Abstract
Ticks are important vectors for the transmission of pathogens including viruses. The viruses carried by ticks also known as tick-borne viruses (TBVs), contain a large group of viruses with diverse genetic properties and are concluded in two orders, nine families, and at least 12 genera. Some members of the TBVs are notorious agents causing severe diseases with high mortality rates in humans and livestock, while some others may pose risks to public health that are still unclear to us. Herein, we review the current knowledge of TBVs with emphases on the history of virus isolation and identification, tick vectors, and potential pathogenicity to humans and animals, including assigned species as well as the recently discovered and unassigned species. All these will promote our understanding of the diversity of TBVs, and will facilitate the further investigation of TBVs in association with both ticks and vertebrate hosts.
Keywords: Ticks, Tick-borne viruses (TBVs), Isolation, Identification
Introduction
Ticks are highly specialized obligate haematophagous ectoparasites. There are over 900 species of ticks in the world, and many of them are capable of transmitting pathogenic agents (Horak et al. 2002). Tick-borne viruses (TBVs) are a major risk from tick bites which could result in viral infectious diseases among animals and humans (Parola and Raoult 2001). Ticks first draw human’s intention for their infestation in animals, which would result in extensive damage to livestock health and production. The role of ticks in transmission of pathogenic viruses has been known for more than 100 years following the discovery of a flavivirus, Louping ill virus, which was identified as being responsible for severe encephalitis in sheep and other livestock (Stockman 1918). Since then, increasing numbers of TBVs have been identified, many of which are known to cause diseases in animals and humans and have been frequently reported to be associated with large epidemics. In 1957, Kyasanur Forest disease virus was first isolated during an outbreak of febrile disease in India, which caused a large number of deaths among monkeys and severe febrile illness among local residents (Holbrook 2012). Crimean–Congo hemorrhagic fever was first noted in 1945 during an epidemic among Soviet military personnel and local inhabitants (Zivcec et al. 2016), which was subsequently confirmed to be caused by Crimean–Congo hemorrhagic fever virus (also called Xinjiang hemorrhagic fever in China) (Butenko et al. 1968). Moreover, the (re-)emergence of some TBV-related epidemics or sporadic cases has also been reported in areas with a history of diseases years ago and in new geographic areas, which keeps reminding us that TBVs have always been a significant public health problem in the world.
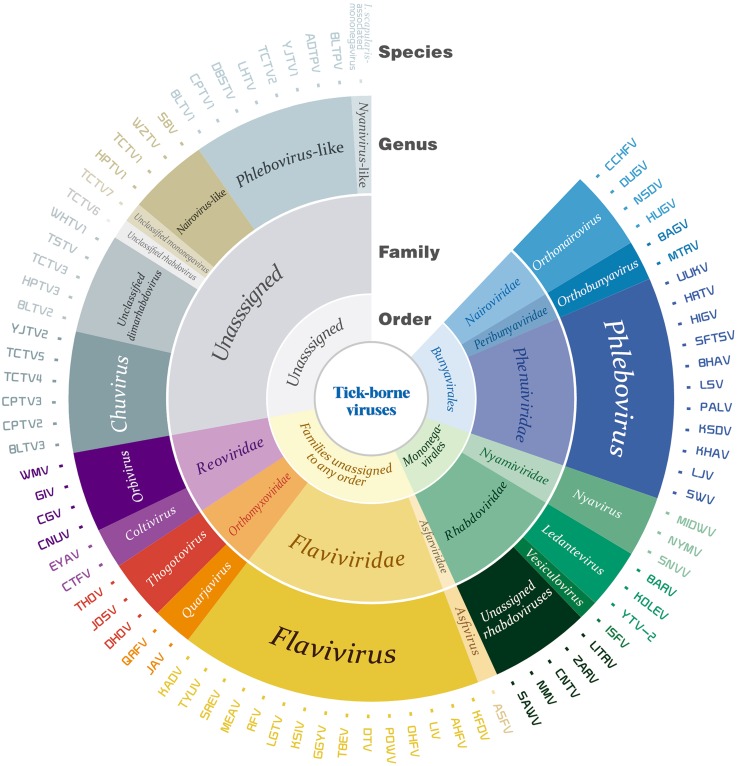
To our knowledge, the known identified TBVs include members of two orders, nine families and at least 12 genera, as well as other unassigned members (Tables 1, 2). Taking advantage of the rapid development of next generation sequencing (NGS) methods in recent years, many novel viral sequences have been identified in ticks of different species distributed in different regions of the world. Some sequences could be assigned into established families and/or genera, while many others were distantly related to known viruses and thus could not be assigned. We herein review the history of TBVs identification and isolation, classification, geographic distribution, and related diseases and epidemics, and discuss the novel viral sequences discovered in ticks recently. Viruses are described in the alphabetical order according to their taxon including Bunyavirales, Mononegavirales, and families unassigned to any order. All these suggest that ticks carry viruses of great diversities and that our current understanding of TBVs might be a tip of iceberg (Fig. 1).
Table 1.
Classification of tick-borne viruses.
| Family | Genus | Species | Main tick vectors | Geographical distributions |
|---|---|---|---|---|
| Bunyavirales | ||||
| Nairoviridae | Orthonairovirus | Crimean–Congo hemorrhagic fever virus | Hy. marginatum, Ixodid spp., R. rossicus | Many countries in Asia and Africa; Parts of Europe (e.g. Albania, Bulgaria) |
| Dugbe virus | Am. variegatum, Hy. truncatum, B. decoloratus, and R. appendiculatus | Sub-Saharan Africa | ||
| Nairobi sheep disease virus Ganjam virus | R. appendiculatus | India and Sri Lanka East and Central Africa | ||
| Farallon virus | Ornithodorus spp. | USA | ||
| Hughes virus | O. denmarki | USA (Florida), Trinidad, Venezuela, Cuba | ||
| Punta Salinas virus | O. amblus | Peru | ||
| Soldado virus | O. maritimus | North Wales, Great Britain, France, Seychelles, and Indian Ocean | ||
| Zirqa virus | Ornithodorus spp., A. cooleyi | Abu Dhabi | ||
| Peribunyaviridae | Orthobunyavirus | Tete orthobunyavirus serogroup | ||
| Bahig virus | Hy. marginatum | Italy | ||
| Matruh virus | Hy. marginatum | Egypt, Italy | ||
| Phenuiviridae | Phlebovirus | Uukunimi group | ||
| Uukuniemi virus | I. ricinus | Finland, Scandinavia, central and eastern of Europe, Azerbaijan in central Asia | ||
| SFTS/Heartland group | ||||
| Heartland virus | Am. americanum | USA | ||
| Hunter island virus | I. eudyptidis | Australia | ||
| Severe fever with thrombocytopenia syndrome virus | H. longicornis | China, South Korea and Japan | ||
| Bhanja group | ||||
| Bhanja virus | H. intermedia | Africa, Asia, southern Europe | ||
| Lone Star virus | Am. americanum | USA (Kentucky) | ||
| Palma virus | H. punctate | Portugal | ||
| Kaisodi group | ||||
| Kaisodi virus | H. spinigera | South India | ||
| Khasan virus | H. longgicornis | Russia | ||
| Lanjan virus | D. auratus | Malaya | ||
| Silverwater virus | H. leporispalustris | Canada (Alberta) and USA (Wisconsin) | ||
| Mononegavirales | ||||
| Nyamiviridae | Nyavirus | Midway virus | Ornithodoros spp. | Central Pacific, Japan |
| Nyamanini virus | A. walkerae, A. arborerus | Nigeria, Egypt, India, Thailand, South Africa | ||
| Sierra Nevada virus | O. coriaceus | USA | ||
| Rhabdoviridae | Ledantevirus | Barur virus | Hy. intermedia | India, Kenya, Somalia |
| Kolente virus | Am. variegatum | Guinea | ||
| Yongjia tick virus 2 | H. hystricis | China | ||
| Vesiculovirus | Isfahan virus | Hy. asiaticum | Turkmenistan, parts of Asia | |
| Unassigned rhabdoviruses | Long Island tick rhabdovirus | Am. americanum | USA | |
| Zahedan rhabdovirus | Hy. anatolicum | Iran | ||
| Sawgrass virus group | ||||
| Connecticut virus | I. dentatus | USA (Connecticut) | ||
| New Minto virus | H. leporispalustris | USA (East central Alaska) | ||
| Sawgrass virus | D. variabilis, H. leporispalustris | USA (Florida) | ||
| Families unassigned to any order | ||||
| Asfarviridae | Asfivirus | African swine fever virus | O. moubata, O. erraticus | Sub-Saharan Africa, Southern Europe, South America |
| Flaviviridae | Flavivirus | Mammalian tick-borne flavivirus group | ||
| Kyasanur Forest disease virus | H. spinigera | India | ||
| Alkhumra hemorrhagic fever virus | O. savignyi | Saudi Arabia | ||
| Louping ill virus | I. ricinus | Ireland, England, Scotland, Wales | ||
| Omsk hemorrhagic fever | D. reticulatus | Russia, Western Siberia | ||
| Powassan virus | Ixodes spp., I. cookei | Canada, USA, Russia | ||
| Deer tick virus | I. scapularis | New England | ||
| Tick-borne encephalitis virus | I. ricinus; I. persulcatus, I. ovatus | Northern Europe Northern Asia, Siberia | ||
| Gadgets Gully virus | I. uriae | Macquarie Island | ||
| Karshi virus | O. papillipes | Uzbek S.S.R, North of Central Asia | ||
| Langat virus | I. granulatus | Malaya | ||
| Royal Farm virus | A. hermanni | Afghanistan | ||
| Seabird tick-borne flavivirus group | ||||
| Meaban virus | O. maritimus | France | ||
| Saumarez Reef virus | O. capensis, I. eudyptidis | Australia | ||
| Tyuleniy virus | I. putus | Tuleniy Island | ||
| Putative third group | ||||
| Kadam virus | R. pravus | Uganda | ||
| Orthomyxoviridae | Quarjavirus | Johnston Atoll virus | O. capensis | Australia, New Zealand and Hawaii, central Pacific |
| Quaranfil virus | A. arboreus | Egypt, South Africa, Nigeria, Afghanistan, Kuwait, Iraq, Yemen and Iran | ||
| Thogotovirus | Dhori virus | Hyalomma spp. | India, eastern Russia, Egypt | |
| Jos virus | Amblyomma spp. and Rhipicephalus spp. | Nigeria Ethiopic, Guinea, Central Africa Republic, Nigeria, Ivory Coast and Senegal | ||
| Thogoto virus | Rhipicephalus spp., Boophilus spp., Hyalomma spp., and Am. variegatum | Central and East Africa Southern Europe, Southern Portugal | ||
| Reoviridae | Coltivirus (Spinareovirinae) | Colorado tick fever virus | D. andersoni, D. occidentalis, D. albipictus, D. arumapertus, H. leporispalustris, Ot. lagophilus, I. sculptus, and I. spinipalpis | USA |
| Eyach virus | I. ricinus, I. ventalloi | Germany, France | ||
| Orbivirus (Sedoreovirinae) | Chenuda virus species | |||
| Baku virus | O. maritimus | Caspian Sea, Uzbekistan | ||
| Chenuda virus | A. hermanni | Egypt, Uzbekistan | ||
| Essaouira virus | O. maritimus | Morocco | ||
| Huacho virus | O. amblus | Peru | ||
| Kala Iris virus | O. maritimus | Morocco | ||
| Mono Lake virus | A. cooleyi | USA (Califonia) | ||
| Sixgun city virus | A. cooleyi | USA | ||
| Chobar Gorge virus species | ||||
| Chobar Gorge virus | Ornithodoros spp. | Nepal | ||
| Great Island virus species | ||||
| Great Island virus | I. uriae | Canada (Newfoundland) | ||
| Kemerovo virus | I. persulcatus, I. ricinus | Russia, Slovakia | ||
| Lipovnik virus | I. ricinus | Slovakia, Czech Republic | ||
| Tribec virus | I. ricinus, H. punctata | Slovakia, Italy, Belorussia | ||
| St Croix River virus | I. scapularis, R. appendiculatus | N/Aa | ||
| Wad Medani virus species | ||||
| Seletar virus | B. microplus | Malaysia, Singapore | ||
| Wad Medani virus | R. sanguineus, Hyalomma spp. | East Africa, Asia, Jamaica | ||
aSt Croix River virus was identified from established tick cell lines. The geographic distribution of this virus was unclear to us.
Table 2.
The unassigned viruses detected in ticks by NGS.
| Viruses | Putative classificationa | Putative tick host | Closest relative (aa identity) |
|---|---|---|---|
| Bole tick virus 3 | Chuvirus | Hy. asiaticum | Midway virus (17.1%) |
| Changping tick virus 2 | Chuvirus | Dermacentor spp. | Midway virus (17.6%) |
| Changping tick virus 3 | Chuvirus | Dermacentor spp. | Midway virus (16.5%) |
| Tacheng tick virus 4 | Chuvirus | A. miniatus | Midway virus (17.5%) |
| Tacheng tick virus 5 | Chuvirus | D. marginatus | Midway virus (16.8%) |
| Yongjia tick virus 2 | Chuvirus | H. hystricis | Nishimuro virus (54.2%) |
| Bole tick virus 2 | Unclassified dimarhabdovirus | Hy. asiaticum | Isfahan virus (38.1%) |
| Huangpi tick virus 3 | Unclassified dimarhabdovirus | H. doenitzi | Eel virus European X (40%) |
| Tacheng tick virus 3 | Unclassified dimarhabdovirus | D. marginatus | Eel virus European X (39.8%) |
| Taishun tick virus | Unclassified dimarhabdovirus | H. hystricis | Vesicular stomatitis Indiana virus (36.6%) |
| Wuhan tick virus 1 | Unclassified dimarhabdovirus | R. microplus | Eel virus European X (38.3%) |
| Tacheng tick virus 6 | Unclassified mononegavirus | A. miniatus | Maize mosaic virus (20.6%) |
| Tacheng tick virus 7 | Unclassified rhabdovirus | A. miniatus | Orchid fleck virus (24.5%) |
| Huangpi tick virus 1 | Nairovirus like | H. doenitzi | Hazara virus (39.5%) |
| Tacheng tick virus 1 | Nairovirus like | D. marginatus | Hazara virus (39.5%) |
| Wenzhou tick virus | Nairovirus like | H. hystricis | Crimean–Congo hemorrhagic fever virus (39.1%) |
| South Bay virus | Nairovirus | I. scapularis | Crimean–Congo hemorrhagic fever virus (37.1%) |
| Bole tick virus 1 | Phlebovirus | Hy. asiaticum | Uukuniemi virus (37.9%) |
| Changping tick virus 1 | Phlebovirus | Dermacentor spp. | Uukuniemi virus (37.9%) |
| Dabieshan tick virus | Phlebovirus | H. longicornis | Uukuniemi virus (39.2%) |
| Lihan tick virus | Phlebovirus | R. microplus | Uukuniemi virus (38.6%) |
| Tacheng tick virus 2 | Phlebovirus | D. marginatus | Uukuniemi virus (39.0%) |
| Yongjia tick virus 1 | Phlebovirus | H. hystricis | Uukuniemi virus (40.5%) |
| American dog tick phlebovirus | Phlebovirus | D. variabilis-associated | Precarious point virus (30.8%) |
| Blacklegged tick phlebovirus | Phlebovirus | I. scapularis | Precarious point virus (30.6%) |
| I. scapularis-associated mononegavirus | Nyamiviridae | I. scapularis | Midway viruses (17%) |
aChuvirus represents the novel group of RNA viruses which are phylogenetically related but are unassigned currently according to the results from Zhang’s study (Lin et al. 2015).
Fig. 1.
Taxonomy of tick-borne viruses. The classification of currently known tick-borne viruses was summarized in open circles which present orders, families, genera, and species of the viruses from the inner to the outer circle, respectively.
Bunyavirales
Bunyavirales is a recently proposed order according to the 10th ICTV report, which includes nine families. Morphological and genomic similarities like spherical virion with lipid bilayer envelope and trisegmented negative single-stranded RNA are shared by most bunyaviruses. Typical bunyaviruses are mainly arthropod-borne viruses, most of which are reported to be associated with viral diseases in vertebrates. TBVs are mainly involved in three families, Nairoviridae, Peribunyaviridae and Phenuiviridae.
Nairoviridae
The family Nairoviridae has one genus Orthonairovirus consisting of at least 35 viruses assigned to seven serogroups as approved species (Crimean–Congo hemorrhagic fever virus, Dera Ghazi Khan virus, Hughes virus, Nairobi sheep disease, Qalyub virus, Sakhalin virus, and Thiafora virus) (Lasecka and Baron 2014; Walker et al. 2016). These nairoviruses are transmitted primarily by ticks and associated with natural hosts like birds, bats, rodents, and other animals.
