Abstract
Gastric intestinal metaplasia is a precancerous change of the mucosa of the stomach with intestinal epithelium, and is associated with an increased risk of dysplasia and cancer. The pathogenesis to gastric cancer is proposed by the Correa hypothesis as the transition from normal gastric epithelium to invasive cancer via inflammation followed by intramucosal cancer and invasion. Multiple risk factors have been associated with the development of gastric intestinal metaplasia interplay, including Helicobacter pylori infection and associated genomics, host genetic factors, environmental milieu, rheumatologic disorders, diet, and intestinal microbiota. Globally, screening guidelines have been established in countries with high incidence. In the United States, no such guidelines have been developed due to lower, albeit increasing, incidence. The American Society for Gastrointestinal Endoscopy recommends a case-by-case patient assessment based upon epidemiology, genetics, and environmental risk factors. Studies have examined the use of a serologic biopsy to stratify risk based upon factors such as H pylori status and virulence factors, along with serologic markers of chronic inflammation including pepsinogen I, pepsinogen II, and gastrin. High-risk patients may then be advised to undergo endoscopic evaluation with mapping biopsies from the antrum (greater curvature, lesser curvature), incisura angularis, and corpus (greater curvature, lesser curvature). Surveillance guidelines have not been firmly established for patients with known gastric intestinal metaplasia, but include repeat endoscopy at intervals according to the histologic risk for malignant transformation.
Keywords: Gastric intestinal metaplasia, Helicobacter pylori, gastric cancer surveillance, intestinal-type gastric cancer, serologic biopsy, spasmolytic polypeptide-expressing metaplasia
Metaplastic changes in the esophagus and the stomach are both associated with an increased risk of cancer in their respective locations.1 Barrett esophagus, or the replacement of stratified squamous epithelium by metaplastic columnar epithelium, is known to result in a 30-fold increased risk of esophageal cancer above that of the general population.1 Although there is debate about the cost-effectiveness of Barrett esophagus screening and surveillance, guidelines are available for the management of this condition.2 Despite the fact that the estimated number of new cancer cases in the United States in 2017 was higher for gastric cancer at 28,000 than esophageal cancer at 16,940 (based upon Surveillance, Epidemiology, and End Results [SEER] data), no similar management guidelines are available in the United States for gastric cancer.3 This article reviews the pathogenesis and risk factors, along with the current screening and surveillance strategies, for gastric intestinal metaplasia.
Background on Gastric Intestinal Metaplasia and Gastric Cancer
Epidemiology
Gastric cancer has seen a steady decline since the 1930s, which may be partially attributable to the advent of widespread food refrigeration that has replaced smoking meat as a means of preservation. The smoking process has been determined to promote carcinogens.4 However, despite global refrigeration, gastric cancer remains the fifth most common malignancy and third leading cause of cancer death worldwide with 723,000 deaths in 2012, according to the World Health Organization (WHO).5 Although the prevalence of gastric intestinal metaplasia (GIM) worldwide is largely unknown, a positive correlation, although not causation, has been associated with regional gastric cancer incidence.6 A large retrospective study performed by Sonnenberg and colleagues reviewed 78,985 patients who underwent upper endoscopy in the United States, and found a 7% prevalence of GIM.7
Pathogenesis
Gastric adenocarcinoma is characterized into 2 subtypes: intestinal and diffuse. The intestinal type is highly correlated with intestinal metaplasia, and the diffuse type is considered to be primarily genetically determined and less associated with environmental factors and the inflammatory cascade.8 Intestinal-type adenocarcinoma accounts for the vast majority of gastric cancer.9
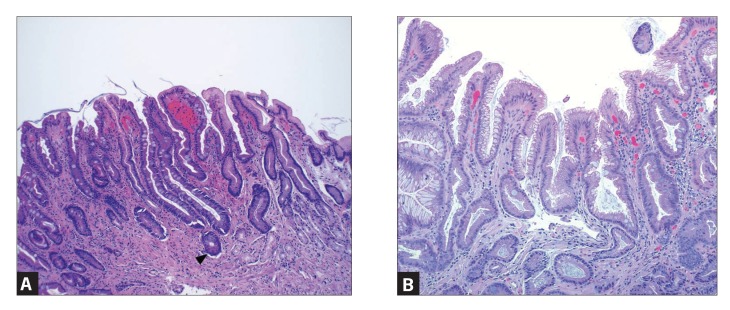
GIM is a precancerous lesion defined as the replacement of surface, foveolar, and/or glandular epithelium in the oxyntic or antral mucosa by intestinal epithelium (Figure 1).10 GIM is categorized anatomically as limited if it is confined to 1 region of the stomach or as extensive if 2 regions of the stomach are involved. Histologically, GIM is considered to be either complete or incomplete.10 Complete (type I) intestinal metaplasia is defined by small intestinal-type mucosa with mature absorptive cells, goblet cells, and a brush border. Incomplete (type II) intestinal metaplasia secretes sialomucins and is similar to colonic epithelium with columnar “intermediate” cells in various stages of differentiation, irregular mucin droplets, and the absence of a brush border.11,12 The highest risk of gastric cancer is associated with incomplete and/or extensive GIM.13 A systematic review that included 10 observational studies throughout Europe, Asia, and Latin America ranging from 10 months to 19 years of follow-up showed the risk of gastric cancer to be 4- to 11-fold higher with incomplete metaplasia compared to without incomplete metaplasia.14
Figure 1.
Intestinal metaplasia is characterized by goblet cells similar to those seen in the small and large intestines. The goblet cells have blue-tinged to clear mucin vacuoles, which compress the nucleus to one side of the cell. Goblet cells may be focal (incomplete) or diffuse, and the gastric mucosa may even resemble small intestinal mucosa (complete). Paneth cells similar to those seen in the small and large intestines may also be seen (arrowhead). Figure 1A shows complete intestinal metaplasia, and Figure 1B shows incomplete intestinal metaplasia.
Figures provided courtesy of Brian Theisen, MD.
