Abstract
Objectives
Assess geographic variation in breast cancer racial mortality disparity by age cohorts in US and ten cities with large African American populations.
Methods
Non-Hispanic Black (NHB) and Non-Hispanic White (NHW) female breast cancer mortality rates and NHB:NHW rate ratio (RR) (disparity) were calculated by four age group categories: < 40, 40–49, 50–64 and 65+ with time period 1999–2013.
Results
In all 10 cities and the US, the most pronounced breast cancer disparities, measured by RR, were seen among younger women. In age group < 40, the RR ranges from 1.71 in Houston to 5.37 in Washington, DC. For age group 50–64, the disparity was less pronounced, ranging from 1.24 in New York to 1.72 in Chicago. For 65+ age group, there was wide city to city variation in breast cancer mortality disparity. Three cities had higher mortality for NHW compared to NHB; Baltimore 0.78, Washington DC 0.94 and New York 0.98. One city had no statistically significant racial variation in breast cancer mortality in this age group and six cities had increased NHB: NHW mortality disparities.
Conclusions
While the mortality rate for breast cancer is lower among younger women, the NHB:NHW disparities, as measured by rate ratios, are most pronounced in these age groups. Given the absence of available data regarding incidence, stage and subtypes, further research is necessary and such research is important, given the possible policy implications of these results with respect to screening guidelines and coverage for mammography and breast cancer treatment in particular for younger NHB women.
Keywords: Breast cancer, Mortality, Age cohorts, Racial disparities, Geographic variation
1. Introduction
With an estimated 40,160 deaths to occur in 2017, breast cancer is the second leading cause of cancer death among women in the US with Non-Hispanic Black (NHB) women experiencing the highest mortality rate compared to other race/ethnic groups [1,2]. Breast cancer mortality among younger NHB women, (< 50) in particular, is higher compared to that of younger Non-Hispanic White (NHW) women [3,4]. The latest data also suggest that, among women 20–49 years of age, the Black:White disparity in breast cancer mortality is the largest disparity among cancer-specific diseases and has widened over the past 30 years [5].
Recent analyses have documented significant variation in NHB and NHW breast cancer mortality and disparity across the US and its largest cities [6,7]. This is the first study to address age specific racial breast cancer mortality disparity at the city level. Analyses at the city level are necessary as certain public health systems, interventions and policies are organized at a city level. Also, as access to care in cities can vary from neighborhood to neighborhood, because of historical patterns of segregation and structural racism in America’s largest cities, we hypothesized that these conditions could result in variation in age specific racial breast cancer disparity rates.
The current study explores breast cancer mortality disparities by age group (< 40, 40–49, 50–64, and 65+) and geographic location building upon prior work [7] that looked at city-level geographic variation overall without breaking out age cohorts. These city-specific data can help inform local health officials and contribute to more tailored public health interventions and policies.
2. Methods
2.1. Population
In addition to the US, 10 cities were included in this analysis based on the following criteria: 1) total population of at least 500,000 and 2) the largest number of African Americans (the US Census 2010 “The Black Population” Table 6). Cities that met these criteria were: Baltimore, MD; Chicago, IL; Dallas, TX; Detroit, MI; Houston, TX; Los Angeles, CA; Memphis, TN; New York City, NY; Philadelphia, PA; and Washington, District of Columbia (DC). Deaths where the cause was malignant neoplasm of the breast (ICD-10 C50.0-C50.9) were extracted from the mortality data files maintained by the National Center for Health Statistics for the period 1999–2013. The extracted death cases were restricted to Non-Hispanic White (NHW) and Non-Hispanic Black (NHB) women. The Person-years (P-years) were obtained from the US Census Bureau. Population by 5-year age groups for the individual 15 years of our study was available for Baltimore, New York City, Philadelphia, Washington, DC, and the US. For Chicago, Dallas, Detroit, Houston, Los Angeles, and Memphis, the P-years were estimated using linear extrapolation and interpolation of the 2000 and 2010 population data from the US Census Bureau by 5-year age group.