The most notorious and focused upon nairovirus is Crimean–Congo hemorrhagic fever virus (CCHFV), which causes an acute febrile illness accompanied with severe bleeding named Crimean–Congo hemorrhagic fever (CCHF) in humans. CCHF was first brought to modern medical attention in 1945 when over 200 Soviet military troops and local inhabitants contracted the disease and about 10% of the patients died (Zivcec et al. 2016). For more than 20 years after the recognition of CCHF, researches in laboratories were limited due to the failure to isolate CCHFV. In 1968, Butenko and his colleagues isolated CCHFV via inoculating of serum from a patient in Russia into newborn mice (Butenko et al. 1968). CCHFV was recognized as a highly pathogenic virus to humans with a mortality rate of 30% (Guo et al. 2012). The recorded case of CCHFV infection presented with a wide geographic distribution including areas in western and central Asia including Xinjianng Province, China, the Middle East, south-eastern Europe and Africa (Burney et al. 1980; Drosten et al. 2002; Dunster et al. 2002; Fang et al. 2015; Nabeth et al. 2004). CCHFV could be transmitted by ticks in an enzootic cycle involving vertebrates and humans. Ixodid tick species from the genus Hyalomma and another species Rhipicephalus rossicus are thought to be the principle vectors for CCHFV transmission (Charrel et al. 2004; Hoogstraal 1979). CCHFV transmission to humans can occur via tick bite or exposure to body fluids from viremic animals or humans (Bente et al. 2013). Humans infected with CCHFV present non-specific symptoms, including a rapid onset high-grade fever, fatigue, and myalgia, frequently accompanied by vomiting and diarrhea. Subsequently, most patients will develop severe disease characterized by thrombocytopenia, elevated circulating liver enzymes, and hemorrhagic manifestation (Ergonul 2006; Whitehouse 2004). A fatal outcome is typically the result of disseminated intravascular coagulopathy, shock and/or multi-organ failure (Ergonul 2006; Vorou et al. 2007). At present, antiviral strategies to treat CCHFV infection still remain controversial or in the experimental stage. The most widely used antiviral medicine, Ribavirin, has been shown to be effective against CCHFV in vitro and in animal models, but its clinical benefit remains unproven (Duygu et al. 2012; Koksal et al. 2010). Dugbe virus (DUGV) belongs to CCHFV group and was first isolated from Amblyomma variegatum in Nigeria in 1964 (Karabatsos 1978). Then the virus was isolated from other tick species including Hyalomma truncatum, Boophilus decoloratus, and Rhipicephalus appendiculatus. DUGV is one of the most commonly found TBVs in Africa and is considered to be endemic in arid regions, because it was frequently isolated from ticks infesting market livestock (Sang et al. 2006; Burt et al. 1996). It can persist and replicate trans-stadially in orally infected Am. variegatum ticks and can be subsequently transmitted by tick feeding to a vertebrate host. As it is less pathogenic than CCHFV, but at the same time it is antigenically and genetically related to CCHFV which requires a high biosafety level laboratory, DUGV is considered an appropriate model for experimental studies on nairovirus, especially on the CCHFV infection mechanism (Coates and Sweet 1990).
Nairobi sheep disease (NSD) was first identified near Nairobi, Kenya in 1910, and Nairobi sheep disease virus (NSDV) was not found to be the causative agent until 1917 (Montgomery 1917). This virus can be transmitted by R. appendiculatus ticks, and causes acute hemorrhagic gastroenteritis in sheep and goats with mortality rates reaching over 90% (Idede 2000). Therefore, NSDV may cause serious damage to the farming industry. NSDV was originally thought to be endemic only in East Africa, but a recent study showed that an Asian variant of NSDV called Ganjam virus (GV), can also be found in other places including India and Sri Lanka (Dandawate et al. 1969; Marczinke and Nichol 2002). So to date, NSDV has been reported in a wide region including East and Central Africa, Ethiopia, Somalia, Botswana, and Mozambique. Sheep and goats are the only known vertebrate reservoirs and amplifying hosts of NSDV/GV. Human infections have also been considered because serological surveys in India were suggestive of a widespread occurrence of infection with GV (Dandawate et al. 1969), and human infection through a needle-stick injury, when researchers handled the virus in the laboratory, was described, which led to mild febrile illness (Rao et al. 1981). A study demonstrated that NSDV/GV was able to block the innate immune system (Holzer et al. 2011). However, compared with CCHFV, research on NSDV/GV is still limited.
The approved species Hughes virus includes five viruses, Farallon virus (FARV), Hughes virus (HUGV), Punta Salinas virus (PSV), Soldado virus (SOLV), and Zirqa virus (ZIRV). They are all TBVs vectored by soft ticks (Kohls et al. 1965). FARV was isolated from Ornithodorus spp. in 1965 from California, USA (Rodovsky and Stiller 1967). HUGV was first isolated from O. denmarki ticks collected in 1962 in Florida and subsequently from the same tick species from Soldado Rock (Gould et al. 1983). PSV was isolated from O. amblus ticks in 1967 from Peru (Converse et al. 1981). SOLV was isolated from O. maritimus in the 1960s–1970s in North Wales, Great Britain, France, Seychelles, and Indian Ocean (Chastel et al. 1979, 1988, 1990; Johnson et al. 1979). ZIRV was isolated from bird-infesting soft ticks (Ornithodorus spp. or Argas. cooleyi) in 1969 from Abu Dhabi (United Arab Emirates) (Varma et al. 1973). So this reveals a remarkably extensive distribution of HUGV members. However, knowledge of HUGV is limited.
Peribunyaviridae
Family Peribunyaviridae consists of the genera Herbevirus and Orthobunyavirus. Genus Orthobunyavirus contains more than 220 viruses assigned to 48 distinct species based on serological relatedness by complement fixation test or hemagglutination inhibition and neutralization tests (https://talk.ictvonline.org/ictv-reports/ictv_online_report/). Most members of genus Orthobunyavirus are arboviruses transmitted by mosquitoes or culicoid flies except for a few that are vectored by ticks (Elliott 2014). At present, Bahig virus (BAGV) and Matruh virus (MTRV) within the Tete orthobunyavirus serogroup are the only known orthobunyaviruses transmitted by ticks (Shchetinin et al. 2015). The prototype of BAGV strain EgB-90 was originally isolated from the blood of Oriolus orilus caught at Bahig village in Egypt in 1966 (Watson et al. 1972). BAGV was subsequently found in migrating birds in Italy and the larvae of Hy. marginatum ticks on a northward migrating Oenanthe oenanthe in Egypt (Balducci et al. 1973). MTRV was first isolated from migrating passerines in Egypt, in 1961 and subsequently found in Italy from migrating birds (Balducci et al. 1973). Both BAGV and MTRV are vectored by Hy. marginatum ticks. And no human and animal disease have been associated with BAGV and MTRV yet (Hubalek and Rudolf 2012).
Phenuiviridae
The family Phenuiviridae contains four genera: Goukovirus, Phasivirus, Phlebovirus, and Tenuivirus. Most viruses from the four genera are arboviruses. Only genus Phlebovirus including a large group of virus members are associated with ticks, which were usually named tick-borne phleboviruses (TBPVs).
Phlebovirus
Phleboviruses are a large group of arboviruses with transmission vectors including ticks, mosquitoes, midges, and flies. A recent study has divided phleboviruses into five phylogenetically related groups: the Sandfly/Mosquito-borne group, the Uukuniemi group, the SFTS/Heartland group, the Bhanja group, and the Kaisodi group (Matsuno et al. 2015). With the exception being the vectors of the Sandfly/Mosquito-borne group viruses are mainly sandflies and mosquitoes, the other groups are mostly TBPVs. The Uukuniemi group includes at least 17 species of TBPVs, which were identified in Europe, Africa, Middle Asia, Australia, and America decades ago (Palacios et al. 2013). The prototype of the Uukuniemi group, Uukuniemi virus (UUKV), was first isolated in 1960 from a pool of Ixodes ricinus ticks collected in southern Finland (Saikku and Brummer-Korvenkontio 1973). Subsequently, the virus was isolated from ticks in Scandinavia, Central and Eastern Europe, and Azerbaijan in central Asia (CDC Arbovirus catalog [http://wwwn.cdc.gov/arbocat/]). The UUKV group was not suggested to pose threat to public health, although antibodies to some members were detected in humans (Palacios et al. 2013).
In recent years, newly emerging TBPVs able to induce severe diseases in humans have attracted increasing attention. Severe fever with thrombocytopenia syndrome virus (SFTSV) is one of the novel pathogenic TBPVs and was first identified in China from patients with severe fever, thrombocytopenia, and leukocytopenia accompanied with gastrointestinal symptoms, chills, joint pain, and myalgia (Fang et al. 2015; Yu et al. 2011). The disease is so called severe fever with thrombocytopenia syndrome (SFTS) with an initially reported fatality rate of up to 30% (Yu et al. 2011). Some patients reported a history of tick bite, and SFTSV was detected mainly in Haemaphysalis longicornis ticks from where the patients lived (Luo et al. 2015). Human-to-human transmission through direct blood contact was also reported in clusters of SFTS patients (Bao et al. 2011; Liu et al. 2012), and other potential transmission routes among persons were also supposed but need further investigation (Gong et al. 2015; Huang et al. 2017; Jeong et al. 2016). From 2010 to 2016, 23 provinces in China have reported over 10,000 SFTS cases with a mean mortality rate of 5.3% (Zhan et al. 2017). After SFTSV identification in China, SFTSV infection was also reported in Japan and South Korea (Hiraki et al. 2014; Kim et al. 2013; Yoo et al. 2016; Yoshikawa et al. 2015). Phylogenetic analyses showed that the genotypes of SFTSV strains were associated with geographic distributions (Shi et al. 2017; Yoshikawa et al. 2015). Recombination and reassortment had happened among different strains and genotypes due to the migration of SFTSV from different locations, which might result in increasing genetic diversity of SFTSV (Liu et al. 2014; Shi et al. 2017). Heartland virus (HRTV) was first isolated from two patients in Missouri, USA, who had diseases with similar symptoms to SFTS (McMullan et al. 2012). HRTV shares similarity of about 60%–70% to SFTSV, and was phylogenetically related to SFTSV (McMullan et al. 2012). Detection of HRTV RNA and isolation of HRTV from Am. americanum ticks revealed Am. americanum may be the primary vector for HRTV transmission. To date, over 30 cases of HRTV infection, including two deaths, have been reported in the United States (Bosco-Lauth et al. 2015; Fill et al. 2017; Muehlenbachs et al. 2014; Riemersma and Komar 2015; Westover et al. 2017), while no other countries nor territories have reported cases associated with HRTV (Savage et al. 2013). So far, studies on the pathogenesis of both SFTSV and HRTV are limited, as virus infection could not induce significant clinical signs in experimental animals. Several autopsy cases with SFTSV and HRTV infection were reported from Japan and America, reflecting different pathological findings but this was probably due to the personal variations of the deaths (Fill et al. 2017; Hiraki et al. 2014; Muehlenbachs et al. 2014). Hunter Island group virus (HIGV) was isolated from I. eudyptidis ticks collected from a village in Australia (Wang et al. 2014). However, HIGV was not suggested to be related to the disease in the village where an outbreak occurred in 2002. Phylogenetic analyses showed that HIGV, HRTV, and SFTSV constitute the SFTS/Heartland group, indicating that HIGV might be a pathogen with zoonotic potential (Matsuno et al. 2015; Wang et al. 2014).
Since the identification of TBPVs related to human diseases, increasing attention has been paid to other TBPVs that were identified years ago but have not been characterized yet. Bhanja virus (BHAV) was first isolated from H. intermedia ticks in India in 1954 and then in Africa and Europe (Hubalek 1987b; Hubalek et al. 1988). It was confirmed as a neurotropic virus to be able to cause disease in ruminants and humans (Balducci et al. 1970; Hubalek 1987a). Palma virus (PALV) was isolated from H. punctata ticks in Portugal in 1992 (Filipe et al. 1994). The phylogenetic relationships of BHAV and PALV were not clarified until 2012, when the complete genome of both viruses was sequenced. The results suggested that BHAV and PALV are novel TBPVs distinct from Uukuniemi group and the SFTS/Heartland group, and form a new group termed as the Bhanja group (Matsuno et al. 2015). Lone star virus (LSV) was originally isolated from Am. americanum (the lone star tick) in Kentucky in 1967 (Kokernot et al. 1969), and was unclassified until 2013 when the sequence was reported (Swei et al. 2013). LSV can infect human (HeLa) and monkey (Vero) cells, but no evidence for human infection has been reported (Labuda and Nuttall 2004). Phylogenetic analysis showed that LSV also belonged to Bhanja group (Matsuno et al. 2015).
The Kaisodi group includes four members, including Kaisodi virus (KSDV) from H. spinigera ticks in 1957 (Bhatt et al. 1966), Khasan virus (KHAV) from H. longicornis ticks in 1971 (Al’khovskii et al. 2013), Lanjan virus (LJV) from Dermacentor auratus ticks in 1960 (Tan et al. 1967), and Silverwater virus (SWV) isolated from H. leporispalustris ticks in 1960 (Hoff et al. 1971). After the full-length genomes of these viruses were obtained recently, benefiting from NGS, these viruses have been identified as being novel TBPVs (Matsuno et al. 2015). So far, no infectious cases have been reported to be associated with members of the Kaisodi group.
Mononegavirales
The viral order Mononegavirales accommodates viruses with nonsegmented, linear, single-stranded negative-sense RNA genomes (Afonso et al. 2016). Two families in Monogegavirales, including Nyamiviridae and Rhabdoviridae, contain members of TBVs.
Nyamiviridae
The family Nyamiviridae is a newly proposed taxon belonging to Mononegavirales, which currently consists of three genera (Nyavirus, Peropuvirus, and Socyvirus) (Kuhn et al. 2013a, b). Three species in genus Nyavirus are TBVs, Midway nyavirus (MIDWV), Nyamanini nyavirus (NYMV), and Sierra Nevada nyavirus (SNVV) (Kuhn et al. 2013a). MIDWV was first isolated in 1966 from seabird ticks Ornithodoros spp. collected on the Midway, Kure, and Manana islands in the Central Pacific and from northern Honshu, Japan. Additionally, two species of nestling seabirds, Larus crassirostris and Nycticorax nycticorax, were found to have specific antibody to MIDWV. MIDWV was confirmed to be pathogenic to newborn Swiss mice. (Takahashi et al. 1982). NYMV was first isolated in 1957 from a cattle egret in South Africa (Taylor et al. 1966b). Then it was then repeatedly isolated from cattle egrets and A. walkerae soft ticks in Nigeria, Egypt, India, and Thailand (Kaiser 1966; Kemp et al. 1975; Taylor et al. 1966b). NYMV is considered primarily a bird virus and postulated to be transovarially transmitted by A. arborerus ticks (Kaiser 1966). No human infection or disease was associated with NYMV. Suckling mice succumbed to NYMV infection 7 days after intracerebral inoculation, and NYMV can productively infects a variety of mammalian cells (Kaiser 1966). MIDWV and NYMV are antigenically related to each other, but they have different geographic distribution and host spectrum (Takahashi et al. 1982). Both viruses are negative-stranded RNA viruses sharing ~ 63% genomic identity, and show a high genetic divergence from all other tested viruses (Mihindukulasuriya et al. 2009). They were therefore proposed to be two novel virus species, creating the novel genus Nyavirus (Kuhn et al. 2013b). SNVV was first isolated in 1975 from soft ticks O. coriaceus collected during the investigation of an outbreak of epizootic bovine abortion (EBA) in northern California, USA. However, it was not the causative agent of EBA. SNVV has about 50% similarity to NYMV and MIDWV, and is phylogenetically related to them (Rogers et al. 2014). It is presently unknown whether SNVV naturally infects birds or mammals.
Rhabdoviridae
The family Rhabdoviridae is composed of a large and diverse group of viruses that can infect a wide range of vertebrates, invertebrates, and plants (Kuzmin et al. 2009). According to the latest official report of ICTV in 2016, the family Rhabdoviridae can be taxonomically classified into 18 genera (Almendravirus, Curiovirus, Cytorhabdovirus, Dichorhavirus, Ephemerovirus, Hapavirus, Ledantevirus, Lyssavirus, Novirhabdovirus, Nucleorhabdovirus, Perhabdovirus, Sigmavirus, Sprivivirus, Sripuvirus, Tibrovirus, Tupavirus, Varicosavirus, and Vesiculovirus) and other unassigned rhabdoviruses. The virions of rhabdoviruses are characteristically bullet-shaped particles with the length of 100–430 nm and the diameter of 45–100 nm (Dilcher et al. 2015). Rhabdoviruses are non-segmented, negative-sense RNA viruses with 11–15 kbp genomes coding for at least five transcription units: nucleoprotein (N), phosphprotein (P), matrix protein (M), glycoprotein (G) and the RNA-dependent RNA polymerase (RdRp) (King et al. 2012). Rhabdoviruses are mainly transmitted by arthropod vectors, and a few are TBV species which are summarized below.
