Abstract
The 1981 Lancet paper by Beasley, et al., "Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22707 men in Taiwan" is a seminal publication that clearly demonstrated that chronic infection with hepatitis B virus (HBV), as measured by seropositivity for the hepatitis B surface antigen (HBsAg), preceded the development of hepatocellular carcinoma (HCC). In doing so, this study paved the way for liver cancer prevention efforts through the implementation of hepatitis B vaccination programs. In this commentary, we will describe the discovery of HBV, which led to the study by Beasley, et al.; summarize the major findings of the Beasley paper and its implications; discuss the importance of well-designed cohort studies for prevention activities; and consider the ramifications of the Beasley study and the work that has followed since.
Keywords: liver cancer, hepatocellular carcinoma, hepatitis B infection, HBV vaccination, aflatoxin
More than 2 billion people worldwide are thought to be seropositive for past or current hepatitis B virus (HBV) infection, and over 250 million people have chronic infection [27, 28]. Of those who become chronically infected, 10–25% will develop hepatocellular carcinoma (HCC) [29]. Given this prevalence and associated disease, prevention of HBV infection is a public health imperative. The identification of a link between HBV and HCC allowed the development of public health strategies that targeted HBV and prevented cancer, as well as end stage liver disease. The seminal 1981 Lancet paper by Beasley, et al., "Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22707 men in Taiwan" [3], provided key evidence that propelled the implementation of liver cancer prevention efforts.
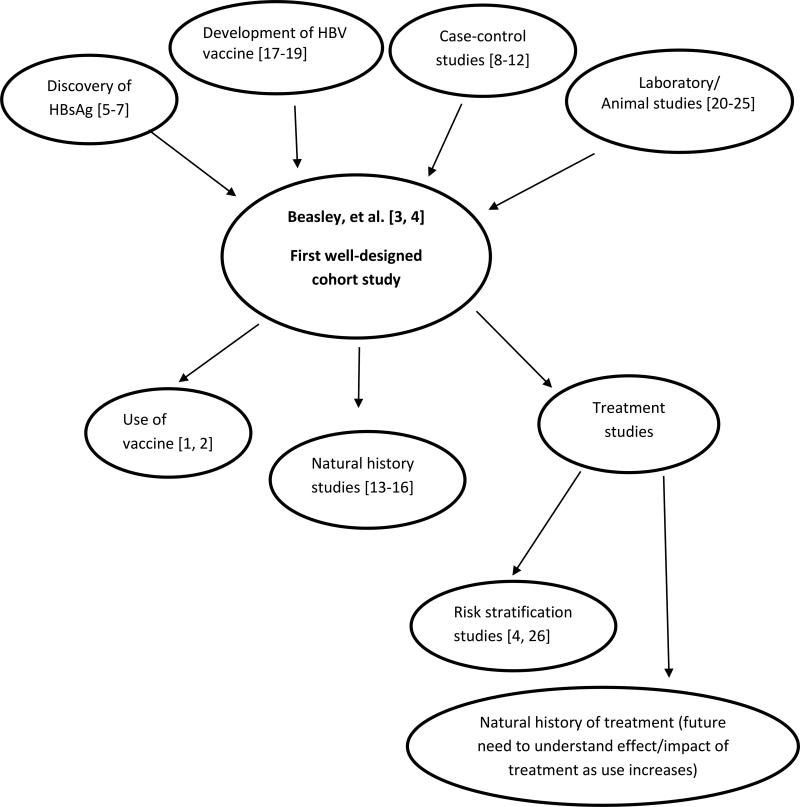
In this commentary, we will describe the discovery of HBV, which led to the study by Beasley, et al.; summarize the major findings of the Beasley paper and its implications; discuss the importance of well-designed cohort studies for prevention activities; and consider the ramifications of the Beasley study and the work that has followed since, including implementation of vaccination programs, natural history studies, and the need for studies of the impact of treatment in the future (Figure 1). Throughout, we will illustrate how scientific teams subsequently addressed the implications of the Beasley paper over the course of years by systematically planning and implementing follow-up studies that could evaluate and build on those implications. This effort is particularly well illustrated by work in Taiwan, where a national vaccination program was implemented, allowing Taiwanese investigators to be the first to show that vaccination reduces cancer risk [1, 30]. Taiwanese investigators also established a cohort of individuals chronically infected with HBV to enable evaluation of the natural history of disease, impact of treatment, and strategies for screening and treatment [14].
