mass, and fat free mass by ~50% at both time points but the biggest impact was on fat mass at 9 months where the MR group deposited only 38% as much fat as Controls (Table 2). Despite the difference in BW of the two groups, rats in the MR group consumed 80% and 73% of the food consumed by Controls at 3 and 9 months, respectively (Table 2). However, expressed per unit of BW, food consumption in the MR group was 48% higher than Controls at 3 months and 53% higher than Controls at 9 months (Table 2). These data show that rats in both groups were growing and depositing new tissue, although the MR group was growing at a significantly slower rate. Moreover, based on the high rate of energy intake per unit BW in the MR group, the data suggest that the energy costs of maintaining existing tissue and/or adding new tissue in this group were significantly higher.
Table 2.
Experiment 1, Juvenile Study. Effects of dietary methionine on food consumption and body composition over time in rats after initiation of the MR diet at 6 weeks of age1
| Time on Diets | ||||||
|---|---|---|---|---|---|---|
| Pre-intervention Measures | 3 Mo | 9 Mo | ||||
| Control | MR | Control | MR | Control | MR | |
| Body Weight (g) | 84 ± 2 | 84 ± 2 | 328 ± 8 | 175 ± 4* | 431 ± 9 | 215 ± 5* |
| Fat Mass (g) | 13.9 ± 0.4 | 13.6 ± 0.4 | 75.1 ± 2.8 | 36.5 ± 1.1* | 114 ± 4 | 44.0 ± 1.5* |
| % Adiposity2 | 16.5 ± 0.2 | 16.1 ± 0.2 | 22.8 ± 0.4 | 20.7 ± 0.2* | 26.4 ± 0.4 | 20.5 ± 0.3* |
| Fat Free Mass (g) | 67.8 ± 1.5 | 68.2 ± 1.5 | 235 ± 5 | 132 ± 3* | 288 ± 5 | 157 ± 4* |
| % Fat Free Mass2 | 80.6 ± 0.4 | 80.9 ± 0.4 | 71.9 ± 0.5 | 75.1 ± 0.3* | 67.0 ± 0.4 | 73.1 ± 0.3* |
| Food Intake3 (kJ/rat/d) | 241 ± 5 | 244 ± 6 | 279 ± 11 | 223 ± 12* | 250 ± 6 | 182 ± 15* |
| Food Intake4 (kJ/d/g BW) | 1.62 ± 0.05 | 1.61 ± 0.04 | 0.85 ± 0.02 | 1.26 ± 0.06* | 0.53 ± 0.02 | 0.81 ± 0.05* |
Thirty-two 5 wk old male rats were singly housed and fed the Control diet for 7 d and then half the rats were randomized to receive the MR diet while the remaining rats continued to receive the Control diet. Body weight, body composition, and food intake were measured at weekly intervals. Response variables at each time point were compared using a 2-way ANOVA and least squares means of each variable were compared using residual variance as the error term and the Bonferroni correction. Means annotated with an asterisk differ from the Control group means at P<0.05.
The % Adiposity was calculated as the fat mass/body weight x 100 and % fat free mass was calculated as fat free mass divided by body weight x 100.
The average food intake less spillage was determined over a 24 h period in rats each diet, converted to kJ, and expressed as kJ/rat/day.
The average energy intake per day expressed per unit of body weight.