Ledantevirus
Genus Ledantevirus comprises 14 species. Three of them are TBVs, including Barur virus (BARV), Kolente virus (KOLEV), and Yongjia tick virus 2 (YTV-2) (Kazimirova et al. 2017). BARV was isolates from Hy. intermedia ticks in India, Kenya, and Somalia (Butenko et al. 1981; Johnson et al. 1977). Monkeys could be infected by BARV, which induced typical localization of lesions in the brain and resulted in the death of the experimental monkeys (Abramova et al. 1987). KOLEV was firstly isolated in Guinea in 1985 from Jones’s leaf-nosed bat and a pool of ticks (Am. variegatum) (Butenko 1996; Konstantinov et al. 2006). Significant cytopathic effect (CPE) could be induced by KOLEV infection in baby hamster kidney (BHK-21) cells. Newborn mice inoculated intracranially with KOLEV showed signs of illness including loss of balance, paralysis, and lethargy (Konstantinov et al. 2006). YTV-2 was identified by NGS from hard ticks (H. hystricis) collected from wild and domestic animals in Zhejiang Province, China, however, the virus was not isolated. Phylogenetic analysis showed that YTV-2 shares high amino acid similarity to all other ledanteviruses (Li et al. 2015). Little is known about these tick-borne rhabdoviruses in association with human or animal disease (Ghedin et al. 2013).
Vesiculovirus
The members of genus Vesiculovirus are mainly arthropod-borne viruses that are transmitted by biting insects, most likely sandflies (Nasar et al. 2017). The Isfahan virus (ISFV) is the unique member in genus Vesiculovirus that has been isolated from Hy. asiaticum ticks in Turkmenistan (Kazimirova et al. 2017; Labuda and Nuttall 2004). It was first isolated from phlebotomine sandflies in Isfahan Province, Iran in 1975, and is endemic in parts of Asia, including Iran, Turkmenistan and the central Asia republics (Tesh et al. 1977). Serological surveys in Iran following the isolation of ISFV detected neutralizing antibodies in humans and in rodents but not in sheep, goats, cattle, chickens or pigeons. Such results suggest that ISFV is not readily transmitted to the latter species and is very likely restricted to a limited range of hosts (Wilks and House 1986). Infection by ISFV has not been linked to any illness in natural infection among domestic animals and human (Gaidamovich et al. 1978). Compared with other vesiculoviruses much milder illness was induced by ISFV in the experimental infection of domestic animals, including pony, steer, sheep, goat and pig (Wilks and House 1986).
Unassigned rhabdoviruses
The only other rhabdoviruses isolated from ticks are all unassigned to any genera presently including two genetically related viruses (Long Island tick rhabdovirus [LITRV] and Zahedan rhabdovirus [ZARV]), and the Sawgrass virus group containing three TBVs.
LITRV was identified in long star ticks (Am. americanum) collected in New York in a program for viral surveillance and discovered in ticks through high-throughput sequencing (HTS) (Tokarz et al. 2014a). ZARV was isolated from Hy. anatolicum ticks from Iran (Dilcher et al. 2015). However, sequence comparisons and phylogenetic analyses do not associate LITRV and ZARV with any one of the recognized species or genera of this family, but these two viruses form a monophyletic clade with an unassigned mosquito-borne rhabdovirus from Côte d’Ivoire, which is proposed to be a unique taxonomic group within Rhabdoviridae (Tokarz et al. 2014a). ZARV can cause typical CPE when inoculated on Vero cells, but no disease developed after subcutaneous and intraperitoneal inoculation into newborn mice. However, important questions about the role of ticks in enzootic transmission of LITRV and ZARV, and the pathogenicity of these viruses to mammalian hosts are unanswered (King et al. 2012; Tokarz et al. 2014a).
The Sawgrass virus group consists of Connecticut virus (CNTV), New Minto virus (NMV), and Sawgrass virus (SAWV), which were first isolated years ago in D. variabilis ticks, H. leporispalustris ticks, and I. dentatus ticks from the USA, respectively (Ritter et al. 1978; Sather et al. 1970; Walker et al. 2015). However, further studies are required to clarify whether ticks can transmit these viruses and their pathogenicity to humans and animals.
Families Unassigned to Any Order
Asfarviridae
The family Asfarviridae has one genus, the genus Asfivirus, and comprises only single species, African swine fever virus (ASFV). It is the only known DNA arbovirus transmitted by ticks, and could infect and cause disease in swine. The African swine fever disease (ASF) caused by ASFV was first identified in Kenya in the 1920s (Montgomery 1921). At this point, it was confined to Africa, but then spread to Europe in the middle of the last century, and later to South America and the Caribbean (Galindo and Alonso 2017). ASF was eradicated from Europe, except for Sardinia, in the 1990s via drastic control and eradication programs. However, in 2007, the disease spread again out of Africa into Caucasus, especially Georgia, and in 2014 it reached the eastern territory of the European Union (EU). The latest reports of the disease include an increasing list of EU countries, Poland and the three Baltic republics and Moldova (Pejsak et al. 2014; Wozniakowski et al. 2016). This re-emerge may be a consequence of the ASFV increase in Africa in combination with the globalization and usage of contaminated garbage/swill to feed pigs (Sanchez-Vizcaino et al. 2013).
ASFV isolates differ in virulence, and may produce acute, chronic, or even inapparent infections. Virulent isolates can cause 100% mortality within 7–10 days, while less virulent isolates may produce a mild disease. A number of infected swine were found to become ASFV carriers after they recovered from the disease (Vinuela 1985). Soft ticks from the genus Ornithodoros are the main vectors of ASFV. O. moubata ticks are involved in the sylvatic transmission cycle of ASFV in sub- Saharan Africa, and O. erraticus is involved in Europe (Anderson et al. 1998). ASFV can be transmitted in tick trans-stadially, transovarially, and sexually (Kleiboeker et al. 1999). It can also be transmitted by a direct oral route in wart hogs, giant forest hogs, and bush pigs, and results in asymptomatic disease or disease with persistent infection, while high mortality and hemorrhage in domestic and wild pigs is usually related to ASFV infection (Detray 1957). Transmission between domestic animals can also occur by ingestion of infected meat and fomites, or mechanically by biting flies (Costard et al. 2013; Guinat et al. 2016). A striking feature of ASFV infections is the absence of neutralizing antibody production. This has severely hampered attempts to produce an effective vaccine although the use of gene deleted virus strains has shown promise in protecting against virulent strains (Zakaryan and Revilla 2016).
Flaviviridae
Family Flaviviridae consists of four genera, Flavivirus, Hepacivirus, Pegivirus, and Pestivirus (Fukuhara et al. 2017). The genomes of Flaviviridae viruses commonly contain a single-strand positive RNA encoding a polyprotein which will be cleaved into 2–4 structural proteins and 7–9 non-structural proteins (Chambers et al. 1990). The genus Flavivirus is a large group of arboviruses able to infect many vertebrates, they can be transmitted by mosquitos, ticks, or specific arthropod vectors. According to the different types of vectors, flaviviruses can be divided in to the tick-borne flavivirus (TBFV) group, the mosquito-borne flavivirus (MBFV) group, and the no known vector group (NKV) (Valarcher et al. 2015; Weaver and Barrett 2004). At least 12 species of TBFVs currently have been recognized and divided into the mammalian tick-borne flavivirus group (M-TBFV) and seabird tick-borne flavivirus group (S-TBFV) (Gritsun et al. 2003). In the M-TBFV group, six important pathogens of humans or animals are known as the “tick-borne encephalitis (TBE) serocomplex” including Kyasanur Forest disease virus, Louping ill virus, Omsk hemorrhagic fever virus, Powassan virus, and Tick-borne encephalitis virus. The Kyasanur Forest disease virus and Omsk hemorrhagic fever virus cause hemorrhagic fever in humans, while the others are encephalitic viruses (Grard et al. 2007).
Tick-borne encephalitis virus (TBEV) is the most notorious members of genus Flavivirus. It is the etiological agent of a severe human neurological infectious disease (Tick borne encephalitis, TBE). Terrible neurological symptoms were observed in patients with severe TBE, including muscle paralysis, disturbance of consciousness, and difficulties in swallowing, and verbal communication. Torturous sequelae were quite common among severe TBE patients (Solomon et al. 2007). Nearly half of the patients of TBE reported difficulties with memory and concentration (Gritsun et al. 2003; Marjelund et al. 2004). TBE was first discovered by Soviet scientists in the 1930s in the Far East under extremely harsh conditions. Some of the scientists paid for this pioneering work with their health and even lives (Zlobin et al. 2017). Since then, areas ranging from northern China and Japan, through far-eastern Russia to Europe have been afflicted by thousands of human cases of TBEV infection each year (Dumpis et al. 1999; Gould et al. 2006). TBEV can be classified into three genotypes in association with their geographic distributions, the European (TBEV-Eu), Siberian (TBEV-Sib), and Far-eastern (TBEV-FE) subtypes. The TBEV-Eu subtype is predominantly epidemic in Europe and Russia; the TBEV-Sib subtype is mainly present in Siberian in Russia; and the TBEV-FE subtype is endemic mainly in China, Japan and far-eastern Russia. Other areas like Lithuania, Sweden, and Denmark, where no TBE cases have been reported, were confirmed to have TBEV-specific antibodies circulating in animal and human populations (Paulsen et al. 2015; Pettersson et al. 2014). The transmitting vectors of TBEV are verified according to the subtypes. The TBEV-Eu subtype is mainly transmitted by I. ricinus, while TBEV-FE and TBEV-Sib are associated with I. persulcatus. Wild mammalian hosts are also involved in the maintenance and circulation of TBEV in nature, especially rodents which act as maintenance and amplifying hosts and the reservoir hosts for TBEV (Suss 2003). Human infections with the TBEV-FE are usually more severe than infection with the other two subtypes, with more frequent encephalitis signs and a higher fatality rate estimated as 5%–35%, compared with 1%–2% for TBEV-Eu and 6%–8% for TBEV-Sib (Gritsun et al. 2003). Presently, only the Far Eastern subtype is endemic in China (Gao et al. 2010; Lu et al. 2008). Several provinces including Xinjiang, Tibet, Inner Mongolia, Jilin, Heilongjiang, Sichuan, and Yunnan have reported cases of TBEV infection (Zhang et al. 2013; Zhao et al. 2012). I. persulcatus ticks are the predominant tick species in China and serve as the major transmission vector of TBEV (Lindquist and Vapalahti 2008; Sun et al. 2017). Other tick species like I. ovatus is responsible for TBEV transmission in Yunan Province (Lu et al. 2008).
The virus species Kyasanur Forest disease virus consists of two subtypes: Kyasanur Forest disease virus (KFDV) and Alkhumra hemorrhagic fever virus (AHFV). KFDV is the prototype of this species and is a zoonotic tick-borne viral disease that causes significant morbidity and mortality in human and monkey populations. It was known to exist in Kyasanur Forest of Karnataka State, India, since 1957 and has spread beyond into surrounding states (Awate et al. 2016; Work et al. 1957). Variants have also emerged in Saudi Arabia and Egypt and possibly in China (Musso et al. 2015; Wang et al. 2009). KFDV is maintained in nature through small mammals, shrews, bats, and monkeys and also in ticks (Pattnaik 2006). It is mainly transmitted to monkeys and humans through tick bites (Sreenivasan et al. 1986). The number of monkeys affected by this disease has increased substantially in the past few decades (Awate et al. 2016; Jeffries et al. 2014). In India, human cases with KFDV infection have been continuously reported. Especially in 2014 and 2015, human/monkey deaths associated with KFDV infection were identified (Sadanandane et al. 2017). Annual numbers of human cases of KFDV are estimated at 400–500 with a fatality rate of 3%–5% (Holbrook 2012). The status of KFDV as a high-risk containment level-4 agent has restricted research on its pathogenicity and the development of therapeutics (Cook et al. 2016). AHFV was first isolated from the blood of six male butchers in Saudi Arabia in 1995, among who two died and the other four recovered. All AHFV infected patients had similar manifestations including fever, headache, generalized body aches, arthralgias, anorexia, vomiting, leucopenia, thrombocytopenia, elevated liver enzymes, elevated creatinine phosphokinase, and elevated blood urea. It was suggested that the human infections by AHFV may be related to contact with infected sheep or ticks feeding on sheep (Dodd et al. 2011; Zaki 1997). Genetic analysis showed that AHFV was highly related to KFDV and it was recommended as a subtype of KFDV (Charrel et al. 2001).
Louping ill disease is a tick borne viral infection that predominantly affects sheep causing neurological disease with the reported morbidity ranging from 5% to 60% in different areas. Typical manifestations included diffuse non-suppurative meningoencephalitis with ataxia, pyrexia, seizures and opisthotonus, posterior paralysis, coma, and death (Scott et al. 2007). Louping ill virus (LIV) is the causative agent which was first isolated in Scotland in 1929 and was the first isolated arthropod-borne virus in Europe (Doherty et al. 1971). It is mainly detected in sheep, cattle, red grouse and ticks in upland areas of the British Isles, particularly in Scotland, Cumbria, Wales, Devon and Ireland (Jeffries et al. 2014). LIV was also detected in other animals, including goats, dogs, pigs, horses, deer, llamas, alpacas, and mountain hares (Jeffries et al. 2014; Reid et al. 1978; Timoney et al. 1976). I. ricinus is the major transmission vector and is associated with the distribution of LIV (Gilbert 2010). Seasonal occurrence, presenting with a high prevalence in spring and autumn, is consistent with the activity of the tick vector (Randolph et al. 2002). The first incidence of possible human LIV infection was reported in 1934, and since then there have been 44 further published reports of clinical disease in man. Most reported LIV infections occurred through occupational exposure to infected livestock including in stockmen, abattoir workers, butchers, and veterinarians who have frequent contact with sheep or other potentially infectious species (Williams and Thorburn 1962).
Omsk hemorrhagic fever virus (OHFV) was identified to be the causative agent of Omsk hemorrhagic fever (OHF) which was first diagnosed in 1940s in Russia. OHFV was first isolated from a patient’s blood in 1947, and later from D. reticulatus ticks, muskrats, and other vertebrates and arthropods. OHFV is genetically related to TBEV, but was suggested to be a unique species based on antigenic tests (Gaidamovich et al. 1989). In contrast to TBEV infection, OHFV infection does not invade the central nervous system, but results in mild flu-like symptoms. OHFV infection also causes hemorrhage, including nosebleeds, bleeding gums, vomiting of blood, blood in the lungs, and non-menstrual bleeding of the uterus. The case-fatality rate varies from 0.5% to 2.5%, and recovery from OHF is generally slow (Ruzek et al. 2010). Different to the widespread of TBEV, OHFV had remained restricted to four Siberian provinces during hundreds of years of evolution (Karan et al. 2014). It is still unclear why the OHFV endemic area is much smaller than where the major vectors and hosts (Dermacentor spp. ticks and muskrats) are distributed.
Powassan virus (POWV) is a tick-borne flavivirus transmitted by Ixodes spp. and can cause a fatal neuroinvasive disease in human. The virus was considered as a human pathogen when it was first isolated from the brain of a young boy who died of encephalitis in the town of Powassan (Canada) (McLean and Larke 1963). Since then, human cases of POWV have been documented in the United States, Canada, and Russia. The majority of symptomatic POWV cases typically involve an initial febrile illness. During the primary phase, sore throat, drowsiness, headache, and disorientation are commonly present (Smith et al. 1974). In severe cases, the most common clinical presentations of disease are encephalitis, meningoencephalitis, and aseptic meningitis (Smith et al. 1974). Approximately 10% of POWV encephalitis cases are fatal, and severe and long-lasting neurological sequelae are present in over 50% of survivors (Ebel 2010). In recent years, the incidence of human infection of POWV appears to be rising probably due to the enhanced surveillance and testing for arthropod-borne viruses or actual emergence of the disease (Ebel et al. 2001; Hinten et al. 2008; Piantadosi et al. 2016). However, compared with other human pathogenic flaviviruses, POWV has been overlooked. POWV was considered as a TBE serocomplex because of the high similarity to TBEV. Much of the understanding of POWV comes from research conducted with TBEV (Kuno et al. 2001). In 1997, a novel virus that was similar but distinct from POWV was isolated from I. scapularis tick (deer tick) in New England (USA) and was named deer tick virus (DTV) (Telford et al. 1997). Sequence alignment revealed a high level of genetic similarity between DTV and POWV, with 84% nucleotide sequence identity and 94% amino acid identity. After that, the POWV strains were divided into two genetic lineages or genotypes, the POWV lineage and the DTV lineage (Beasley et al. 2001). Although experimental evidence from mouse infection was suggestive that DTV was less infectious than other members of the TBE serocomplex viruses including TBEV and LIV (Telford et al. 1997), it could cause neuroinvasiveness in mice similar to POWV infection in humans. So the public significance of DTV deserves further investigation (Beasley et al. 2001).