Figure 1. Contributions to understanding of liver cancer etiology and prevention.
Abbreviations: HBsAg, hepatitis B surface antigen; HBV, hepatitis B
Before 1970, several studies observed that patients with “serum hepatitis” had detectable levels of “Australia antigen” or “serum hepatitis” [5–7], now known as HBV surface antigen (HBsAg) (Figure 1). Baruch Blumberg and Harvey Alter discovered HBsAg in 1963 [5], and Chung et al. independently identified this antigen [6]. Subsequent reports implicated HBsAg in the development of viral hepatitis [31, 32]. These findings inspired Irving Millman and Blumberg to devise and patent a concept to use HBsAg from human plasma to prepare a hepatitis B vaccine in 1969 [17]. This concept was groundbreaking in that it was the first time a vaccine was proposed that was not prepared from tissue culture [17]. Hilleman used the idea to develop a vaccine against HBV, which was licensed in 1976 [18], and Blumberg won the Nobel Prize in 1976 for both describing HBV and devising the novel concept of a plasma-based vaccine. In addition to Millman and Blumberg’s initial technological advance through the development of a vaccine based on plasma-derived HBsAg rather than human tissue, mass production of the vaccine involved another innovation: development of the first genetically engineered vaccine. Charnay, et al., used the recently cloned HBV genome to identify the gene that coded for HBsAg, fused the HBsAg coding sequence with the beta-galactosidase gene in bacteriophage lambda, and infected Escherichia coli with this bacteriophage to biosynthesize the HBsAg protein [19].
In the 1970s, laboratory and animal experiments provided evidence that HBV infection was involved in hepatocellular carcinogenesis. In vitro studies suggested that cell-mediated immunity was involved both in the control of HBV infection and in the generation of chronic liver damage [20, 21]. Animal studies demonstrated the infectious nature of HBV, the development of liver disease and cancer after transmission of HBV, and the prevention of HBV infection after vaccination [22–25]. In addition, a number of case-control studies reported a higher prevalence of HBV antigens among patients with HCC [8–12]. One of the major limitations of the case-control design is its potential reverse causation. The exposure information is collected at disease onset; thus, whether the exposure is a cause of HCC or rather a consequence of HCC onset cannot be definitively demonstrated. For example, it could be postulated that the presence of a liver tumor might lead to a heightened immune response resulting in greater HBsAg seropositivity. Nevertheless, these previous studies laid the ground work for the Beasley paper, which is considered the final word on the association between HBV and liver cancer.