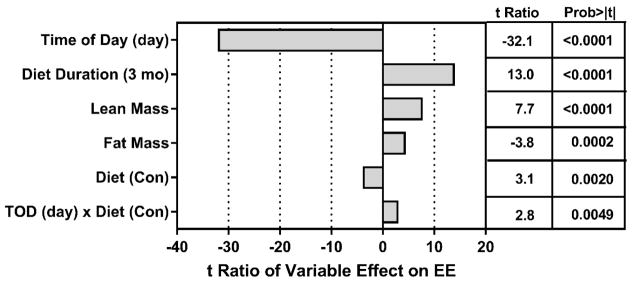
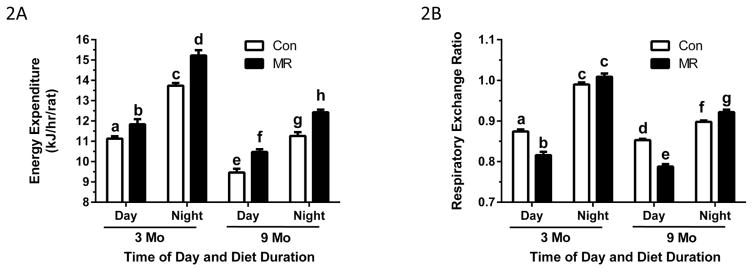
The compiled measurements of EE were analyzed by ANCOVA to assess the impact of Diet, Diet Duration, Time of Day, lean mass, and fat mass on variation in EE between groups. The relative contributions of model components to variation in EE is shown as the t-ratio of each variable’s impact on total variation in EE (Fig. 1). Although all model components had significant t-ratios, Fig. 1 shows that Time of Day was the largest contributor to variation in EE among rats. The negative t-ratio for Time of Day indicates that EE was significantly lower during the day, irrespective of Diet or Diet Duration. Diet Duration had a significant independent effect on EE, as EE decreased with age in rats on both diets. Lean mass and fat mass had significant positive effects on variation in EE, and as predicted, the Control diet had a negative effect on EE relative to the MR diet (Fig. 1). Lastly, a significant Time of Day x Diet interaction was detected based on the differential effect of the MR diet on EE measured during the day versus at night. This corresponds with increased nighttime activity and feeding, and the larger effect of MR on nighttime EE versus its daytime effect. These effects are shown graphically in Fig. 2A where the impact of Diet Duration, irrespective of Time of Day and Diet is evident. Fig. 2A also illustrates the independent effects of Diet and Time of Day on EE at the two time points. The day-to-night increase in EE in Controls at 3 months was 24% while the corresponding increase in the MR group was 29%. At 9 months, EE in the MR group was 11% higher than Controls during the day and 10% higher than Controls during the night (Fig. 2A).
Figure 1. Assessment of components contributing to variation in energy expenditure (EE) in the Juvenile Study.
The relative contributions of model components in accounting for variation in total EE is shown as the t ratio of each variable’s impact on EE and was calculated by Analysis of Covariance. EE was measured in rats by indirect calorimetry after consuming the Control or MR diet for 3 months or 9 months beginning at 6 weeks of age. At each time point, the rats were acclimated in the metabolic chambers for 24 h prior to measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2) at 48-min intervals for 72 h. EE was calculated as described in the Materials and Methods.
Figure 2. Effect of diet duration and time of day on energy expenditure (2A) and respiratory exchange ratio (2B) in rats in the Juvenile Study.
The rats were acclimated in the metabolic chambers for 24 h prior to measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2) at 48-min intervals for 72 h after the rats consumed the Control or MR diet for 3 months or 9 months beginning at 6 weeks of age. Least squares means of EE and RER for the time of day x diet duration interaction for each diet were calculated by Analysis of Covariance. EE and RER were calculated from VO2 and VCO2 as described in the Materials and Methods. Least square means ± SEM are presented in the bar graph and means annotated with different letters differ at P<0.05.
RER provides a real time index of substrate utilization during the metabolic cycle, and is based on the molar ratios of O2 consumed and CO2 produced during the oxidation of glucose (1.00), lipid (0.70), and protein (0.80) (10–12). RERs typically range towards 1 during the switch to glucose utilization in the fed state and towards 0.7 during the switch to fat utilization during fasting. Fig. 2B shows the expected diurnal fluctuations in RER in each group, approaching 1 at night when rats consume 75–85% of their daily intake (13, 14). The nighttime RER in the MR group slightly exceeded 1 after 3 months (Fig. 2B), which occurs when glucose is used to support de novo lipogenesis (10, 12). During the day-time post-absorptive state, RERs in the MR group dropped to ~0.80 at both 3 and 9 months, indicating a greater shift to lipid as fuel than Controls. In addition, the range of day-to-night excursions in RER are considered a measure of metabolic flexibility and were significantly larger in the MR group after both Diet Durations (Fig. 2B). Considered together, the MR group oxidized more fat during the day than Controls and the nighttime shift from fat to carbohydrate was more complete.