Four other M-TBFVs, Gadgets Gully virus (GGYV), Karshi virus (KSIV), Langat virus (LGTV) and Royal Farm virus (RFV) are not considered human pathogens at present. Knowledge about their ecology and epidemiology is limited. GGYV was first isolated from pools of seabird ticks (I. uriae) collected between 1975 and 1979 at Macquarie Island (St George et al. 1985). After near 30 years, a new strain with 96% identity to the first one was isolated from I. uriae ticks collected in the same island, suggesting this flavivirus has maintained remarkable genetic stability for over 30 years. Phylogenetic analysis showed that GGYV was more related to the M-TBFV groups although it was discovered from seabird ticks (Major et al. 2009). KSIV was first isolated from O. papillipes ticks collected in 1971 from Karshinsk steppe, Uzbekistan (Lvov et al. 1976). Subsequently, it was isolated from H. asiaticum ticks collected in the north of Central Asia (Alma-Ata region of the Kazakh Soviet Socialist Republic) in 1976 (Khutoretskaya et al. 1985). KSIV was experimentally proved to be transmitted both by ticks and mosquitoes (Khutoretskaya et al. 1985). So far, it is not known to cause disease in humans (Grard et al. 2007). LGTV was firstly isolated via intracerebral inoculation into suckling mice from a pool of hard ticks (I. granulatus) from forest rats caught near Kuala Lumpur in Malaya. LGTV can cause paralysis in adult mice and induce fever in macaque monkeys (Smith 1956). No human disease has been reported to be associated with LGTV (Campbell and Pletnev 2000). RFV was isolated from A. hermanni ticks collected in Afghanistan. Complement fixation testing showed it was closely related to POWV. The virus was pathogenic for hamster but not for weanling guinea pigs and rabbits (Williams et al. 1972).
Seabirds are hosts of at least 29 tick species and thus may play roles in dispersal of virus due to their large population size, wide geographic distributions, and high mobility (Dietrich et al. 2011). The S-TBFV group includes three species: Meaban virus (MEAV), Saumarez Reef virus (SREV), and Tyuleniy virus (TYUV). MEAV was first isolated from O. maritimus ticks collected in 1981 and 1982 in the nests of herring gulls on islands of South Brittany, France. No antibody to MEAV was detected in sera collected from local residents (Chastel et al. 1985). SREV was isolated from seabird ticks of species O. capensis collected from the nests of Sooty Terns in 1974 and I. eudyptidis collected from two dead Silver Gulls (Larus) in 1826 (St George et al. 1977). TYUV was originally isolated from I. putus ticks collected from rifts in rocks at a seabird colony on Tuleniy Island (Lvov et al. 1971). Though no human nor other mammalian animal disease were related with these S-TBFVs, all these viruses can cause paralysis in suckling mouse, and SREV can even cause death in mice (Chastel et al. 1985; Lvov et al. 1971; St George et al. 1977).
Kadam virus (KADV) was originally isolated from R. pravus ticks collected from a cow in Uganda (Henderson et al. 1970). Subsequently, it was isolated in ticks from the Ar-Riyadh region in Saudi Arabia, and Uganda (Al-Khalifa et al. 2007). KADV was initially assigned to the M-TBFV group (Labuda and Nuttall 2004), but analysis of genetic distances showed that KADV constitutes a putative third group of tick-borne viruses in addition to the M-TBFV and S-TBFV groups (Grard et al. 2007).
Orthomyxoviridae
The family Orthomyxoviridae comprises viruses characterized with six to eight segments of linear, negative-sense RNA genomes (King et al. 2012). One most notorious representative in this family Orthomyxoviridae is influenza virus which attracted significant attentions from physicians and researchers (Urbaniak et al. 2014). In this family, two recently proposed genera, Quarjavirus and genus Thogotovirus, contain members of TBVs (Da Silva et al. 2005; King et al. 2012; Presti et al. 2009).
Quaranjavirus
Two species, Johnston Atoll virus (JAV) and Quaranfil virus (QRFV) are included in genus Quaranjavirus (Presti et al. 2009). JAV was originally isolated from soft ticks (O. capensis) collected in 1964 from a Noddy Tern (Anous stolidus) nest, Sand Island, Johnston Atoll in the Central Pacific (Clifford et al. 1968). Since then, Eastern Australia, New Zealand, and Hawaii have reported successful isolation of JAV (Austin 1978). To date, no human disease has been reported to be associated with JAV, but it is lethal to newborn mice after subcutaneous inoculation (Clifford et al. 1968). QRFV was first isolated in 1953 from children with mild febrile illness in Egypt. Subsequently, it was also isolated from ticks (A. arboreus) and birds collected in Egypt, South Africa, Afghanistan, Nigeria, Kuwait, Iraq, Yemen, and Iran (Converse and Moussa 1982; Kemp et al. 1975). Human serological studies showed approximately 8% of the local population had neutralizing antibodies to QRFV, suggesting the potential for infection by QRFV in humans (Taylor et al. 1966b). Experimental QRFV infection in laboratory mice caused a lethal respiratory disease and meningoencephalitis, indicating the pathogenic ability of ORFV infection in animals (Baskerville and Lloyd 1976). So the characteristics and pathogenesis of the novel tick-borne orthomyxoviruses require further investigations.
Thogotovirus
Thogoto virus (THOV) is the type species of genus Thogotovirus, and it was identified and isolated from Boophilus spp. and Rhipicephalus spp. ticks in Kenya (Africa) (Karabatsos 1978; Sang et al. 2006) and Sicily (Europe), from Am. variegatum in Nigeria, and from Hyalomma spp. ticks in both Nigeria and Egypt (Calisher et al. 1987; Karabatsos 1978). THOV infection has been reported to be pathogenic for sheep and has been associated with high level of abortion. Other livestock animals, including cattle, goats, and mongoose, can also be infected (Service 2001). Two human cases with natural infections of THOV have been reported with typical clinical manifestations including fever, encephalitis or meningoencephalitis, and one death was reported (Moore et al. 1975). Dhori virus (DHOV) was isolated from Hyalomma spp. ticks and was reported in India, eastern Russia, Egypt, and Southern Portugal (Anderson and Casals 1973; Filipe and Casals 1979; L’Vov et al. 2002). Five human cases with DHOV infection were identified who were febrile and had encephalitis, indicative of the potential of thogotoviruses to induce human diseases (Butenko et al. 1987). Jos virus (JOSV) was originally isolated from cow serum in Nigeria in 1967, and was then isolated from Amblyomma spp. and Rhipicephalus spp. ticks from Ethiopic, Guinea, Central Africa Republic, Nigeria, Ivory Coast, and Senegal (Bussetti et al. 2012; Karabatsos 1978). Though no diseased cases associated with JOSV have been reported for human and livestock, several studies have confirmed that JOSV could cause a fatal illness with acute hepatocellular necrosis when inoculated into newborn mice, suggestive of the potential health threat to animals (Bussetti et al. 2012; Fagbami and Ikede 1978).
Interestingly, thogotovirus surface glycoproteins show no similarities to any influenza viral proteins but have striking sequence homology to a baculovirus surface glycoprotein (Freedman-Faulstich and Fuller 1990; Morse et al. 1992). This significant difference between thogotovirus and other influenza viruses may be responsible for the ability of different host adaption in tick and avian (Freedman-Faulstich and Fuller 1990; Kimble et al. 2010). Significantly, the high structural and biochemical similarities of thogotovirus to influenza virus, their abundance and wide distribution in the world, and the high possibility and ability of orthomyxoviruses to undergo reassortment together make thogotoviruses deserving of more attention in the future in case of large epidemics.
Reoviridae
Reoviridae represents the largest family of dsRNA viruses containing viruses isolated from a wide range of vertebrates, invertebrates, plants, insects, and bacteria. Virus members of Reoviridae have genomes composed of 9–12 segments of linear dsRNA. The virus particles have icosahedral symmetry with a diameter of approximately 60–85 nm comprising three concentric protein layers which are designated as outer capsid, subcore, and core structures from the outer layer to the inner layer, respectively (Hill et al. 1999; Nason et al. 2004). Reoviridae includes 75 virus species and 30 further tentative species (Brussaard et al. 2004). According to differences in the morphological and genetic features, Reoviridae is divided into two subfamilies, Spinareovirinae and Sedoreovirinae. Subfamily Spinareovirinae describes the genera including spiked or turreted viruses, and Sedoreovirinae characterizes the genera containing the non-turreted viruses with relatively smooth morphology. Fifteen genera are included in this family, six belonging to the Sedoreovirinae subfamily (Cardoreovirus, Mimoreovirus, Orbivirus, Phytoreovirus, Rotavirus, Seadornavirus) and nine belonging to the Spinareovirinae subfamily (Aquareovirus, Coltivirus, Cypovirus, Dinovernavirus, Fijivirus, Idnoreovirus, Mycoreovirus, Orthoreovirus, Oryzavirus). Coltiviruses belonging to the Spinareovirinae subfamily and orbiviruses belonging to the Sedoreovirinae subfamily are arboviruses including TBVs.
Coltivirus
Presently, the genus Coltivirus contains Colorado tick fever virus (CTFV), California hare coltivirus (CTFV-Ca, a serotype of CTFV), and Eyach virus (EYAV) (King et al. 2012). Coltiviruses are mainly transmitted by ticks of family Ixodidae. CTFV is the typical species of genus Coltivirus. It causes an endemic disease named Colorado tick fever (CTF) in humans in northwestern America. It was initially confused with a mild form of Rocky Mountain spotted fever caused by Rickettsia rickettsii, until in 1946 when CTFV was isolated from human serum (Florio et al. 1946). CTFV is transmitted mainly by the wood tick D. andersoni, and other ticks such as D. occidentalis, D. albipictus, D. arumapertus, H. leporispalustris, Ot. lagophilus, I. sculptus, and I. spinipalpis are also associated with CTFV infection (Attoui et al. 2005). CTFV has a wide host range including ground squirrels, chipmunks, wild mice, wood rats, wild rabbits and hares, porcupines, marmots, deer, elk, sheep, and coyotes. Patients with CTFV infection have clinical signs including an abrupt onset of fever, chills, headache, retroorbital pain, photophobia, myalgia, abdominal pain, and generalized malaise. Patients with severe symptoms may have infection of the central nervous system (CNS) or hemorrhagic fever, pericarditis, myocarditis, and orchitis, which have been mainly observed in children (Attoui et al. 2005). EYAV is a distinct viral species but antigenically related to CTFV (Rehse-Kupper et al. 1976). It was first isolated in 1976 from I. ricinus ticks in southwestern Germany and from I. ricinus and I. ventalloi in 1981 (Chastel et al. 1984). The reservoir of EYAV is thought to be the European rabbit (Oryctolagus cunniculus), but the natural cycle of the virus is still unclear. Serological tests have indicated that EYAV is associated with meningoencephalitis in humans (Labuda and Nuttall 2004).
Orbivirus
The genus Orbivirus contains 22 recognized virus species (Roy and Noad 2006). Most orbiviruses are vertebrate-infecting viruses transmitted by blood-feeding arthropod vectors (Hubalek and Rudolf 2012). Five orbivirus species are TBVs: Chenuda virus, Chobar Gorge virus, Great Island virus, St Croix River virus, and Wad Medani virus. These tick-borne orboviruses (TBOVs) share low level identities with and are distantly related to other orbiviruses (Belaganahalli et al. 2015).
The species Chenuda virus includes seven serotypes: Baku virus (BAKUV), Chenuda virus (CNUV), Essaouira virus (ESSV), Huacho virus (HUAV), Kala Iris virus (KIRV), Mono Lake virus (MLV), and Sixgun City virus (SCV). BAKUV was isolated in 1970 in the Soviet Union (Karabatsos 1978). CNUV was isolated in 1954 from ticks in Egypt, with serological evidence of infection in birds, camels, pigs, buffalo, dogs, donkeys and rodents (Karabatsos 1978). ESSV and KIRV were isolated from O. maritimus from Morocco in 1979 and 1981, respectively (Chastel et al. 1993). HUAV was isolated in Peru in 1967 (Clifford et al. 1980), while MLV and SCV were isolated in 1966 and 1969, respectively, in the United States (Calisher et al. 1988; Labuda and Nuttall 2004). The species Chobar Gorge virus is associated with bats and includes two serotypes. The prototype Chobar Gorge virus (CGV) was isolated in 1970 from Ornithodoros spp. ticks in Nepal. Serological evidence revealed the potential for infection by CGV to cattle, horses, sheep, buffalo, and humans (Karabatsos 1978). Presently, Great Island virus represents the single species containing members of 36 serotypes, which are assigned as the GIV group. Viral members of GIV group are quite widespread in Europe, North America, the Russian Far East, and a sub-antarctic island (Hubalek and Rudolf 2012; Main et al. 1973; Nunn et al. 2006). The prototype, Great Island virus (GIV), was originally isolated from the I. uriae ticks and the seabird host Fratercula arctica in Newfoundland, Canada. Neutralizing antibodies were identified in sera from other seabirds (Uria aalge), suggesting possible infection of GIV in seabirds (Moss et al. 1988; Nunn et al. 2006). Kemerovo virus (KEMV) was isolated from I. persulcatus female ticks collected in the Kemerovo region (Russia) (Libikova et al. 1970). Neutralizing antibodies to KEMV were found in sera from humans, livestock, wild rodents, and birds (Libikova et al. 1970, 1978). Another two members of the GIV group, Lipovnik virus (LIPV) and Tribec virus (TRBV), which are quite closely related to KEMV, were isolated from adult I. ricinus ticks collected in Czechoslovakia in 1963 (Libikova et al. 1964). In subsequent years, KEMV, LIPV, TRBV, and other viral members with similar antigenic properties were identified and isolated from ticks in different continents, which comprise the GIV group of up to 36 members (Dedkov et al. 2014). The species Wad Medani virus includes two serotypes: Seletar virus (SELV) and Wad Medani virus (WMV). SELV was isolated from B. microplus ticks collected in the Seletar district, Singapore, in 1961. Serological evidence suggested the infection in cattle, camel, pigs, buffalo and rodents (Karabatsos 1978; Taylor et al. 1966a). WMV was first isolated from ticks collected at Wad Medani in Sudan in 1952, and subsequently from sheep and ixodid ticks from in the Sudan, West Pakistan, India, Russia, and Jamaica. St Croix River virus (SCRV) was the first recognized endogenous virus isolated from tick cell lines (IDE2) established in 1994 from eggs of I. scapularis ticks (Attoui et al. 2001). Then, it was found in other tick cell lines, IDE8 (I. scapularis), and RA243 and RA257 (R. appendiculatus) (Alberdi et al. 2012). SCRV is considered as a “tick only” virus because it fails to infect any other non-tick cells including the cell lines derived from mosquitoes, amphibians, and mammals (Bell-Sakyi and Attoui 2013). Phylogenetic analysis indicates that SCRV represents a lineage ancestral to other known TBOVs (Bell-Sakyi and Attoui 2013). It strongly indicated that SCRV is unlikely to be an arbovirus.
TBVs Unassigned to Families
Researchers are making efforts to identify, isolate, and characterize novel TBVs, and to determine whether these TBVs pose threats to public health, so that they can prepare for a rapid response to newly emerging diseases caused by novel TBVs. Taking advantage of NGS, a large number of novel virus-related sequences have been identified in different tick species; however, researchers failed to isolate these viruses (Li et al. 2015; Tokarz et al. 2014b). Phylogenetic analyses showed some of the novel TBVs are close to assigned virus families and genera, but most others are distantly related to defined families (15.8%–54.2%) and thus could not be assigned (Table 2) (Li et al. 2015; Qin et al. 2014; Tokarz et al. 2014b). A novel group of segmented RNA viruses was discovered taking Jingmen tick virus (JMTV) as the representative. The genome of JMTV is partly related to flaviviruses, but employs a different genome organization (Callister et al. 2008; Qin et al. 2014). The discovery of JMTV and other related segmented RNA viruses might reveal an unanticipated evolutionary link between segmented and unsegmented RNA viruses (Qin et al. 2014). Some other TBVs have employed different strategies for genome organization like circular genomes and segmented circular genomes, which have been rarely described before. These viruses locate between the segmented and unsegmented linear RNA viruses according to their phylogenic relationships (Li et al. 2015). All these data have shown the great diversity of TBVs and indicated there might be much more diversity than we previously thought.
Conclusion
Since the discovery of first tick-borne pathogenic virus over 100 years ago, diversified TBVs with global distribution have been discovered and isolated belonging to at least 2 orders, 9 families, and 12 genera. In recent years, the rapid development of NGS has boosted the discovery of novel TBVs. An unexpected role of TBVs in the evolutions of RNA viruses has been revealed, which also suggests the significant role of ticks in circulating and transmitting TBVs. The known TBVs may just represent a tip of the iceberg, the most and rest part of which remains to be explored.
Our current knowledge about the association of TBVs and tick species are still limited. Hopefully, ongoing researches from worldwide scientists on the immunomodulation mechanism of tick salivary, interactions between tick viruses and the transmitted-vector tick species, and anti-tick vaccines will have great significance in controlling of tick-borne viruses and protecting humans and livestock from pathogenic TBVs.
Acknowledgements
This work was supported by Science and Technology Basic Work Program (2013FY113500) from the Ministry of Science and Technology of China and the European Union’s Horizon 2020 Research and Innovation Programme (No. 653316), the Strategic Bio-resource Service Network Plan and Building the Biogenetic Resource Preserving Capacity Program from the Chinese Academy of Sciences (ZSSB-002), and the Hubei Provincial program of scientific and technological condition platform establishment (2017BEC003).