The pathogenesis of the progression from precancerous lesions to intestinal-type gastric adenocarcinoma has been proposed as the progression from normal gastric epithelium to inflammation, atrophy, intramucosal carcinoma, and finally invasive carcinoma.15 A multifactorial interplay between Helicobacter pylori genomics, host genetic factors, environmental milieu, diet, and intestinal microbiota predispose the gastric mucosa to an inflammatory cascade with cancerous potential.16
The first histologic change in the cascade is active chronic inflammation with either nonatrophic chronic gastritis characterized by the presence of glands, or multi-focal atrophic gastritis. The subsequent histologic changes progress through complete metaplasia, incomplete metaplasia, and low- and high-grade dysplasia, followed by carcinoma.14 A nationwide cohort study was performed in the Netherlands to evaluate the risk of gastric cancer associated with the various premalignant gastric lesions. Atrophic gastritis, intestinal metaplasia, mild-moderate dysplasia, and severe dysplasia were associated with annual incidences of gastric cancer of 0.1%, 0.25%, 0.6%, and 6.0%, respectively.17 The incidence of gastric cancer associated with GIM ranges from 0% to 10% in systematic reviews, with the variable range attributable to various sample sizes and follow-up periods.18,19
Molecular Patterns
Recent research is delving into spasmolytic polypeptide-expressing metaplasia (SPEM) as a possible precursor lesion vs a commensal precancerous change with GIM.20 SPEM is a metaplastic mucous cell phenotype with histologic characteristics of the deep antral gland cells that has previously been described as pseudopyloric metaplasia, mucous metaplasia, and antralization of the corpus.21,22 Thought to be a response to inflammation in terms of wound healing,20 it has been found that atrophic changes of the gastric body related to SPEM can be strongly associated with the development of gastric cancer in that SPEM may be a precursor to GIM,21,22 with the biomarker HE4 appearing to be a specific marker of the process of SPEM.23 Overall, it is most likely that gastric cancer stems from a chronic inflammatory state with changes accumulating into a hyperproliferative state vulnerable to deleterious mutations in stem or progenitor cell populations, and that GIM and SPEM may be indirect markers of this malignant transformation.21
Risk Factors of Gastric Intestinal Metaplasia and Gastric Cancer
Helicobacter pylori Infection
Precancerous gastric lesions are highly associated with H pylori infection. In fact, H pylori was recognized as a class I carcinogen by the WHO in 1994,24 and a meta-analysis has shown that H pylori infection results in a 2- to 3-fold increase in the risk of gastric cancer.25 It is estimated that approximately 75% of the global gastric cancer burden is attributed to H pylori–induced inflammation.26
The virulence effects of H pylori have been shown to derive from bacterium factors causing alterations in gastric epithelial cells. Once the bacterial cell signaling protein cagA reaches the host cytosol, it is capable of altering subsequent generations of progenitor cells, leading to the development of cancer through changes in mitotic activity, apoptosis, cellular assembly, and signaling.26 Although it should be noted that while the presence of the cagA protein doubles the risk of gastric cancer, cagA-negative strands also increase the risk of distal gastric cancer.27 Additional H pylori virulence factors include babA2, which encodes bacterial adhesion with gastric epithelial cells, and vacuolating cytotoxin A, which is encoded by the gene vacA.28,29 H pylori strains carrying some combination of the babA2, cagA, and vacA genes were associated with the highest risk of developing intestinal metaplasia. The risk of developing more serious gastric lesions was directly proportional to the number of virulence factors contained in the genotype of a given H pylori strain.28
A longitudinal cohort study followed 4655 healthy, asymptomatic subjects for 7.7 years, with gastric cancer developing in 45 patients.30 No cancer developed in the H pylori–negative/cagA-negative group. The risk of gastric cancer increased progressively from H pylori–negative/cagA-negative to H pylori–positive/cagA-negative, H pylori–positive/cagA-positive, and finally H pylori–negative/cagA-positive, as loss of H pylori can be seen with extensive intestinal metaplasia.30 The implication of this study is that severe gastritis with extensive intestinal metaplasia, rather than simply H pylori infection, is the key risk factor for gastric cancer.
H pylori infection has been shown to be associated with iron deficiency anemia (IDA), and there is evidence that markers of IDA correlate with an elevated risk of gastric cancer.31,32 It is proposed that the chronic inflammatory state induced by H pylori infection results in upregulation of hepcidin and the development of IDA. The most virulent and proinflammatory strains of H pylori, cagA and vacA, are best equipped to live in an iron-deficient environment. Therefore, iron deficiency further selects for a population of predominantly cagA-positive and/or vacA-positive strains, which creates a more inflammatory and proneoplastic environment.26
Autoimmune Gastritis
Autoimmune gastritis (AIG) is caused by the destruction of parietal cells; thus, unlike alternate causes of chronic atrophic gastritis (eg, H pylori, drug-induced), it is only found in the corpus and fundus, where parietal cells are exclusively located. Loss of functional parietal cells results in decreased gastric acid and elevation of the gastric pH above that required for the absorption of inorganic ions leading to iron deficiency, and loss of intrinsic factor contributes to malabsorption of vitamin B12 and the development of pernicious anemia.33-35
AIG has been associated with 2 distinct types of gastric cancer, one of which is gastric adenocarcinoma. However, H pylori–negative AIG is not thought to result in the previously described Correa cascade from normal gastric epithelium to invasive carcinoma, raising the question of whether AIG is independently sufficient to result in gastric adenocarcinoma.33 Unlike other causes of chronic atrophic gastritis, AIG is associated with type I gastric carcinoid tumors. Gastric carcinoid tumors are the result of enterochromaffin cell hyperplasia, which is caused by overproduction of gastrin by antral G cells due to chronic achlorhydria.36 Gastric carcinoids are relatively rare (<1%) compared to adenocarcinomas; however, approximately 50% of all gastric carcinoid tumors are associated with pernicious anemia.37
Rheumatologic Disorders
Certain rheumatologic disorders have been correlated with risk for gastric cancer. Sjögren syndrome (SS) has a well-known association with mucosa-associated lymphoid tissue lymphoma, and the stomach is the most common location of extraglandular lymphoma involvement in SS. In addition to lymphomatous gastric risk, SS has been associated with solid tumor risk, specifically gastric adenocarcinoma. A recent Spanish study has found a standardized incidence ratio of 2.53 (95% CI, 1.05-6.07) in women with SS. This risk was not seen in men in this study, as fewer men were included because this disease is more commonly associated with women.38 Additionally, SS has an established association with atrophic gastritis.39 However, this association has been more commonly found with mild atrophy as opposed to more severe atrophic forms.40 Nevertheless, the chronic atrophic gastritis featured in SS has a positive severity correlation with known systemic serologic parameters with established SS disease inflammatory severity markers, such as sedimentation rate, immunoglobulin A level, and SS-B antibody.41 Furthermore, serum pepsinogen I levels, discussed later as a statistically significant marker with negative correlation with precancerous lesions and serologic biopsies, held a similarly negative correlation with the aforementioned inflammatory markers titer.41 Overall, given the association of SS with increased risk of atrophic gastritis and gastric cancer, it would be reasonable to take this disease into consideration when deciding to screen patients, particularly in patients with correlating clinical and biochemical markers portending an increased risk of precancerous lesions.