Experiment 2 – Adult Study
In Experiment 2, the starting weight of all rats was ~390 g and BW increased in Controls by 13% at 3 months to 438 ± 7 g and by 22% at 6 months to 472 ± 12 g (Table 3). Fat mass increased by a modest 20% in Controls at 3 months and by 38% at 9 months, while fat-free mass increased by 9% and 15% over this period (Table 3). Thus, fat mass accumulation outpaced accumulation of fat-free mass and BW between 6 and 12 months in Controls. In contrast, BW was essentially stable in the MR group during the study, with the MR group losing only 18 g or 5% of BW over 6 months (Table 3). Fat mass was also constant during this period, with final (84 ± 2.3 g) and starting fat mass (88 ± 1.7 g) not differing (Table 3). Fat free mass at the end was 96% of its starting value (Table 3), indicating that on average, BW and composition of the MR group remained almost constant during the study. Despite the difference in BW between Control and MR rats at 3 and 6 months, food consumption did not differ between groups at either time point (Table 3). However, when food intake was expressed per unit BW, energy intake in the MR group was 17% higher than Controls at 3 months and 23% higher at 6 months (Table 3). In contrast to the Juvenile Study, where energy intake was used to support maintenance and growth in both groups, the MR group in the Adult Study used essentially 100% of their intake for maintenance. By definition, the MR group was relatively close to being in energy balance. Given that Control rats continued to grow at the same level of energy intake, we conclude that energy costs of maintaining existing tissue were significantly higher in the MR group.
Table 3.
Experiment 2, Adult Study. Effects of dietary methionine on food consumption and body composition over time in rats after initiation of the MR diet at 6 months of age
| Time on Diets | ||||||
|---|---|---|---|---|---|---|
| Pre-intervention Measures | 3 mo | 6 Mo | ||||
| Control | MR | Control | MR | Control | MR | |
| Body Weight (g) | 387 ± 6 | 388 ± 5 | 438 ± 7 | 376 ± 4* | 472 ± 12 | 370 ± 6* |
| Fat Mass (g) | 90.5 ± 2.1 | 88.4 ± 1.7 | 109 ± 3 | 85.6 ± 1.6* | 125 ± 5 | 84.3 ± 2.3* |
| % Adiposity1 | 23.3 ± 0.3 | 22.8 ± 0.3 | 25.5 ± 0.3 | 23.2 ± 0.2* | 26.3 ± 0.4 | 22.7 ± 0.4* |
| Fat Free Mass (g) | 273 ± 4 | 274 ± 3 | 297 ± 4 | 266 ± 2* | 314 ± 6 | 264 ± 4* |
| % Fat Free Mass2 | 70.4 ± 0.3 | 70.7 ± 0.3 | 67.9 ± 0.3 | 70.7 ± 0.2* | 66.7 ± 0.5 | 71.3 ± 0.3* |
| Food Intake3 (kJ/rat/d) | 298 ± 7 | 297 ± 7 | 281 ± 4 | 284 ± 6 | 263 ± 9 | 255 ± 6 |
| Food Intake4 (kJ/d/g BW) | 0.74 ± 0.02 | 0.75 ± 0.02 | 0.64 ± 0.01 | 0.75 ± 0.02* | 0.56 ± 0.01 | 0.69 ± 0.02* |
Thirty-two 6 mo old male rats were singly housed and fed the Control diet for 7 d and then half the rats were randomized to receive the MR diet while the remaining rats continued to receive the Control diet. Body weight, body composition, and food intake were measured at weekly intervals. Response variables at each time point were compared using a 2-way ANOVA and least squares means of each variable were compared using residual variance as the error term and the Bonferroni correction. Means annotated with an asterisk differ from the Control group means at P<0.05.
The % Adiposity was calculated as the fat mass/body weight x 100 and % fat free mass was calculated as fat free mass divided by body weight x 100.
The average food intake less spillage was determined over a 24 h period in rats on each diet, converted to kJ, and expressed as kJ/rat/day.
The average energy intake per day was expressed per unit of body weight.