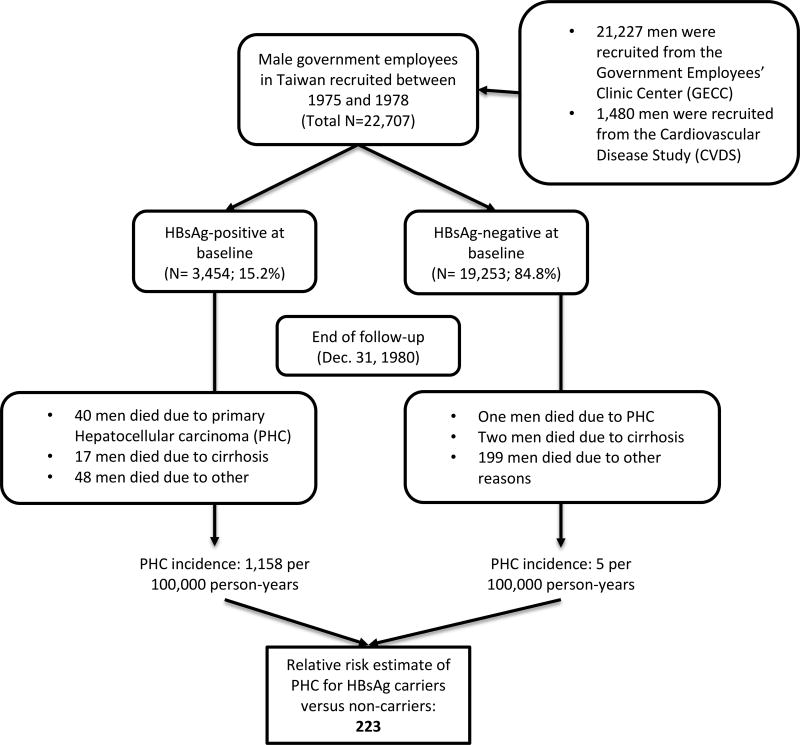
Beasley, et al., made use of the life and health insurance system used by Chinese government employees in Taiwan to enroll 22,707 male participants and follow them for HCC (Figure 2). They focused on men because the incidence of HCC is higher in men than women, government employees were typically male, and male employees tended to be older and to stay in service longer. Deaths were identified through health and life insurance, and the cause of death was verified through medical record review. Of the 41 deaths due to HCC, 19 had histological confirmation and the remainder had elevated serum alpha fetoprotein (AFP) levels, imaging indicative of HCC, or both. HBV markers were measured through commercial radioimmunoassay kits.
Figure 2. Study design and main result of the seminal 1981 Lancet paper by Beasley, et al.
Of the 22,707 men in the cohort, 3,454 (15.2%) were HBsAg-positive, and 19,253 (84.8%) were HBsAg-negative at study entry. After an average of 3.3 years of follow-up per man, there were 307 deaths, 105 (34%) of which were among HBsAg-positive men. Of these 105 deaths, more than 50% were due to HCC (n=40, 38.1%) or cirrhosis (n=17, 16.2%). The incidence for HCC was 1158 per 100,000 person-years, compared to only 5 per 100,000 person-years in HBsAg-negative participants, resulting in a relative risk of 223 (95% CI: 28–1479). The magnitude of this association is quite striking for a study of cancer epidemiology, highlighting the powerful role that chronic HBV infection plays in HCC development.
This study was particularly well-considered in its design. It took advantage of an existing life and health insurance system with routine exams, as well as an existing prospective study, allowing the investigators to recruit and follow participants for HCC outcomes in a timely and efficient manner. While the study needed a large number of participants to identify a sufficient number of outcomes, the anti-hepatitis B core antigen (anti-HBc) test was too expensive to test on everyone, so the investigators tested a sample of HBsAg-seronegative men for anti-HBc and used the results from the sample to estimate the prevalence of anti-HBc-seropositivity in the entire cohort. As with all epidemiology studies, the investigation by Beasley et al. was not without limitations. This study did not have the benefit of the modeling approaches that are commonly used today to control for confounders, such as age and aflatoxins (liver-damaging metabolites produced by certain molds that contaminate foods like corn and peanuts). The modest number of outcomes at the time of publication (e.g., 41 deaths from liver cancer) also limited the ability to fully account for possible confounding factors.
Despite these limitations, this study demonstrated that HCC incidence increased dramatically with age. Given that most HBV infection in Asia occurs at birth and that age thus acts as a surrogate for the duration of infection, these findings imply that the risk of HCC depends on the duration of HBV infection. Taken together, the Beasley study provided strong evidence for temporality of the association between HBV infection and development of HCC, confirmed the increased risk of HCC indicated by the case-control studies, and established the incidence rate for HCC in chronic HBV carriers. The findings from this study had a major impact on our understanding of the etiology of liver cancer, as well as on cancer prevention efforts. This paper was the first well-designed cohort study to prospectively demonstrate that chronic infection with HBV (i.e., seropositivity for HBsAg) preceded liver cancer and to estimate the potential impact of that exposure.