As for other rheumatologic disorders, patients with osteoarthritis and women with rheumatoid arthritis have been associated with a decreased risk of stomach cancer. This decreased risk has been attributed to nonsteroidal anti-inflammatory drugs (NSAIDs) and other medications that may bestow a common protective effect.42
Host Genetic Factors
Cancer involving the gastric cardia appears to be a distinct entity from gastric cancer limited to the distal, noncardia stomach. Shared risk factors for both types of gastric cancer include advanced age, male sex, smoking tobacco, and family history. Approximately 70% of gastric cancers are diagnosed between the ages of 55 and 84 years, and men have a 5- and 2-fold increased risk of cardia and noncardia types, respectively.18,43 The mechanism by which men are predisposed to developing gastric cancer is likely multifactorial. Men have historically been more likely to smoke tobacco, but it has also been proposed that estrogen is protective, as both delayed menopause and increased fertility lower the risk of gastric cancer.44 Family history is well known to be a key risk factor for the development of gastric cancer. Although there is variation across studies, the odds ratio of a first-degree relative developing gastric cancer ranges from 2 to 10 depending upon the demographics of the population studied.18,45
The risk factors for gastric cancer developing in the cardia closely mirror those for Barrett esophagus and esophageal adenocarcinoma. Characteristics of the at-risk population include white race, obesity, and gastroesophageal reflux disease. Whites are approximately 2 times as likely to develop gastric cancer in the cardia but half as likely to develop gastric cancer in the noncardia stomach.43
The overwhelming majority of noncardia gastric cancer develops in people of East Asian, Pacific Islander, Hispanic, and African-American descent. According to data from the SEER registries, the incidences of gastric cancer (per 100,000/year) for Asians/Pacific Islanders (men: 20.8, women: 11.7), African Americans (men: 18.4, women: 9.2), and Hispanics (men: 17.1, women: 10.0) are nearly twice as high compared to whites (men: 10.7, women: 5.0).3 Of the Asian-American subgroups, Korean and Japanese Americans have particularly high incidence rates.18,46
This predominance of nonwhite, noncardia gastric cancer was reflected in a national pathology database in the United States that reviewed 800,000 subjects with gastric biopsies. The prevalence of GIM in people of East Asian descent (Korean, Japanese, Chinese, Vietnamese) and Hispanic descent was significantly higher than that of all other ethnic backgrounds at 20% and 12% vs 8%, respectively.47
In 2010, Edgren and colleagues evaluated the association between ABO blood types and gastric cancer and peptic ulcer disease in a prospective study of Swedish and Danish blood donors.48 The study monitored 1,089,022 donors for up to 35 years and found that the blood group A conferred an incidence rate ratio of 1.20 (95% CI, 1.02-1.42). Although this study was limited by a particularly homogenous and relatively low-risk population for gastric cancer, the conclusion was consistent with the association between blood group A and gastric cancer that has been observed since the 1950s across the world.49–51
Tobacco Smoking
In 2004, after an extensive review of the available evidence, the WHO–International Agency for Research on Cancer made the determination that tobacco smoking has a causal role in the development of gastric cancer.52 In a meta-analysis of 40 studies examining the relationship between smoking tobacco and gastric cancer, the risk of stomach cancer among smokers was 1.5 to 1.6 times higher than in nonsmokers. It was estimated that over 80,000 cases of gastric cancer, or 11% of all estimated cases, are attributable to tobacco smoking annually.53 Analyses have shown variation in the relationship between tobacco and sex, with 13% to 16% and 4% to 7% of gastric cancer being related to tobacco use in men and women, respectively.54 The proposed mechanism of tobacco use contributing to gastric cancer is an increased risk of transition to dysplasia. A population-based study in China involving 3000 residents showed that cigarette smoking nearly doubled the risk of transition to dysplasia, with a mild association of transition to intestinal metaplasia. The risk of transition to dysplasia was highly correlated to a family history of stomach cancer and blood type A.55
Bile Acid Reflux
A high concentration of bile acids, as seen with a diet high in fat or with excess bile acid reflux into the gastric lumen, is thought to predispose patients to gastric cancer via promotion of gastric mucosal injury. In a prospective study of 767 patients, bile acid was shown to promote intestinal metaplasia and gastric carcinogenesis in H pylori–positive patients.56 Bile acids indirectly damage DNA by induction of oxidative stress and also induce frequent apoptosis. Both mechanisms promote constitutive mutations, which over time lead to selective mutations and increase the risk of gastric cancer.57
Diet
In an effort to better understand preventive strategies for gastric cancer, the World Cancer Research Fund/American Institute of Cancer Research (WCRF/AICR) performed an extensive review of the impact of diet on gastric cancer. The WCRF/AICR concluded that vegetables and fruits were likely protective, and high-salt/salt-preserved diets and animal meats smoked to a high degree were likely associated with gastric cancer.58
High-salt consumption has long been associated with an elevated risk of gastric cancer due to an increased risk of H pylori infection, as well as promotion of H pylori virulence via cagA.59,60 A study by Loh and colleagues demonstrated that a high-salt concentration upregulates expression of cagA for select H pylori strains with a unique DNA motif.61 A follow-up animal study demonstrated that cagA-positive H pylori strains were needed to cause an inflammatory reaction in response to high-salt concentrations.62
Dietary exposure to N-nitroso–containing compounds has been shown to increase the risk of noncardia gastric cancer via promotion of gastric carcinogenesis.58 Processed meats, or those that have undergone salt preservation, smoking, or fermentation, are positively associated with noncardia gastric cancer in a dose-dependent manner.63 The mechanism by which processed meats are carcinogenic is multifactorial and related to the high-salt burden and nitrite and nitrate additives.64 N-nitroso is the exogenous carcinogenic byproduct of nitrites and nitrates when combined with amino acids.64 N-nitroso is also formed endogenously from the haem iron found in red meat, which promotes H pylori growth via oxidative stress and DNA damage.65
An additional dietary factor that may alter the virulence of H pylori infection is folic acid, as folic acid supplementation has been shown to reduce gastric inflammation and dysplasia in murine models.26,66
Microbiota
The gastrointestinal microbiota, specifically the relationship between H pylori and a microbiota predominated by Firmicutes or alternate enterohepatic Helicobacter species, can potentiate the carcinogenic effect of H pylori infection, and Bacteroidetes can mitigate this effect.26,67
The risk factors are summarized and categorized in the Table.