2.2. Statistical analysis
The data were stratified by four age groups: < 40, 40–49, 50–64 and 65+. For these categories, truncated age standardization was used to obtain the mortality rates. Using the NHB:NHW rate ratio (RR) with 95% confidence intervals (CI), the disparity was assessed by age group for the US and the 10 cities over the 15-year study period. A 15-year period was chosen to increase the stability of the city-level data, especially in younger age groups, but the overall trend in the disparity by age group for each individual year was calculated for the US and is displayed in Fig. 1a. A RR of 1.00 indicates no disparity between NHB and NHW mortality rates and it represents the target to reach. A RR greater than 1 indicates higher mortality rates among NHB compared to NHW, and a RR less than 1 indicates that the mortality rates is lower among NHB compared to NHW.
Fig. 1.
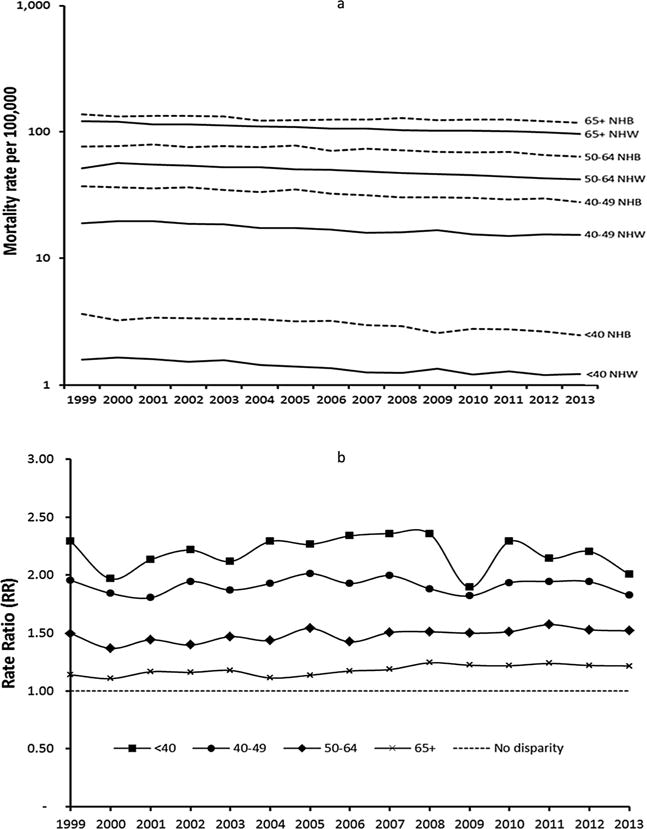
a) Non-Hispanic Black (NHB) and Non-Hispanic White (NHW) breast cancer mortality rates by year and age group in the US (Logarithmic scale has been used). b) Non-Hispanic Black (NHB) and Non-Hispanic White (NHW) breast cancer rate ratios by year and age group in the US.
Another measure of disparity, the mortality risk differences (RD) with 95% confidence interval, were calculated across the different age groups. Excess deaths among NHB stemming from the NHB:NHW disparity were obtained by applying the age-specific NHW breast cancer mortality rate per 100,000 to the age-specific NHB population for the entire 15 years of the study. These were then totaled and subtracted from the NHB observed number of deaths and the difference represents the excess breast cancer mortality deaths due to the disparity [7]. The excess deaths were only calculated for age group and cities where statistically significant disparities were observed. The analyses were not stratified or controlled by breast cancer incidence, subtypes or stage as the mortality data files do not include any of these variables and they are not available elsewhere at the city level. All statistical analyses were conducted with STATA.14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.)
3. Results
Table 1 presents the 15-year NHB and NHW breast cancer mortality rates and rate ratios by age group for the US and the 10 study cities. In the US, the disparity is statistically significantly different between each age cohort and largest among women < 40 (RR of 2.17, 95% CI [2.10–2.25], second largest for the 40–49 age cohort RR 1.90, CI [1.86–1.93], with the 50–64 showing a lower RR 1.55, CI[1.53–1.57] and the lowest RR 1.18, CI[1.17–1.19] for the 65+ age cohort.
Table 1.