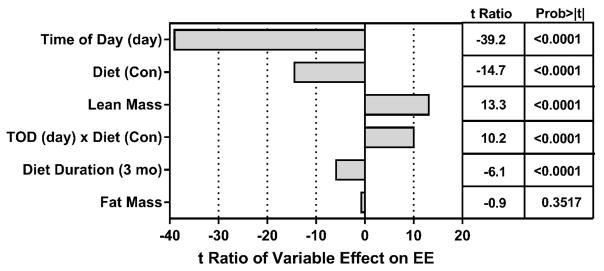
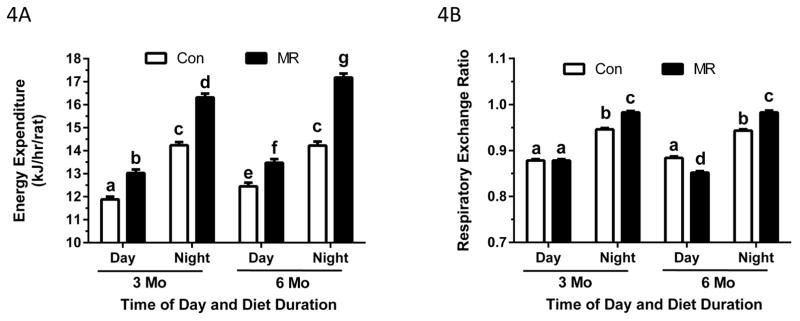
As in Experiment 1, the relative contributions of Diet, Diet Duration, Time of Day, lean mass, and fat mass to variation in EE was assessed by ANCOVA and is shown as the t-ratio of each variable’s impact on total variation in EE. Fig. 3 illustrates that Time of Day was the largest contributor to variation in EE, and as expected, EE was much lower in both groups during the day. Diet Duration also had a significant effect on EE, but in contrast to the Juvenile Study, EE was significantly higher at 6 months than 3 months in both groups (Fig. 3). Lean mass had a significant positive effect on variation in EE as predicted, but in this experiment, fat mass had no effect on variation in EE (Fig. 3). As predicted from the data in Table 3, EE in the MR group, averaged across Time of Day and Diet Duration, was significantly higher than Controls (Fig. 3). Lastly, a significant Time of Day x Diet interaction was detected based on the differential effect of the diets on nighttime EE averaged across Diet Duration. This is illustrated graphically in Fig. 4A, which shows a modest increase in EE between 3 and 6 months, irrespective of Time of Day and Diet. The least square means presented in Fig. 4A also illustrate the independent effects of Diet and Time of Day on EE at both times, with MR producing a 10% increase in EE over Controls during the day at 3 months and a 15% increase over Controls at night. After 6 months, EE in the MR group was 8% higher than Controls during the day and 21% higher than Controls at night (Fig. 4A). These data show that dietary MR produced a consistent increase in EE over Controls between 3 and 6 months, and that the nighttime increase was approximately twice that of the daytime effect (Fig. 4A).
Figure 3. Assessment of components contributing to variation in EE in the Adult Study.
The relative contributions of model components in accounting for variation in total EE is shown as the t ratio of each variable’s impact on EE and was calculated by Analysis of Covariance. EE was measured by indirect calorimetry after rats had consumed the Control or MR diet for 3 months or 6 months beginning at 6 months of age. The rats were acclimated in the metabolic chambers for 24 h prior to measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2) at 48-min intervals for 72 h.
Figure 4. Effect of diet duration and time of day on EE (4A) and RER (4B) in rats in the Adult Study.
The rats were acclimated in the metabolic chambers for 24 h prior to measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2) at 48-min intervals for 72 h after the rats consumed the Control or MR diet for 3 months or 6 months beginning at 6 months of age. Least squares means of EE and RER for the time of day x diet duration interaction for each diet were calculated by Analysis of Covariance. EE and RER were calculated from VO2 and VCO2 as described in the Materials and Methods. Least square means ± SEM are presented in the bar graph and means annotated with different letters differ at P<0.05.
RERs were comparable between groups during the day at 3 months, but MR produced a larger nighttime increase in RER compared to Controls (Fig. 4B). This is indicative of greater metabolic flexibility in the MR group. Diet Duration was without effect in either group, but the daytime RER was slightly lower in the MR group compared to Controls at 6 months (Fig. 4B). The nighttime increase in RER in the MR group was also significantly greater than Controls after 6 months (Fig. 4B). Therefore, although substrate selection between groups was comparable during the day, the nighttime shift to carbohydrate oxidation in the MR group was more complete at both time points.