Table.
Risk Factors Associated With Gastric Cancer
| Environmental Factors |
|---|
| Helicobacter pylori Infection |
| BabA2 oncogene |
| CagA oncogene |
| VacA oncogene |
| Tobacco Smoking |
| Bile Acid Reflux |
| Diet |
| Folic acid (decreased risk) |
| Vegetables (decreased risk) |
| Fruits (decreased risk) |
| Salt |
| Smoked foods |
| High fat |
| N-nitroso–containing compounds |
| Microbiota |
| Firmicutes |
| Enterohepatic Helicobacter species |
| Bacteroidetes (decreased risk) |
| Host-Related Factors |
| Host Genetic Factors |
| Advanced age |
| Male sex |
| Family history |
| Blood type A |
| Ethnicity |
| East Asian |
| Pacific Islander |
| Hispanic |
| African American |
| Rheumatologic Disorders |
| Sjögren syndrome |
| Rheumatoid arthritis (decreased risk) |
| Osteoarthritis (decreased risk) |
| Environmental Factor and Host-Related Factor |
| Autoimmune Gastritis (with coexisting H pylori infection) |
Diagnosis, Screening, and Surveillance of Gastric Intestinal Metaplasia
Population-Based Screening
Gastric cancer screening en masse has been shown to be effective in countries with a high incidence of gastric cancer. The characteristics of gastric cancer that lend to screening include an adequate lag time of 44 months to progress from an early to an advanced stage, as well as a significant improvement of mortality with early intervention.68 National screening programs in both Japan and Korea recommend endoscopy for all men and women over 40 years of age. Several uncontrolled trials have suggested that these screening programs have resulted in a reduction of mortality due to gastric cancer.68,69 However, a single global screening strategy would not realistically be cost-effective due to the high variability of gastric cancer burden worldwide. In countries with a low incidence of gastric cancer, such as the United States, a stepwise screening approach may be more reasonable.
Serologic Biopsy
In countries with a low incidence of gastric cancer, it has been proposed to first triage a subject based upon epidemiology, genetics, and environmental risk factors. Those individuals at increased risk can then be further stratified based upon H pylori status and virulence factors, along with serologic markers of chronic inflammation such as pepsinogen I, pepsinogen II, and gastrin.70
Pepsinogen is a proenzyme of pepsin, an endoproteinase of gastric juice.71 Serum pepsinogen levels reflect the morphologic and functional status of the stomach mucosa, and serve as a marker of chronic atrophic gastritis.8 Pepsinogen is characterized into pepsinogen I and II. Pepsinogen I is secreted exclusively by chief and mucous neck cells in the fundic glands of the fundus and body. Pepsinogen II is secreted by the entire stomach and duodenum.9 Patterns have been established of pepsinogen levels reflecting mucosal change, as gastric inflammation leads to a decrease in pepsinogen I and an increase in pepsinogen II, thus coined a serologic biopsy.72
An additional marker of interest is gastrin, an enzyme synthesized and secreted almost exclusively by G cells located in the antrum. Gastrin is released by the G cells in the antrum of the stomach in response to low acidity. Chronic H pylori infection results in increased gastrin levels due to hyperplasia of the G cells. The effect on gastrin levels from modulation of acid secretion is dependent upon the region of the stomach that is affected. Gastrin levels are increased when the corpus mucosa is predominantly involved, and are decreased with antral-predominant atrophic gastritis.9
Tu and colleagues compared these serologic biomarkers to traditional risk factors (age, sex, smoking, family history, symptoms) in a large Chinese population-based screening analysis.70 Pepsinogen I and II, as well as the pepsinogen I/II ratio, were assessed in combination with a gastrin subset gastrin-17 and H pylori antibody (HP IgG). A serologic biomarker score was established using multivariate odds ratio risk points. Pepsinogen II, pepsinogen I/II ratio, and HP IgG were associated with predicting precancerous or cancerous lesions at baseline, and pepsinogen I, pepsinogen I/II ratio, and gastrin-17 were associated with follow-up surveillance benefit. Thus, as the inflammatory cascade progresses from nonatrophic gastritis through the atrophic gastritis–intestinal metaplasia–neoplastic spectrum, it was found that these 5 specific markers were statistically significant in predicting precancerous lesions in comparison to traditional risk factors (receiver operating characteristic, 0.803 vs 0.580).70 As discussed previously, intestinal-type gastric cancer is more strongly associated with the atrophic gastritis and intestinal metaplasia cascade, thus explaining the closer correlation with intestinal-type gastric cancer and the serologic biopsy than diffuse-type gastric cancer.