Cohort studies are critical not only because they demonstrate temporality (i.e., that the exposure precedes the outcome), but also because they allow us to calculate public health measures that public health authorities can use to evaluate the magnitude of the problem and how best to attack it. For example, incidence rates and absolute risks among exposed and unexposed individuals can be calculated in cohort studies. By determining how quickly the outcomes occur and the proportion of people affected by them, public health workers can consider how best to apply interventions. Cohort studies are also singular in the amount of time and energy required to run them. They require years of follow-up, necessitating extensive staff and logistical frameworks to follow individuals for years and to collect data and specimens. This kind of effort requires a great deal of foresight and planning. The fruits of these efforts can be quite substantial, however, as was the case in the cohort study by Beasley, et al.
The idea of carcinogenesis as a multistage process was relatively new at the time of the Beasley paper [33]. Some felt that HBV could not cause liver cancer since HCC also developed in HBsAg-negative individuals. In addition, awareness of aflatoxins as hepatocarcinogens was increasing, and many thought aflatoxins were the primary etiologic agent [27]. Aflatoxins had been discovered and hypothesized to cause liver cancer in the 1960s [34], around the same time as the discovery of HBV. A series of epidemiologic studies identified an association between aflatoxin and liver cancer in numerous African and Asian countries, and the role of aflatoxin and HBV in liver carcinogenesis was strongly debated [34]. While the Beasley paper but did not rule out the etiologic role of aflatoxin in the HCC, it solidified the acceptance of HBV as an independent causal factor in liver carcinogenesis, providing the impetus needed to initiate national HBV vaccination programs.
Taiwan was a leader in this area and instituted a national vaccination program in 1984. Given that primary infection with HBV is an early event and occurs frequently from perinatal mother-to-infant infection, for the first 2 years the vaccination program covered only neonates born to mothers who were HBsAg carriers, and then in July 1986 the program was extended to all neonates [1]. Taiwanese investigators capitalized on the Taiwanese National Cancer Registry to quickly evaluate the impact of this program. In 1997, they demonstrated for the first time that the incidence of HCC declined in children aged 6 to 14 years after initiation of the universal HBV vaccination program [1]. In 2009, they demonstrated that this protection extended to adolescence [35], and later they showed that young adults were also protected [36]. When the vaccine failed, it failed most often because of poor compliance or because of transmission from highly infectious mothers [35, 36], which is why vaccination is recommended within 24 hours of birth today [2].
Thus, work by Beasley, et al., laid the groundwork for effective implementation of the HBV vaccine in public health. Following the Beasley study, HBV vaccination programs were implemented in numerous countries in addition to Taiwan, such as the Republic of The Gambia, Tunisia, and South Africa, with substantial seroprotection and reduction of chronic HBV infection [37, 38]. This protection clearly led to a reduction in HCC, as demonstrated by the Taiwanese vaccination program [36, 39]. In the United States (US), the HBV vaccine was first licensed in 1981, but vaccination efforts initially focused on individuals at high risk for HBV infection (e.g., health care workers, men who have sex with men, injection drug users, hemodialysis patients, individuals with multiple sex partners, infants born to HBV-infected women) [38]. Rates of HBV infection were not significantly reduced until the US adopted universal infant HBV immunization in 1991 and began routine immunization of infants in 1992 [38]. Laws requiring immunization prior to school entry had a major impact [40], and most states today have mandatory school vaccination [41]. On a global scale, the World Health Organization (WHO) recommended in 1992 that endemic countries incorporate the HBV vaccine in their national immunization programs by 1995 and that all other countries add it by 1997, resulting in greater than 78% of WHO member states adopting universal childhood HBV vaccination by 2004 [38]. These efforts were driven by the knowledge that HBV infection has a major impact on morbidity and mortality, which was first demonstrated through the Beasley paper. In addition, these efforts proved the HBV vaccine’s place in history as the first vaccine to prevent cancer.