Non-Hispanic Black (NHB) and Non-Hispanic White (NHW) breast cancer mortality rates (1999–2013) and rate ratios by age group for the US and 10 study cities.
| < 40
|
40–49
|
50–64
|
65+
|
|||||
|---|---|---|---|---|---|---|---|---|
| NHW | NHB | NHW | NHB | NHW | NHB | NHW | NHB | |
| US | 1.32 | 2.87 | 16.11 | 30.53 | 40.99 | 63.65 | 101.38 | 119.40 |
| Baltimore, MD | 2.45 | 6.73 | 28.14 | 37.17 | 49.38 | 61.15 | 136.84 | 106.20 |
| Chicago, IL | 1.27 | 3.26 | 18.69 | 36.13 | 41.96 | 71.98 | 114.67 | 136.49 |
| Dallas, TX | 0.82 | 2.96 | 17.63 | 36.22 | 37.74 | 63.71 | 100.46 | 145.90 |
| Detroit, MI | 1.42 | 3.79 | 22.07 | 31.69 | 54.86 | 69.21 | 124.46 | 125.76 |
| Houston, TX | 2.22 | 3.80 | 23.36 | 46.15 | 54.49 | 86.80 | 123.02 | 142.03 |
| Los Angeles, CA | 1.39 | 3.58 | 19.89 | 40.41 | 46.76 | 80.05 | 111.55 | 150.80 |
| Memphis, TN | 1.12 | 3.43 | 12.26 | 38.05 | 45.22 | 74.54 | 95.16 | 152.72 |
| New York City, NY | 1.25 | 2.59 | 18.10 | 28.23 | 45.98 | 57.25 | 113.07 | 110.95 |
| Philadelphia, PA | 1.33 | 3.08 | 19.03 | 28.90 | 51.23 | 70.35 | 127.15 | 136.33 |
| Washington, DC | 0.55 | 2.96 | 13.72 | 37.15 | 41.66 | 66.65 | 134.36 | 126.34 |
| NHB:NHW rate ratio and 95% CI*
|
||||||||
|---|---|---|---|---|---|---|---|---|
| < 40
|
40–49
|
50–64
|
65+
|
|||||
| US | 2.17 | 2.10–2.25 | 1.90 | 1.86–1.93 | 1.55 | 1.53–1.57 | 1.18 | 1.17–1.19 |
| Baltimore, MD | 2.75 | 1.69–4.46 | 1.32 | 0.98–1.78 | 1.24 | 1.02–1.51 | 0.78 | 0.68–0.89 |
| Chicago, IL | 2.57 | 1.87–3.52 | 1.93 | 1.62–2.31 | 1.72 | 1.54–1.91 | 1.19 | 1.11–1.28 |
| Dallas, TX | 3.59 | 1.96–6.56 | 2.06 | 1.55–2.72 | 1.69 | 1.42–2.01 | 1.45 | 1.28–1.65 |
| Detroit, MI | 2.67 | 0.99–7.24 | 1.44 | 0.88–2.35 | 1.26 | 0.97–1.63 | 1.01 | 0.86–1.19 |
| Houston, TX | 1.71 | 1.21–2.41 | 1.98 | 1.62–2.41 | 1.59 | 1.42–1.79 | 1.15 | 1.04–1.28 |
| Los Angeles, CA | 2.57 | 1.81–3.65 | 2.03 | 1.69–2.45 | 1.71 | 1.53–1.92 | 1.35 | 1.24–1.47 |
| Memphis, TN | 3.06 | 1.47–6.38 | 3.10 | 2.07–4.65 | 1.65 | 1.37–1.98 | 1.60 | 1.40–1.84 |
| New York City, NY | 2.07 | 1.69–2.53 | 1.56 | 1.40–1.74 | 1.24 | 1.17–1.33 | 0.98 | 0.94–1.03 |
| Philadelphia, PA | 2.32 | 1.53–3.51 | 1.52 | 1.22–1.89 | 1.37 | 1.22–1.55 | 1.07 | 0.99–1.16 |
| Washington, DC | 5.37 | 2.28–12.67 | 2.71 | 1.73–4.23 | 1.60 | 1.28–2.00 | 0.94 | 0.81–1.09 |
Some cities are doing better than the national level and are doing better than the other. There is a geographic variation as some cities are doing better than the others.
All confidence intervals that include 1 do not display a statistically significant result. For example, Detroit in the age < 40 doesn’t show a statistically significant result.
The pattern of larger disparities among younger age groups (< 40 and 40–49) is observed across all 10 study cities. For example, in Chicago, the disparity is largest among women younger than 40 years old (RR 2.57, 95% CI [1.87; 3.52]) and among women 40–49 (RR 1.93, 95% CI [1.62; 2.31]). It is then followed by women 50–64 (RR 1.72, 95% CI [1.54; 1.91]) and 65+ (RR 1.19, 95% CI [1.11; 1.28]). Across the 10 study cities, the largest disparities in NHB:NHW breast cancer mortality rates were observed in the < 40 age group in Washington, DC (RR 5.37, 95% CI [2.28; 12.67]) and the 40–49 age group in Memphis (RR 3.10, 95% CI [2.07; 4.65]).