Additionally, antiparietal cell antibodies (APCAs), which target the α- and β-subunits of proton pumps, are markers of autoimmune corpus atrophic gastritis.73 It is postulated that APCAs are induced via molecular mimicry with oxyntic glands after H pylori infection has caused sufficient corpus atrophy.74 A Japanese study in H pylori–positive patients demonstrated good concordance between pepsinogen I/II ratio and APCA levels in the prediction of corpus atrophy.75
Endoscopic Screening
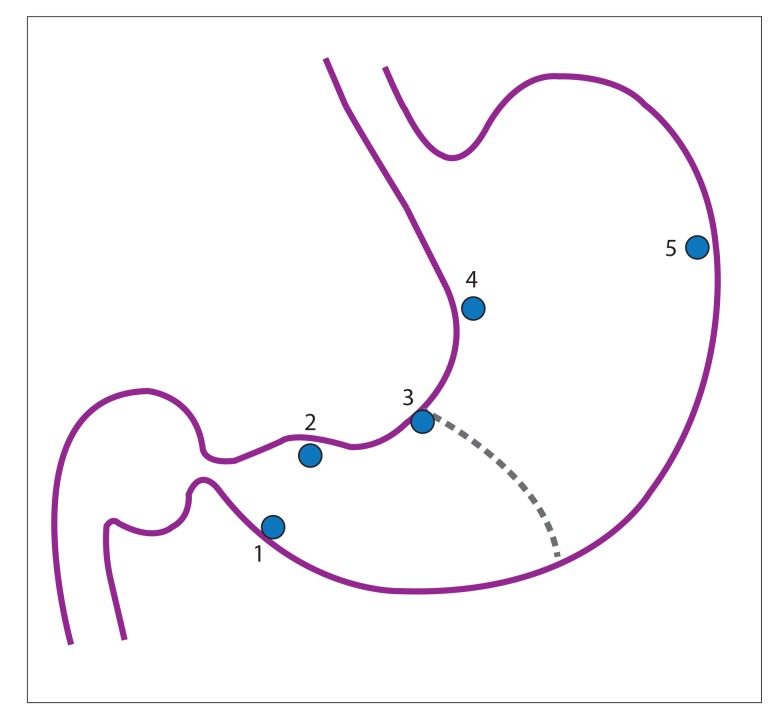
When a patient from a low-incidence pool is determined to be at high risk for a premalignant gastric lesion, it is reasonable to proceed to endoscopy with histologic evaluation. The Modified Sydney System (MSS) has been accepted as the standardized classification of gastritis since it was established in 1994.10 The MSS has both endoscopic and histologic arms, with the histologic arm focusing on combining topographic, morphologic, and etiologic information, which is both reproducible and clinically useful. Mapping studies were evaluated, and it was determined that along with focused biopsies of mucosal abnormalities, nonspecific biopsies from the antrum (greater curvature, lesser curvature), incisura angularis, and corpus (greater curvature, lesser curvature) for a total of 5 biopsies had a high probability of establishing an accurate H pylori status. Of note, corpus biopsies were especially valuable after prolonged treatment with proton pump inhibitors, as the oxyntic mucosa may be exclusively infected. Biopsies from the incisura angularis are also particularly important, as these are the most likely to reveal maximal atrophy, intestinal metaplasia, and premalignant dysplasia (Figure 2).10,76,77
Figure 2.
Anatomic locations recommended for gastric biopsy mapping protocol: (1) antrum, greater curvature within 3 to 5 cm of the pylorus; (2) antrum, lesser curvature within 3 to 5 cm of the pylorus; (3) incisura angularis; (4) corpus, lesser curvature; and (5) corpus, greater curvature.
Reproduced from Dixon et al.10
Although white-light endoscopy is the most readily available method of upper endoscopy, other modalities exist that may allow for a more detailed gastric endoscopic evaluation. Narrow-band imaging (NBI) is a high- resolution endoscopic technique that enhances the mucosal surface, and is currently being used most frequently in the evaluation of Barrett esophagus and inflammatory bowel disease. Using specific filtered blue and green wavelengths, fine detailed mucosal changes are made more prominent, with a specific light blue crest pattern suggestive of intestinal metaplasia.78 Various studies have examined NBI vs white-light endoscopy in the surveillance of GIM, noting that NBI has sensitivities and specificities ranging from 70% to 90% compared to approximately 50% with white-light endoscopy.77–79 Other methods that allow a more detailed examination compared to white-light endoscopy are chromoendoscopy, which entails topical application of stains or pigments to improve tissue characterization with magnification endoscopy, and confocal endomicroscopy, which involves scattered light image reconstruction to create increased detail and resolution. Overall, further studies are needed to validate NBI, chromoendoscopy, and confocal endomicroscopy patterns in GIM screening and surveillance.