The findings of the Beasley study also led to a number of studies of the natural history of HBV and its interaction with other risk factors. In Taiwan, for example, a team of investigators designed and implemented of the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) study [13]. This landmark study followed chronically HBV-infected patients who were at least 30 years old, the age at which HCC incidence increases dramatically [3, 37], for virologic and liver disease outcomes. In this study, hepatitis B e antigen (HBeAg) was strongly associated with increased risk of HCC [13]. Furthermore, the incidence of HCC and cirrhosis increased with increasing HBV DNA levels, while declining HBV DNA levels were associated with seroclearance of virologic markers [14]. HBV genotype C was also associated with increased HCC risk [15].
In addition to these natural history studies, the strong association between HBsAg and HCC, together with the associations observed between aflatoxin and liver cancer and improved biomarkers for aflatoxin [42], eventually led to the joint evaluation of HBV and aflatoxin as risk factors for HCC. As described in a recent review [16], prospective cohort and molecular pathology studies demonstrated an interaction between HBV and aflatoxin, with odds ratios ranging from 7.3 (95% CI: 2.2–24) to 22.8 (3.6–143.4) for HBsAg alone, 1.7 (95% CI: 0.3–10.8) to 5.5 (95% CI: 1.3–23.4) for aflatoxin alone, and 59.4 (95% CI: 16.6–212) to 129 (95% CI: 25–659) for HBsAg and aflatoxin together. These findings highlight the importance of the HBV vaccine since the effects of aflatoxin are most pronounced among chronic HBV carriers. In addition, although health officials set limits on aflatoxin levels in food products soon after the toxic effects of aflatoxin were discovered, those limits are often exceeded in developing countries even today [34], reiterating the need for timely HBV vaccination (i.e., within 24 hours of birth [2]).
The acceptance of HBV as an etiologic agent in hepatocellular carcinogenesis also led to increased research into the mechanisms by which HBV caused HCC and liver disease. This research in turn led to the identification of drugs that could be used to treat HBV infection. Initially, interferon (IFN)α-2b and then pegylated-IFNα-2a were approved for the treatment of chronic HBV infection [43, 44]. Today, entecavir (approved in 2005) and tenofovir (approved in 2008) are considered first-line treatments since HBV resistance is rare with these drugs [37]. As treatments have come on line and are increasingly used, the need arises to evaluate and understand the effect and impact of such interventions.
These drugs are not fully effective against HBV, however, and there is a need to identify the segment of the population that is at highest risk for developing HCC. For example, in Taiwan, the REVEAL-HBV investigators created an HCC nomogram using HBeAg, HBV genotype C, gender, age, family history of HCC, alcohol consumption, and serum alanine aminotransferase (ALT) [26]. This approach was then refined through a development effort in REVEAL-HBV that was then validated through a consortium of international cohorts, resulting in the 17-point Risk Estimation for Hepatocellular Carcinoma in Chronic Hepatitis B (REACH-B) risk score [4]. This risk score is currently used for risk prediction in Asian clinical guidelines [45], whereas US guidelines tend to focus on non-biological measures of high risk, such as those born outside the US [46].
HBV screening, vaccination, and treatment is also important for the prevention of end-stage liver disease. As shown by Beasley, et al., a substantial proportion of patients with chronic HBV infection die from liver cirrhosis [3]. HBV treatment has been shown to reduce fibrosis and cirrhosis incidence and can also lead to regression of cirrhosis [44]. Thus, screening to identify chronic HBV carriers for treatment and vaccination to prevent HBV infection in the first place are important not only for the prevention of HCC, but also for the prevention of other forms of liver disease.