In all cities, the lowest disparities in NHB:NHW breast cancer were found for the age group 65+. However, for this age cohort, there was considerable variation between cities regarding the level and direction of this disparity. In Baltimore, NHB women aged 65+ had a statistically significantly lower breast cancer mortality rate as compared to NHW (Table 1). In several other cities (New York, Detroit, Philadelphia, and Washington DC), there was an absence of mortality disparity as measured by Rate Ratios for this 65+ age group. In contrast, Memphis, Dallas and Los Angeles retained significantly higher disparities at this 65+ age stage (Memphis RR 1.60, 95% CI [1.40–1.84], Dallas RR 1.45 95% CI [1.28–1.65], Los Angeles RR 1.35, 95% CI [1.24–1.47] compared to New York, Philadelphia, Washington DC and Baltimore.
These data are illustrated in Fig. 1a and b, which show mortality rates and disparity trends over the 15 years of the study in the US as a whole. While the lowest mortality rates occur among women < 40 and 40–49, these are the age groups for which the largest disparity in mortality outcomes is observed. Conversely, the highest mortality rates are observed among women 65+ and this is the age group for which the smallest disparity in mortality rates is observed. Similar results were found with different age group cut-offs (< 50, 50–69 and 70+) (Supplemental Fig. 1).
Table 2 contains the mortality rate difference and the number of excess NHB deaths by age group for the period 1999–2013. In the US, the number of excess NHB deaths among women < 40 was 2832, followed by 6479 excess death in the age group 40–49. Among age groups 50–64 and 65+, there were 10,775 and 5583 excess NHB deaths, respectively. In Chicago, 93 excess NHB deaths were seen among women < 40, 197 among women 40–49. In the 50–65 age group, 418 excess NHB deaths were observed, and in the 65+ age group, 234 excess NHB deaths were calculated. As expected in Baltimore, NHW women in the age group 65+ had a higher number of excess deaths compared to NHB. Similar results were found with different age group cut-offs (< 50, 50–69 and 70+) (Supplemental Table 2).
Table 2.
Risk difference (RD) and annual excess Non-Hispanic Black deaths by age group for the US and the 10 study cities with statistically significant rate ratios, 1999–2013. All confidence intervals that include 0 do not display a statistically significant result.
| < 40
|
40–49
|
50–64
|
65+
|
|||||
|---|---|---|---|---|---|---|---|---|
| RD | CI | RD | CI | RD | CI | RD | CI | |
| US | 1.55 | −0.41 to 3.51 | 14.43 | 12.47–16.39 | 22.65 | 20.69–24.61 | 18.02 | 16.07–19.99 |
| Baltimore, MD | 4.28 | 2.32–6.24 | 9.03 | 7.11–11.03 | 11.77 | 9.94–13.86 | −30.64 | −34.58–−30.66 |
| Chicago, IL | 1.99 | 0.03–3.95 | 17.44 | 15.5–19.42 | 30.02 | 28.16–32.08 | 21.82 | 20.28–24.20 |
| Dallas, TX | 2.13 | 0.17–4.09 | 18.60 | 16.69–20.61 | 25.98 | 24.23–28.15 | 45.44 | 46.72–50.64 |
| Detroit, MI | 2.37 | 0.41–4.33 | 9.62 | 7.71–11.63 | 14.35 | 12.71–16.63 | 1.30 | −0.52 to 3.40 |
| Houston, TX | 1.57 | −0.39 to 3.53 | 22.79 | 20.9–24.82 | 32.31 | 30.66–34.58 | 19.01 | 17.91–21.83 |
| Los Angeles, CA | 2.19 | 0.23–4.15 | 20.52 | 18.61–22.53 | 33.29 | 31.63–35.55 | 39.25 | 38.91–42.83 |
| Memphis, TN | 2.31 | 0.35–4.27 | 25.79 | 23.9–27.82 | 29.31 | 27.69–31.61 | 57.55 | 59.64–63.56 |
| New York City, NY | 1.34 | −0.62 to 3.30 | 10.13 | 8.17–12.09 | 11.27 | 9.32–13.24 | −2.13 | −4.10 to −0.18 |
| Philadelphia, PA | 1.75 | −0.21 to 3.71 | 9.87 | 7.92–11.84 | 19.12 | 17.27–21.19 | 9.18 | 7.48–11.40 |
| Washington, DC | 2.41 | 0.45–4.37 | 23.43 | 21.54–25.46 | 24.99 | 23.31–27.23 | −8.02 | −10.80 to −6.88 |
| Excess Death
|
||||
|---|---|---|---|---|
| < 40 | 40–49 | 50–64 | 65+ | |
|
|
|
|
|
|
| US | 2832 | 6479 | 10,775 | 5583 |
| Baltimore, MD | 119 | – | 72 | −137 |
| Chicago, IL | 93 | 197 | 418 | 234 |
| Dallas, TX | 33 | 68 | 107 | 117 |
| Detroit, MI | – | – | – | – |
| Houston, TX | 39 | 131 | 219 | 82 |
| Los Angeles, CA | 37 | 96 | 182 | 174 |
| Memphis, TN | 50 | 125 | 156 | 183 |
| New York City, NY | 182 | 266 | 338 | – |
| Philadelphia, PA | 81 | 81 | 180 | – |
| Washington, DC | 51 | 92 | 128 | – |
“–“ No Statistically significant NHB/NHW rate ratio (see Table 2).