Discussion
The most significant finding from the present work is that the impact of MR on energy balance depends on the age and/or size of the animal when the diet is introduced. In young growing animals, the hyperphagic effect of MR provided an increase in energy intake that was sufficient to overcome the simultaneous increase in EE, leaving sufficient net energy to support continued, albeit slowed deposition of new tissue. Energy intake in excess of expenditure is defined as net energy, and is partitioned between fat and protein synthesis in a manner that determines the relative composition of tissue deposition during growth to maturity(15–17). However, net energy is always a remainder term determined by the difference between total energy intake and the energy required to meet daily maintenance requirements. For example, on any given day the proportion of energy intake required for maintenance defines the energy available to support new growth. In practical terms, physiological changes that increase EE reduce energetic efficiency and effectively reduce the availability of net energy. It follows that energy intake and energetic efficiency are critical terms in the energy balance equation. The indirect calorimetry data in our Juvenile Study clearly establish that MR increases EE and therefore the energy required to support maintenance requirements. Maintenance energy is most simply defined as the energy intake required to maintain constant body weight and composition(15–18), and is summative in the sense that it includes the energy costs of basal metabolism, thermoregulation, activity, and assimilation of food. Although activity was not measured here, previous studies have found no evidence that dietary MR affects EE by increasing activity (2, 3, 9, 19). In fact, earlier work showed that MR increases EE by increasing uncoupled respiration and enhancing futile substrate cycling (2, 9). However, even with the increase in EE in the MR group, the simultaneous increase in their energy intake compensated for increased maintenance costs and provided sufficient net energy to support a continued, yet slower rate of growth. It is also possible that the reduced amount of methionine in the MR diet limited the rate of growth independently of the diet’s effect on EE. This seems unlikely based on the ability of the MR diet to sustain a much higher BW and lean mass in the Adult Study.
In the Adult Study, MR produced an increase in EE that completely compensated for their increase in energy intake and put the animals in a state close to energy balance. For example, the MR group lost only 15 g of their initial BW of 388 g (e.g., <4%) over the subsequent six months, and their body composition was essentially unchanged. Energy balance is defined as the point when energy intake and expenditure are equal, and it occurs in practice upon attainment of physical maturity when the rate of energy intake is sufficient to maintain a constant body weight and composition. The data presented here make a compelling case that the MR diet, through a combination of increased energy intake (per unit BW) and EE, brought the rats in this group into energy balance. Over the same 6 month period, the Control group continued to grow and deposited 40 additional g of protein, 37 additional g of fat, and 85 additional g of BW while consuming the same amount of food per rat as the MR group (see Table 3). These findings illustrate the importance of the MR-dependent increase in weight-adjusted energy intake, for without it, the MR-dependent increase in EE would have put the rats in a state of significant negative energy balance. It is worth noting that rats in the Adult Study were not obese prior to introduction of MR, so from a translational perspective an important question is whether MR would induce weight loss if animals were obese when MR was introduced. The answer is probably yes based on recent work where mice were initially fed high fat diets to produce obesity, followed by 8 weeks of dietary MR (20). MR reduced BW from 44 g to 27 g and adiposity from 32% to 17% over the 8 week study (20). These findings argue that dietary MR would be effective in producing weight loss in the context of obesity and weight stability in non-obese individuals.
The analysis of EE by ANCOVA provided several additional insights into how diet, age, time of day, and body composition influence variation in EE among the rats. In both studies the most important effector of variation in EE was time of day, with daytime having a significant negative effect that was independent of diet and age. This is not surprising because rodents sleep during the day and are active at night. Therefore, the large impact of time of day on EE reflects the fact that it encompasses variation in EE associated with the nighttime increase in activity and food consumption of rodents in both diets at all ages. Second, diet duration had opposite effects on EE in the two studies, with increased age having a significant negative effect in the Juvenile Study, and a modest positive effect in the Adult Study (see Figs. 1, 2A, 3, and 4A). No diet x diet duration interactions were detected, indicating that the observed effects of diet duration on EE were common to both diets in each study. It is interesting that lean mass and fat mass had significant positive effects on variation in EE in the Juvenile Study, indicating that deposition of each tissue type was positively related to and contributing to EE (Fig. 1). However, the twofold higher t-ratio for lean mass compared to fat mass is consistent with the expected greater impact of lean tissue on EE than fat tissue. In contrast, variation in fat mass had no significant effect on variation in EE in the Adult Study (Fig. 3), while lean mass had the predicted positive effect. The reasons for the difference between studies are unclear, but may relate to the fact that rats in the MR group, despite their significant increase in EE, were weight stable with unchanging fat mass over the 6 month study. Thus, it is possible that the combination of stable fat mass in the MR group and modest increase in adiposity of Controls provided insufficient variation to detect a co-variation of fat mass with EE between groups. Considered as a whole, the most important finding from this analysis is that the MR diet had a significant positive effect on EE regardless of the age when it was initiated, the Diet Duration, or the Time of Day when it was measured.