As described by Beasley, et al., their study “establishes beyond any doubt that the HBsAg carrier state commonly precedes [HCC].” This observation provided critical data needed to motivate wide-scale use of the HBV vaccine, develop treatments, and identify risk-stratification methods. Given that HBV accounts for 23% of the HCC in developed countries and 59% in developing countries [47], these efforts have had a major impact. It has been estimated that in the year 2000, HBV led to 620,000 deaths worldwide, 94% due to cirrhosis and HCC but that without HBV vaccination, 1.4 million would have died [48]. HBV vaccination is estimated to avert 8.3 deaths per 1,000 people vaccinated [49]. However, much work remains to be done. For example, HBV-related HCC and mortality is projected to increase in Australia, Vietnam, and Spain [29], and hepatitis D infection is increasingly understood as an important co-infection with HBV that can accelerate hepatocarcinogenesis [50]. Additional research, new innovations, and improved implementation are needed. The paper by Beasley, et al., will no doubt continue to be an important source of motivation for these efforts.
Highlights.
"Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22707 men in Taiwan" by Beasley, et al., is a seminal publication that clearly demonstrated that chronic infection with hepatitis B virus (HBV) preceded the development of hepatocellular carcinoma (HCC)
The results of this study facilitated the implementation of liver cancer prevention programs, particularly through HBV vaccination
The study design took advantage of existing systems to prospectively evaluate HBV and HCC through a well-designed cohort study
This effort led to a larger team-science approach in Taiwan that enabled investigators to evaluate the impact of HBV vaccination and define the natural history of HBV infection.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Abbreviations
- AFP
alpha fetoprotein
- ALT
alanine aminotransferase
- anti-HBc
anti-hepatitis B core antigen
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IFN
interferon
- REVEAL-HBV
Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship contribution statement
All authors participated in the drafting of this manuscript. All authors reviewed and approved the final manuscript.
Conflicts of Interest: none.
References
- 1.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336(26):1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 2.Bleich LM, Swenson ES. Prevention of neonatal hepatitis B virus transmission. J Clin Gastroenterol. 2014;48(9):765–72. doi: 10.1097/MCG.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 3.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2(8256):1129–33. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 4.Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK R.-B.W. Group. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. The Lancet. Oncology. 2011;12(6):568–74. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg BS, Alter HJ, Visnich S. A "New" Antigen in Leukemia Sera. JAMA. 1965;191:541–6. doi: 10.1001/jama.1965.03080070025007. [DOI] [PubMed] [Google Scholar]
- 6.Chung WK, Moon SK, Gershon RK, Prince AM, Popper H. Anicteric Hepatitis in Korea. Ii. Serial Histologic Studies. Arch Intern Med. 1964;113:535–42. doi: 10.1001/archinte.1964.00280100043008. [DOI] [PubMed] [Google Scholar]
- 7.Australia antigen and hepatitis. Br Med J. 1970;1(5691):247–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel CL, Anthony PP, Mody N, Barker LF. Hepatitis-associated antigen in Ugandan patients with hepatocellular carcinoma. Lancet. 1970;2(7674):621–4. doi: 10.1016/s0140-6736(70)91396-6. [DOI] [PubMed] [Google Scholar]
- 9.Maupas P, Werner B, Larouze B, Millman I, London WT, O'Connell A, Blumberg BS. Antibody to hepatitis-B core antigen in patients with primary hepatic carcinoma. Lancet. 1975;2(7923):9–11. doi: 10.1016/s0140-6736(75)92951-7. [DOI] [PubMed] [Google Scholar]