A negative number means there were no excess deaths among NHB women as they had s statistically significant lower mortality rate. The death rate was per 100,000 women.
4. Discussion
The findings of this study supplements prior city level analysis of racial disparity on breast cancer mortality [7], adding comparison of mortality disparities by age cohorts between the 10 cities with the largest African American populations and the US. There is a statistically significant gradient in the NHB:NHW breast cancer mortality disparity across age groups with the largest disparities observed among women less than 40 years old and aged 40–49 and the smallest among women aged 50–64 and those 65+. All 10 cities demonstrated the same trend. However, the direction and magnitude of disparities varied not only by age group, but by geographic location. Some cities have less racial breast cancer mortality disparity than other cities across age groups. For example, US Eastern cities such as New York, Philadelphia, Washington and Baltimore exhibited no or lower breast cancer mortality disparities among women 65+ compared to the US or other cities. Lastly, almost two-thirds of excess deaths were observed among women in < 50.
These results were not controlled for incidence, stage, and subtype due to the lack of such data. NHB women in their forties are known to have a higher incidence of breast cancer compared to NHW. Also, there is a higher proportion of estrogen negative breast cancer among NHB women, especially NHB women < 50 [8]. Both of these factors might explain the larger disparity observed among women in their forties. However NHB women in older age groups also have a significantly higher proportion of estrogen negative breast cancer compared to NHW women, but the disparity is smaller in this age group and non-existent in some cities. Nevertheless, the convergence of breast cancer incidence among older NHW and NHB [4] could have played a role in the lower disparity observed among women age 65+. While variation in incidence and tumor biology may contribute to the observed mortality disparities, the considerable variation between cities and the US as a whole suggests structural factors and not biologic variation as an explanation.
Certain public policies (public insurance coverage, eligibility for certain preventive health screening programs) vary by age and geography. Eligibility rules for enrollment in federal Medicare do not vary from state to state and provide access to health insurance for most individuals over the age of 65, though there are well demonstrated racial variations in care delivery in the Medicare covered population. State coverage through Medicaid has been subject to considerably more variation between states as well as temporal changes in eligibility related to state budgets and state policy decisions. Over the past 2 decades, including the 15 years considered in this paper which precedes implementation of the Affordable Care Act states have varied considerably in their approaches to expanding Medicaid [9]. New York and Illinois have implemented extensive coverage expansions prior to the Affordable Care Act, as has Pennsylvania. Tennessee in the mid–2000 s had the largest contraction of its Medicaid program in its history due to budgetary challenges [10,11]. A new paper published on breast cancer outcomes in Tennessee post Medicaid contraction indicates that post contraction of the Tennessee Medicaid program, there were increases in delays in accessing treatment and an increase in later stage of diagnosis for breast cancer [12]. Further study is needed to examine how closely the regional variations in disparity correlate with variations in provision of health insurance.