The ultimate goal of our work is to translate the documented preclinical efficacy of dietary MR into a therapeutic diet based on dietary MR. The most applicable context would be adults who are overweight and present with elements of metabolic disease. We have undertaken an initial proof of concept study in humans with metabolic syndrome (21). The approach involved elimination of meat, poultry, dairy and grains from the diet and replacement of 100% of daily protein requirements with a commercial food (e.g., Hominex-2®) containing a semi-synthetic mixture of L-amino acids lacking methionine. In practice, we found that Hominex-2® meal replacements were supplying ~75% of daily energy requirements. Study participants were instructed to make up the calorie deficiency with unlimited fruit and vegetable intake, and limited intake of grains. In our study, the Hominex-2®-based approach to dietary MR increased 24h fat oxidation and reduced hepatic lipid content as predicted (21). However, a retrospective analysis revealed a key limitation of the Hominex-2®-based approach that limited its overall efficacy. Aside from poor palatability, Hominex-2® contains 0.9 g of cystine/100 g and based on the well-documented methionine-sparing effects of cystine (22), we believe that the cystine in Hominex-2® compromised the reduction in methionine produced by the diet. In subsequent preclinical work, we have established that MR is only effective within a defined concentration range (23), and that addition of as little as 0.1% cystine to the MR diet reversed all but the direct transcriptional effects of MR on specific hepatic genes associated with de novo lipogenesis (e.g., SCD-1) (19, 24). This could explain why the presence of cystine in Hominex-2® limited the diet’s effects to those on hepatic lipid metabolism (21). Viewed together, these findings argue that using MR to treat metabolic disease will involve developing palatable foods that eliminate cysteine and provide methionine within the defined range shown to be biologically effective.
What is already known about this subject?
Initiation of dietary methionine restriction in young rats or mice simultaneously increases energy intake and energy expenditure, but the impact on energy balance is such that the animals maintain a modest positive energy balance and continue to grow, although at a slower rate than Control animals.
It is unclear how initiating dietary methionine restriction after attainment of physical maturity will affect the components of energy balance and body composition in rats over time.
It is unclear whether dietary methionine restriction affects the age-dependent changes in the components contributing to variation in energy expenditure and energy balance.
What does your study add?
Initiation of dietary methionine restriction after attainment of physical maturity increases energy intake and energy expenditure in a coordinated manner that produces animals that are in energy balance with respect to both body weight and body composition.
Initiation of dietary methionine restriction after attainment of ~80% of physical maturity effectively clamps the body weight and body composition of rats.
Initiation of dietary methionine restriction in young growing animals produces an increase in energy intake that is sufficient to overcome the increase in energy expenditure and provide net energy to support continued albeit slowed growth for 9 months after weaning.
The age-dependent decrease in energy expenditure that typically occurs in rats between 4 months of age and 10 months of age is not modified by dietary methionine restriction.