- 10.Prince AM, Szmuness W, Michon J, Demaille J, Diebolt G, Linhard J, Quenum C, Sankale M. A case/control study of the association between primary liver cancer and hepatitis B infection in Senegal. Int J Cancer. 1975;16(3):376–83. doi: 10.1002/ijc.2910160304. [DOI] [PubMed] [Google Scholar]
- 11.Larouze B, Saimot G, Lustbader ED, London WT, Werner BG, Payet M. Host responses to hepatitis-B infection in patients with primary hepatic carcinoma and their families. A case/control study in Senegal, West Africa. Lancet. 1976;2(7985):534–8. doi: 10.1016/s0140-6736(76)91792-x. [DOI] [PubMed] [Google Scholar]
- 12.Trichopoulos D, Tabor E, Gerety RJ, Xirouchaki E, Sparros L, Munoz N, Linsell CA. Hepatitis B and primary hepatocellular carcinoma in a European population. Lancet. 1978;2(8102):1217–9. doi: 10.1016/s0140-6736(78)92097-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH R.-H.S. Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26(4):628–38. doi: 10.1111/j.1440-1746.2011.06695.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang HI, Yeh SH, Chen PJ, Iloeje UH, Jen CL, Su J, Wang LY, Lu SN, You SL, Chen DS, Liaw YF, Chen CJ R.-H.S. Group. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(16):1134–43. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wild CP, Montesano R. A model of interaction: aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Lett. 2009;286(1):22–8. doi: 10.1016/j.canlet.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 17.Trepo C. A brief history of hepatitis milestones. Liver Int. 2014;34(Suppl 1):29–37. doi: 10.1111/liv.12409. [DOI] [PubMed] [Google Scholar]
- 18.Buynak EB, Roehm RR, Tytell AA, Bertland AU, 2nd, Lampson GP, Hilleman MR. Vaccine against human hepatitis B. JAMA. 1976;235(26):2832–4. [PubMed] [Google Scholar]
- 19.Charnay P, Gervais M, Louise A, Galibert F, Tiollais P. Biosynthesis of hepatitis B virus surface antigen in Escherichia coli. Nature. 1980;286(5776):893–5. doi: 10.1038/286893a0. [DOI] [PubMed] [Google Scholar]
- 20.Dudley FJ, Giustino V, Sherlock S. Cell-mediated immunity in patients positive for hepatitis-associated antigen. Br Med J. 1972;4(5843):754–6. doi: 10.1136/bmj.4.5843.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klatskin G. Persistent HB antigenemia: associated clinical manifestations and hepatic lesions. Am J Med Sci. 1975;270(1):33–40. doi: 10.1097/00000441-197507000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Barker LF, Chisari FV, McGrath PP, Dalgard DW, Kirschstein RL, Almeida JD, Edington TS, Sharp DG, Peterson MR. Transmission of type B viral hepatitis to chimpanzees. J Infect Dis. 1973;127(6):648–62. doi: 10.1093/infdis/127.6.648. [DOI] [PubMed] [Google Scholar]
- 23.Barker LF, Maynard JE, Purcell RH, Hoofnagle JH, Berquist KR, London WT, Gerety RJ, Krushak DH. Hepatitis B virus infection in chimpanzees: titration of subtypes. J Infect Dis. 1975;132(4):451–8. doi: 10.1093/infdis/132.4.451. [DOI] [PubMed] [Google Scholar]
- 24.Gyorkey F, Melnick JL, Mirkovic R, Cabral GA, Gyorkey P, Hollinger FB. Experimental carcinoma of liver in macaque monkeys exposed to diethylnitrosamine and hepatitis B virus. J Natl Cancer Inst. 1977;59(5):1451–67. doi: 10.1093/jnci/59.5.1451. [DOI] [PubMed] [Google Scholar]
- 25.McAuliffe VJ, Purcell RH, Gerin JL. Type B hepatitis: a review of current prospects for a safe and effective vaccine. Rev Infect Dis. 1980;2(3):470–92. doi: 10.1093/clinids/2.3.470. [DOI] [PubMed] [Google Scholar]
- 26.Yang HI, Sherman M, Su J, Chen PJ, Liaw YF, Iloeje UH, Chen CJ. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(14):2437–44. doi: 10.1200/JCO.2009.27.4456. [DOI] [PubMed] [Google Scholar]
- 27.Kane MA. Global control of primary hepatocellular carcinoma with hepatitis B vaccine: the contributions of research in Taiwan. Cancer Epidemiol Biomarkers Prev. 2003;12(1):2–3. [PubMed] [Google Scholar]