Abbreviations of generic name of tick
- A
Argas
- Am
Amblyomma
- B
Boophilus
- D
Dermacentor
- Hy
Hyalomma
- H
Haemaphysalis
- I
Ixodes
- O
Ornithodoros
- Ot
Otobius
- R
Rhipicephalus
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Fei Deng, Phone: +86-27-87198465, Email: df@wh.iov.cn.
Shu Shen, Phone: +86-27-87199229, Email: shenshu@wh.iov.cn.
References
- Abramova LF, Terskikh II, Terskikh IF, Gromashevskii VL, Gromashevskii VF, L’Vov DK. Comparative characteristics of the pathogenicity of 2 viruses of the family Rhabdoviridae for monkeys. Vopr Virusol. 1987;2:357–359. [PubMed] [Google Scholar]
- Afonso CL, Amarasinghe GK, Banyai K, Bao Y, Basler CF, Bavari S, Bejerman N, Blasdell KR, Briand FX, Briese T, Bukreyev A, Calisher CH, Chandran K, Cheng J, Clawson AN, Collins PL, Dietzgen RG, Dolnik O, Domier LL, Durrwald R, Dye JM, Easton AJ, Ebihara H, Farkas SL, Freitas-Astua J, Formenty P, Fouchier RA, Fu Y, Ghedin E, Goodin MM, Hewson R, Horie M, Hyndman TH, Jiang D, Kitajima EW, Kobinger GP, Kondo H, Kurath G, Lamb RA, Lenardon S, Leroy EM, Li CX, Lin XD, Liu L, Longdon B, Marton S, Maisner A, Muhlberger E, Netesov SV, Nowotny N, Patterson JL, Payne SL, Paweska JT, Randall RE, Rima BK, Rota P, Rubbenstroth D, Schwemmle M, Shi M, Smither SJ, Stenglein MD, Stone DM, Takada A, Terregino C, Tesh RB, Tian JH, Tomonaga K, Tordo N, Towner JS, Vasilakis N, Verbeek M, Volchkov VE, Wahl-Jensen V, Walsh JA, Walker PJ, Wang D, Wang LF, Wetzel T, Whitfield AE, Xie JT, Yuen KY, Zhang YZ, Kuhn JH. Taxonomy of the order Mononegavirales: update 2016. Arch Virol. 2016;161:2351–2360. doi: 10.1007/s00705-016-2880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi MP, Dalby MJ, Rodriguez-Andres J, Fazakerley JK, Kohl A, Bell-Sakyi L. Detection and identification of putative bacterial endosymbionts and endogenous viruses in tick cell lines. Ticks Tick Borne Dis. 2012;3:137–146. doi: 10.1016/j.ttbdis.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khalifa MS, Diab FM, Khalil GM. Man-threatening viruses isolated from ticks in Saudi Arabia. Saudi Med J. 2007;28:1864–1867. [PubMed] [Google Scholar]
- Al’khovskii SV, L’Vov DK, Shchelkanov M, Shchetinin AM, Deriabin PG, Samokhvalov EI, Gitel’man AK, Botikov AG. The taxonomy of the Khasan virus (KHAV), a new representative of phlebovirus genera (Bunyaviridae), isolated from the ticks haemaphysalis longicornis (Neumann, 1901) in the Maritime Territory (Russia) Vopr Virusol. 2013;58:15–18. [PubMed] [Google Scholar]
- Anderson CR, Casals J. Dhori virus, a new agent isolated from Hyalomma dromedarii in India. Indian J Med Res. 1973;61:1416–1420. [PubMed] [Google Scholar]
- Anderson EC, Hutchings GH, Mukarati N, Wilkinson PJ. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet Microbiol. 1998;62:1–15. doi: 10.1016/S0378-1135(98)00187-4. [DOI] [PubMed] [Google Scholar]
- Attoui H, Stirling JM, Munderloh UG, Billoir F, Brookes SM, Burroughs JN, de Micco P, Mertens PP, de Lamballerie X. Complete sequence characterization of the genome of the St Croix River virus, a new orbivirus isolated from cells of Ixodes scapularis. J Gen Virol. 2001;82:795–804. doi: 10.1099/0022-1317-82-4-795. [DOI] [PubMed] [Google Scholar]
- Attoui H, Mohd Jaafar F, de Micco P, de Lamballerie X. Coltiviruses and seadornaviruses in North America, Europe, and Asia. Emerg Infect Dis. 2005;11:1673–1679. doi: 10.3201/eid1111.050868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin FJ. Johnston Atoll virus (Quaranfil group) from Ornithodoros capensis (Ixodoidea: Argasidae) infesting a gannet colony in New Zealand. Am J Trop Med Hyg. 1978;27:1045–1048. doi: 10.4269/ajtmh.1978.27.1045. [DOI] [PubMed] [Google Scholar]
- Awate P, Yadav P, Patil D, Shete A, Kumar V, Kore P, Dolare J, Deshpande M, Bagde S, Sapkal G, Gurav Y, Mourya DT. Outbreak of Kyasanur Forest disease (monkey fever) in Sindhudurg, Maharashtra State, India, 2016. J Infect. 2016;72:759–761. doi: 10.1016/j.jinf.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Balducci M, Verani P, Lopes MC, Nardi F. Experimental pathogenicity of Bhanja virus for white mice and Macaca mulatta monkeys. Acta Virol. 1970;14:237–243. [PubMed] [Google Scholar]
- Balducci M, Verani P, Lopes MC, Gregorio B. Isolation in Italy of Bahig and Matruh viruses (Tete group) from migratory birds. BMC Vet Res. 1973;11:244. [Google Scholar]
- Bao CJ, Guo XL, Qi X, Hu JL, Zhou MH, Varma JK, Cui LB, Yang HT, Jiao YJ, Klena JD, Li LX, Tao WY, Li X, Chen Y, Zhu Z, Xu K, Shen AH, Wu T, Peng HY, Li ZF, Shan J, Shi ZY, Wang H. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin Infect Dis. 2011;53:1208–1214. doi: 10.1093/cid/cir732. [DOI] [PubMed] [Google Scholar]
- Baskerville A, Lloyd G. The pathogenesis and pathology of experimental Quaranfil virus infection. Br J Exp Pathol. 1976;57:152–156. [PMC free article] [PubMed] [Google Scholar]
- Beasley DW, Suderman MT, Holbrook MR, Barrett AD. Nucleotide sequencing and serological evidence that the recently recognized deer tick virus is a genotype of Powassan virus. Virus Res. 2001;79:81–89. doi: 10.1016/S0168-1702(01)00330-6. [DOI] [PubMed] [Google Scholar]
- Belaganahalli M, Maan S, Maan N, Brownlie J, Tesh R, Attoui H, Mertens P. Genetic characterization of the tick-borne orbiviruses. Viruses. 2015;7:2185. doi: 10.3390/v7052185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Sakyi L, Attoui H. Endogenous tick viruses and modulation of tick-borne pathogen growth. Front Cell Infect Microbiol. 2013;3:25. doi: 10.3389/fcimb.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean–Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Bhatt PN, Kulkarni KG, Boshell J, Rajagopalan PK, Patil AP, Goverdhan MK, Pavri KM. Kaisodi virus, a new agent isolated from Haemaphysalis spinigera in Mysore State, South India. I. Isolation of strains. Am J Trop Med Hyg. 1966;15:958–960. doi: 10.4269/ajtmh.1966.15.958. [DOI] [PubMed] [Google Scholar]
- Bosco-Lauth AM, Panella NA, Root JJ, Gidlewski T, Lash RR, Harmon JR, Burkhalter KL, Godsey MS, Savage HM, Nicholson WL, Komar N, Brault A. Serological investigation of heartland virus (Bunyaviridae: Phlebovirus) exposure in wild and domestic animals adjacent to human case sites in Missouri 2012–2013. Am J Trop Med Hyg. 2015;92:1163–1167. doi: 10.4269/ajtmh.14-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard CP, Noordeloos AA, Sandaa RA, Heldal M, Bratbak G. Discovery of a dsRNA virus infecting the marine photosynthetic protist Micromonas pusilla. Virology. 2004;319:280–291. doi: 10.1016/j.virol.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Burney MI, Ghafoor A, Saleen M, Webb PA, Casals J. Nosocomial outbreak of viral hemorrhagic fever caused by Crimean Hemorrhagic fever-Congo virus in Pakistan, January 1976. Am J Trop Med Hyg. 1980;29:941–947. doi: 10.4269/ajtmh.1980.29.941. [DOI] [PubMed] [Google Scholar]
- Burt FJ, Spencer DC, Leman PA, Patterson B, Swanepoel R. Investigation of tick-borne viruses as pathogens of humans in South Africa and evidence of Dugbe virus infection in a patient with prolonged thrombocytopenia. Epidemiol Infect. 1996;116:353–361. doi: 10.1017/S0950268800052687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussetti AV, Palacios G, Travassos da Rosa A, Savji N, Jain K, Guzman H, Hutchison S, Popov VL, Tesh RB, Lipkin WI. Genomic and antigenic characterization of Jos virus. J Gen Virol. 2012;93:293–298. doi: 10.1099/vir.0.035121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko AM. Arbovirus circulation in the Republic of Guinea. Med Parazitol (Mosk) 1996;2:40–45. [PubMed] [Google Scholar]
- Butenko AM, Chumakov MP, Rubin VN, Stolbov DN. Isolation and investigation of astrakhan strain (“drozdov”) of Crimean hemorrhagic fever virus and data on serodiagnosis of this infection. Mater XV Nauch Sess Inst Poliom Virus Encefal (Moskva) 1968;3:88–90. [Google Scholar]
- Butenko AM, Gromashevsky VL, L’Vov DK, Popov VF. First isolations of Barur virus (Rhabdoviridae) from ticks (Acari: Ixodidae) in Africa. J Med Entomol. 1981;18:232–234. doi: 10.1093/jmedent/18.3.232. [DOI] [PubMed] [Google Scholar]
- Butenko AM, Leshchinskaia EV, Semashko IV, Donets MA, Mart’ianova LI. Dhori virus—a causative agent of human disease. 5 Cases of laboratory infection. Vopr Virusol. 1987;32(6):724–729. [PubMed] [Google Scholar]
- Calisher CH, Karabatsos N, Filipe AR. Antigenic uniformity of topotype strains of Thogoto virus from Africa, Europe, and Asia. Am J Trop Med Hyg. 1987;37:670–673. doi: 10.4269/ajtmh.1987.37.670. [DOI] [PubMed] [Google Scholar]
- Calisher CH, Schwan TG, Lazuick JS, Eads RB, Francy DB. Isolation of Mono Lake virus (family Reoviridae, genus Orbivirus, Kemerovo serogroup) from Argas cooleyi (Acari: Argasidae) collected in Colorado. J Med Entomol. 1988;25:388–390. doi: 10.1093/jmedent/25.5.388. [DOI] [PubMed] [Google Scholar]
- Callister DM, Winter AD, Page AP, Maizels RM. Four abundant novel transcript genes from Toxocara canis with unrelated coding sequences share untranslated region tracts implicated in the control of gene expression. Mol Biochem Parasitol. 2008;162:60–70. doi: 10.1016/j.molbiopara.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Campbell MS, Pletnev AG. Infectious cDNA clones of Langat tick-borne flavivirus that differ from their parent in peripheral neurovirulence. Virology. 2000;269:225–237. doi: 10.1006/viro.2000.0220. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Hahn CS, Galler R, Rice CM. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- Charrel RN, Zaki AM, Attoui H, Fakeeh M, Billoir F, Yousef AI, de Chesse R, De Micco P, Gould EA, de Lamballerie X. Complete coding sequence of the Alkhurma virus, a tick-borne flavivirus causing severe hemorrhagic fever in humans in Saudi Arabia. Biochem Biophys Res Commun. 2001;287:455–461. doi: 10.1006/bbrc.2001.5610. [DOI] [PubMed] [Google Scholar]
- Charrel RN, Attoui H, Butenko AM, Clegg JC, Deubel V, Frolova TV, Gould EA, Gritsun TS, Heinz FX, Labuda M, Lashkevich VA, Loktev V, Lundkvist A, Lvov DV, Mandl CW, Niedrig M, Papa A, Petrov VS, Plyusnin A, Randolph S, Suss J, Zlobin VI, de Lamballerie X. Tick-borne virus diseases of human interest in Europe. Clin Microbiol Infect. 2004;10:1040–1055. doi: 10.1111/j.1469-0691.2004.01022.x. [DOI] [PubMed] [Google Scholar]
- Chastel C, Launay H, Rogues G, Beaucournu JC. Isolation in France of Soldado virus (arbovirus, Hughes serogroup) from Ornithodoros (A.) maritimus Vermeil and Marguet 1967. C R Seances Acad Sci D. 1979;288:559–561. [PubMed] [Google Scholar]
- Chastel C, Main AJ, Couatarmanac’h A, Le Lay G, Knudson DL, Quillien MC, Beaucournu JC. Isolation of Eyach virus (Reoviridae, Colorado tick fever group) from Ixodes ricinus and I. ventalloi ticks in France. Arch Virol. 1984;82:161–171. doi: 10.1007/BF01311160. [DOI] [PubMed] [Google Scholar]
- Chastel C, Main AJ, Guiguen C, le Lay G, Quillien MC, Monnat JY, Beaucournu JC. The isolation of Meaban virus, a new Flavivirus from the seabird tick Ornithodoros (Alectorobius) maritimus in France. Arch Virol. 1985;83:129–140. doi: 10.1007/BF01309911. [DOI] [PubMed] [Google Scholar]
- Chastel C, Guiguen C, Le Lay G, Le Goff F. First isolation of Soldado virus in southern France. Acta Virol. 1988;32:191. [PubMed] [Google Scholar]
- Chastel C, Le Lay G, Le Goff F, Monnat JY. Isolation of Soldado virus from a seabird (Rissa tridactyla L.) in France. Acta Virol. 1990;34:109. [PubMed] [Google Scholar]
- Chastel C, Main AJ, Bailly-Choumara H, Le Goff F, Le Lay G. Essaouira and Kala iris: two new orbiviruses of the Kemerovo serogroup, Chenuda complex, isolated from Ornithodoros (Alectorobius) maritimus ticks in Morocco. Acta Virol. 1993;37:484–492. [PubMed] [Google Scholar]
- Clifford CM, Thomas LA, Hughes LE, Kohls GM, Philip CB. Identification and comparison of two viruses isolated from ticks of the genus Ornithodoros. Am J Trop Med Hyg. 1968;17:881–885. doi: 10.4269/ajtmh.1968.17.881. [DOI] [PubMed] [Google Scholar]
- Clifford CM, Hoogstraal H, Radovsky FJ, Stiller D, Keirans JE. Ornithodoros (alectorobius) amblus (Acarina: Ixodoidea: Argasidae): identity, marine bird and human hosts, virus infections, and distribution in Peru. J Parasitol. 1980;66:312–323. doi: 10.2307/3280825. [DOI] [PubMed] [Google Scholar]
- Coates DM, Sweet C. Studies on the pathogenicity of a nairovirus, Dugbe virus, in normal and immunosuppressed mice. J Gen Virol. 1990;71(Pt 2):325–332. doi: 10.1099/0022-1317-71-2-325. [DOI] [PubMed] [Google Scholar]
- Converse JD, Moussa MI. Quaranfil virus from Hyalomma dromedarii (Acari: Ixodoidea) collected in Kuwait, Iraq and Yemen. J Med Entomol. 1982;19:209–210. doi: 10.1093/jmedent/19.2.209. [DOI] [PubMed] [Google Scholar]
- Converse JD, Moussa MI, Easton ER, Casals J. Punta Salinas virus (Hughes group) from Argas arboreus (Ixodoidea: Argasidae) in Tanzania. Trans R Soc Trop Med Hyg. 1981;75:755–756. doi: 10.1016/0035-9203(81)90175-9. [DOI] [PubMed] [Google Scholar]
- Cook BW, Nikiforuk AM, Cutts TA, Kobasa D, Court DA, Theriault SS. Development of a subgenomic clone system for Kyasanur Forest disease virus. Ticks Tick Borne Dis. 2016;7:1047–1051. doi: 10.1016/j.ttbdis.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Costard S, Mur L, Lubroth J, Sanchez-Vizcaino JM, Pfeiffer DU. Epidemiology of African swine fever virus. Virus Res. 2013;173:191–197. doi: 10.1016/j.virusres.2012.10.030. [DOI] [PubMed] [Google Scholar]
- Da Silva EV, Da Rosa AP, Nunes MR, Diniz JA, Tesh RB, Cruz AC, Vieira CM, Vasconcelos PF. Araguari virus, a new member of the family Orthomyxoviridae: serologic, ultrastructural, and molecular characterization. Am J Trop Med Hyg. 2005;73:1050–1058. [PubMed] [Google Scholar]
- Dandawate CN, Work TH, Webb JK, Shah KV. Isolation of Ganjam virus from a human case of febrile illness: a report of a laboratory infection and serological survey of human sera from three different states of India. Indian J Med Res. 1969;57:975–982. [PubMed] [Google Scholar]
- Dedkov VG, Markelov ML, Gridneva KA, Bekova MV, Gmyl AP, Kozlovskaya LI, Karganova GG, Romanova L, Pogodina VV, Yakimenko VV, Shipulin GA. Prevalence of Kemerovo virus in ixodid ticks from the Russian Federation. Ticks Tick Borne Dis. 2014;5:651–655. doi: 10.1016/j.ttbdis.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Detray DE. African swine fever in wart hogs (Phacochoerus aethiopicus) J Am Vet Med Assoc. 1957;130:537–540. [PubMed] [Google Scholar]