Acknowledgments
This work was supported in part by ADA 1-12-BS-58 (TWG), NIH DK-096311 (TWG), and NIH P30 GM118430 (TWG). This research project also used the Transgenic and Animal Phenotyping core facilities that are supported in part by the NORC (NIH P30 DK072476) center grant from the NIH and NIH U54 GM104940 which funds the Louisiana Clinical and Translational Science Center (WDJ). DW was supported by NIH NRSA 1 F32 DK098918.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
Reference List
- 1.Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5(4):305–14. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 2.Hasek BE, Stewart LK, Henagan TM, et al. Dietary methionine restriction enhances metabolic flexibility and increases uncoupled respiration in both fed and fasted states. Am J Physiol Regul Integr Comp Physiol. 2010;299:R728–R739. doi: 10.1152/ajpregu.00837.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plaisance EP, Henagan TM, Echlin H, et al. Role of β-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am J Physiol Regul Integr Comp Physiol. 2010;299:R740–R750. doi: 10.1152/ajpregu.00838.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wanders D, Ghosh S, Stone K, Van NT, Gettys TW. Transcriptional impact of dietary methionine restriction on systemic inflammation: Relevance to biomarkers of metabolic disease during aging. Biofactors. 2013;40:13–26. doi: 10.1002/biof.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasek BE, Boudreau A, Shin J, et al. Remodeling the integration of lipid metabolism between liver and adipose tissue by dietary methionine restriction in rats. Diabetes. 2013;62:3362–72. doi: 10.2337/db13-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes. 2014;63:3721–33. doi: 10.2337/db14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ables GP, Ouattara A, Hampton TG, et al. Dietary methionine restriction in mice elicits an adaptive cardiovascular response to hyperhomocysteinemia. Sci Rep. 2015;5:8886. doi: 10.1038/srep08886.:8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrone CE, Malloy VL, Orentreich DS, Orentreich N. Metabolic Adaptations to Methionine Restriction that Benefit Health and Lifespan in Rodents. Exp Gerontol. 2012;48:654–60. doi: 10.1016/j.exger.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Wanders D, Burk DH, Cortez CC, et al. UCP1 is an essential mediator of the effects of methionine restrictin on energy balance but not insulin sensitivity. FASEB J. 2015 Jun;29:2603–15. doi: 10.1096/fj.14-270348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elia M, Livesey G. Theory and validity of indirect calorimetry during net lipid synthesis. Am J Clin Nutr. 1988;47(4):591–607. doi: 10.1093/ajcn/47.4.591. [DOI] [PubMed] [Google Scholar]
- 11.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 12.Simonson DC, DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. Am J Physiol. 1990;258(3 Pt 1):E399–E412. doi: 10.1152/ajpendo.1990.258.3.E399. [DOI] [PubMed] [Google Scholar]
- 13.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res. 2003;11(7):845–51. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Shen L, Liu Y, et al. Diurnal rhythm of apolipoprotein A-IV in rat hypothalamus and its relation to food intake and corticosterone. Endocrinology. 2004;145(7):3232–8. doi: 10.1210/en.2003-1554. [DOI] [PubMed] [Google Scholar]
- 15.Brody S. Bioenergetics and Growth. New York: Reinhold; 1945. [Google Scholar]
- 16.Brody S. Nutrition. Annu Rev Biochem. 1935;4:383–401. [Google Scholar]
- 17.Kleiber M. An introduction to animal energetics. New York: John Wiley & Sons, Inc; 1961. The fire of life. [Google Scholar]
- 18.Blaxter KL, Wainman FW. The fasting metabolism of cattle. Br J Nutr. 1966;20(1):103–11. doi: 10.1079/bjn19660012. [DOI] [PubMed] [Google Scholar]
- 19.Wanders D, Stone KP, Forney LA, et al. Role of GCN2-independent signaling through a non-canonical PERK/NRF2 pathway in the physiological responses to dietary methionine restriction. Diabetes. 2016;65(6):1499–510. doi: 10.2337/db15-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wanders D, Forney LA, Stone KP, Burk DH, Pierse A, Gettys TW. FGF21 Mediates the Thermogenic and Insulin-Sensitizing Effects of Dietary Methionine Restriction but not its Effects on Hepatic Lipid Metabolism. Diabetes. 2017;66:858–67. doi: 10.2337/db16-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plaisance EP, Greenway FL, Boudreau A, et al. Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(5):E836–E840. doi: 10.1210/jc.2010-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Buono M, Wykes LJ, Ball RO, Pencharz PB. Dietary cysteine reduces the methionine requirement in men. Am J Clin Nutr. 2001;74(6):761–6. doi: 10.1093/ajcn/74.6.761. [DOI] [PubMed] [Google Scholar]
- 23.Forney LA, Wanders D, Stone KP, Pierse A, Gettys TW. Concentration-dependent linkage of dietary methionine restriction to the components of its metabolic phenotype. Obesity (Silver Spring) 2017;25:730–8. doi: 10.1002/oby.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, et al. Cysteine supplementation reverses methionine restriction effects on rat adiposity: significance of stearoyl-coenzyme A desaturase. J Lipid Res. 2011;52(1):104–12. doi: 10.1194/jlr.M010215. [DOI] [PMC free article] [PubMed] [Google Scholar]