Endoscopic Surveillance
De Vries and colleagues performed a prospective, multicenter study in 2010 in an effort to determine the appropriate biopsy regimen for surveillance of premalignant gastric lesions in patients previously diagnosed with intestinal metaplasia or dysplasia.80 This study included 112 patients with intestinal metaplasia or either low- or high-grade dysplasia. Seven or 9 nontargeted biopsies were taken from the antrum, incisura angularis, corpus, and cardia, and were compared to a control arm with 5 biopsy sites based upon the MSS. Obtaining 12 biopsies was considered to be the gold standard.9 Biopsies based upon the MSS detected 90% of the patients with GIM and 50% of those with dysplasia, as opposed to 97% with GIM and 100% with dysplasia when at least 7 biopsies were obtained. De Vries and colleagues concluded that at least 9 biopsies, including from the cardia, were required for sufficient surveillance of premalignant lesions in a population with a low incidence of gastric cancer.80
Current American Society for Gastrointestinal Endoscopy guidelines do not recommend blanket surveillance for individuals with GIM “unless other risk factors for gastric cancer are present, such as a family history of gastric cancer and Asian heritage.”81 However, the European counterpart, the European Society of Gastrointestinal Endoscopy, recommends H pylori treatment if a patient is found to be infected with the bacteria, followed by surveillance with mapping biopsies for dysplasia every 3 years.82 If low-grade dysplasia is detected in a patient with GIM, surveillance esophagogastroduodenoscopy (EGD) with mapping should be performed within 1 year to assess for endoscopically visible lesions. Patients with confirmed high-grade dysplasia in the absence of an endoscopically visible lesion are recommended to undergo repeat EGD within 6 to 12 months for surveillance of visible lesions. Those patients with lesions should undergo proper staging along with surgical or endoscopic resection due to the high probability of coexisting invasive adenocarcinoma, as 25% of patients with high-grade dysplasia may progress to adenocarcinoma within 1 year.13
Chemoprevention and Treatment
There are currently no recommended medical therapies for treatment or prevention of GIM. NSAIDs have been examined for chemopreventive effects in different types of malignancies, and although further studies are needed, there has been some evidence suggesting that NSAIDs may have a preventive nature in the course of gastric cancer. In 2010, a meta-analysis of 21 studies demonstrated a potential protective effect of NSAIDs when adjusted for known risk factors.83 Furthermore, small studies have examined the role of the NSAID celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor. Patients with H pylori eradication therapy followed by celecoxib treatment for 8 to 12 weeks demonstrated regression in gastric precancerous lesions, including atrophic gastritis, intestinal metaplasia, and low-grade dysplasia.84,85 The proposed mechanism for the protective effect of selective COX-2 inhibitors is related to induction of apoptosis and suppression of cell proliferation and angiogenesis. Further randomized, controlled trials are needed to elaborate on this potential effect.85
Conclusion
Overall, GIM carries a significant potential risk of malignancy and is becoming increasingly recognized, as well as the epidemiologic, genetic, and environmental risk factors associated with its development. Currently, there are no blanket screening guidelines, which leaves the assessment of patients to a case-by-case basis. This lack of consensus places the decision-making burden onto individual physicians. As the health care needs of a diverse population continue to emerge in the United States, cost-effective screening and risk assessment methods such as serologic biopsy and H pylori evaluation will become ever more important. The availability and advancement of means of screening and detection for gastric adenocarcinoma need to be broadened to allow more gastroenterologists to develop both the ability and knowledge of how to endoscopically locate and manage precursor lesions. Further investigation is needed on methods for prevention and treatment of a disease that continues to carry a high burden of morbidity and mortality.
References
- 1.Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut. 1989;30(1):14–18. doi: 10.1136/gut.30.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014 National Cancer Institute. [Accessed January 23, 2018]. https://seer.cancer.gov/csr/1975_2014/ eds. Published April 2017. Updated June 28, 2017.
- 4.La Vecchia C, Negri E, D’Avanzo B, Franceschi S. Electric refrigerator use and gastric cancer risk. Br J Cancer. 1990;62(1):136–137. doi: 10.1038/bjc.1990.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer, World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [Accessed January 23, 2018]. http://globocan.iarc.fr/Default.aspx
- 6.Sipponen P, Kimura K. Intestinal metaplasia, atrophic gastritis and stomach cancer: trends over time. Eur J Gastroenterol Hepatol. 1994;6(suppl 1):S79–S83. [PubMed] [Google Scholar]
- 7.Sonnenberg A, Lash RH, Genta RM. A national study of Helicobactor pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139(6):1894–1901.e2. doi: 10.1053/j.gastro.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Oishi Y, Kiyohara Y, Kubo M, et al. The serum pepsinogen test as a predictor of gastric cancer: the Hisayama study. Am J Epidemiol. 2006;163(7):629–637. doi: 10.1093/aje/kwj088. [DOI] [PubMed] [Google Scholar]
- 9.de Vries AC, Kuipers EJ. Epidemiology of premalignant gastric lesions: implications for the development of screening and surveillance strategies. Helicobacter. 2007;12(suppl 2):22–31. doi: 10.1111/j.1523-5378.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 10.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71(7):1150–1158. doi: 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Olmez S, Aslan M, Erten R, Sayar S, Bayram I. The prevalence of gastric intestinal metaplasia and distribution of Helicobacter pylori infection, atrophy, dysplasia, and cancer in its subtypes. Gastroenterol Res Pract. 2015;2015:434039. doi: 10.1155/2015/434039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassaro M, Rugge M, Gutierrez O, Leandro G, Graham DY, Genta RM. Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol. 2000;95(6):1431–1438. doi: 10.1111/j.1572-0241.2000.02074.x. [DOI] [PubMed] [Google Scholar]