- Dietrich M, Gomez-Diaz E, McCoy KD. Worldwide distribution and diversity of seabird ticks: implications for the ecology and epidemiology of tick-borne pathogens. Vector Borne Zoonotic Dis. 2011;11:453–470. doi: 10.1089/vbz.2010.0009. [DOI] [PubMed] [Google Scholar]
- Dilcher M, Faye O, Faye O, Weber F, Koch A, Sadegh C, Weidmann M, Sall AA. Zahedan rhabdovirus, a novel virus detected in ticks from Iran. Virol J. 2015;12:183. doi: 10.1186/s12985-015-0410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd KA, Bird BH, Khristova ML, Albarino CG, Carroll SA, Comer JA, Erickson BR, Rollin PE, Nichol ST. Ancient ancestry of KFDV and AHFV revealed by complete genome analyses of viruses isolated from ticks and mammalian hosts. PLoS Negl Trop Dis. 2011;5:e1352. doi: 10.1371/journal.pntd.0001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty PC, Reid HW, Smith W. Louping-ill encephalomyelitis in the sheep. IV. Nature of the perivascular inflammatory reaction. J Comp Pathol. 1971;81:545–549. doi: 10.1016/0021-9975(71)90083-1. [DOI] [PubMed] [Google Scholar]
- Drosten C, Minnak D, Emmerich P, Schmitz H, Reinicke T. Crimean–Congo hemorrhagic fever in Kosovo. J Clin Microbiol. 2002;40:1122–1123. doi: 10.1128/JCM.40.3.1122-1123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumpis U, Crook D, Oksi J. Tick-borne encephalitis. Clin Infect Dis. 1999;28:882–890. doi: 10.1086/515195. [DOI] [PubMed] [Google Scholar]
- Dunster L, Dunster M, Ofula V, Beti D, Kazooba-Voskamp F, Burt F, Swanepoel R, DeCock KM. First documentation of human Crimean–Congo hemorrhagic fever, Kenya. Emerg Infect Dis. 2002;8:1005–1006. doi: 10.3201/eid0809.010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duygu F, Kaya T, Baysan P. Re-evaluation of 400 Crimean–Congo hemorrhagic fever cases in an endemic area: is ribavirin treatment suitable? Vector Borne Zoonotic Dis. 2012;12:812–816. doi: 10.1089/vbz.2011.0694. [DOI] [PubMed] [Google Scholar]
- Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol. 2010;55:95–110. doi: 10.1146/annurev-ento-112408-085446. [DOI] [PubMed] [Google Scholar]
- Ebel GD, Spielman A, Telford SR., 3rd Phylogeny of North American Powassan virus. J Gen Virol. 2001;82:1657–1665. doi: 10.1099/0022-1317-82-7-1657. [DOI] [PubMed] [Google Scholar]
- Elliott RM. Orthobunyaviruses: recent genetic and structural insights. Nat Rev Microbiol. 2014;12:673–685. doi: 10.1038/nrmicro3332. [DOI] [PubMed] [Google Scholar]
- Ergonul O. Crimean–Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–214. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagbami AH, Ikede BO. Pathogenicity and pathology of Jos virus infection in mice and tissue culture. Microbios. 1978;21:81–88. [PubMed] [Google Scholar]
- Fang LQ, Liu K, Li XL, Liang S, Yang Y, Yao HW, Sun RX, Sun Y, Chen WJ, Zuo SQ, Ma MJ, Li H, Jiang JF, Liu W, Yang XF, Gray GC, Krause PJ, Cao WC. Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect Dis. 2015;15:1467–1479. doi: 10.1016/S1473-3099(15)00177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe AR, Casals J. Isolation of Dhori virus from Hyalomma marginatum ticks in Portugal. Intervirology. 1979;11:124–127. doi: 10.1159/000149023. [DOI] [PubMed] [Google Scholar]
- Filipe AR, Alves MJ, Karabatsos N, de Matos AP, Nuncio MS, Bacellar F. Palma virus, a new bunyaviridae isolated from ticks in Portugal. Intervirology. 1994;37:348–351. doi: 10.1159/000150399. [DOI] [PubMed] [Google Scholar]
- Fill MA, Compton ML, McDonald EC, Moncayo AC, Dunn JR, Schaffner W, Bhatnagar J, Zaki SR, Jones TF, Shieh WJ. Novel clinical and pathologic findings in a heartland virus-associated death. Clin Infect Dis. 2017;64:510–512. doi: 10.1093/cid/ciw766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio L, Stewart MO, Mugrage ER. The etiology of colorado tick fever. J Exp Med. 1946;83:1–10. doi: 10.1084/jem.83.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman-Faulstich EZ, Fuller FJ. Nucleotide sequence of the tick-borne, orthomyxo-like Dhori/Indian/1313/61 virus envelope gene. Virology. 1990;175:10–18. doi: 10.1016/0042-6822(90)90181-P. [DOI] [PubMed] [Google Scholar]
- Fukuhara T, Tamura T, Ono C, Shiokawa M, Mori H, Uemura K, Yamamoto S, Kurihara T, Okamoto T, Suzuki R, Yoshii K, Kurosu T, Igarashi M, Aoki H, Sakoda Y, Matsuura Y. Host-derived apolipoproteins play comparable roles with viral secretory proteins Erns and NS1 in the infectious particle formation of Flaviviridae. PLoS Pathog. 2017;13:e1006475. doi: 10.1371/journal.ppat.1006475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidamovich S, Obukhova VR, Sveshnikova NA, Cherednichenko Iu N, Kostiukov MA (1978) Natural foci of viruses borne by Phlebotomus papatasi in the USSR according to a serologic study of the population. Vopr Virusol, pp 556–560. (in Russian) [PubMed]
- Gaidamovich S, Sveshnikova NA, Stephenson JR, Lee JM, Mel’nikova EE. Antigenic analysis of tick-borne encephalitis virus group using monoclonal antibodies and immunofluorescence. Vopr Virusol. 1989;34:684–689. [PubMed] [Google Scholar]
- Galindo I, Alonso C. African swine fever virus: a review. Viruses. 2017 doi: 10.3390/v9050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Nasci R, Liang G. The neglected arboviral infections in mainland China. PLoS Negl Trop Dis. 2010;4:e624. doi: 10.1371/journal.pntd.0000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghedin E, Rogers MB, Widen SG, Guzman H, Travassos da Rosa AP, Wood TG, Fitch A, Popov V, Holmes EC, Walker PJ, Vasilakis N, Tesh RB. Kolente virus, a rhabdovirus species isolated from ticks and bats in the Republic of Guinea. J Gen Virol. 2013;94:2609–2615. doi: 10.1099/vir.0.055939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L. Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases? Oecologia. 2010;162:217–225. doi: 10.1007/s00442-009-1430-x. [DOI] [PubMed] [Google Scholar]
- Gong Z, Gu S, Zhang Y, Sun J, Wu X, Ling F, Shi W, Zhang P, Li D, Mao H, Zhang L, Wen D, Zhou B, Zhang H, Huang Y, Zhang R, Jiang J, Lin J, Xia S, Chen E, Chen Z. Probable aerosol transmission of severe fever with thrombocytopenia syndrome virus in southeastern China. Clin Microbiol Infect. 2015;21:1115–1120. doi: 10.1016/j.cmi.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Gould EF, Chanas AC, Chanas AF, Buckley A, Buckley AF, Varma MG, Varma MG. Immunofluorescence studies on the antigenic interrelationships of the Hughes virus serogroup (genus Nairovirus) and identification of a new strain. J Gen Virol. 1983;64:739–742. doi: 10.1099/0022-1317-64-3-739. [DOI] [PubMed] [Google Scholar]
- Gould EA, Higgs S, Buckley A, Gritsun TS. Potential arbovirus emergence and implications for the United Kingdom. Emerg Infect Dis. 2006;12:549–555. doi: 10.3201/eid1204.051010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grard G, Moureau G, Charrel RN, Lemasson J-J, Gonzalez J-P, Gallian P, Gritsun TS, Holmes EC, Gould EA, de Lamballerie X. Genetic characterization of tick-borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology. 2007;361:80–92. doi: 10.1016/j.virol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Gritsun TS, Lashkevich VA, Gould EA. Tick-borne encephalitis. Antiviral Res. 2003;57:129–146. doi: 10.1016/S0166-3542(02)00206-1. [DOI] [PubMed] [Google Scholar]
- Guinat C, Gogin A, Blome S, Keil G, Pollin R, Pfeiffer DU, Dixon L. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Vet Rec. 2016;178:262–267. doi: 10.1136/vr.103593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang W, Ji W, Deng M, Sun Y, Zhou H, Yang C, Deng F, Wang H, Hu Z, Lou Z, Rao Z. Crimean–Congo hemorrhagic fever virus nucleoprotein reveals endonuclease activity in bunyaviruses. Proc Natl Acad Sci USA. 2012;109:5046–5051. doi: 10.1073/pnas.1200808109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BE, Tukei PM, McCrae AW, Ssenkubuge Y, Mugo WN. Virus isolations from Ixodid ticks in Uganda. II. Kadam virus—a new member of arbovirus group B isolated from Rhipicephalus pravus Dontiz. East Afr Med J. 1970;47:273–276. [PubMed] [Google Scholar]
- Hill CL, Booth TF, Prasad BV, Grimes JM, Mertens PP, Sutton GC, Stuart DI. The structure of a cypovirus and the functional organization of dsRNA viruses. Nat Struct Biol. 1999;6:565–568. doi: 10.1038/9347. [DOI] [PubMed] [Google Scholar]
- Hinten SR, Beckett GA, Gensheimer KF, Pritchard E, Courtney TM, Sears SD, Woytowicz JM, Preston DG, Smith RP, Jr, Rand PW, Lacombe EH, Holman MS, Lubelczyk CB, Kelso PT, Beelen AP, Stobierski MG, Sotir MJ, Wong S, Ebel G, Kosoy O, Piesman J, Campbell GL, Marfin AA. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis. 2008;8:733–740. doi: 10.1089/vbz.2008.0022. [DOI] [PubMed] [Google Scholar]
- Hiraki T, Yoshimitsu M, Suzuki T, Goto Y, Higashi M, Yokoyama S, Tabuchi T, Futatsuki T, Nakamura K, Hasegawa H, Saijo M, Kakihana Y, Arima N, Yonezawa S. Two autopsy cases of severe fever with thrombocytopenia syndrome (SFTS) in Japan: a pathognomonic histological feature and unique complication of SFTS. Pathol Int. 2014;64:569–575. doi: 10.1111/pin.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff GL, Iversen JO, Yuill TM, Anslow RO, Jackson JO, Hanson RP. Isolations of Silverwater virus from naturally infected snowshoe hares and Haemaphysalis ticks from Alberta and Wisconsin. Am J Trop Med Hyg. 1971;20:320–325. doi: 10.4269/ajtmh.1971.20.320. [DOI] [PubMed] [Google Scholar]
- Holbrook MR. Kyasanur forest disease. Antiviral Res. 2012;96:353–362. doi: 10.1016/j.antiviral.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer B, Bakshi S, Bridgen A, Baron MD. Inhibition of interferon induction and action by the nairovirus Nairobi sheep disease virus/Ganjam virus. PLoS ONE. 2011;6:e28594. doi: 10.1371/journal.pone.0028594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstraal H. The epidemiology of tick-borne Crimean–Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15:307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- Horak IG, Camicas JL, Keirans JE. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida): a world list of valid tick names. Exp Appl Acarol. 2002;28:27–54. doi: 10.1023/A:1025381712339. [DOI] [PubMed] [Google Scholar]
- Huang D, Jiang Y, Liu X, Wang B, Shi J, Su Z, Wang H, Wang T, Tang S, Liu H, Hu Z, Deng F, Shen S. A cluster of symptomatic and asymptomatic infections of severe fever with thrombocytopenia syndrome caused by person-to-person transmission. Am J Trop Med Hyg. 2017;97:396–402. doi: 10.4269/ajtmh.17-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubalek Z. Experimental pathogenicity of Bhanja virus. Zentralbl Bakteriol Mikrobiol Hyg A. 1987;266:284–291. doi: 10.1016/s0176-6724(87)80042-1. [DOI] [PubMed] [Google Scholar]
- Hubalek Z. Geographic distribution of Bhanja virus. Folia Parasitol (Praha) 1987;34:77–86. [PubMed] [Google Scholar]
- Hubalek Z, Rudolf I. Tick-borne viruses in Europe. Parasitol Res. 2012;111:9–36. doi: 10.1007/s00436-012-2910-1. [DOI] [PubMed] [Google Scholar]
- Hubalek Z, Mittermayer T, Halouzka J, Cerny V. Isolation of “exotic” Bhanja virus (Bunyaviridae) from ticks in the temperate zone. Arch Virol. 1988;101:191–197. doi: 10.1007/BF01311000. [DOI] [PubMed] [Google Scholar]
- Idede BO. Nairobi sheep disease. Parassitologia. 2000;39:95–98. [PubMed] [Google Scholar]
- Jeffries CL, Mansfield KL, Phipps LP, Wakeley PR, Mearns R, Schock A, Bell S, Breed AC, Fooks AR, Johnson N. Louping ill virus: an endemic tick-borne disease of Great Britain. J Gen Virol. 2014;95:1005–1014. doi: 10.1099/vir.0.062356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong EJ, Song JY, Lim CS, Lee I, Park MS, Choi MJ, Jeon JH, Kang SH, Jung BK, Yoon JG, Hyun HJ, Noh JY, Cheong HJ, Kim WJ. Viral shedding from diverse body fluids in a patient with severe fever with thrombocytopenia syndrome. J Clin Virol. 2016;80:33–35. doi: 10.1016/j.jcv.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Johnson BK, Shockley P, Chanas AC, Squires EJ, Gardner P, Wallace C, Simpson DI, Bowen ET, Platt GS, Way H, Chandler JA, Highton RB, Hill MN. Arbovirus isolations from mosquitoes: Kano Plain, Kenya. Trans R Soc Trop Med Hyg. 1977;71:518–521. doi: 10.1016/0035-9203(77)90147-X. [DOI] [PubMed] [Google Scholar]
- Johnson BK, Chanas AC, Shockley P, Squires EJ, Varma MG, Leake CJ, Simpson DI. Investigation of the ecology of Soldado virus on Puffin Island, North Wales. Acta Virol. 1979;23:428–432. [PubMed] [Google Scholar]
- Kaiser MN. Viruses in ticks. I. Natural infections of Argas (Persicargas) arboreus by Quaranfil and Nyamanini viruses and absence of infections in A. (p.) persicus in Egypt. Am J Trop Med Hyg. 1966;15:964–975. doi: 10.4269/ajtmh.1966.15.964. [DOI] [PubMed] [Google Scholar]
- Karabatsos N. Supplement to International Catalogue of Arboviruses including certain other viruses of vertebrates. Am J Trop Med Hyg. 1978;27:372–440. doi: 10.4269/ajtmh.1978.27.372. [DOI] [PubMed] [Google Scholar]
- Karan LS, Ciccozzi M, Yakimenko VV, Lo Presti A, Cella E, Zehender G, Rezza G, Platonov AE. The deduced evolution history of Omsk hemorrhagic fever virus. J Med Virol. 2014;86:1181–1187. doi: 10.1002/jmv.23856. [DOI] [PubMed] [Google Scholar]
- Kazimirova M, Thangamani S, Bartikova P, Hermance M, Holikova V, Stibraniova I, Nuttall PA. Tick-borne viruses and biological processes at the tick-host-virus interface. Front Cell Infect Microbiol. 2017;7:339. doi: 10.3389/fcimb.2017.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp GE, Lee VH, Moore DL. Isolation of Nyamanini and Quaranfil viruses from Argas (Persicargas) arboreus ticks in Nigeria. J Med Entomol. 1975;12:535–537. doi: 10.1093/jmedent/12.5.535. [DOI] [PubMed] [Google Scholar]
- Khutoretskaya NV, Aristova VA, Rogovaya SG, Lvov DK, Karimov SK, Skvortsova TM, Kondrashina NG. Experimental study of the reproduction of Karshi virus (Togaviridae, flavivirus) in some species of mosquitoes and ticks. Acta Virol. 1985;29:231–236. [PubMed] [Google Scholar]
- Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013;19:1892–1894. doi: 10.3201/eid1911.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble B, Nieto GR, Perez DR. Characterization of influenza virus sialic acid receptors in minor poultry species. Virol J. 2010;7:365. doi: 10.1186/1743-422X-7-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AMQ, Carstens EB, Lefkowitz EJ (2012) In virus taxonomy: ninth report of the international committee on taxonomy of viruses. 10.1016/b978-0-12-384684-6.x0001-8