- 28.World Health Organization. Hepatitis B Fact sheet. 2017 Accessible at: http://www.who.int/mediacentre/factsheets/fs204/en/
- 29.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16(7):453–63. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee CL, Ko YC. Hepatitis B vaccination and hepatocellular carcinoma in Taiwan. Pediatrics. 1997;99(3):351–3. doi: 10.1542/peds.99.3.351. [DOI] [PubMed] [Google Scholar]
- 31.Okochi K, Murakami S. Observations on Australia antigen in Japanese. Vox Sang. 1968;15(5):374–85. doi: 10.1111/j.1423-0410.1968.tb04078.x. [DOI] [PubMed] [Google Scholar]
- 32.Prince AM. An antigen detected in the blood during the incubation period of serum hepatitis. Proc Natl Acad Sci U S A. 1968;60(3):814–21. doi: 10.1073/pnas.60.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peto R. Carcinogenesis as a multistage process--evidence from human studies. IARC Sci Publ. 1982;(39):27–8. [PubMed] [Google Scholar]
- 34.Pitt JI, Miller JD. A Concise History of Mycotoxin Research. J Agric Food Chem. 2016 doi: 10.1021/acs.jafc.6b04494. [DOI] [PubMed] [Google Scholar]
- 35.Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, Chen DS G. Taiwan Hepatoma Study. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101(19):1348–55. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- 36.Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, Wang TE, Chen BW, Chuang WL, Soon MS, Lin CY, Chiou ST, Kuo HS, Chen DS G. Taiwan Hepatoma Study. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology. 2016;151(3):472–480 e1. doi: 10.1053/j.gastro.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy PTF, Litwin S, Dolman GE, Bertoletti A, Mason WS. Immune Tolerant Chronic Hepatitis B: The Unrecognized Risks. Viruses. 2017;9(5) doi: 10.3390/v9050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112–25. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 39.Sherman M. Hepatitis B Vaccination: Putting Hepatologists Out of Business? Gastroenterology. 2016;151(3):390–2. doi: 10.1053/j.gastro.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 40.C. Centers for Disease. Prevention, Effectiveness of a middle school vaccination law--California, 1999–2001. MMWR Morb Mortal Wkly Rep. 2001;50(31):660–3. [PubMed] [Google Scholar]
- 41.Immunization Action Coalition. [Accessed 4 August 2017];Hepatitis B Prevention Mandates for Daycare and K-12. http://www.immunize.org/laws/hepb.htm.
- 42.Groopman JD, Johnson D, Kensler TW. Aflatoxin and hepatitis B virus biomarkers: a paradigm for complex environmental exposures and cancer risk. Cancer Biomark. 2005;1(1):5–14. doi: 10.3233/cbm-2005-1103. [DOI] [PubMed] [Google Scholar]
- 43.De Clercq E. Current treatment of hepatitis B virus infections. Rev Med Virol. 2015;25(6):354–65. doi: 10.1002/rmv.1849. [DOI] [PubMed] [Google Scholar]
- 44.Halegoua-De Marzio D, Hann HW. Then and now: the progress in hepatitis B treatment over the past 20 years. World J Gastroenterol. 2014;20(2):401–13. doi: 10.3748/wjg.v20.i2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeFevre ML, Force USPST. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(1):58–66. doi: 10.7326/M14-1018. [DOI] [PubMed] [Google Scholar]
- 47.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34(6):1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 49.Lee LA, Franzel L, Atwell J, Datta SD, Friberg IK, Goldie SJ, Reef SE, Schwalbe N, Simons E, Strebel PM, Sweet S, Suraratdecha C, Tam Y, Vynnycky E, Walker N, Walker DG, Hansen PM. The estimated mortality impact of vaccinations forecast to be administered during 2011–2020 in 73 countries supported by the GAVI Alliance. Vaccine. 2013;31(Suppl 2):B61–72. doi: 10.1016/j.vaccine.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 50.Shirvani-Dastgerdi E, Schwartz RE, Ploss A. Hepatocarcinogenesis associated with hepatitis B, delta and C viruses. Curr Opin Virol. 2016;20:1–10. doi: 10.1016/j.coviro.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]