- 14.González CA, Sanz-Anquela JM, Gisbert JP, Correa P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int J Cancer. 2013;133(5):1023–1032. doi: 10.1002/ijc.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13(1):2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10(6):403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vries AC, Van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134(4):945–952. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 18.Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc. 2016;84(1):18–28. doi: 10.1016/j.gie.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Rugge M, Correa P, Dixon MF, et al. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24(2):167–176. doi: 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Spechler SJ, Merchant JL, Wang TC, et al. A summary of the 2016 James W. Freston Conference of the American Gastroenterological Association: intestinal metaplasia in the esophagus and stomach: origins, differences, similarities and significance. Gastroenterology. 2017;153(1):e6–e13. doi: 10.1053/j.gastro.2017.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138(7):2207–2210.e1. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer. 2009;12(4):189–197. doi: 10.1007/s10120-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134(2):511–522. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 25.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114(6):1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 26.Amieva M, Peek RM., Jr Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150(1):64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kikuchi S, Crabtree JE, Forman D, Kurosawa M Research Group on Prevention of Gastric Carcinoma Among Young Adults. Association between infections with CagA-positive or -negative strains of Helicobacter pylori and risk for gastric cancer in young adults. Am J Gastroenterol. 1999;94(12):3455–3459. doi: 10.1111/j.1572-0241.1999.01607.x. [DOI] [PubMed] [Google Scholar]
- 28.Höcker M, Hohenberger P. Helicobacter pylori virulence factors—one part of a big picture. Lancet. 2003;362(9391):1231–1233. doi: 10.1016/S0140-6736(03)14547-3. [DOI] [PubMed] [Google Scholar]
- 29.Palframan SL, Kwok T, Gabriel K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front Cell Infect Microbiol. 2012;2:92. doi: 10.3389/fcimb.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109(1):138–143. doi: 10.1002/ijc.11680. [DOI] [PubMed] [Google Scholar]
- 31.Yamanouchi J, Azuma T, Yakushijin Y, Hato T, Yasukawa M. Dramatic and prompt efficacy of Helicobacter pylori eradication in the treatment of severe refractory iron deficiency anemia in adults. Ann Hematol. 2014;93(10):1779–1780. doi: 10.1007/s00277-014-2052-x. [DOI] [PubMed] [Google Scholar]
- 32.Noto JM, Gaddy JA, Lee JY, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123(1):479–492. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minalyan A, Benhammou JN, Artashesyan A, Lewis MS, Pisegna JR. Autoimmune atrophic gastritis: current perspectives. Clin Exp Gastroenterol. 2017;10:19–27. doi: 10.2147/CEG.S109123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toh BH, Sentry JW, Alderuccio F. The causative H+/K+ ATPase antigen in the pathogenesis of autoimmune gastritis. Immunol Today. 2000;21(7):348–354. doi: 10.1016/s0167-5699(00)01653-4. [DOI] [PubMed] [Google Scholar]
- 35.Faber K. Achylia gastrica mit anamie. Med Klin. 1909;5:1310. [Google Scholar]
- 36.Creutzfeldt W. The achlorhydria-carcinoid sequence: role of gastrin. Digestion. 1988;39(2):61–79. doi: 10.1159/000199609. [DOI] [PubMed] [Google Scholar]
- 37.Nikou GC, Angelopoulos TP. Current concepts on gastric carcinoid tumors. Gastroenterol Res Pract. 2012;2012:287825. doi: 10.1155/2012/287825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brito-Zerón P, Kostov B, Fraile G, et al. SS Study Group GEAS-SEMI. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J Hematol Oncol. 2017;10(1):90. doi: 10.1186/s13045-017-0464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pokorny G, Karácsony G, Lonovics J, Hudák J, Németh J, Varró V. Types of atrophic gastritis in patients with primary Sjögren’s syndrome. Ann Rheum Dis. 1991;50(2):97–100. doi: 10.1136/ard.50.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collin P, Karvonen AL, Korpela M, Laippala P, Helin H. Gastritis classified in accordance with the Sydney system in patients with primary Sjögren’s syndrome. Scand J Gastroenterol. 1997;32(2):108–111. doi: 10.3109/00365529709000179. [DOI] [PubMed] [Google Scholar]
- 41.Maury CP, Törnroth T, Teppo AM. Atrophic gastritis in Sjögren’s syndrome. Morphologic, biochemical, and immunologic findings. Arthritis Rheum. 1985;28(4):388–394. doi: 10.1002/art.1780280406. [DOI] [PubMed] [Google Scholar]
- 42.Thomas E, Brewster DH, Black RJ, Macfarlane GJ. Risk of malignancy among patients with rheumatic conditions. Int J Cancer. 2000;88(3):497–502. [PubMed] [Google Scholar]
- 43.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11(2):235–256. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 44.Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58(1):16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- 45.Shin CM, Kim N, Yang HJ, et al. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol. 2010;44(2):e34–e39. doi: 10.1097/MCG.0b013e3181a159c4. [DOI] [PubMed] [Google Scholar]
- 46.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19(3):227–256. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song H, Ekheden IG, Zheng Z, Ericsson J, Nyrén O, Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867. doi: 10.1136/bmj.h3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edgren G, Hjalgrim H, Rostgaard K, et al. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010;172(11):1280–1285. doi: 10.1093/aje/kwq299. [DOI] [PubMed] [Google Scholar]
- 49.Aird I, Bentall HH, Roberts JA. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1(4814):799–801. doi: 10.1136/bmj.1.4814.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Liu L, Ji J, et al. ABO blood group system and gastric cancer: a case-control study and meta-analysis. Int J Mol Sci. 2012;13(10):13308–13321. doi: 10.3390/ijms131013308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rizzato C, Kato I, Plummer M, et al. Risk of advanced gastric precancerous lesions in Helicobacter pylori infected subjects is influenced by ABO blood group and cagA status. Int J Cancer. 2013;133(2):315–322. doi: 10.1002/ijc.28019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 53.Trédaniel J, Boffetta P, Buiatti E, Saracci R, Hirsch A. Tobacco smoking and gastric cancer: review and meta-analysis. Int J Cancer. 1997;72(4):565–573. doi: 10.1002/(sici)1097-0215(19970807)72:4<565::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 54.Peleteiro B, Castro C, Morais S, Ferro A, Lunet N. Worldwide burden of gastric cancer attributable to tobacco smoking in 2012 and predictions for 2020. Dig Dis Sci. 2015;60(8):2470–2476. doi: 10.1007/s10620-015-3624-x. [DOI] [PubMed] [Google Scholar]