For the 65+ age group, variation regarding insurance coverage is less, due to the universality of Medicare coverage in all states, for most though not all residents. However, geographic variations in the mortality disparity remain, though the magnitude is less pronounced, with several cities demonstrating mortality rates favoring NHBs and others displaying virtually no disparity at all. Cities such as Memphis, Dallas and Los Angeles, have elevated rate ratios, suggesting that structural factors beyond insurance coverage are likely at play. Certain public health systems and public policies are organized at a city level and distribution of resources/access points across a city varies. Social factors such as structural racism, as well as intersectionality of regional variations in access to high quality screening, diagnostics, and treatment exist and might have played a role in the gradient of disparities within cities [13–16]. However further research is necessary given the lack of available data to control for incidence, state, and breast cancer sub-types.
Extant research has established that regular screening mammography, early diagnosis, and timely treatment initiation reduce the morbidity and mortality associated with breast cancer [17–20]. To promote early stage breast cancer diagnosis and treatment initiation, regular screening is strongly advocated by all national clinical guidelines [17,21–23]. However, over the past several years, disagreement has arisen over the age at which a patient should initiate routine screening with the United States Preventive Services Task Force (USPSTF) recommending that average risk women undergo routine screening biennially at age 50 [23,24,22]. Although our study does not provide data on whether the cancer was screen-detected and we cannot directly link the observed disparities to mammography screening policies, we believe that recommendations by the USPSTF may increase racial disparity in outcomes among women in their forties, though additional research is needed that could control for variation in incidence, stage, and subtypes. Notably, 29% of all NHB breast cancer deaths occurred among women < 50, compared to 20% among NHW women and two third of excess deaths were observed among women in their forties.
5. Limitations
One limitation of the study is the lack of information on breast cancer death by subtype and stage at diagnosis and incidence from the mortality data files. Although there are no such data available by age group and at the city level, available data that have assessed breast cancer incidence (2010–2014) and mortality (2011–2015) rates at the states level have shown that, with the exception of Maryland and Tennessee where NHB/NHW incidence rate ratios were not statistically significant, the NHB/NHW incidence rate ratios were significantly lower in all the states of the cities included in this study while mortality rate ratios on its own were significantly higher in all these states; ranging from 1.32 in Maryland to 1.52 in Texas and Tennessee [25]. Also, the findings of our study might not necessarily be generalizable to non-metropolitan areas, where the disparities could go in either direction.
6. Conclusion
In summary, this work has possible implications for health insurance, mammography and breast cancer treatment coverage, which is particularly timely given our current national debate and state’s decision-making based on budgetary needs and possible federal cuts in support to the Medicaid program. While more research is necessary to understand how variation in incidence, stage, and subtype of breast cancer affects such disparities, the public health implications of the age variation in breast cancer disparities are significant and with further research might trigger further examination of current conflicting breast cancer screening guidelines and the models used to generate the guidelines, possibly leading to more nuanced and targeted guidelines.
Supplementary Material
Acknowledgments
Hunt’s work was supported by the Avon Breast Cancer Crusade (02-2015-020). Drs Sighoko and Murphy and Ms. Irizarry are supported by grants from the Avon Breast Cancer Crusade (05-2015-016) and were supported by Susan G. Komen.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.canep.2018.02.003.
Footnotes
Authorship contributions
Dominique Sighoko as first author acquired all data necessary for the study, conceived of the analysis, performed the analysis, worked with other authors on interpretation of analysis and drafted the paper.
Bijou Hunt contributed significantly to the writing of the manuscript and was involved in editing and interpretation of the analysis.
Bethliz Irizarry was involved in editing and writing the manuscript.
Karriem Watson contributed to reviewing and writing the manuscript.
David Ansell was involved in conceptualizing the research, assisting with drafting the manuscript, interpreting the data and editing the manuscript.
Anne Marie Murphy was involved in conceptualizing the research, drafting the manuscript in particular the discussion as it relates to policy implications, interpreting the data, reviewing and editing the manuscript.
Conflicts of interest
None.
Contributor Information
Bijou R. Hunt, Email: Bijou.hunt@sinai.org.
Bethliz Irizarry, Email: Bethliz_irizarry@rush.edu.
Karriem Watson, Email: Kswatson@uic.edu.