- Kleiboeker SB, Scoles GA, Burrage TG, Sur J. African swine fever virus replication in the midgut epithelium is required for infection of Ornithodoros ticks. J Virol. 1999;73:8587–8598. doi: 10.1128/jvi.73.10.8587-8598.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls GM, Sonenshine DE, Clifford CM. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions of three new species. Ann Entomol Soc Am. 1965;58:331–364. doi: 10.1093/aesa/58.3.331. [DOI] [PubMed] [Google Scholar]
- Kokernot RH, Calisher CH, Stannard LJ, Hayes J. Arbovirus studies in the Ohio-Mississippi Basin, 1964–1967. VII. Lone Star virus, a hitherto unknown agent isolated from the tick Amblyomma americanum (Linn) Am J Trop Med Hyg. 1969;18:789–795. doi: 10.4269/ajtmh.1969.18.789. [DOI] [PubMed] [Google Scholar]
- Koksal I, Yilmaz G, Aksoy F, Aydin H, Yavuz I, Iskender S, Akcay K, Erensoy S, Caylan R, Aydin K. The efficacy of ribavirin in the treatment of Crimean–Congo hemorrhagic fever in Eastern Black Sea region in Turkey. J Clin Virol. 2010;47:65–68. doi: 10.1016/j.jcv.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Konstantinov OF, Diallo SM, Diallo SF, Inapogi AP, Inapogi AF, Ba A, Ba AF, Kamara SK, Kamara SK. The mammals of Guinea as reservoirs and carriers of arboviruses. Med Parazitol (Mosk) 2006;1:34–39. [PubMed] [Google Scholar]
- Kuhn JH, Bekal S, Caì Y, Clawson AN, Domier LL, Herrel M, Jahrling PB, Kondo H, Lambert KN, Mihindukulasuriya KA, Nowotny N, Radoshitzky SR, Schneider U, Staeheli P, Suzuki N, Tesh RB, Wang D, Wang LF, Dietzgen RG (2013a) Create a family named Nyamiviridae, with 1 genus and 3 species, in the order Mononegavirales. ICTV [International Committee for Taxonomy of Viruses] Proposal (Taxoprop) No. 2013.002a-hV. 10.13140/rg.2.1.2621.5524
- Kuhn JH, Bekal S, Cai Y, Clawson AN, Domier LL, Herrel M, Jahrling PB, Kondo H, Lambert KN, Mihindukulasuriya KA, Nowotny N, Radoshitzky SR, Schneider U, Staeheli P, Suzuki N, Tesh RB, Wang D, Wang LF, Dietzgen RG. Nyamiviridae: proposal for a new family in the order Mononegavirales. Arch Virol. 2013;158:2209–2226. doi: 10.1007/s00705-013-1674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno G, Artsob H, Karabatsos N, Tsuchiya KR, Chang GJ. Genomic sequencing of deer tick virus and phylogeny of powassan-related viruses of North America. Am J Trop Med Hyg. 2001;65:671–676. doi: 10.4269/ajtmh.2001.65.671. [DOI] [PubMed] [Google Scholar]
- Kuzmin IV, Novella IS, Dietzgen RG, Padhi A, Rupprecht CE. The rhabdoviruses: biodiversity, phylogenetics, and evolution. Infect Genet Evol. 2009;9:541–553. doi: 10.1016/j.meegid.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Labuda M, Nuttall PA. Tick-borne viruses. Parasitology. 2004;129(Suppl):S221–S245. doi: 10.1017/S0031182004005220. [DOI] [PubMed] [Google Scholar]
- Lasecka L, Baron MD. The molecular biology of nairoviruses, an emerging group of tick-borne arboviruses. Arch Virol. 2014;159:1249–1265. doi: 10.1007/s00705-013-1940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Shi M, Tian JH, Lin XD, Kang YJ, Chen LJ, Qin XC, Xu J, Holmes EC, Zhang YZ. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife. 2015 doi: 10.7554/eLife.05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libikova H, Rehacek J, Gresikova M, Kozuch O, Somogyiova J, Ernek E. Cytopathic Viruses Isolated from Ixodes Ricinus Ticks in Czechoslovakia. Acta Virol. 1964;8:96. [PubMed] [Google Scholar]
- Libikova H, Tesarova J, Rajcani J. Experimental infection of monkeys with Kemerovo virus. Acta Virol. 1970;14:64–69. [PubMed] [Google Scholar]
- Libikova H, Heinz F, Ujhazyova D, Stunzner D. Orbiviruses of the Kemerovo complex and neurological diseases. Med Microbiol Immunol. 1978;166:255–263. doi: 10.1007/BF02121159. [DOI] [PubMed] [Google Scholar]
- Lin T, Cai XZ, Shi MM, Ying ZM, Hu B, Zhou CH, Wang W, Shi ZL, Yan SG. In vitro and in vivo evaluation of vancomycin-loaded PMMA cement in combination with ultrasound and microbubbles-mediated ultrasound. Biomed Res Int. 2015;2015:309739. doi: 10.1155/2015/309739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet. 2008;371:1861–1871. doi: 10.1016/S0140-6736(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Walker DH, Ren J, Wang Y, Yu XJ. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis. 2012;12:156–160. doi: 10.1089/vbz.2011.0758. [DOI] [PubMed] [Google Scholar]
- Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14:763–772. doi: 10.1016/S1473-3099(14)70718-2. [DOI] [PubMed] [Google Scholar]
- Lu Z, Broker M, Liang G. Tick-borne encephalitis in mainland China. Vector Borne Zoonotic Dis. 2008;8:713–720. doi: 10.1089/vbz.2008.0028. [DOI] [PubMed] [Google Scholar]
- Luo LM, Zhao L, Wen HL, Zhang ZT, Liu JW, Fang LZ, Xue ZF, Ma DQ, Zhang XS, Ding SJ, Lei XY, Yu XJ. Haemaphysalis longicornis Ticks as Reservoir and Vector of Severe Fever with Thrombocytopenia Syndrome Virus in China. Emerg Infect Dis. 2015;21:1770–1776. doi: 10.3201/eid2110.150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lvov DK, Timopheeva AA, Chervonski VI, Gromashevski VL, Klisenko GA, Gostinshchikova GV, Kostyrko IN. Tuleniy virus. A new Group B arbovirus isolated from Ixodes (Ceratixodes) putus Pick.-Camb. 1878 collected on Tuleniy Island, Sea of Okhotsk. Am J Trop Med Hyg. 1971;20:456–460. doi: 10.4269/ajtmh.1971.20.456. [DOI] [PubMed] [Google Scholar]
- Lvov DK, Neronov VM, Gromashevsky VL, Skvortsova TM, Berezina LK, Sidorova GA, Zhmaeva ZM, Gofman YA, Klimenko SM, Fomina KB. “Karshi” virus, a new flavivirus (Togaviridae) isolated from Ornithodoros papillipes (Birula, 1895) ticks in Uzbek S.S.R. Arch Virol. 1976;50:29–36. doi: 10.1007/BF01317998. [DOI] [PubMed] [Google Scholar]
- L’Vov DN, Dzharkenov AF, Aristova VA, Kovtunov AI, Gromashevskii VL, Vyshemirskii OI, Galkina IV, Larichev VF, Butenko AM, L’Vov DK. The isolation of Dhori viruses (Orthomyxoviridae, Thogotovirus) and Crimean–Congo hemorrhagic fever virus (Bunyaviridae, Nairovirus) from the hare (Lepus europaeus) and its ticks Hyalomma marginatum in the middle zone of the Volga delta, Astrakhan region, 2001. Vopr Virusol. 2002;47:32–36. [PubMed] [Google Scholar]
- Main AJ, Downs WG, Shope RE, Wallis RC. Great Island and Bauline: two new Kemerovo group orbiviruses from Ixodes uriae in eastern Canada. J Med Entomol. 1973;10:229–235. doi: 10.1093/jmedent/10.3.229. [DOI] [PubMed] [Google Scholar]
- Major L, Linn ML, Slade RW, Schroder WA, Hyatt AD, Gardner J, Cowley J, Suhrbier A. Ticks associated with macquarie island penguins carry arboviruses from four genera. PLoS ONE. 2009;4:e4375. doi: 10.1371/journal.pone.0004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinke BI, Nichol ST. Nairobi Sheep Disease Virus, an Important Tick-Borne Pathogen of Sheep and Goats in Africa, Is Also Present in Asia. Virology. 2002;303:146–151. doi: 10.1006/viro.2002.1514. [DOI] [PubMed] [Google Scholar]
- Marjelund S, Tikkakoski TF, Tuisku S, Tuisku SF, Raisanen S, Raisanen S. Magnetic resonance imaging findings and outcome in severe tick-borne encephalitis. Report of four cases and review of the literature. Acta Radiol. 2004;45:88–94. doi: 10.1080/02841850410003356. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Weisend C, Kajihara M, Matysiak C, Williamson BN, Simuunza M, Mweene AS, Takada A, Tesh RB, Ebihara H. Comprehensive molecular detection of tick-borne phleboviruses leads to the retrospective identification of taxonomically unassigned bunyaviruses and the discovery of a novel member of the genus phlebovirus. J Virol. 2015;89:594–604. doi: 10.1128/JVI.02704-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DM, Larke RP. Powassan and Silverwater viruses: ecology of two Ontario arboviruses. Can Med Assoc J. 1963;88:182–185. [PMC free article] [PubMed] [Google Scholar]
- McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albarino CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- Mihindukulasuriya KA, Nguyen NL, Wu G, Huang HV, da Rosa AP, Popov VL, Tesh RB, Wang D. Nyamanini and midway viruses define a novel taxon of RNA viruses in the order Mononegavirales. J Virol. 2009;83:5109–5116. doi: 10.1128/JVI.02667-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery E. On a tick-borne gastro-enteritis of sheep and goats occurring in British East Africa. J Comp Pathol Ther. 1917;30:28–57. doi: 10.1016/S0368-1742(17)80002-3. [DOI] [Google Scholar]
- Montgomery RE. On a form of swine fever occurring in british east Africa (Kenya Colony) J Comp Pathol Ther. 1921;34:159–191. doi: 10.1016/S0368-1742(21)80031-4. [DOI] [Google Scholar]
- Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM, David-West TS, Kemp GE. Arthropod-borne viral infections of man in Nigeria, 1964-1970. Ann Trop Med Parasitol. 1975;69:49–64. doi: 10.1080/00034983.1975.11686983. [DOI] [PubMed] [Google Scholar]
- Morse MA, Marriott AC, Nuttall PA. The glycoprotein of Thogoto virus (a tick-borne orthomyxo-like virus) is related to the baculovirus glycoprotein GP64. Virology. 1992;186:640–646. doi: 10.1016/0042-6822(92)90030-S. [DOI] [PubMed] [Google Scholar]
- Moss SR, Ayres CM, Nuttall PA. The Great Island subgroup of tick-borne orbiviruses represents a single gene pool. J Gen Virol. 1988;69(Pt 11):2721–2727. doi: 10.1099/0022-1317-69-11-2721. [DOI] [PubMed] [Google Scholar]
- Muehlenbachs A, Fata CR, Lambert AJ, Paddock CD, Velez JO, Blau DM, Staples JE, Karlekar MB, Bhatnagar J, Nasci RS, Zaki SR. Heartland virus-associated death in tennessee. Clin Infect Dis. 2014;59:845–850. doi: 10.1093/cid/ciu434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso M, Galati V, Stella MC, Capone A. A case of Alkhumra virus infection. J Clin Virol. 2015;66:12–14. doi: 10.1016/j.jcv.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Nabeth P, Thior M, Faye O, Simon F. Human Crimean–Congo hemorrhagic fever, Senegal. Emerg Infect Dis. 2004;10:1881–1882. doi: 10.3201/eid1010.040586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasar F, Matassov D, Seymour RL, Latham T, Gorchakov RV, Nowak RM, Leal G, Hamm S, Eldridge JH, Tesh RB, Clarke DK, Weaver SC. Recombinant Isfahan virus and vesicular stomatitis virus vaccine vectors provide durable, multivalent, single-dose protection against lethal alphavirus challenge. J Virol. 2017;91:pii: e01729-16. doi: 10.1128/JVI.01729-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nason EL, Rothagel R, Mukherjee SK, Kar AK, Forzan M, Prasad BV, Roy P. Interactions between the inner and outer capsids of bluetongue virus. J Virol. 2004;78:8059–8067. doi: 10.1128/JVI.78.15.8059-8067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn MA, Barton TR, Wanless S, Hails RS, Harris MP, Nuttall PA. Tick-borne Great Island Virus: (I) Identification of seabird host and evidence for co-feeding and viraemic transmission. Parasitology. 2006;132(pt2):233–240. doi: 10.1017/S0031182005008930. [DOI] [PubMed] [Google Scholar]
- Palacios G, Savji N, Travassos da Rosa A, Guzman H, Yu X, Desai A, Rosen GE, Hutchison S, Lipkin WI, Tesh R. Characterization of the Uukuniemi virus group (Phlebovirus: Bunyaviridae): evidence for seven distinct species. J Virol. 2013;87:3187–3195. doi: 10.1128/JVI.02719-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, Raoult D. Tick-borne bacterial diseases emerging in Europe. Clin Microbiol Infect. 2001;7:80–83. doi: 10.1046/j.1469-0691.2001.00200.x. [DOI] [PubMed] [Google Scholar]
- Pattnaik P. Kyasanur forest disease: an epidemiological view in India. Rev Med Virol. 2006;16:151–165. doi: 10.1002/rmv.495. [DOI] [PubMed] [Google Scholar]
- Paulsen KM, Pedersen BN, Soleng A, Okbaldet YB, Pettersson JH, Dudman SG, Ottesen P, Vik IS, Vainio K, Andreassen A. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks from three islands in north-western Norway. APMIS. 2015;123:759–764. doi: 10.1111/apm.12412. [DOI] [PubMed] [Google Scholar]
- Pejsak Z, Truszczynski M, Niemczuk K, Kozak E, Markowska-Daniel I. Epidemiology of African Swine Fever in Poland since the detection of the first case. Pol J Vet Sci. 2014;17:665–672. doi: 10.2478/pjvs-2014-0097. [DOI] [PubMed] [Google Scholar]
- Pettersson JH, Golovljova I, Vene S, Jaenson TG. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks in northern Europe with particular reference to Southern Sweden. Parasit Vectors. 2014;7:102. doi: 10.1186/1756-3305-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi A, Rubin DB, McQuillen DP, Hsu L, Lederer PA, Ashbaugh CD, Duffalo C, Duncan R, Thon J, Bhattacharyya S, Basgoz N, Feske SK, Lyons JL. Emerging cases of powassan virus encephalitis in new england: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2016;62:707–713. doi: 10.1093/cid/civ1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti RM, Zhao G, Beatty WL, Mihindukulasuriya KA, da Rosa AP, Popov VL, Tesh RB, Virgin HW, Wang D. Quaranfil, Johnston Atoll, and Lake Chad viruses are novel members of the family Orthomyxoviridae. J Virol. 2009;83:11599–11606. doi: 10.1128/JVI.00677-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XC, Shi M, Tian JH, Lin XD, Gao DY, He JR, Wang JB, Li CX, Kang YJ, Yu B, Zhou DJ, Xu J, Plyusnin A, Holmes EC, Zhang YZ. A tick-borne segmented RNA virus contains genome segments derived from unsegmented viral ancestors. Proc Natl Acad Sci USA. 2014;111:6744–6749. doi: 10.1073/pnas.1324194111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE, Green RM, Hoodless AN, Peacey MF. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int J Parasitol. 2002;32:979–989. doi: 10.1016/S0020-7519(02)00030-9. [DOI] [PubMed] [Google Scholar]
- Rao CV, Dandawate CN, Rodrigues JJ, Rao GL, Mandke VB, Ghalsasi GR, Pinto BD. Laboratory infections with Ganjam virus. Indian J Med Res. 1981;74:319–324. [PubMed] [Google Scholar]
- Rehse-Kupper B, Casals J, Rehse E, Ackermann R. Eyach–an arthropod-borne virus related to Colorado tick fever virus in the Federal Republic of Germany. Acta Virol. 1976;20:339–342. [PubMed] [Google Scholar]
- Reid HW, Barlow RM, Pow I. Isolation of louping-ill virus from red deer (Cervus elaphus) Vet Rec. 1978;102:463–464. doi: 10.1136/vr.102.21.463. [DOI] [PubMed] [Google Scholar]
- Riemersma KK, Komar N. Heartland virus neutralizing antibodies in vertebrate wildlife, United States, 2009–2014. Emerg Infect Dis. 2015;21:1830–1833. doi: 10.3201/eid2110.150380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter DG, Calisher CH, Muth DJ, Shope RE, Murphy FA, Whitfield SG. New Minto virus: a new rhabdovirus from ticks in Alaska. Can J Microbiol. 1978;24:422–426. doi: 10.1139/m78-069. [DOI] [PubMed] [Google Scholar]