- 55.Kneller RW, You WC, Chang YS, et al. Cigarette smoking and other risk factors for progression of precancerous stomach lesions. J Natl Cancer Inst. 1992;84(16):1261–1266. doi: 10.1093/jnci/84.16.1261. [DOI] [PubMed] [Google Scholar]
- 56.Tatsugami M, Ito M, Tanaka S, et al. Bile acid promotes intestinal metaplasia and gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2101–2107. doi: 10.1158/1055-9965.EPI-12-0730. [DOI] [PubMed] [Google Scholar]
- 57.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589(1):47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Wiseman M The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 59.Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. 2003;13(3):162–168. doi: 10.2188/jea.13.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59(19):4823–4828. [PubMed] [Google Scholar]
- 61.Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67(10):4709–4715. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- 62.Gaddy JA, Radin JN, Loh JT, et al. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81(6):2258–2267. doi: 10.1128/IAI.01271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Cancer Research Fund International. Diet, nutrition, physical activity and stomach cancer. CUP: Continuous Update Project. [Accessed January 23, 2018]. http://www.wcrf.org/sites/default/files/Stomach-Cancer-2016-Report.pdf
- 64.Takahashi M, Nishikawa A, Furukawa F, Enami T, Hasegawa T, Hayashi Y. Dose-dependent promoting effects of sodium chloride (NaCl) on rat glandular stomach carcinogenesis initiated with N-methyl-N’-nitro-N-nitrosoguanidine. Carcinogenesis. 1994;15(7):1429–1432. doi: 10.1093/carcin/15.7.1429. [DOI] [PubMed] [Google Scholar]
- 65.Cross AJ, Pollock JRA, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63(10):2358–2360. [PubMed] [Google Scholar]
- 66.Gonda TA, Kim YI, Salas MC, et al. Folic acid increases global DNA methylation and reduces inflammation to prevent Helicobacter-associated gastric cancer in mice. Gastroenterology. 2012;142(4):824–833.e7. doi: 10.1053/j.gastro.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 67.Ge Z, Feng Y, Muthupalani S, et al. Coinfection with enterohepatic Helicobacter species can ameliorate or promote Helicobacter pylori-induced gastric pathology in C57BL/6 mice. Infect Immun. 2011;79(10):3861–3871. doi: 10.1128/IAI.05357-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki S, Tsugane S JPHC Study Group. Gastric cancer screening and subsequent risk of gastric cancer: a large-scale population-based cohort study, with a 13-year follow-up in Japan. Int J Cancer. 2006;118(9):2315–2321. doi: 10.1002/ijc.21664. [DOI] [PubMed] [Google Scholar]
- 69.Lin JT. Screening of gastric cancer: who, when, and how. Clin Gastroenterol Hepatol. 2014;12(1):135–138. doi: 10.1016/j.cgh.2013.09.064. [DOI] [PubMed] [Google Scholar]
- 70.Tu H, Sun L, Dong X, et al. A serological biopsy using five stomach-specific circulating biomarkers for gastric cancer risk assessment: a multi-phase study. Am J Gastroenterol. 2017;112(5):704–715. doi: 10.1038/ajg.2017.55. [DOI] [PubMed] [Google Scholar]
- 71.Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, et al. Validity of serum pepsinogen I/II ratio for the diagnosis of gastric epithelial dysplasia and intestinal metaplasia during the follow-up of patients at risk for intestinal-type gastric adenocarcinoma. Neoplasia. 2004;6(5):449–456. doi: 10.1593/neo.03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982;83(1 pt 2):204–209. [PubMed] [Google Scholar]
- 73.Karlsson FA, Burman P, Lööf L, Mårdh S. Major parietal cell antigen in autoimmune gastritis with pernicious anemia is the acid-producing H+,K+-adenosine triphosphatase of the stomach. J Clin Invest. 1988;81(2):475–479. doi: 10.1172/JCI113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Negrini R, Savio A, Poiesi C, et al. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996;111(3):655–665. doi: 10.1053/gast.1996.v111.pm8780570. [DOI] [PubMed] [Google Scholar]
- 75.Ito M, Haruma K, Kaya S, et al. Implication of anti-parietal cell antibody levels in gastrointestinal diseases, including gastric carcinogenesis. Dig Dis Sci. 2002;47(5):1080–1085. doi: 10.1023/a:1015042208224. [DOI] [PubMed] [Google Scholar]
- 76.Fennerty MB, Emerson JC, Sampliner RE, McGee DL, Hixson LJ, Garewal HS. Gastric intestinal metaplasia in ethnic groups in the southwestern United States. Cancer Epidemiol Biomarkers Prev. 1992;1(4):293–296. [PubMed] [Google Scholar]
- 77.Capelle LG, Haringsma J, de Vries AC, et al. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig Dis Sci. 2010;55(12):3442–3448. doi: 10.1007/s10620-010-1189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38(8):819–824. doi: 10.1055/s-2006-944632. [DOI] [PubMed] [Google Scholar]
- 79.Gonen C, Simsek I, Sarioglu S, Akpinar H. Comparison of high resolution magnifying endoscopy and standard videoendoscopy for the diagnosis of Helicobacter pylori gastritis in routine clinical practice: a prospective study. Helicobacter. 2009;14(1):12–21. doi: 10.1111/j.1523-5378.2009.00650.x. [DOI] [PubMed] [Google Scholar]
- 80.de Vries AC, Haringsma J, de Vries RA, et al. Biopsy strategies for endoscopic surveillance of pre-malignant gastric lesions. Helicobacter. 2010;15(4):259–264. doi: 10.1111/j.1523-5378.2010.00760.x. [DOI] [PubMed] [Google Scholar]
- 81.Evans JA, Chandrasekhara V, Chathadi KV, et al. ASGE Standards of Practice Committee. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc. 2015;82(1):1–8. doi: 10.1016/j.gie.2015.03.1967. [DOI] [PubMed] [Google Scholar]
- 82.Dinis-Ribeiro M, Areia M, de Vries AC, et al. European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Endoscopy. 2012;44(1):74–94. doi: 10.1055/s-0031-1291491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tian W, Zhao Y, Liu S, Li X. Meta-analysis on the relationship between nonsteroidal anti-inflammatory drug use and gastric cancer. Eur J Cancer Prev. 2010;19(4):288–298. doi: 10.1097/CEJ.0b013e328339648c. [DOI] [PubMed] [Google Scholar]
- 84.Hung KH, Yang HB, Cheng HC, Wu JJ, Sheu BS. Short-term celecoxib to regress long-term persistent gastric intestinal metaplasia after Helicobacter pylori eradication. J Gastroenterol Hepatol. 2010;25(1):48–53. doi: 10.1111/j.1440-1746.2009.05974.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang LJ, Wang SY, Huo XH, et al. Anti-Helicobacter pylori therapy followed by celecoxib on progression of gastric precancerous lesions. World J Gastroenterol. 2009;15(22):2731–2738. doi: 10.3748/wjg.15.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]