David Ansell, Email: David_ansell@rush.edu.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 3.Ekwueme DU, Rim GP, Guy SH, Jr, White A, Hall IJ, Fairley TL, Dean HD. Health and economic impact of breast cancer mortality in young women: 1970–2008. Am J Prev Med. 2014;46(1):71–79. doi: 10.1016/j.amepre.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sighoko D, Fackenthal Hainaut JDP. Changes in the pattern of breast cancer burden among African American women: evidence based on 29 states and District of Columbiaduring 1998 to 2010. Ann Epidemiol. 2015;25(1):15–25 (e10). doi: 10.1016/j.annepidem.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy AM, Yang J, Armstrong K. Increasing disparities in breast cancer mortality from 1979 to 2010 for US black women aged 20 to 49 years. Am J Public Health. 2015;105:S446–S448. doi: 10.2105/AJPH.2014.302297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt BR, Hurlbert MS. Black:white disparities in breast cancer mortality in the 50 largest cities in the United States, 2005–2014. Cancer Epidemiol. 45(2016):169–173. doi: 10.1016/j.canep.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Sighoko D, Murphy AM, Irizarry B, Rauscher G, Ferrans C, Ansell D. Changes in the Racial Disparity in Breast Cancer Mortality in the Ten US Cities with the Largest African American Populations from 1999 to 2013: The Reduction in Breast Cancer Mortality Disparity in Chicago. Cancer Causes Control. 2017 doi: 10.1007/s10552-017-0878-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sighoko D, Rauscher G, Murphy A. Male breast cancer: are there racial disparities in incidence and tumor characteristics? Med Res Arch. 2017;5(5) [Google Scholar]
- 9.Guy GP, Jr, Ketsche MJEP, Joski P, Adams EK. The role of public and private insurance expansions and premiums for low-income parents: lessons from state experiences. Med Care. 2017;55(3):236–243. doi: 10.1097/MLR.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 10.Emerson JS, Hull PC, Cain VA, Novotny M, Stanley RE, Levine RS. TennCare disenrollment and avoidable hospital visits in Davidson County, Tennessee. J Health Care Poor Underserved. 2012;23(1):425–445. doi: 10.1353/hpu.2012.0002. [DOI] [PubMed] [Google Scholar]
- 11.Tarazi WW, Green TL, Sabik LM. Medicaid disenrollment and disparities in access to care: evidence from Tennessee. Health Serv Res. 2016 doi: 10.1111/1475-6773.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarazi WW, Bradley CJ, Bear HD, Harless DW, Sabik LM. Impact of Medicaid disenrollment in Tennessee on breast cancer stage at diagnosis and treatment. Cancer. 2017 doi: 10.1002/cncr.30771. [DOI] [PubMed] [Google Scholar]
- 13.Rauscher GH, Murphy AM, Orsi JM, Dupuy DM, Grabler PM, Weldon CB. Beyond the mammography quality standards act: measuring the quality of breast cancer screening programs. AJR Am J Roentgenol. 2014;202(1):145–151. doi: 10.2214/AJR.13.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina Y, Silva A, Rauscher GH. Racial/ethnic disparities in time to a Breast cancer diagnosis: the mediating effects of health care facility factors. Med Care. 2015;53(10):872–878. doi: 10.1097/MLR.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortel M, Rauscher GH, Murphy AM, Hoskins K, Warnecke RB. Racial and ethnic disparity in symptomatic Breast cancer awareness despite a recent screen: the role of tumor biology and mammography facility characteristics. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1599–1606. doi: 10.1158/1055-9965.EPI-15-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauscher GH, Khan JA, Berbaum ML, Conant EF. Potentially missed detection with screening mammography: does the quality of radiologist’s interpretation vary by patient socioeconomic advantage/disadvantage? Ann Epidemiol. 2013;23(4):210–214. doi: 10.1016/j.annepidem.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–26. doi: 10.7326/0003-4819-151-10-200911170-00008. W-236. [DOI] [PubMed] [Google Scholar]
- 18.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 19.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(5 Part 1):347–360. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 20.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 21.Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, Dempsey PJ, Farrar WB, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096. doi: 10.6004/jnccn.2009.0070. [DOI] [PubMed] [Google Scholar]
- 22.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan B, Nygren P, Humphrey L. Screening for Breast Cancer: Systematic Evidence Review Update for the US Preventive Services Task Force. Rockville, MD: 2009. [PubMed] [Google Scholar]
- 23.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, Walter LC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American cancer society. JAMA. 2015;314(15):1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson HD, Cantor A, Humphrey L, Fu R, Pappas M, Daeges M, Griffin J. Screening for Breast Cancer: A Systematic Review to Update the 2009 U.S. Preventive Services Task Force Recommendation. Rockville, MD: 2016. [PubMed] [Google Scholar]
- 25.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics: 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