- Rodovsky FJ, Stiller D. Descriptive notes on Ornithodoros ticks from gull nests on the Farallon islands and isolation of a variant of Hughes virus. J Parasitol. 1967;53:890–892. doi: 10.2307/3276795. [DOI] [PubMed] [Google Scholar]
- Rogers MB, Cui L, Fitch A, Popov V, Travassos da Rosa AP, Vasilakis N, Tesh RB, Ghedin E. Whole genome analysis of sierra nevada virus, a novel mononegavirus in the family nyamiviridae. Am J Trop Med Hyg. 2014;91:159–164. doi: 10.4269/ajtmh.14-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P, Noad R. Bluetongue virus assembly and morphogenesis. Curr Top Microbiol Immunol. 2006;309:87–116. doi: 10.1007/3-540-30773-7_4. [DOI] [PubMed] [Google Scholar]
- Ruzek D, Yakimenko VV, Karan LS, Tkachev SE. Omsk haemorrhagic fever. Lancet. 2010;376:2104–2113. doi: 10.1016/S0140-6736(10)61120-8. [DOI] [PubMed] [Google Scholar]
- Sadanandane C, Elango A, Marja N, Sasidharan PV, Raju KH, Jambulingam P. An outbreak of Kyasanur forest disease in the Wayanad and Malappuram districts of Kerala, India. Ticks Tick Borne Dis. 2017;8:25–30. doi: 10.1016/j.ttbdis.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Saikku P, Brummer-Korvenkontio M. Arboviruses in Finland. II. Isolation and characterization of Uukuniemi virus, a virus associated with ticks and birds. Am J Trop Med Hyg. 1973;22:390–399. doi: 10.4269/ajtmh.1973.22.390. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vizcaino JM, Mur L, Martinez-Lopez B. African swine fever (ASF): five years around Europe. Vet Microbiol. 2013;165:45–50. doi: 10.1016/j.vetmic.2012.11.030. [DOI] [PubMed] [Google Scholar]
- Sang R, Onyango C, Gachoya J, Mabinda E, Konongoi S, Ofula V, Dunster L, Okoth F, Coldren R, Tesh R, da Rossa AT, Finkbeiner S, Wang D, Crabtree M, Miller B. Tickborne arbovirus surveillance in market livestock, Nairobi, Kenya. Emerg Infect Dis. 2006;12:1074–1080. doi: 10.3201/eid1207.060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather GE, Lewis AL, Jennings W, Bond JO, Hammon WM. Sawgrass virus: a newly described arbovirus in Florida. Am J Trop Med Hyg. 1970;19:319–326. doi: 10.4269/ajtmh.1970.19.319. [DOI] [PubMed] [Google Scholar]
- Savage HM, Godsey MS, Lambert A, Panella NA, Burkhalter KL, Harmon JR, Lash RR, Ashley DC, Nicholson WL. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PR, Sargison ND, Wilson DJ. The potential for improving welfare standards and productivity in United Kingdom sheep flocks using veterinary flock health plans. Vet J. 2007;173:522–531. doi: 10.1016/j.tvjl.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Service MW. In the encyclopedia of arthropod transmitted infections. Wallingford: CABI Publising; 2001. pp. 504–506. [Google Scholar]
- Shchetinin AM, Lvov DK, Deriabin PG, Botikov AG, Gitelman AK, Kuhn JH, Alkhovsky SV. Genetic and phylogenetic characterization of tataguine and witwatersrand viruses and other orthobunyaviruses of the anopheles a, capim, guama, koongol, mapputta, tete, and turlock serogroups. Viruses. 2015;7:5987–6008. doi: 10.3390/v7112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Hu S, Liu X, Yang J, Liu D, Wu L, Wang H, Hu Z, Deng F, Shen S. Migration, recombination, and reassortment are involved in the evolution of severe fever with thrombocytopenia syndrome bunyavirus. Infect Genet Evol. 2017;47:109–117. doi: 10.1016/j.meegid.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Smith CE. A virus resembling Russian spring-summer encephalitis virus from an Ixodid tick in Malaya. Nature. 1956;178:581–582. doi: 10.1038/178581a0. [DOI] [PubMed] [Google Scholar]
- Smith R, Woodall JP, Whitney E, Deibel R, Gross MA, Smith V, Bast TF. Powassan virus infection. A report of three human cases of encephalitis. Am J Dis Child. 1974;127:691–693. doi: 10.1001/archpedi.1974.02110240077010. [DOI] [PubMed] [Google Scholar]
- Solomon T, Hart IJ, Beeching NJ. Viral encephalitis: a clinician’s guide. Practical Neurology. 2007;7:288. doi: 10.1136/jnnp.2007.129098. [DOI] [PubMed] [Google Scholar]
- Sreenivasan MA, Bhat HR, Rajagopalan PK. The epizootics of Kyasanur Forest disease in wild monkeys during 1964–1973. Trans R Soc Trop Med Hyg. 1986;80:810–814. doi: 10.1016/0035-9203(86)90390-1. [DOI] [PubMed] [Google Scholar]
- St George TD, Standfast HA, Doherty RL, Carley JG, Fillipich C, Brandsma J. The isolation of Saumarez Reef virus, a new flavivirus, from bird ticks Ornithodoros capensis and Ixodes eudyptidis in Australia. Aust J Exp Biol Med Sci. 1977;55:493–499. doi: 10.1038/icb.1977.49. [DOI] [PubMed] [Google Scholar]
- St George TD, Doherty RL, Carley JG, Filippich C, Brescia A, Casals J, Kemp DH, Brothers N. The isolation of arboviruses including a new flavivirus and a new Bunyavirus from Ixodes (Ceratixodes) uriae (Ixodoidea: Ixodidae) collected at Macquarie Island, Australia, 1975-1979. Am J Trop Med Hyg. 1985;34:406–412. doi: 10.4269/ajtmh.1985.34.406. [DOI] [PubMed] [Google Scholar]
- Stockman S. Louping-ill. J Comp Pathol Ther. 1918;31:137–193. doi: 10.1016/S0368-1742(18)80019-4. [DOI] [Google Scholar]
- Sun RX, Lai SJ, Yang Y, Li XL, Liu K, Yao HW, Zhou H, Li Y, Wang LP, Mu D, Yin WW, Fang LQ, Yu HJ, Cao WC. Mapping the distribution of tick-borne encephalitis in mainland China. Ticks Tick Borne Dis. 2017;8:631–639. doi: 10.1016/j.ttbdis.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Suss J. Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine. 2003;21:S19–S35. doi: 10.1016/S0264-410X(02)00812-5. [DOI] [PubMed] [Google Scholar]
- Swei A, Russell BJ, Naccache SN, Kabre B, Veeraraghavan N, Pilgard MA, Johnson BJ, Chiu CY. The genome sequence of Lone Star virus, a highly divergent bunyavirus found in the Amblyomma americanum tick. PLoS ONE. 2013;8:e62083. doi: 10.1371/journal.pone.0062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Yunker CE, Clifford CM, Nakano W, Fujino N, Tanifuji K, Thomas LA. Isolation and characterization of Midway virus: a new tick-borne virus related to Nyamanini. J Med Virol. 1982;10:181–193. doi: 10.1002/jmv.1890100304. [DOI] [PubMed] [Google Scholar]
- Tan DS, Smith CE, McMahon DA, Bowen ET. Lanjan virus, a new agent isolated from Dermacentor auratus in Malaya. Nature. 1967;214:1154–1155. doi: 10.1038/2141154a0. [DOI] [PubMed] [Google Scholar]
- Taylor RM, Hoogstraal H, Hurlbut HS. Isolation of a virus (Wad Medani) from Rhipicephalus sanguineus collected in Sudan. Am J Trop Med Hyg. 1966;15:75. doi: 10.4269/ajtmh.1966.15.75. [DOI] [PubMed] [Google Scholar]
- Taylor RM, Hurlbut HS, Work TH, Kingston JR, Hoogstraal H. Arboviruses isolated from ARGAS TICKS IN Egypt: Quaranfil, Chenuda, and Nyamanini. Am J Trop Med Hyg. 1966;15:76–86. doi: 10.4269/ajtmh.1966.15.76. [DOI] [PubMed] [Google Scholar]
- Telford SR, 3rd, Armstrong PM, Katavolos P, Foppa I, Garcia AS, Wilson ML, Spielman A. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis. 1997;3:165–170. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh R, Saidi S, Javadian E, Loh P, Nadim A. Isfahan virus, a new vesiculovirus infecting humans, gerbils, and sandflies in Iran. Am J Trop Med Hyg. 1977;26:299–306. doi: 10.4269/ajtmh.1977.26.299. [DOI] [PubMed] [Google Scholar]
- Timoney PJ, Donnelly WJ, Clements LO, Fenlon M. Encephalitis caused by louping ill virus in a group of horses in Ireland. Equine Vet J. 1976;8:113–117. doi: 10.1111/j.2042-3306.1976.tb03311.x. [DOI] [PubMed] [Google Scholar]
- Tokarz R, Sameroff S, Leon MS, Jain K, Lipkin WI. Genome characterization of Long Island tick rhabdovirus, a new virus identified in Amblyomma americanum ticks. Virol J. 2014;11:26. doi: 10.1186/1743-422X-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarz R, Williams SH, Sameroff S, Sanchez Leon M, Jain K, Lipkin WI. Virome analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks reveals novel highly divergent vertebrate and invertebrate viruses. J Virol. 2014;88:11480–11492. doi: 10.1128/JVI.01858-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaniak K, Kowalczyk A, Markowska-Daniel I. Influenza A viruses of avian origin circulating in pigs and other mammals. Acta Biochim Pol. 2014;61:433–439. [PubMed] [Google Scholar]
- Valarcher JF, Hagglund S, Juremalm M, Blomqvist G, Renstrom L, Zohari S, Leijon M, Chirico J. Tick-borne encephalitis. Rev Sci Tech. 2015;34:453–466. doi: 10.20506/rst.34.2.2371. [DOI] [PubMed] [Google Scholar]
- Varma MG, Bowen ET, Simpson DI, Casals J. Zirga virus, a new arbovirus isolated from bird-infesting ticks. Nature. 1973;244:452. doi: 10.1038/244452a0. [DOI] [PubMed] [Google Scholar]
- Vinuela E. African swine fever virus. Curr Top Microbiol Immunol. 1985;116:151–170. doi: 10.1007/978-3-642-70280-8_8. [DOI] [PubMed] [Google Scholar]
- Vorou R, Pierroutsakos IN, Maltezou HC. Crimean–Congo hemorrhagic fever. Curr Opin Infect Dis. 2007;20:495–500. doi: 10.1097/QCO.0b013e3282a56a0a. [DOI] [PubMed] [Google Scholar]
- Walker PJ, Firth C, Widen SG, Blasdell KR, Guzman H, Wood TG, Paradkar PN, Holmes EC, Tesh RB, Vasilakis N. Evolution of genome size and complexity in the rhabdoviridae. PLoS Pathog. 2015;11:e1004664. doi: 10.1371/journal.ppat.1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PJ, Widen SG, Wood TG, Guzman H, Tesh RB, Vasilakis N. A global genomic characterization of nairoviruses identifies nine discrete genogroups with distinctive structural characteristics and host-vector associations. Am J Trop Med Hyg. 2016;94:1107–1122. doi: 10.4269/ajtmh.15-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang H, Fu S, Wang H, Ni D, Nasci R, Tang Q, Liang G. Isolation of kyasanur forest disease virus from febrile patient, yunnan, china. Emerg Infect Dis. 2009;15:326–328. doi: 10.3201/eid1502.080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Selleck P, Yu M, Ha W, Rootes C, Gales R, Wise T, Crameri S, Chen H, Broz I, Hyatt A, Woods R, Meehan B, McCullough S, Wang LF. Novel phlebovirus with zoonotic potential isolated from ticks, Australia. Emerg Infect Dis. 2014;20:1040–1043. doi: 10.3201/eid2006.140003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GE, Shope RE, Kaiser MN. An ectoparasite and virus survey of migratory birds in the eastern Mediterranean. In: Cherepanov AI, editor. Transcontinental connections of migratory birds and their role in the distribution of arboviruses. Novosibirsk: Nauka; 1972. pp. 176–180. [Google Scholar]
- Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. 2004;2:789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westover JB, Rigas JD, Van Wettere AJ, Li R, Hickerson BT, Jung KH, Miao J, Reynolds ES, Conrad BL, Nielson S, Furuta Y, Thangamani S, Wang Z, Gowen BB. Heartland virus infection in hamsters deficient in type I interferon signaling: protracted disease course ameliorated by favipiravir. Virology. 2017;511:175–183. doi: 10.1016/j.virol.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse CA. Crimean–Congo hemorrhagic fever. Antiviral Res. 2004;64:145–160. doi: 10.1016/S0166-3542(04)00163-9. [DOI] [PubMed] [Google Scholar]
- Wilks CR, House JA. Susceptibility of various animals to the vesiculoviruses Isfahan and Chandipura. J Hyg (Lond) 1986;97:359–368. doi: 10.1017/S002217240006544X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, Thorburn H. Serum antibodies to louping-ill virus. Scott Med J. 1962;7:353–355. doi: 10.1177/003693306200700803. [DOI] [PubMed] [Google Scholar]
- Williams RE, Casals J, Moussa MI, Hoogstraal H. Royal farm virus: a new tickborne group B agent related to the RSSE complex. Am J Trop Med Hyg. 1972;21:582–586. doi: 10.4269/ajtmh.1972.21.582. [DOI] [PubMed] [Google Scholar]
- Work TH, Trapido H, Murthy DP, Rao RL, Bhatt PN, Kulkarni KG. Kyasanur forest disease. III. A preliminary report on the nature of the infection and clinical manifestations in human beings. Indian J Med Sci. 1957;11:619–645. [PubMed] [Google Scholar]
- Wozniakowski G, Kozak E, Kowalczyk A, Lyjak M, Pomorska-Mol M, Niemczuk K, Pejsak Z. Current status of African swine fever virus in a population of wild boar in eastern Poland (2014-2015) Arch Virol. 2016;161:189–195. doi: 10.1007/s00705-015-2650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JR, Heo ST, Park D, Kim H, Fukuma A, Fukushi S, Shimojima M, Lee KH. Family cluster analysis of severe fever with thrombocytopenia syndrome virus infection in Korea. Am J Trop Med Hyg. 2016;95:1351–1357. doi: 10.4269/ajtmh.16-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Shimojima M, Fukushi S, Tani H, Fukuma A, Taniguchi S, Singh H, Suda Y, Shirabe K, Toda S, Shimazu Y, Nomachi T, Gokuden M, Morimitsu T, Ando K, Yoshikawa A, Kan M, Uramoto M, Osako H, Kida K, Takimoto H, Kitamoto H, Terasoma F, Honda A, Maeda K, Takahashi T, Yamagishi T, Oishi K, Morikawa S, Saijo M. Phylogenetic and geographic relationships of severe fever with thrombocytopenia syndrome virus in China, South Korea, and Japan. J Infect Dis. 2015;212:889–898. doi: 10.1093/infdis/jiv144. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaryan H, Revilla Y. African swine fever virus: current state and future perspectives in vaccine and antiviral research. Vet Microbiol. 2016;185:15–19. doi: 10.1016/j.vetmic.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Zaki AM. Isolation of a flavivirus related to the tick-borne encephalitis complex from human cases in Saudi Arabia. Trans R Soc Trop Med Hyg. 1997;91:179–181. doi: 10.1016/S0035-9203(97)90215-7. [DOI] [PubMed] [Google Scholar]
- Zhan J, Wang Q, Cheng J, Hu B, Li J, Zhan F, Song Y, Guo D. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin. 2017;32:51–62. doi: 10.1007/s12250-016-3931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GL, Liu R, Sun X, Zheng Y, Liu XM, Zhao Y, Dang RL, Liu SK, Xia J, Zheng Z, Yang YH. Investigation on the endemic foci of new emerged tick-borne encephalitis in Charles Hilary, Xinjiang. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34:438–442. [PubMed] [Google Scholar]
- Zhao JW, Wang HY, Wang Y. Regional distribution profiles of tick-borne pathogens in China. Chin J Vector Biol Control. 2012;23:445–448. [Google Scholar]
- Zivcec M, Scholte FE, Spiropoulou CF, Spengler JR, Bergeron E. Molecular insights into Crimean–Congo hemorrhagic fever virus. Viruses. 2016;8:106. doi: 10.3390/v8040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlobin VI, Pogodina VV, Kahl O. A brief history of the discovery of tick-borne encephalitis virus in the late 1930s (based on reminiscences of members of the expeditions, their colleagues, and relatives) Ticks Tick Borne Dis. 2017;8:813–820. doi: 10.1016/j.ttbdis.2017.05.001. [DOI] [PubMed] [Google Scholar]